Abstract
Helicobacter pylori infection is the major cause of gastroduodenal pathologies, but only a minority of infected patients develop gastric B-cell lymphoma, gastric autoimmunity, or other life threatening diseases, as gastric cancer or peptic ulcer. The type of host immune response against H. pylori, particularly the cytolytic effector functions of T cells, is crucial for the outcome of the infection. T cells are potentially able to kill a target via different mechanisms, such as perforins or Fas-Fas ligand interaction. In H. pylori-infected patients with gastric autoimmunity cytolytic T cells, that cross-recognize different epitopes of H. pylori proteins and H+K+-ATPase autoantigen, infiltrate the gastric mucosa and lead to gastric atrophy via long-lasting activation of Fas ligand-mediated appotosis and perforin-induced cytotoxicity. On the other hand, gastric T cells from MALT lymphoma exhibit defective perforin- and Fas-Fas ligand-mediated killing of B cells, with consequent abnormal help for B-cell proliferation, suggesting that deregulated and exhaustive H. pylori-induced T cell-dependent B-cell activation can support both the onset and the promotion of low-grade B-cell lymphoma.
1. Introduction
Helicobacter pylori is a Gram-negative gastrointestinal bacterium that has coevolved with its human host for at least 58.000 years, and worldwide still half of the human population is infected [1]. H. pylori infection leads to chronic inflammation of the gastric mucosa, which remains often without clinical symptoms. However, serious diseases as peptic ulcer disease, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric adenocarcinoma develop in 15 percent of the infected population [2]. Whereas H. pylori infection triggers a vigorous immune response, resulting in high titres of H. pylori-specific antibodies, this response is not sufficient for eradication of the pathogen and when remaining untreated with antibiotics, infection will remain for life. Therefore, H. pylori is often regarded a model organism for persistent bacterial infection in man [3].
H. pylori is heterogeneous and outcome of infection depends on both bacterial virulence factors and host genetics, as reviewed in [4]. Whereas appropriate host immune responses lead to asymptomatic persistent colonization, H. pylori-associated pathology is the result of aberrant inflammation.
1.1. The Immune Response to H. pylori Infection
H. pylori induces a strong innate immune response that involves various components of the innate immune system, including nucleotide-binding oligomerization domain protein I (Nod1) [5] and secretion of antimicrobial peptides [6]. In addition, studies report that H. pylori induces pro-inflammatory gene expression in host cells via Toll-like receptor (TLR)2, TLR4, TLR5, and TLR9 [7–10]. However, several of these reports present conflicting data and this area requires further research, which should take into account TLR expression dynamics [11] and the actual repertoire of expressed TLR in the infected gastric mucosa in vivo. The innate immune response to H. pylori results in inflammation of the gastric epithelium (gastritis) and induces an influx of neutrophils and other immune cell though the release of chemokines and cytokines, as reviewed in [12], thereby being essential for the initiation of an acquired immune response to H. pylori.
Immunity to Helicobacter is dependent on T cells. The murine H. felis infection model provides a useful model resembling gastric pathological changes in human H. pylori infection. H. felis infection of B and T cell-deficient (RAG-1−/−) mice and T cell-deficient (TCRβδ−/−) mice does not result in gastric pathology despite high levels of colonization, whereas infection of B cell-deficient mice results in severe gastric alteration, identical to those seen in immunocompetent mice [13]. These results indicate that T cells are required for protection against Helicobacter, but also that gastric immune mediated damage is dependent on T cells, and not by B cells or antibody secretion.
CD4+ T cells can be categorized according to their cytokine-secretion profile and cytotoxic potential. There are two main subsets of T helper (Th) cells. Th1 cells secrete tumour necrosis factor (TNF) and interferon-γ (IFN-γ), lyse antigen-loaded target cells through mechanisms that are mediated by perforin or FAS (also known as CD95), and elicit macrophage activation. Th2 cells secrete interleukin-4 (IL-4), IL-5, and IL-10, are involved in down regulation of Th1cell-mediated inflammatory events, and facilitate production of antibodies by B cells.
The host cytokine response is regarded an important determinant in onset of gastritis and the development of disease. Vaccination studies in different mouse strains have shown that, dependent on mouse strain or Helicobacter species, both Th2 [14, 15] and Th1 [16, 17] cytokine responses are involved in the adaptive immune response against H. pylori and that protection from disease may require the ability of the host to mount a balanced Th1/Th2 response upon infection.
Whereas the severity of gastritis due to innate immunity influences the risk of disease, it seems that the pattern of inflammation in the stomach determines which disease will develop [2]: chronic antral-predominant inflammation is associated with increased acid production and predisposes tot duodenal ulceration, whereas corpus-predominant or pan-gastritis is associated with reduced acid production and predisposes to gastric ulceration and gastric adenocarcinoma. Whether H. pylori-associated gastroduodenal disease develops, or asymptomatic chronic gastritis, is related to the predominant T-helper phenotype of the acquired immune response that eventually arises upon infection. Human gastric T cells in H. pylori infection have a predominant Th1 phenotype [18, 19], which is associated with pathology [20].
Dendritic cells (DC) in the gastric mucosa take up antigens and migrate to nearby lymph nodes where they activate naïve T cells and orchestrate the subsequent immune response [21]. In vitro, DC cultured in the presence of H. pylori are activated and secrete cytokines, including interleukin (IL)-6, IL-8, IL-10, IL-12, IL-1β, and TNFα [22, 23]. H. pylori-pulsed DC induce increased IL-2, IFN-γ, and TNFα in CD4+CD45RA+ naïve T cells, which is in agreement with the Th1 cytokine profile produced by T cells from gastric mucosal biopsies [18, 19, 24]. Virulence factors that contribute to the predominance of Th1 responses in H. pylori infection include the H. pylori neutrophil activating protein (HP-NAP), which induces IL-12 secretion by neutrophils and monocytes and strongly promotes Th1-skewing of antigen-specific CD4+ T cells [25], and a plasticity region locus jhp0947-jhp0949 that is associated with duodenal ulcer disease [26]. In addition, the Th1/Th2 balance is influenced by H. pylori genomic DNA recombination [27] and phase-variable expression of LPS Lewis blood-group antigens which bind to the DC receptor DC-SIGN [28], further enhancing H. pylori capacity to adapt to the host immune system in order to achieve persistent infection.
