Abstract
Lectins, proteins which selectively recognize carbohydrates, have been used in histochemistry for the evaluation of changes in glycosylation in processes of cellular differentiation and/or dedifferentiation. Cratylia mollis seed lectins (Cramoll 1,4 and Cramoll 3), conjugated to horseradish peroxidase, were used as histochemical probes in human prostate tissues: normal (NP), hyperplasia (BPH), and prostate carcinoma (PCa). The staining pattern of Con-A and Cramoll 1,4 in BPH was more intense than in NP. These lectins also showed staining differences between BPH and PCa; the latter showing decreased staining intensity with an increased degree of malignancy. PNA and Cramoll 3 stained epithelial cells similarly in all diagnoses although they did present intense staining of PCa glands lumen. Corpora amylacea were not differentially recognized by any of the lectins. Cramoll 1,4 and Cramoll 3 seed lectins present themselves as candidates for histochemical probes for prostate pathologies when compared to commercial lectins such as Con-A and PNA.
1. Introduction
Prostate cancer is the most diagnosed male malignancy and the second leading cause of cancer-related death among American men. Fortunately, mortality rates have increased less than incidence rates in many countries [1]. In Brazil, according to the National Cancer Institute [2], prostate carcinoma (PCa) is the second most common cancer, in incidence, after skin tumors (non-melanoma), and, in terms of mortality, it is exceeded only by lung cancer. The etiologic factors associated with prostate cancer are varied, encompassing both host genetic and environmental influences [3].
Glycosylation is one of the most common co- or posttranslational modifications. Inside a cell, complex glycosylation pathways assemble these oligosaccharides and attach them to proteins and lipids as they travel to the cell surface [4]. Furthermore, by virtue of their peripheral location, particular oligosaccharide epitopes on proteins or lipids exert key functions in important intercellular communication processes such as fertilization [5], immune response, pathogen anchoring, or metastasis [6]. These particular carbohydrate epitopes are recognized by membrane-anchored carbohydrate-recognition domains of different molecules such as receptors, enzymes, antibodies, or lectins [6]. The latter class comprises proteins of nonimmune origin that display specificity for terminal or subterminal carbohydrates, forming non-covalent bonds. On account of these specific affinities, lectins have been extensively used as histochemical probes to characterize various cell types at various stages of differentiation and maturation of cancer [4, 6, 7]. Thus, modern lectin histochemistry has become a valuable instrument to analyze patterns of glucide composition in glycoconjugates and their modifications in the cell during malignant transformation in tumors [8].
Cratylia mollis (camaratu bean) is a native forage from the semiarid region of the Northeast of Brazil, and its seeds are considered an important lectin source (Cramoll), supplying multiple molecular forms (Cramoll 1 to 4) with different carbohydrate specificities [9, 10].
The aim of the present investigation was to evaluate the binding pattern of two preparations containing isoforms from C. mollis seed lectin (Cramoll 1,4, a preparation containing Cramoll 1 and Cramoll 4 glucose/mannose-specific and Cramoll 3 galactose-specific) in human prostate tissues. Con A and PNA were used for binding comparison since they possess the same carbohydrate specificity as Cramoll 1,4 and Cramoll 3, respectively.
2. Materials and Methods
2.1. Tissue Sections
Formalin-fixed, paraffin-embedded tissue blocks of eight cases of normal human prostate were obtained from the Obit Identification Service at the Federal University of Pernambuco, Brazil; 61 cases of BPH and 82 cases of PCa were obtained from the Tissue Bank of the University Hospital of the Federal University of Pernambuco, Brazil. Patient ages varied between 31 and 69 years (mean 44) at the time of diagnosis for normal tissues, 46 and 92 years (mean 69) for BPH, and 52 and 88 years (mean 66) for PCa.
