Abstract
I. viscosa has been used for years in folk medicine for its anti-inflammatory, antipyretic, antiseptic, and paper antiphlogistic activities. In this study, cytotoxic and genotoxic effects of I. viscosa leaf extracts on the root meristem cells of Allium cepa have been examined. Onion bulbs were exposed to 2.5 mg/ml, 5 mg/ml, and 10 mg/ml concentrations of the extracts for macroscopic and microscopic analysis. Tap water has been used as a negative control and Ethyl methanesulfonate (EMS) (2 · 10−2 M) has been used as a positive control. The test concentrations have been determined according to doses which are recommended for use in alternative medicine. There has been statistically significant (P < .05) inhibition of root growth depending on concentration by the extracts when compared with the control groups. All the tested extracts have been observed to have cytotoxic effects on cell division in A. cepa. I. viscosa leaf extract induces the total number of chromosomal aberrations and micronuclei (MNC) formations in A. cepa root tip cells significantly when compared with control groups. Also, this paper shows for the first time the induction of cell death, ghost cells, cells with membrane damage, and binucleated cells by extract treatment. These results suggest the cytotoxic and genotoxic effects of the I. viscosa leaf extracts on A. cepa.
1. Introduction
Medicinal herbs have been used in folk medicine for millennia. Simply, in recent times, scientific study of their effects has flourished. Nevertheless, some of them can cause adverse effects or have the potential to interact with other medications [1]; moreover, there is little information on the potential risk to health of such herbs [2]. Based on their long-term use by humans, one might expect herbs used in traditional medicine to have low toxicity. It is known that green plants in general are a primary source of antimutagens as well as natural toxic agents [3], and many plants contain cytotoxic and genotoxic substances. Recent investigations have revealed that many plants used as food or in traditional medicine have mutagenic effects and cytotoxic and genotoxic effects in vitro and in vivo assays [4–7]. This raises concern about the potential mutagenic or genotoxic hazards resulting from the long-term use of such plants. Many plants contain mutagenic and/or carcinogenic substances [8, 9] and their use has been correlated with high rate of tumor formation in some human populations [8, 10–13].
Inula viscosa (L.) Aiton (syn. Cupularia viscosa G. et G., Dittrichia viscosa Greuter) (Compositae); common name, “sticky fleabane” is a perennial weed that is found in most of the Mediterranean basin [14–16]. I. viscosa has been used for years in folk medicine for its antiinflammatory [17], antipyretic, antiseptic, and antiphlogistic activities [18, 19], and in the treatment of diabetes [20]. Aqueous extracts of I. viscosa exhibit antifungal activity in vitro [21, 22] and it has been demonstrated that some of its organic solvent extracts are antibacterial [23]. Cohen et al. [24] provided evidence for the antifungal activity in plant extracts made with organic solvents, including methanol, ethanol, ethyl acetate, acetone, chloroform, and n-hexane. In addition, this herb has been used in Spanish folk medicine for treating gastroduodenal disorders [25]. I. viscosa has antiulcerogenic effects [26], causes abortion [16, 27, 28], prevents zygote implantation in mammals [16], prevents growth of pathogenic fungi [29], has a strong antioxidant activity [30]. There is published evidence that I. viscosa has also nematicidal/antihelmynth properties [31].
I. viscosa contains some pharmacologically active compounds [32, 33] including sesquiterpenes, sesquiterpenes acids [34], azulenes, lactones, flavonoids, and essential oils [18] which are isolated and identified in its leaves. Currently, there is no published data on the cytotoxicity and genotoxicity of I. viscosa leaf extracts. The purpose of this study is to investigate cytotoxic and genotoxic effects of I. viscosa leaf extracts using the Allium Test.
2. Materials and Methods
2.1. Chemicals
Ethyl methanesulfonate (EMS) (CAS No: M-0880) was purchased from Sigma (Sigma Chemical Co., St Louis, MO).
2.2. Collection of Inula viscosa (L) Ait
I. viscosa specimens were collected from vicinities of Söke-Kuşadası/Aydın (Turkey) during flowering (November 2007). The plant was identified by Dr. Özkan EREN, botanist, Department of Biology; University of Adnan Menderes and voucher specimen was deposited at the Herbarium of Department of Biology, Adnan Menderes University.