1.2. Polarization and Properties of CD4+ T Cells in H. pylori-Associated Peptic Ulcer Disease
In H. pylori- infected individuals, peptic ulcer disease (PUD) is associated with Th1 polarization of gastric CD4+ T cell responses, whereas uncomplicated chronic gastritis (UCG) is associated with a mixed Th1/Th2 response [20]. T cell clones from the gastric antral mucosa of patients with or without peptic ulcer display a different preferential antigen-specificity. Approximately half of the H. pylori-reactive Th cell clones derived from peptic ulcer patients were specific for the Cytotoxin-associated protein (CagA), whereas approximately one forth of H. pylori-reactive Th clones from nonulcer gastritis patients were specific for H. pylori Urease [18, 20]. Upon antigen-specific stimulation, over eighty percent of the H. pylori-reactive Th cells from peptic ulcer disease patients showed a polarized Th1 profile, with high production of IFN-γ and no IL-4. In contrast, in non-ulcer gastritis patients about two-third of the H. pylori-specific antral clones displayed a mixed Th1/Th2 cytokine profile, whereas only one-third was polarized to Th1 [20]. These results are in agreement with several studies that indicate that Th1 polarization of the H. pylori-specific T cell response is associated with more severe disease [18, 19, 24, 26, 29, 30]. In the H. pylori-infected gastric mucosa, IL-12 drives the Th1 signalling pathway [31].
The pathogenic capacity of polarized Th1 responses in H. pylori infection is indirectly supported by the phenomenon often referred to as “the African enigma” [32], that is, despite a high prevalence of H. pylori infection in Africa, peptic ulcer disease and gastric cancer are uncommon. The notion that concurrent Th2 responses could reduce Th1-mediated gastro-duodenal pathology and development of gastric cancer is supported by the observation that coinfection of mice with H. felis and a natural murine helminth (Heligmosomoides polygyrus) reduced Th1-mediated gastric atrophy, which is regarded as a premalignant lesion [33]. Therefore, one possible explanation for the low prevalence of H. pylori-associated diseases in Africa is that the endemic high parasitic burden causes predominant Th2 responses that protect H. pylori-individuals from developing peptic ulcers and gastric cancer.
1.3. CD4+CD25+ Regulatory T Cells in H. pylori-Induced Gastric Inflammation
An accumulating body of evidence indicates that CD4+CD25+ regulatory T cells (Treg) do not only prevent development of autoimmune diseases, but also regulate pathogen- induced inflammation and disease, including in H. pylori infection [34].
In mice, CD4+CD25+ Treg reduce gastric immunopathology, despite increased H. pylori colonization of the gastric mucosa [35], and in vitro suppress antigen-specific proliferation of H. pylori-specific CD4+ T effector cells [36]. In vivo, depletion of CD4+CD25+Foxp3+ Treg in H. pylori infected mice leads to severe gastritis, influx of B cells, T cells, and macrophages into the gastric mucosa, increased titers of H. pylori-specific IgG1, and IgG2c and, subsequently, reduced bacterial load [37]. The mechanisms of effector T cell suppression by CD4+CD25+ Treg remain to be elucidated, but they include CTLA-4-mediated anergy [38] and TGF-β secretion [39]. Once CD4+ T cells are rendered anergic by CD4+CD25+ Treg, the presence of these Treg is not required to maintain hyporesponsiveness [40].
Also in humans, natural CD4+CD25+Foxp3+ Treg are recruited during H. pylori infection and correlate with increased bacterial colonization and mucosal TGF-β gene expression [41]. When compared to adults, H. pylori-infected children have increased numbers of Treg and increased expression of the Treg-cytokines TGF-β and IL-10 and lower level of gastritis, despite similar levels of H. pylori colonization [39]. In addition, CD4+CD25+Foxp3+ Treg cell numbers are increased in areas of duodenal gastric metaplasia in duodenal ulcer patients where they are localized in CD4+ T cell aggregates [42]. Together, these results suggest that H. pylori infection induces CD4+CD25+Foxp3+ Treg that contribute to the asymptomatic persistent infection, seen in the majority of people, and that individuals with inadequate regulatory T cell responses will develop gastro-duodenal pathology [43].
This profound role of regulatory T cells in outcome of H. pylori infection may provide another explanation for the “African enigma”: in endemic areas long-term helminth infections induce a robust anti-inflammatory, regulatory T cell network [44] that protects H. pylori-infected individuals from peptic ulcer disease and gastric cancer.
2. H. pylori Infection, Molecular Mimicry, and Gastric Autoimmunity
H. pylori infection is strongly associated with gastric autoimmunity. Several clinical and epidemiological studies indicate that most patients with autoimmune gastritis (AIG) have or have had H. pylori infection [45]. AIG is characterized by autoimmune- mediated destruction of the secretory glands in the corpus of the stomach, leading to loss of gastric acid producing parietal cells and zymogenic cells, referred to as corpus atrophy. The end point of AIG is pernicious anemia (PA) [46]. There are striking similarities between classical AIG and H. pylori-induced corpus atrophic gastritis [47], and histologically defined preclinical stages of AIG can be successfully treated by H. pylori eradication [48–50].
H. pylori induces autoantibodies reactive with gastric mucosal antigens in approximately half of the infected individuals [51–55]. Gastric H+K+-ATPase, the proton pump located in the secretory canaliculi of the parietal cells, is the autoantigen that drives immunopathology in AIG [56]. H+K+-ATPase has also been identified as the single major autoantigen in chronic H. pylori gastritis with corpus atrophy [57].
In mice, H. pylori infection induces gastric autoantibodies through mimicry between LPS Lewis blood-group antigens and Lewis antigens on the glycosylated β-subunit of H+K+-ATPase [58]. However, in humans, H. pylori-associated anti-H+K+-ATPase autoantibodies are directed against protein epitopes, and their formation does not involve Lewis mimicry [47]. In humans, H. pylori infection leads to the activation of gastric T cells, of which a proportion is cross-reactive against both H. pylori peptide antigens and H+K+-ATPase [59], and circulating antigastric autoantibodies are not pathogenic themselves, but mark ongoing T cell-mediated gastric autoimmunity.