2.2. Horseradish Peroxidase (HRP) Lectin Conjugation
Cramoll 1,4 and Cramoll 3 were extracted, isolated, and purified from seeds of C. mollis according to Correia and Coelho [10] and Paiva and Coelho [9] at the Glycoprotein Laboratory at the Biochemistry Department of the Federal University of Pernambuco, Brazil. Cramoll 1,4 and Cramoll 3 HRP conjugation was performed according to Beltrão et al. [11]. Canavalia ensiformis agglutinin (Concanavalin A (Con-A)) and Arachis hypogea (peanut) agglutinin (PNA) conjugated to HRP (Con A-HRP and PNA-HRP) were purchased from Sigma (St. Louis, MO, USA).
2.3. Polyacrylamide Gel Electrophoresis
Basic and native polyacrylamide gel electrophoresis (10% w/v) was carried out with conjugated and nonconjugated C. mollis isoforms. Protocols were followed as described by Paiva and Coelho [9].
2.4. Lectin Histochemistry
Tissue slices (4 μm) were cut, adhered to albumin-treated slides, deparaffinized in xylol, and hydrated in graded ethanol (100%–70%). Slices were treated with 0.1% (w/v) trypsin solution for 2 minutes at 37°C and afterwards with 0.3% (v/v) methanolic hydrogen peroxide solution for 15 minutes at 25°C. Sections were incubated with HRP-conjugated lectins (Con-A-HRP, Cramoll 1,4-HRP, PNA-HRP, and Cramoll 3-HRP, at various concentrations −8, 15, 25, 30 and 60 μg/mL) for 2 hours at 4°C. Slices were washed (twice for 5 minutes) with 10 mM phosphate buffer saline solution (PBS) pH 7.2, containing 0.15 M NaCl, after each step. Lectin staining was visualized with 3,3′-diaminobenzidine- (DAB-) hydrogen peroxide in PBS for 4 minutes at 25°C [11]. Thereafter, tissues were rinsed in distilled water, counterstained with haematoxylin, dehydrated in graded ethanol, cleared in xylol, and mounted. Inhibition lectin-carbohydrate binding controls were performed using the specific sugars at a final concentration of 0.3 M for each lectin (methyl-α-D-mannopyranoside for Cramoll 1,4 and Con-A; D-galactose for Cramoll 3 and PNA). The rest of the protocol was as described above.
2.5. Light Microscopy
Tissue sections were examined using an optical microscope (Nikon Eclipse 50i, USA). Staining intensity was determined as the pattern observed in at least 20% of cells with cytoplasm or membrane staining, in luminal secretion, corpora amylacea, and stroma and scored in four categories: 0—no staining, 1—weak staining, 2—moderate staining, and 3—intense staining. For image acquisition, an Image Analyses System (software NIS—Elements F version 2.30—Nikon, USA) was used.
2.6. Statistical Analysis
Staining intensity was analyzed by nonparametric tests (Mann-Whitney test and Kruskal-Wallis, followed by multiple comparison posttest of Dunn) with a significance level of 95% (P < .05) using GraphPad Prism version 5.00.
3. Results
3.1. Horseradish Peroxidase (HRP) Lectin Conjugation
Conjugation efficiency of Cramoll 3 and Cramoll 1,4 to HRP was evaluated using gel electrophoresis for basic and native proteins. Nonconjugated samples were also included. The results showed that conjugated lectins migrated more slowly than their respective nonconjugated counterparts (Figure 1). Lectin conjugates maintained their hemagglutinating activities (data not shown).
Figure 1.