2.3. Preparation of the Aqueous Extracts of I. viscosa Leaves
The extracts were prepared according to the traditional use in Turkey (decoction) and we used in this study crude extracts of I. viscosa leaves. Studying with crude extracts is appropriate because traditional medicinal herbs are generally used as crude extracts. The I. viscosa leaves were rinsed with water, dried in a ventilated oven at 55°C for 24 h and subsequently milled to a fine powder by ground into fine powder using a kitchen blender. The powder was placed in small plastic bags (100 g each) and stored at 4°C until use. The extract was prepared by boiling 20 g powdered plant material mixed with 200 ml distilled water for covered beaker (10% stock solution) for 5 min and, cooled to room temperature for 10 min. Thereafter, the extract was filtered through a filter paper (ISO Lab. Quantitative Filter Paper) to remove particulate matter. Stock solution was diluted with distilled water to 2.5 mg/ml, 5 mg/ml, and 10 mg/ml concentrations. Fresh extract was prepared daily for each experiment, just before administration.
2.4. Allium Test
Small bulbs (1.5–2.0 cm in diameter) of the common onion, A. cepa, (2n = 16) were purchased at a local supermarket. Prior to initiating the test, the outer scales of the bulbs and the dry bottom plate were removed without destroying the root primordia. For each extract sample, a series of six bulbs were placed in tap water (pH 7.3) for 48 h and then onion roots were treated with the leaves extracts at 2.5 mg/ml, 5 mg/ml, and 10 mg/ml concentrations of I. viscosa. The test tubes were kept in an incubator at 22 ± 1°C and the test samples were changed daily at the same time. Several of the newly formed root tips were then cut from each bulb and examined for any visible morphological abnormalities. The bulbs with satisfactory root lengths (2–2.5 cm) were used in the study, while those with exceptionally long or short roots were discarded (on average 2-3 bulbs). Therefore, individual sets of five bulbs were used for each extract sample. Tap water (pH 7.3) was used as a negative control [35, 36] and Ethyl methanesulfonate (EMS, 2.10−2 M) used as a positive control mutagen. EMS has been used in a wide variety of biological test systems in studies of mutation effects [37–39]. EMS induces DNA damage by a direct mechanism, acting at various sites as a monofunctional ethylating agent of nucleotides [40]. After 24 h of exposure, several root tips were removed from the bulbs, fixed in 3 : 1 (v/v) ethanol : glacial acetic acid and stored overnight at 4°C. The next day they were placed in 70% (v/v) aqueous alcohol and refrigerated until used. An average of five slides was made for each bulb using five roottips which hydrolyzed in 1 N hydrochloric acid (HCl) for 3 min and microscope slides were prepared by squashing the stained root tips in 2% (w/v) acetic orcein. Five slide was prepared per bulb, and each slide was examined using Olympus BX51 at a total magnification of 40 × 10.
The following parameters were used for determination of cytotoxicity and genotoxicity: (i) the mitotic index (MI) was calculated as the ratio between the number of mitotic cells and the total number of cells scored and expressed as percentage and (ii) chromatin aberrations (stickiness, breaks and polar deviation) were used as endpoints for determination of cytogenetic effects and micronuclei (MNC) were scored in interphase cells per 1000 cells (‰ MNC) [41]. The most frequent abnormalities are shown in microphotographs.
After 72 h of exposure to the I. viscosa leaf extract samples, the root lengths were measured and used as an index of general toxicity. The results for mitotic index and root length are expressed as percent of the negative and positive control. Visible morphological modifications, such as changes in root consistency and color as well as the presence of swelling (c-tumors), hooks or twists in the roots were also observed.
2.5. Statistical Analysis
Statistical analyses were performed using the SPSS 11.5 software package programme. Data on physicochemical parameters, root length, root growth, and mitotic index and chromosomal aberrations were compared using analysis of variance (ANOVA) to confirm the variability of the data and validity of results. Differences between corresponding controls and exposure treatments were considered statistically significant at P < .05.