2.1. Animal Models for Human AIG
Our knowledge of the pathogenesis of human AIG is based largely on mouse models of experimental autoimmune gastritis (EAIG) [60]. EAIG spontaneously develops in T cell receptor transgenic (TCR-Tg) mice in which the CD4+ T cells express an αβTCR that recognize an epitope of the H+K+-ATPase α-subunit (630–641) [61] or in neonatally thymectomized mice [56]. In EAIG and AIG, the gastric inflammatory infiltrate comprises CD4+ and CD8+ T cells, macrophages and B cells [56], and the histopathological lesions in mouse models are similar to those observed in humans with chronic AIG [62, 63]. EAIG is mediated by H+K+-ATPase-specific CD4+ T cells [64] and gastritogenic T cell epitopes on both α and β subunits on H+K+-ATPase have been identified [65–67]. In human AIG, a proportion of autoreactive gastric T cells recognize H+K+-ATPase epitopes identical or partly overlapping with those in EAIG [68, 69]. The relevance of such partly conserved epitopes in (E)AIG remains unclear, but availability of an epitope for TCR recognition, rather than affinity for the TCR, enhances the gastritogenic potential of autoreactive T cells [70]. EAIG can be induced by either Th1, Th2 or Th17 (IL-17 secreting) cells specific for H+K+-ATPase [71]. However, a major role has been attributed to IFN-γ [72], and gastric mucosa infiltrating Th1 cells in onset and pathology of EAIG [73]. This is in concordance with our observation that in AIG, the majority (87%) of H+K+-ATPase-specific T cell clones produced IFN-γ upon stimulation with the appropriate antigenic peptide epitope [69]. These striking similarities between EAIG and AIG indicate that EAIG is an excellent model for human AIG. Since EAIG is one of the best studied animal models for human autoimmunity, advances in this model may contribute to our knowledge of organ-specific autoimmune disease in general. Recently, the role of naturally occurring CD4+CD25+ Treg in Th1-, Th2-, and Th17-mediated gastritis has been described [71] and TGFβ-induced autoantigen-specific, Foxp3+ regulatory T cells (iTreg) have been shown to prevent autoimmunity by suppressing activation of autoantigen-presenting dendritic cells [74]. Even if data in H. pylori model is still relatively sparce, the balance between IL-12, IL-23, and Treg responses seems to play an important role in the type of inflammatory responses in mouse models of inflammatory bowel disease. The gastrointestinal tract of mammals is inhabited by many different species of commensal micro-organisms that exist in a mutualistic relationship with the host. The colonization of the small intestine of mice with a single commensal microbe, such as segmented filamentous bacterium, was sufficient to induce the appearance of Th cells able to produce IL-17 and IL-22 in the lamina propria with related activation of inflammatory genes [75]. Using indeed mouse models of H. hepaticus-induced T-cell dependent colitis, it was demonstrated that IL-23 and not IL-12 is essential for the development of a severe disease. Although IL-23 has been implicated in the genesis of other inflammatory disorders via the activation of IL-17-secreting Th cells, in this H. hepaticus model of colitis, IL-17 in the absence of IFN-γ did not appear sufficient to induce disease, the maximum intestinal inflammation depending on both IFN-γ and IL-17 [76].
2.2. Gastric T Cells in AIG Patients without and with Concurrent H. pylori Infection
In AIG patients without evidence of previous or actual H. pylori infection, large numbers of T cells are present in the gastric mucosa [68]. Around 25% of the corpus derived T cells recognized gastric H+K+-ATPase, whereas only a few (3%) of the antral T cells proliferated upon stimulation with this autoantigen. As in EAIG, in which administration of depleting anti-CD4 antibodies but not anti-CD8 antibodies reduces the incidence of gastritis [64], all human H+K+-ATPase-specific T cell clones were CD4+ [68, 69]. T cell proliferation to purified H+K+-ATPase is dose-dependent and HLA Class II restricted, and involves different TCR Vβ rearrangements and submolecular epitopes, indicating that human AIG is driven by antigen-specific polyclonal activation of autoreactive T cells, and not due to expansion of a single- autoreactive T cell clone [68].
From AIG patients with a concurrent H. pylori infection, gastric CD4+ T cells reactive to H+K+-ATPase and CD4+ T cells reactive to H. pylori can be cloned, but also CD4+ T cells that recognize both H+K+-ATPase and H. pylori antigens [59]. Identification of these H. pylori/H+K+-ATPase-specific T cells has provided a functional mechanism that can explain the long known association between H. pylori infection and corpus atrophic gastritis.
2.3. Antigen-Specificity and Cross-Reactivity of H. pylori/H+K+-Atpase-Specific T Cells
A library of overlapping 15-mer synthetic peptides, together comprising the entire α and β subunits of human gastric H+K+-ATPase, was used to identify the submolecular epitopes recognized by H+K+-ATPase-specific and H. pylori/H+K+-ATPase-specific CD4+ T cells from H. pylori- infected AIG patients [59, 68, 69]. Subsequently, candidate H. pylori epitopes were predicted by alignment of identified H+K+-ATPase epitopes with the genomes of the H. pylori J99 and 26695 strains, taking into account the MHC class II peptide binding motifs of each individual T cell donor [59]. The repertoire of H+K+-ATPase α and β subunit epitopes recognized by H+K+-ATPase-specific CD4+ T cell clones is similar in H. pylori- infected and noninfected AIG patients [59, 69]. All H. pylori/H+K+-ATPase-cross-reactive T cells find their autoantigenic epitope in the α subunit of H+K+-ATPase [59], which is much longer (1034 amino acids) than the β subunit (270 amino acids long) [69]. Surprisingly, none of the identified H. pylori peptides recognized by the H. pylori/H+K+-ATPase cross-reactive T cells belong to the known immunodominant H. pylori proteins, that is, VacA, CagA, and Urease, which have been identified as major targets of gastric T cells in H. pylori- infected patients with chronic antral gastritis [20] and peptic ulcer [18]. Instead, the H. pylori peptide epitopes recognized by cross-reactive T cells are predominantly derived from products of H. pylori house-hold genes [59]. Antigen-specific stimulation of H. pylori/H+K+-ATPase-cross-reactive T cells is HLA-DR restricted [59]. H. pylori/H+K+-ATPase-reactive T cell clones, derived from two different donors that share the expression of HLA-DRB1*04 and recognize the same H+K+-ATPase peptide, also find their bacterial epitope in the same H. pylori. And vice versa, two T cell clones recognizing the same H+K+-ATPase epitope, but derived from different donors, one expressing HLA-DRB1*0301 and the other expressing HLA-DRB1*1303, find their cross-reactive bacterial peptide in different H. pylori proteins [59].
2.4. Cytokine Profile and Effector Functions of Gastric T Cells in Human AIG without and with Concurrent H. pylori Infection
Upon activation with the appropriate antigenic peptide, all H+K+-ATPase-reactive gastric T cell or H+K+-ATPase/H. pylori-cross-reactive T cell clones from AIG patients have a Th1 phenotype, characterized by production of IFN-γ and neither IL-4 nor IL-5 [59, 68, 69]. After activation, all the autoreactive and cross-reactive H+K+-ATPase-specific Th clones were able to induce cell death via either Fas-Fas ligand(FasL)-mediated apoptosis or perforin-mediated cytotoxicity against target cells [59]. The expression of Fas on gastric epithelial cells is increased by the Th1 cytokines IFN-γ and TNFα [77] and Fas/FasL interactions contribute to death of gastric epithelial cells [78].
Together, these observations are in agreement with at least two possible scenario's of the role of H. pylori infection in the onset or acceleration of gastric autoimmunity [79]. In most infected individuals, as yet not completely understood, interactions between H. pylori and the host immune system lead to asymptomatic chronic inflammation of the gastric mucosa, kept under control by CD4+CD25+ Treg. However, also apparently healthy individuals may harbour H+K+-ATPase-autoreactive CD4+ T cells that have escaped from negative selection in the thymus, as reflected by the occasional presence of H+K+-ATPase-specific autoantibodies [57]. In such individuals, H. pylori-driven chronic Th1-mediated inflammation may be sufficient to abrogate CD4+CD25+ Treg suppression, leading to activation of H+K+-ATPase-specific CD4+ T cells and subsequent gastric autoimmunity. A second scenario also involves thymic escape by H+K+-ATPase-specific autoreactive T cells, but in addition, genetics predisposes a host to develop gastric autoimmunity if this host expresses particular HLA alleles that can facilitate the presentation of both H+K+-ATPase epitopes and H. pylori peptides [45, 79]. This second scenario is supported by the significant association of increased risk for development of PA with expression of HLA-DR2 /HLA-DR4 or HLA-DR4/HLA-DR5 [80], and observations that a substantial proportion of patients with PA are or were infected with H. pylori [81, 82].