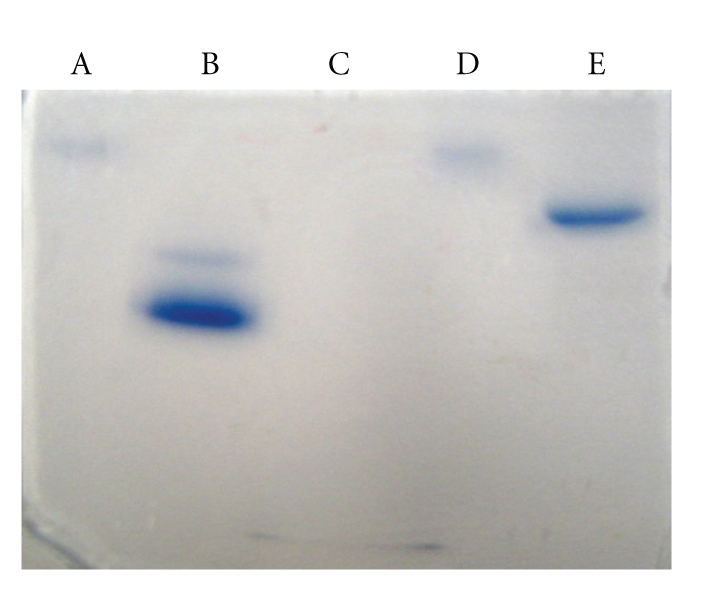
Basic and native protein gel electrophoresis of Cratylia mollis seed lectins and conjugates. (A) Cramoll 1,4-HRP, (B) Cramoll 1,4, (C) cytochrome C, (D) Cramoll 3-HRP, and (E) Cramoll 3.
3.2. Pathologic Findings
Corpora amylacea were observed in 50.8% of the 61 cases of BPH. The Gleason score for PCa (n = 82) was of 2 + 2 = 4 in 3 samples, 2 + 3 = 5 in 1, 3 + 2 = 5 in 1, 3 + 3 = 6 in 24, 3 + 4 = 7 in 36, 4 + 3 = 7 in 3, 3 + 5 = 8 in 3, 4 + 4 = 8 in 2, 4 + 5 = 9 in 3, 5 + 4 = 9 in 3, and 5 + 5 = 10 in 3. Corpora amylacea were visualized in 40.2% of PCa in which the Gleason score was usually 3.
3.3. Lectin Histochemistry
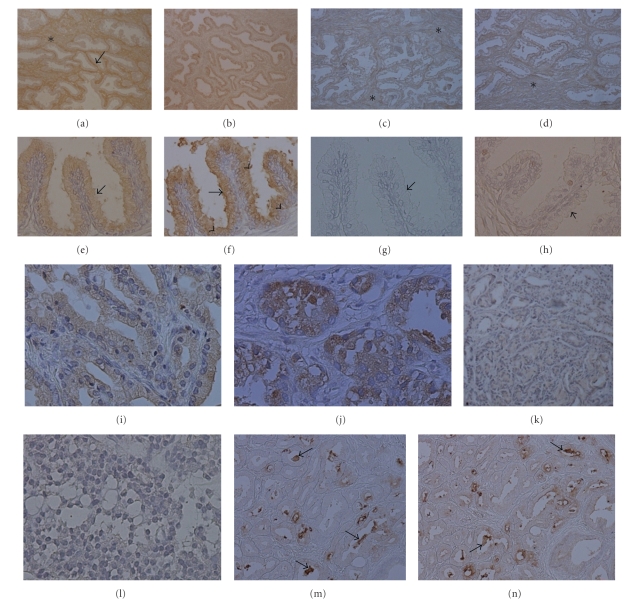
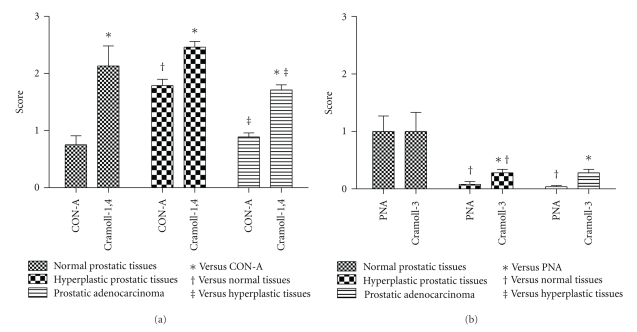
Cytoplasm of normal prostate cells was weakly and moderately stained by Con-A and Cramoll 1,4, both at 15 μg/mL. Normal tissue stroma was moderately stained by Con-A but no staining was observed with Cramoll 1,4 (Figures 2(a) and 2(b)). Hyperplastic epithelial cells were stained by lectins, Con-A, and Cramoll 1,4, mainly in the apical cytoplasm and cell borders. Cramoll 1,4 staining pattern in BPH was intense in most cases (Figures 2(e) and 2(f)). In PCa samples, characterized by heterogeneous Gleason scores, Con-A and Cramoll 1,4 staining intensity decreased while the malignancy degree increased, that is, in well differentiated tissues both lectins showed moderate staining while in undifferentiated tissues no staining was observed. PCa cell staining was more evident using Cramoll 1,4 (Figures 2(i) to 2(l)). Stroma in hyperplastic and neoplastic tissues was not stained by either lectin. Figure 3(a) presents the statistical comparison of the staining patterns of Cramoll 1,4 and Con-A in normal and transformed prostate tissues.