3. H. pylori Infection and MALT Lymphoma
Extranodal marginal zone B cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) represents the third commonest form of non-Hodgkin lymphoma [83, 84]. The most frequent site of MALT lymphomas is the stomach, where they were first recognized as a distinct entity [85]. A link of H. pylori infection with gastric MALT lymphoma was provided by the identification of H. pylori in the majority of the lymphoma specimens [86]. H. pylori-related low-grade gastric MALT lymphoma represents a model for studying the interplay between chronic infection, immune response, and lymphomagenesis. This lymphoma represents the first described neoplasia susceptible of regression following antibiotic therapy resulting in H. pylori eradication [87]. A prerequisite for lymphomagenesis is the development of secondary inflammatory MALT induced by H. pylori. Tumor cells of low-grade gastric MALT lymphoma (MALToma) are memory B cells still responsive to differentiation signals, such as CD40 costimulation and cytokines produced by antigen-stimulated Th cells, and dependent for their growth on the stimulation by H. pylori-specific T cells [88–91]. In early phases, this tumor is sensitive to withdrawal of H. pylori-induced T-cell help, providing an explanation for both the tumor's tendency to remain localized to its primary site and its regression after H. pylori eradication.
3.1. H. pylori-Specific T Cells in Gastric MALT Lymphoma
The analysis of the antigen-induced B-cell help exerted by H. pylori-reactive gastric T-cell clones provided detailed information on the molecular and cellular mechanisms associated with the onset of low-grade gastric MALToma. In the stomach of MALToma patients, a high percentage of Th cells were specific for H. pylori (between 3% and 20% in each case). In particular, 25% were specific for urease, 4% for VacA, and none for CagA or HSP; 71% of Th clones proliferated in response to H. pylori antigens, different from Urease, CagA, VacA, or HSP [91]. Each H. pylori-specific Th clone derived from gastric MALToma produced IL-2 and a variety of B-cell-stimulating cytokines, such as IL-4 and IL-13 [91]. In vitro stimulation with the appropriate H. pylori antigens induced H. pylori-specific Th clones derived from gastric MALToma to express powerful help for B-cell activation and proliferation [91]. B cells from MALToma patients proliferate in response to H. pylori, but the B-cell proliferation induced by H. pylori antigens was strictly T-cell-dependent because it could not take place with H. pylori and without T helper cells [91].
3.2. Effector T Cell Functions in H. pylori-Associated MALT Lymphoma
In chronic gastritis patients, either with or without ulcer, the helper function towards B cells exerted by H. pylori antigen-stimulated gastric T-cell clones was negatively regulated by the concomitant cytolytic killing of B cells [18, 20]. In contrast, gastric T-cell clones from MALToma were unable to downmodulate their antigen-induced help for B-cell proliferation. Indeed, none of these clones was able to express perforin-mediated cytotoxicity against autologous B cells. Moreover, the majority of Th clones from uncomplicated chronic gastritis induced Fas-Fas ligand-mediated apoptosis in target cells, whereas only a small fraction of H. pylori-specific gastric clones from MALToma were able to induce apoptosis in target cells, including autologous B cells [91]. Both defective perforin-mediated cytotoxicity and poor ability to induce Fas-Fas ligand-mediated apoptosis were restricted to MALToma-infiltrating T cells, since H. pylori-specific Th cells derived from the peripheral blood of the same patients expressed the same cytolytic potential and proapoptotic activity as that shown by Th cells from chronic gastritis patients [91].
Accordingly, mice lacking T cell and NK cell cytotoxic effector pathways have also been shown to develop spontaneous tumors (Table 1) [92–101]. For example, mice that lack perforin, a cytotoxic molecule used by cytotoxic cells such as CD8+ T cells and NK cells to form membrane pores in target cells, develop lymphomas with age. These spontaneous lymphomas are of B cell origin, develop in older mice (>1 year of age) regardless of the mouse strain [94, 95], and, when transplanted into WT mice, are rejected by CD8+ T cells [94]. B cell lymphomas also arise in mice lacking both perforin and β2 m, and tumor onset is earlier and occurs with increased prevalence compared with mice lacking only perforin. In addition, B cell lymphomas derived from mice lacking both perforin and β2 m are rejected by either NK cells or γδ T cells following transplantation to WT mice, rather than by CD8+ T cells (as in tumors derived from mice lacking only perforin), demonstrating that cell surface expression of MHC class I molecules by tumor cells can be an important factor in determining which effector cells mediate immune protective effects [96]. Intriguingly, mutations in the gene encoding perforin have also been identified in subsets of lymphoma patients [102], although it is not clear whether this contributes to disease. Mice lacking the death-inducing molecule TNF-related apoptosis-inducing ligand (TRAIL) or expressing a defective mutant form of the death-inducing molecule FasL have also been shown to be susceptible to spontaneous lymphomas that develop with late onset [97, 98]. These aging studies have clearly demonstrated a critical role for cytotoxic pathways in immunoregulation and/or immunosuppression of spontaneous tumor development in mice. The reason why gastric T cells of MALToma, while delivering powerful help to B cells, are deficient in mechanisms involved in the control of B-cell growth still remains unclear. It has been shown that VacA toxin inhibits antigen processing in APCs and T cells, but not the exocytosis of perforin-containing granules of NK cells [103, 104]. It is possible that, in some H. pylori-infected individuals, some bacterial components affect the development or the expression in gastric T cells of regulatory cytotoxic mechanisms on B-cell proliferation, allowing exhaustive and unbalanced B-cell help and lymphomagenesis to occur [91, 105, 106].
Table 1.
Immunodeficient mouse strains that develop spontaneous or induced lymphoma.
| Strain | Description | Phenotype | Ref |
|---|---|---|---|
| Perforin−/− | Lack perforin | Mice develop B cell lymphomas at 14–21 months | [92] |
| IFNg−/− | Lack IFN-γ | Mice develop predominantly T cell lymphomas at 13–19 months | [93] |
| Perforin−/−IFNg−/− | Lack perforin and IFN-γ | Mice develop B cell lymphomas similar to those observed in perforin-deficient mice, but with earlier onset and increased frequency | [93] |
| Perforin−/−B2m−/− | Lack perforin and MHC I | Mice develop B cell lymphomas similar to those observed in perforin-deficient mice, but with earlier onset and increased frequency | [94] |
| Trail−/− | Lack trail | 25% of mice develop lymphomas later in life (> 400 days) | [95] |
| IFNg−/− | Lack IFN-γ | Increased susceptibility to N-methyl-N-nitrosurea-induced lymphomas | [97, 99] |
| IL12p35−/− | Lack IL-12 | Increased susceptibility to N-methyl-N-nitrosurea-induced lymphomas | [97] |
| P53+/−Perforin−/− | Heterozygous for p53 and lack perforin | Accelerated onset of B cell lymphomas | [92] |
| IFNg−/−HTLVTax transgenic | Lack IFN-γ and transgenic for Tax | Accelerated onset of lymphomas or leukemia | [98] |
4. Concluding Remarks
Helicobacter pylori is able to induce a huge variety of antigen-specific T-cell responses in the stomach, due to host genetics, age, sex, different bacterial and environmental factors, or other concomitant infections. The cytolytic effector functions of gastric H. pylori-specific T cells are extremely different between patients with autoimmune gastritis or MALT lymphoma (Figure 1). In some patients, due to genetic and environmental factors not yet fully elucidated, H. pylori infection triggers an abnormal activation at gastric level of cytotoxic, and proapoptotic cross-reactive T cells leading to gastric autoimmunity via molecular mimicry. Conversely in a minority of infected patients, H. pylori is able to induce the development of specific T cells defective of both perforin- and Fas ligand-mediated cytotoxicity, which consequently promotes both B-cell overgrowth and exhaustive B-cell proliferation, finally leading to the onset of low-grade gastric MALT lymphoma.
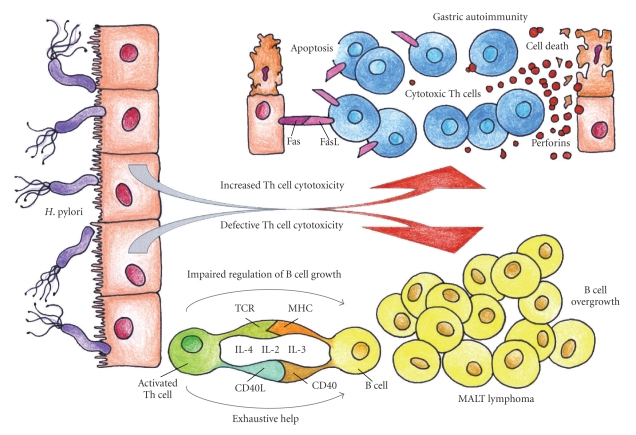
Figure 1.
Different cytotoxic functions of T cells in H. pylori-related gastric autoimmunity and gastric lymphoma. T cells are essential for defence against infection, but inappropriate Th responses can be harmful for the host. In susceptible individuals, H. pylori induces gastric autoimmunity via molecular mimicry by the expansion of H. pylori-specific T cells that cross-react with H+K+-ATPase epitopes. Cross-reactive T cells would result in destruction of gastric mucosa, by the long-lasting activation of both Fas-ligand (FasL)-induced apoptosis and perforin-mediated cytotoxicity. Conversely, in a minority of infected patients, gastric H. pylori-specific Th cells display deficient cytotoxic control (both perforin and Fas-Fas-ligand mediated) of B-cell growth. Such cytolytic defects, associated with the production of cytokines with B-cell growth factor activity and the chronic delivery of costimulatory signals by Th cells, together with chronic exposure to H. pylori antigens, would result in overgrowth of B cells, thus promoting the neoplastic B-cell transformation and the onset of gastric low-grade MALT B-cell lymphoma.
Acknowledgment
The authors thank Ente Cassa di Risparmio di Firenze and the Italian Ministry of University and Research, for their support of their studies, and Dr. Chiara Della Bella for the artwork.
References
- 1.Linz B, Balloux F, Moodley Y, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445(7130):915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annual Review of Pathology. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 3.Merrell DS, Falkow S. Frontal and stealth attack strategies in microbial pathogenesis. Nature. 2004;430(6996):250–256. doi: 10.1038/nature02760. [DOI] [PubMed] [Google Scholar]
- 4.Kusters JG, van Vliet AHM, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clinical Microbiology Reviews. 2006;19(3):449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viala J, Chaput C, Boneca IG, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nature Immunology. 2004;5(11):1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 6.Isomoto H, Mukae H, Ishimoto H, et al. Elevated concentrations of α-defensins in gastric juice of patients with Helicobacter pylori infection. American Journal of Gastroenterology. 2004;99(10):1916–1923. doi: 10.1111/j.1572-0241.2004.40334.x. [DOI] [PubMed] [Google Scholar]
- 7.Ding S-Z, Torok AM, Smith MF, Jr., Goldberg JB. Toll-like receptor 2-mediated gene expression in epithelial cells during Helicobacter pylori infection. Helicobacter. 2005;10(3):193–204. doi: 10.1111/j.1523-5378.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 8.Gewirtz AT, Yu Y, Krishna US, Israel DA, Lyons SL, Peek RM., Jr. Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. The Journal of Infectious Diseases. 2004;189(10):1914–1920. doi: 10.1086/386289. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara S, Rumi MAK, Kadowaki Y, et al. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. The Journal of Immunology. 2004;173(2):1406–1416. doi: 10.4049/jimmunol.173.2.1406. [DOI] [PubMed] [Google Scholar]
- 10.Torok AM, Bouton AH, Goldberg JB. Helicobacter pylori induces interleukin-8 secretion by Toll-like receptor 2- and Toll-like receptor 5-dependent and -independent pathways. Infection and Immunity. 2005;73(3):1523–1531. doi: 10.1128/IAI.73.3.1523-1531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Aubel RAMH, Keestra AM, Krooshoop DJEB, van Eden W, van Putten JPM. Ligand-induced differential cross-regulation of Toll-like receptors 2, 4 and 5 in intestinal epithelial cells. Molecular Immunology. 2007;44(15):3702–3714. doi: 10.1016/j.molimm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Robinson K, Argent RH, Atherton JC. The inflammatory and immune response to Helicobacter pylori infection. Best Practice & Research: Clinical Gastroenterology. 2007;21(2):237–259. doi: 10.1016/j.bpg.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Roth KA, Kapadia SB, Martin SM, Lorenz RG. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. The Journal of Immunology. 1999;163(3):1490–1497. [PubMed] [Google Scholar]
- 14.Gottwein JM, Blanchard TG, Targoni OS, et al. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund’s adjuvant by systemic immunization. The Journal of Infectious Diseases. 2001;184(3):308–314. doi: 10.1086/322032. [DOI] [PubMed] [Google Scholar]
- 15.Saldinger PF, Porta N, Launois P, et al. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology. 1998;115(4):891–897. doi: 10.1016/s0016-5085(98)70261-6. [DOI] [PubMed] [Google Scholar]