Figure 2.
Histochemistry of prostatic tissues using Con A, Cramoll 1,4, PNA, and Cramoll 3. (a) Normal prostate stained with Con-A. Epithelial cells (arrow) present a weak staining while stroma (asterisk) is intensely stained. (b) Moderate staining by Cramoll 1,4 in epithelium of normal glands. (c and d) Normal prostate epithelium and stroma (asterisks) presenting weak staining by PNA and Cramoll 3, respectively. (e) Con A moderate staining of BPH glands (arrow). (f) BPH epithelial cells showing intense staining by Cramoll 1,4 in apical cytoplasm (arrowheads) and membrane (arrow). (g and h) BPH glands (arrows) were not stained or only weakly stained by PNA and Cramoll 3, respectively. (i and j) Gleason score 2 and 3 PCa cells stained with a moderate pattern, respectively. (k) Weak staining in Gleason score 4 PCa cells. (l) Nonstaining cells in Gleason score 5 PCa. (m and n) PCa gland lumens (arrows) presented an intense staining pattern for PNA and Cramoll 3, respectively. Magnification (a, b, c, d, k, m, and n) x100 and (e, f, g, h, i, j, and l) x400.
Figure 3.
Statistical analysis of lectin histochemistry of normal human prostate, BPH, and PCa with Con-A and Cramoll 1,4 (a) and PNA and Cramoll 3 (b) (P < .05).
PNA and Cramoll 3 (25 μg/mL—galactose specific) weakly stained the normal tissue and did not present a different staining pattern between stroma and glandular tissue (Figures 2(c) and 2(d)). BPH and PCa neoplastic cells were not differentially stained by these two lectins, rending a non-staining or a weakly heterogeneous pattern (Figures 2(g) and 2(h)). In PCa PNA and Cramoll 3 presented a focal and intense staining pattern in luminal secretion and apical membrane of glandular tumor cells (Figures 2(m) and 2(n)). PCa stroma was moderately stained by PNA and Cramoll 3. Statistical comparison between staining patterns of Cramoll 3 and PNA in studied samples is abridged in Figure 3(b).
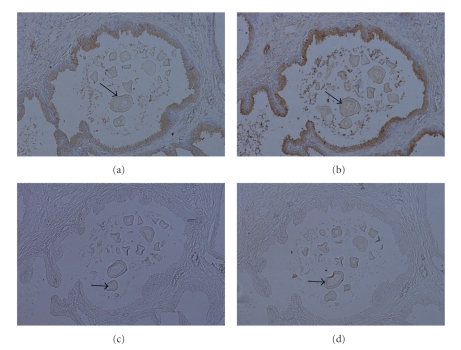
Corpora amylacea in BPH were stained in a heterogeneous pattern for all lectins used. The staining pattern for Cramoll 1,4 and Cramoll 3 was more intense than that observed when commercial lectins were used (Figures 4(a) to 4(d)). In PCa the corpora amylacea were not stained by the four lectins used.