- 16.Akhiani AA, Pappo J, Kabok Z, et al. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. The Journal of Immunology. 2002;169(12):6977–6984. doi: 10.4049/jimmunol.169.12.6977. [DOI] [PubMed] [Google Scholar]
- 17.Garhart CA, Heinzel FP, Czinn SJ, Nedrud JG. Vaccine-induced reduction of Helicobacter pylori colonization in mice is interleukin-12 dependent but gamma interferon and inducible nitric oxide synthase independent. Infection and Immunity. 2003;71(2):910–921. doi: 10.1128/IAI.71.2.910-921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Elios MM, Manghetti M, de Carli M, et al. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. The Journal of Immunology. 1997;158(2):962–967. [PubMed] [Google Scholar]
- 19.Sommer F, Faller G, Konturek P, et al. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infection and Immunity. 1998;66(11):5543–5546. doi: 10.1128/iai.66.11.5543-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Elios MM, Manghetti M, Almerigogna F, et al. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. European Journal of Immunology. 1997;27(7):1751–1755. doi: 10.1002/eji.1830270723. [DOI] [PubMed] [Google Scholar]
- 21.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 22.Hafsi N, Voland P, Schwendy S, et al. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. The Journal of Immunology. 2004;173(2):1249–1257. doi: 10.4049/jimmunol.173.2.1249. [DOI] [PubMed] [Google Scholar]
- 23.Kranzer K, Söllner L, Aigner M, et al. Impact of Helicobacter pylori virulence factors and compounds on activation and maturation of human dendritic cells. Infection and Immunity. 2005;73(7):4180–4189. doi: 10.1128/IAI.73.7.4180-4189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bamford KB, Fan X, Crowe SE, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114(3):482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 25.Amedei A, Cappon A, Codolo G, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. Journal of Clinical Investigation. 2006;116(4):1092–1101. doi: 10.1172/JCI27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jonge R, Kuipers EJ, Langeveld SCL, et al. The Helicobacter pylori plasticity region locus jhp0947-jhp0949 is associated with duodenal ulcer disease and interleukin-12 production in monocyte cells. FEMS Immunology and Medical Microbiology. 2004;41(2):161–167. doi: 10.1016/j.femsim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Robinson K, Loughlin MF, Potter R, Jenks PJ. Host adaptation and immune modulation are mediated by homologous recombination in Helicobacter pylori. The Journal of Infectious Diseases. 2005;191(4):579–587. doi: 10.1086/427657. [DOI] [PubMed] [Google Scholar]
- 28.Bergman MP, Engering A, Smits HH, et al. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. The Journal of Experimental Medicine. 2004;200(8):979–990. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehmann FS, Terracciano L, Carena I, et al. In situ correlation of cytokine secretion and apoptosis in Helicobacter pylori-associated gastritis. American Journal of Physiology. 2002;283(2):G481–G488. doi: 10.1152/ajpgi.00422.2001. [DOI] [PubMed] [Google Scholar]
- 30.Wen S, Felley CP, Bouzourene H, Reimers M, Michetti P, Pan-Hammarström Q. Inflammatory gene profiles in gastric mucosa during Helicobacter pylori infection in humans. The Journal of Immunology. 2004;172(4):2595–2606. doi: 10.4049/jimmunol.172.4.2595. [DOI] [PubMed] [Google Scholar]
- 31.Pellicanò A, Sebkova L, Monteleone G, et al. Interleukin-12 drives the Th1 signaling pathway in Helicobacter pylori-infected human gastric mucosa. Infection and Immunity. 2007;75(4):1738–1744. doi: 10.1128/IAI.01446-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33(4):429–431. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox JG, Beck P, Dangler CA, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces Helicobacter-induced gastric atrophy. Nature Medicine. 2000;6(5):536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 34.Raghavan S, Holmgren J. CD4+CD25+ suppressor T cells regulate pathogen induced inflammation and disease. FEMS Immunology and Medical Microbiology. 2005;44(2):121–127. doi: 10.1016/j.femsim.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Raghavan S, Fredriksson M, Svennerholm A-M, Holmgren J, Suri-Payer E. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clinical and Experimental Immunology. 2003;132(3):393–400. doi: 10.1046/j.1365-2249.2003.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raghavan S, Suri-Payer E, Holmgren J. Antigen-specific in vitro suppression of murine Helicobacter pylori-reactive immunopathological T cells by CD4+CD25+ regulatory T cells. Scandinavian Journal of Immunology. 2004;60(1):82–88. doi: 10.1111/j.0300-9475.2004.01447.x. [DOI] [PubMed] [Google Scholar]
- 37.Rad R, Brenner L, Bauer S, et al. CD25+/FOXP3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131(2):525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Anderson KM, Czinn SJ, Redline RW, Blanchard TG. Induction of CTLA-4-mediated anergy contributes to persistent colonization in the murine model of gastric Helicobacter pylori infection. The Journal of Immunology. 2006;176(9):5306–5313. doi: 10.4049/jimmunol.176.9.5306. [DOI] [PubMed] [Google Scholar]
- 39.Harris PR, Wright SW, Serrano C, et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134(2):491–499. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Stuller KA, Ding H, Redline RW, Czinn SJ, Blanchard TG. CD25+ T cells induce Helicobacter pylori-specific CD25− T-cell anergy but are not required to maintain persistent hyporesponsiveness. European Journal of Immunology. 2008;38(12):3426–3436. doi: 10.1002/eji.200838428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kandulski A, Wex T, Kuester D, et al. Naturally occurring regulatory T cells (CD4+, CD25 high, FOXP3+) in the antrum and cardia are associated with higher H. pylori colonization and increased gene expression of TGF-β1. Helicobacter. 2008;13(4):295–303. doi: 10.1111/j.1523-5378.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- 42.Kindlund B, Sjöling A, Hansson M, et al. FOXP3-expressing CD4+ T-cell numbers increase in areas of duodenal gastric metaplasia and are associated to CD4+ T-cell aggregates in the duodenum of Helicobacter pylori-infected duodenal ulcer patients. Helicobacter. 2009;14(3):192–201. doi: 10.1111/j.1523-5378.2009.00673.x. [DOI] [PubMed] [Google Scholar]
- 43.Robinson K, Kenefeck R, Pidgeon EL, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57(10):1375–1385. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 44.Yazdanbakhsh M, Kremsner PG, van Ree R. Immunology: allergy, parasites, and the hygiene hypothesis. Science. 2002;296(5567):490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 45.D’Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends in Molecular Medicine. 2004;10(7):316–323. doi: 10.1016/j.molmed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Toh B-H, van Driel IR, Gleeson PA. Mechanisms of disease: pernicious anemia. New England Journal of Medicine. 1997;337(20):1441–1448. doi: 10.1056/NEJM199711133372007. [DOI] [PubMed] [Google Scholar]