Figure 4.
Lectin histochemistry of corpora amylacea (arrows) in BPH ((a) to (d)). In glands, corpora amylacea presented weak binding with Con-A (a) and non-staining to PNA (c); BPH glands were Cramoll 1,4 (b) and Cramoll 3 (d) moderately and weakly stained, respectively. Magnification x100.
Lectin staining was completely abolished in all tissues by inhibition of the lectin carbohydrate-binding site with solutions of methyl-α-D-mannopyranoside (0.3 M) for Con-A and Cramoll 1,4 and of D-galactose (0.3 M) for PNA and Cramoll 3.
4. Discussion
Cramoll 3 and Cramoll 1,4 were properly conjugated to HRP. Enzyme conjugation efficiency of in-house purified proteins is very important to assure the quality of histochemical assays [11].
Con-A and Cramoll 1,4 (glucose/mannose specific) data were similar to those reported by Morales et al. [12] using (Galanthus nivalis agglutinin GNA—10 μg/mL) and Con-A (20 μg/mL) for normal and hyperplastic prostate cells. They observed that normal prostatic glands showed low content or nonaccessibility to glucose/mannose residues when compared to the hyperplastic glands in which the high staining pattern indicates a high content or accessibility to these saccharides in secretory cells (apical cytoplasm of epithelium and luminal content). The lectin staining of secreted glycoconjugates with mannose/glucose residues is observed in human hyperplastic prostatic glands.
The increase in glucose/mannose glycocalyx content can be related to high production of glycoconjugates bearing these residues as observed by lectin histochemistry. In prostatic hyperplastic glands the presence of acid phosphatase which contains N-linked high-mannose chains in its structure was observed by Jakob et al. [13]. The present study also suggests that the increased lectin staining in BHP could be related to an increased secretor activity of prostatic cells in this pathology [14].
Con A staining of normal prostate stroma is in disagreement with the results of Arenas et al. [15] that observed a weak stroma staining of normal, BPH, and PCa tissues. This occurred probably because they used different enzymatic treatments that may have cleaved expressed stroma glycoconjugates bearing glucose/mannose residues in contrast to our trypsin treatment.
Con-A and Cramoll 1,4 different staining patterns indicate that the latter has greater diagnostic value for prostate diseases than the commercially available lectin. Such differences in staining can be explained by their similar but not equal quaternary structure which influences the stabilization of the lectin-carbohydrate complex [16]. Thus, the glycoconjugates expressed in pathological prostatic tissues seem to be more likely or prone to form more stable complexes with Cramoll 1,4.
In the present study, PNA and Cramoll 3 increased staining patterns from normal to PCa tissues are in agreement with the results of Janssen et al. [17] who observed that the binding of PNA increases from BPH to cancers. These authors also established a significant positive correlation between the number of PNA acceptors and those of prostatic-specific antigen.
Arenas et al. [15], using PNA, found an increasing staining pattern of epithelial cells in normal and BPH to PCa. We observed that using PNA as well as Cramoll 3 in PCa, galactose residues content increased only in luminal secretion and the apical membrane of malignant tumor glands and not in the cytoplasm of epithelium cells. The absence and/or nonaccessibility of galactose residues in epithelial cell glycoconjugates can be evaluated using a neuraminidase pretreatment instead of or together with trypsin (as used in our work) in order to expose galactose nonaccessible residues due to the presence of sialic acid residues. PNA staining in PCa stroma suggests that the development of PCa is associated with an increase in galactose residues in glycoconjugates at luminal border of prostate cells and stroma. Such results are in agreement with Arenas et al. [15].
The low incidence of corpora amylacea in PCa cannot be used to exclude malignancy as also observed by Christian and collaborators [18]. Corpora amylacea contain an amyloid substance, β2-microglobulin, a double content of sugars (glucosamine and galactose) in relation to proteins and sulfur atoms that suggest the presence of glycosaminoglycans [12, 18]. Our labeling for BPH glands and normal prostate are similar to those observed by Morales et al. [12]. The intense lectin staining in corpora amylacea of secretory cells of BPH is probably due to the accumulation of keratan sulfate after luminal secretion, a component which has been identified in both prostatic secretor cells and corpora amylacea [19]. The origin and function of corpora amylacea have been debated but its function continues to be unknown. Research indicates that corpora amylacea have a secretory origin [12, 20].
Beyond structural roles in increasing the protein stability, protecting from proteolysis, and improving the protein solubility [21], glycosylation promotes also general functional diversity reflected on the high structural possibilities of glycans [22] which can be acting as key events in apoptosis, immunomodulation, and antiproliferative activity in tumor cells [23]. And the lectins remain in focus to decipher these diverse glycan chains, being intensively used as cyto- and histochemistry tool for diagnosis and prognosis of cancer [23].
5. Conclusions
Our results showed that C. mollis lectin isoforms are able to recognize cells and an extracellular structure (corpora amylacea) of prostatic tissues. Proving that lectins bind differently the glycocode of normal and tumor cells and are able to detect subtle neoplastic changes of histologically related or similar pathologies; these two isoforms of a Brazilian Northeast native forage plant can be used as auxiliary tools in prostate cancer diagnosis compared to commercial lectins such as Con-A and PNA. The knowledge of the saccharide identity of cell glycoconjugates enables them to be used as targets for drug delivery and immunomodulation.
Acknowledgments
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for research grants.
References
- 1.Bryant RJ, Hamdy FC. Screening for prostate cancer: an update. European Urology. 2008;53(1):37–44. doi: 10.1016/j.eururo.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Instituto Nacional de Câncer; Ministério da Saúde. Estimativas 2008: incidência de câncer no Brasil. Rio de Janeiro (Brasil): INCA; 2007, http://bvsms.saude.gov.br/bvs/publicacoes/estimativa_incidencia_cancer_2008.pdf.
- 3.Isaacs W, De Marzo A, Nelson WG. Focus on prostate cancer. Cancer Cell. 2002;2(2):113–116. doi: 10.1016/s1535-6108(02)00103-4. [DOI] [PubMed] [Google Scholar]
- 4.Campbell CT, Sampathkumar S-G, Yarema KJ. Metabolic oligosaccharide engineering: perspectives, applications, and future directions. Molecular BioSystems. 2007;3(3):187–194. doi: 10.1039/b614939c. [DOI] [PubMed] [Google Scholar]
- 5.Velásquez JG, Canovas S, Barajas P, et al. Role of sialic acid in bovine sperm-zona pellucida binding. Molecular Reproduction and Development. 2007;74(5):617–628. doi: 10.1002/mrd.20619. [DOI] [PubMed] [Google Scholar]
- 6.Jiménez-Castells C, De La Torre BG, Andreu D, Gutiérrez-Gallego R. Neo-glycopeptides: the importance of sugar core conformation in oxime-linked glycoprobes for interaction studies. Glycoconjugate Journal. 2008;25(9):879–887. doi: 10.1007/s10719-008-9150-8. [DOI] [PubMed] [Google Scholar]
- 7.Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14(11):53–62. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 8.Sharon N. Lectins: carbohydrate-specific reagents and biological recognition molecules. The Journal of Biological Chemistry. 2007;282(5):2753–2764. doi: 10.1074/jbc.X600004200. [DOI] [PubMed] [Google Scholar]