- 47.Bergman MP, vandenbroucke-Grauls CMJE, Appelmelk BJ, et al. The story so far: Helicobacter pylori and gastric autoimmunity. International Reviews of Immunology. 2005;24(1-2):63–91. doi: 10.1080/08830180590884648. [DOI] [PubMed] [Google Scholar]
- 48.Stolte M, Baumann K, Bethke B, Ritter M, Lauer E, Eidt H. Active autoimmune gastritis withouth total atrophy of the glands. Zeitschrift für Gastroenterologie. 1992;30(10):729–735. [PubMed] [Google Scholar]
- 49.Stolte M, Meier E, Meining A. Cure of autoimmune gastritis by Helicobacter pylori eradication in a 21-year-old male. Zeitschrift für Gastroenterologie. 1998;36(8):641–643. [PubMed] [Google Scholar]
- 50.Tucci A, Poli L, Tosetti C, et al. Reversal of fundic atrophy after eradication of Helicobacter pylori. American Journal of Gastroenterology. 1998;93(9):1425–1431. doi: 10.1111/j.1572-0241.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- 51.Faller G, Steininger H, Eck M, Hensen J, Hahn EG, Kirchner T. Antigastric autoantibodies in Helicobacter pylori gastritis: prevalence, in-situ binding sites and clues for clinical relevance. Virchows Archiv. 1996;427(5):483–486. doi: 10.1007/BF00199508. [DOI] [PubMed] [Google Scholar]
- 52.Faller G, Steininger H, Kränzlein J, et al. Antigastric autoantibodies in Helicobacter pylori infection: implications of histological and clinical parameters of gastritis. Gut. 1997;41(5):619–623. doi: 10.1136/gut.41.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Negrini R, Lisato L, Zanella I, et al. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991;101(2):437–445. doi: 10.1016/0016-5085(91)90023-e. [DOI] [PubMed] [Google Scholar]
- 54.Negrini R, Savio A, Poiesi C, et al. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996;111(3):655–665. doi: 10.1053/gast.1996.v111.pm8780570. [DOI] [PubMed] [Google Scholar]
- 55.Steininger H, Faller G, Dewald E, Brabletz T, Jung A, Kirchner T. Apoptosis in chronic gastritis and its correlation with antigastric autoantibodies. Virchows Archiv. 1998;433(1):13–18. doi: 10.1007/s004280050210. [DOI] [PubMed] [Google Scholar]
- 56.Toh B-H, Sentry JW, Alderuccio F. The causative H+/K+ ATPase antigen in the pathogenesis of autoimmune gastritis. Immunology Today. 2000;21(7):348–354. doi: 10.1016/s0167-5699(00)01653-4. [DOI] [PubMed] [Google Scholar]
- 57.Claeys D, Faller G, Appelmelk BJ, Negrini R, Kirchner T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115(2):340–347. doi: 10.1016/s0016-5085(98)70200-8. [DOI] [PubMed] [Google Scholar]
- 58.Appelmelk BJ, Faller G, Claeys D, Kirchner T, vandenbroucke-Grauls CMJE. Bugs on trial: the case of Helicobacter pylori and autoimmunity. Immunology Today. 1998;19(7):296–299. doi: 10.1016/s0167-5699(98)01281-x. [DOI] [PubMed] [Google Scholar]
- 59.Amedei A, Bergman MP, Appelmelk BJ, et al. Molecular mimicry between Helicobacter pylori antigens and H+,K+-adenosine triphosphatase in human gastric autoimmunity. The Journal of Experimental Medicine. 2003;198(8):1147–1156. doi: 10.1084/jem.20030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alderuccio F, Sentry JW, Marshall ACJ, Biondo M, Toh BH. Animal models of human disease: experimental autoimmune gastritis—a model for autoimmune gastritis and pernicious anemia. Clinical Immunology. 2002;102(1):48–58. doi: 10.1006/clim.2001.5134. [DOI] [PubMed] [Google Scholar]
- 61.McHugh RS, Shevach EM, Margulies DH, Natarajan K. A T cell receptor transgenic model of severe, spontaneous organ-specific autoimmunity. European Journal of Immunology. 2001;31(7):2094–2103. doi: 10.1002/1521-4141(200107)31:7<2094::aid-immu2094>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 62.Burman P, Kampe O, Kraaz W, et al. A study of autoimmune gastritis in the postpartum period and at a 5-year follow-up. Gastroenterology. 1992;103(3):934–942. doi: 10.1016/0016-5085(92)90027-v. [DOI] [PubMed] [Google Scholar]
- 63.Martinelli TM, van Driel IR, Alderuccio F, Gleeson PA, Toh B-H. Analysis of mononuclear cell infiltrate and cytokine production in murine autoimmune gastritis. Gastroenterology. 1996;110(6):1791–1802. doi: 10.1053/gast.1996.v110.pm8964405. [DOI] [PubMed] [Google Scholar]
- 64.de Silva HD, van Driel IR, La Gruta N, Toh BH, Gleeson PA. CD4+ T cells, but not CD8+ T cells, are required for the development of experimental autoimmune gastritis. Immunology. 1998;93(3):405–408. doi: 10.1046/j.1365-2567.1998.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Silva HD, Gleeson PA, Toh BH, van Driel IR, Carbone FR. Identification of a gastritogenic epitope of the H/K ATPase β-subunit. Immunology. 1999;96(1):145–151. doi: 10.1046/j.1365-2567.1999.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishio A, Hosono M, Watanabe Y, Sakai M, Okuma M, Masuda T. A conserved epitope on H+,K+-adenosine triphosphatase of parietal cells discerned by a murine gastritogenic T-cell clone. Gastroenterology. 1994;107(5):1408–1414. doi: 10.1016/0016-5085(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 67.Suri-Payer E, Amar AZ, McHugh R, Natarajan K, Margulies DH, Shevach EM. Post-thymectomy autoimmune gastritis: fine specificity and pathogenicity of anti-H/K ATPase-reactive T cells. European Journal of Immunology. 1999;29(2):669–677. doi: 10.1002/(SICI)1521-4141(199902)29:02<669::AID-IMMU669>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 68.D’Elios MM, Bergman MP, Azzurri A, et al. H+,K+-ATPase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology. 2001;120(2):377–386. doi: 10.1053/gast.2001.21187. [DOI] [PubMed] [Google Scholar]
- 69.Bergman MP, Amedei A, D’Elios MM, et al. Characterization of H+,K+-ATPase T cell epitopes in human autoimmune gastritis. European Journal of Immunology. 2003;33(2):539–545. doi: 10.1002/immu.200310030. [DOI] [PubMed] [Google Scholar]
- 70.Levin D, DiPaolo RJ, Brinster C, et al. Availability of autoantigenic epitopes controls phenotype, severity, and penetrance in TCR Tg autoimmune gastritis. European Journal of Immunology. 2008;38(12):3339–3353. doi: 10.1002/eji.200838584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stummvoll GH, DiPaolo RJ, Huter EN, et al. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. The Journal of Immunology. 2008;181(3):1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barrett SP, Gleeson PA, de Silva H, Toh B-H, van Driel IR. Interferon-γ is required during the initiation of an organ-specific autoimmune disease. European Journal of Immunology. 1996;26(7):1652–1655. doi: 10.1002/eji.1830260737. [DOI] [PubMed] [Google Scholar]
- 73.Katakai T, Mori KJ, Masuda T, Shimizu A. Differential localization of Th1 and Th2 cells in autoimmune gastritis. International Immunology. 1998;10(9):1325–1334. doi: 10.1093/intimm/10.9.1325. [DOI] [PubMed] [Google Scholar]