- 9.Paiva PMG, Coelho LCBB. Purification and partial characterization of two lectin isoforms from Cratylia mollis mart. (camaratu bean) Applied Biochemistry and Biotechnology. 1992;36(2):113–118. [Google Scholar]
- 10.Correia MTS, Coelho LCBB. Purification of a glucose/mannose specific lectin, isoform 1, from seeds of Cratylia mollis mart. (camaratu bean) Applied Biochemistry and Biotechnology. 1995;55(3):261–273. doi: 10.1007/BF02786865. [DOI] [PubMed] [Google Scholar]
- 11.Beltrão EIC, Correia MTS, Figuerêdo-Silva J, Coelho LCBB. Binding evaluation of isoform 1 from Cratylia mollis lectin to human mammary tissues. Applied Biochemistry and Biotechnology A. 1998;74(3):125–134. doi: 10.1007/BF02825961. [DOI] [PubMed] [Google Scholar]
- 12.Morales E, Polo LA, Pastor LM, et al. Characterization of corpora amylacea glycoconjugates in normal and hyperplastic glands of human prostate. Journal of Molecular Histology. 2005;36(4):235–242. doi: 10.1007/s10735-005-5784-z. [DOI] [PubMed] [Google Scholar]
- 13.Jakob CG, Lewinski K, Kuciel R, Ostrowski W, Lebioda L. Crystal structure of human prostatic acid phosphatase. Prostate. 2000;42(3):211–218. doi: 10.1002/(sici)1097-0045(20000215)42:3<211::aid-pros7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Carson C, III, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61(4):2–7. doi: 10.1016/s0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 15.Arenas MI, Romo E, de Gaspar I, et al. A lectin histochemistry comparative study in human normal prostate, benign prostatic hyperplasia, and prostatic carcinoma. Glycoconjugate Journal. 1999;16(7):375–382. doi: 10.1023/a:1007012514118. [DOI] [PubMed] [Google Scholar]
- 16.de Souza GA, Oliveira PSL, Trapani S, et al. Amino acid sequence and tertiary structure of Cratylia mollis seed lectin. Glycobiology. 2003;13(12):961–972. doi: 10.1093/glycob/cwg115. [DOI] [PubMed] [Google Scholar]
- 17.Janssen T, Petein M, Van Velthoven R, et al. Differential histochemical peanut agglutinin stain in benign and malignant human prostate tumors: relationship with prostatic specific antigen immunostain and nuclear DNA content. Human Pathology. 1996;27(12):1341–1347. doi: 10.1016/s0046-8177(96)90348-2. [DOI] [PubMed] [Google Scholar]
- 18.Christian JD, Lamm TC, Morrow JF, Bostwick DG. Corpora amylacea in adenocarcinoma of the prostate: incidence and histology within needle core biopsies. Modern Pathology. 2005;18(1):36–39. doi: 10.1038/modpathol.3800250. [DOI] [PubMed] [Google Scholar]
- 19.Cohen RJ, Holland JW, Redmond SL, McNeal JE, Dawkins HJS. Identification of the glycosaminoglycan keratan sulfate in the prostatic secretory cell. Prostate. 2000;44(3):204–209. doi: 10.1002/1097-0045(20000801)44:3<204::aid-pros4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 20.Cohen RJ, McNeal JE, Redmond SL, et al. Luminal contents of benign and malignant prostatic glands: correspondence to altered secretory mechanisms. Human Pathology. 2000;31(1):94–100. doi: 10.1016/s0046-8177(00)80204-x. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld R, Bangio H, Gerwig GJ, et al. A lectin array-based methodology for the analysis of protein glycosylation. Journal of Biochemical and Biophysical Methods. 2007;70(3):415–426. doi: 10.1016/j.jbbm.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Angeloni S, Ridet JL, Kusy N, et al. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology. 2005;15(1):31–41. doi: 10.1093/glycob/cwh143. [DOI] [PubMed] [Google Scholar]
- 23.Końska G, Wójtowicz U, Pituch-Noworolska A. Possible application of lectins in diagnostics and therapy. Part I. Diagnostic application. Przegląd lekarski. 2008;65(4):189–194. [PubMed] [Google Scholar]