- 74.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFβ-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. The Journal of Immunology. 2007;179(7):4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 75.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kullberg MC, Jankovic D, Feng CG, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. The Journal of Experimental Medicine. 2006;203(11):2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Houghton J, Macera-Bloch LS, Harrison L, Kim KH, Korah RM. Tumor necrosis factor alpha and interleukin 1β up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection. Infection and Immunity. 2000;68(3):1189–1195. doi: 10.1128/iai.68.3.1189-1195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, Fan X, Lindholm C, et al. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infection and Immunity. 2000;68(7):4303–4311. doi: 10.1128/iai.68.7.4303-4311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergman M, Del Prete G, van Kooyk Y, Appelmelk B. Helicobacter pylori phase variation, immune modulation and gastric autoimmunity. Nature Reviews Microbiology. 2006;4(2):151–159. doi: 10.1038/nrmicro1344. [DOI] [PubMed] [Google Scholar]
- 80.Ungar B, Mathews JD, Tait BD, Cowling DC. HLA-DR patterns in pernicious anaemia. British Medical Journal. 1981;282(6266):768–770. doi: 10.1136/bmj.282.6266.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karnes WE, Jr., Samloff IM, Siurala M, et al. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology. 1991;101(1):167–174. doi: 10.1016/0016-5085(91)90474-y. [DOI] [PubMed] [Google Scholar]
- 82.Ma J-Y, Borch K, Sjostrand SE, Janzon L, Mardh S. Positive correlation between H,K-Adenosine triphosphatase autoantibodies and Helicobacter pylori antibodies in patients with pernicious anemia. Scandinavian Journal of Gastroenterology. 1994;29(11):961–965. doi: 10.3109/00365529409094870. [DOI] [PubMed] [Google Scholar]
- 83.Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89(11):3909–3918. [PubMed] [Google Scholar]
- 84.Du MQ. MALT lymphoma : recent advances in aetiology and molecular genetics. Journal of Clinical and Experimental Hematopathology. 2007;47(2):31–42. doi: 10.3960/jslrt.47.31. [DOI] [PubMed] [Google Scholar]
- 85.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52(8):1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 86.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. The Lancet. 1991;338(8776):1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 87.Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade-B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. The Lancet. 1993;342(8871):575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 88.Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. The Lancet. 1993;342(8871):571–574. doi: 10.1016/0140-6736(93)91408-e. [DOI] [PubMed] [Google Scholar]
- 89.Hussell T, Isaacson PG, Crabtree JE, Spencer JO. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. The Journal of Pathology. 1996;178(2):122–127. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 90.Greiner A, Knörr C, Qin Y, et al. Low-grade B cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signaling and Th2-type cytokines for in vitro growth and differentiation. American Journal of Pathology. 1997;150(5):1583–1593. [PMC free article] [PubMed] [Google Scholar]
- 91.D’Elios MM, Amedei A, Manghetti M, et al. Impaired T-cell regulation of B-cell growth in Helicobacter pylori-related gastric low-grade MALT lymphoma. Gastroenterology. 1999;117(5):1105–1112. doi: 10.1016/s0016-5085(99)70395-1. [DOI] [PubMed] [Google Scholar]
- 92.Swann JB, Smyth MJ. Immune surveillance of tumors. Journal of Clinical Investigation. 2007;117(5):1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nature Reviews Immunology. 2002;2(10):735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 94.Smyth MJ, Thia KYT, Street SEA, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. The Journal of Experimental Medicine. 2000;192(5):755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Street SEA, Trapani JA, MacGregor D, Smyth MJ. Suppression of lymphoma and epithelial malignancies effected by interferon γ. The Journal of Experimental Medicine. 2002;196(1):129–134. doi: 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Street SEA, Hayakawa Y, Zhan Y, et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and γδ T cells. The Journal of Experimental Medicine. 2004;199(6):879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zerafa N, Westwood JA, Cretney E, et al. Cutting edge: TRAIL deficiency accelerates hematological malignancies. The Journal of Immunology. 2005;175(9):5586–5590. doi: 10.4049/jimmunol.175.9.5586. [DOI] [PubMed] [Google Scholar]
- 98.Davidson WF, Giese T, Fredrickson TN. Spontaneous development of plasmacytoid tumors in mice with defective Fas-Fas ligand interactions. The Journal of Experimental Medicine. 1998;187(11):1825–1838. doi: 10.1084/jem.187.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J, Xiang Z, Ma X. Role of IFN regulatory factor-1 and IL-12 in immunological resistance to pathogenesis of N-methyl-N-nitrosourea-induced T lymphoma. The Journal of Immunology. 2004;173(2):1184–1193. doi: 10.4049/jimmunol.173.2.1184. [DOI] [PubMed] [Google Scholar]
- 100.Mitra-Kaushik S, Harding J, Hess J, Schreiber R, Ratner L. Enhanced tumorigenesis in HTLV-1 tax-transgenic mice deficient in interferon-gamma. Blood. 2004;104(10):3305–3311. doi: 10.1182/blood-2004-01-0266. [DOI] [PubMed] [Google Scholar]
- 101.Street SEA, Cretney E, Smyth MJ. Perforin and interferon-γ activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97(1):192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 102.Clementi R, Locatelli F, Dupre L, et al. A proportion of patients with lymphoma may harnor mutations of the perforin gene. Blood. 2005;105:4424–4428. doi: 10.1182/blood-2004-04-1477. [DOI] [PubMed] [Google Scholar]
- 103.Molinari M, Salio M, Galli C, et al. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. The Journal of Experimental Medicine. 1998;187(1):135–140. doi: 10.1084/jem.187.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boncristiano M, Paccani SR, Barone S, et al. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. The Journal of Experimental Medicine. 2003;198(12):1887–1897. doi: 10.1084/jem.20030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lehours P, Zheng Z, Skoglund A, Megraud F, Engstrand L. Is there a link between the lipopolysaccharide of Helicobacter pylori gastric MALT lymphoma associated strains and lymphoma pathogenesis? PLoS One. 2009;4, article e7297 doi: 10.1371/journal.pone.0007297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lehours P, Dupouy S, Bergey B, et al. Identification of a genetic marker of Helicobacter pylori strains involved in gastric extranodal marginal zone B cell lymphoma of the MALT-type. Gut. 2004;53(7):931–937. doi: 10.1136/gut.2003.028811. [DOI] [PMC free article] [PubMed] [Google Scholar]