3. Results
3.1. Physicochemical Characterization
The levels of the physicochemical parameters (root number and root length) are presented in Table 1. This results show that all tested concentrations of I. viscosa leaf extracts caused significant inhibition in the growth of roots in comparison to negative control and positive control. The inhibition of root number and root length was greater with increasing concentrations of I. viscosa leaf extracts. Measured average root length is 3.58 cm in negative control and 2.74 cm in positive control. However, average root length in 10 mg/ml treatment group was decreased significantly compared to that of the negative control (2.82 cm) (Table 1). Average root lengths in treatment groups were decreased depending on concentration, significantly. The root morphology was nearly normal during the negative control treatment, but at 2.5 mg/ml Inula leaf extract, the roots appeared slightly yellow and at 5 mg/ml Inula leaf extract, the roots appeared a slightly brown. At 10 mg/ml Inula leaf extract, the roots morphology showed an obvious difference in its appearance in that it turned to brownish in colour.
Table 1.
The average root numbers and root lengths in controls and treatment concentrations.
| Treatment groups | Concentrations | Average root number ± SD | Average root lengths (cm) ± SD |
|---|---|---|---|
| Negative control | Tap water | 37.6 ± 4.03 | 3.58 ± 0.61 |
| Positive Control (EMS) | 2 × 10−2 M | 28.6 ± 4.72* | 2.74 ± 0.42* |
| Inula1 | 2.5 mg/ml | 22.0 ± 5.52* | 3.36 ± 0.52 |
| Inula2 | 5 mg/ml | 20.6 ± 2.30* | 3.12 ± 0.33 |
| Inula3 | 10 mg/ml | 19.2 ± 5.97* | 2.82 ± 0.53* |
* P < .05 in One Way ANOVA.
3.2. Cytogenetic Analysis
With the objective of investigating the possible mechanism involved in root growth inhibition, cytogenetic analysis was performed. I. viscosa leaf extracts provoked strong inhibition of the mitotic index, where a statistically significant difference in relation to the control and the decrease in the mitotic index was positively correlated with increasing concentration of the I. viscosa leaf extracts (Table 2).
Table 2.
The dividing and total cells counted in microscopic observations and mitotic values in control and in treatment concentrations.
| Treatment groups | Concentrations | Total cells | Dividing cells | MI (%) ± SD |
|---|---|---|---|---|
| Negative control | Tap water | 25000 | 1687 | 6.748 ± 1.17 |
| Pozitive control (EMS) | 2 × 10−2 M | 25000 | 481 | 1.924 ± 0.91* |
| Inula1 | 2.5 mg/ml | 25000 | 800 | 3.200 ± 0.60* |
| Inula2 | 5 mg/ml | 25000 | 518 | 1.984 ± 0.75* |
| Inula3 | 10 mg/ml | 25000 | 22 | 0.088 ± 0.05* |
*P < .05 in One Way ANOVA.
Cytogenetic alterations were investigated and the results can be seen in Table 3. I. viscosa leaf extracts induced chromosome and cytological alterations both in treatment and control groups. An analysis of chromosome aberrations showed that most of the fragments detected in the different treatments were of chromosome type (Figure 1(a)). The observation of chromosome breaks showed the clastogenic effect of I. viscosa leaf extracts. The occurrence of chromosome fragments allows observation of statistically significant differences at I. viscosa leaf extracts.
Table 3.
Chromosome and mitotic aberrations in the root meristem cells of Allium cepa after I. viscosa leaf extract treatment.
| Treatment groups | Concentrations | Chromosome breaks (%) ± SD | Stickiness (%) ± SD | Polar deviations (%) ± SD | Aberrant cell (%) ± SD | MNC (‰) ± SD |
|---|---|---|---|---|---|---|
| Negative control | Tap water | — | 0.69 ± 0.91 | 7.65 ± 1.97 | 8.34 ± 1.85 | 0.28 ± 0.18 |
| Positive control (EMS) | 2 × 10−2 M | — | 31.63 ± 12.88* | 8.97 ± 6.18 | 40.60 ± 9,94* | 0.68 ± 0.18 |
| Inula1 | 2.5 mg/ml | 7.22 ± 2.61 | 17.32 ± 2.52* | 6.09 ± 1.36 | 30.63 ± 5.03* | 0.64 ± 0.17 |
| Inula2 | 5 mg/ml | 0.95 ± 0.91 | 28.74 ± 8.18* | 10.14 ± 1.33 | 39.83 ± 7.10* | 0.48 ± 0.41 |
| Inula3 | 10 mg/ml | — | 8.89 ± 1.44* | 9.44 ± 12.96 | 18.33 ± 6.93* | 0.04 ± 0.08* |
*P < .05 in One Way ANOVA.
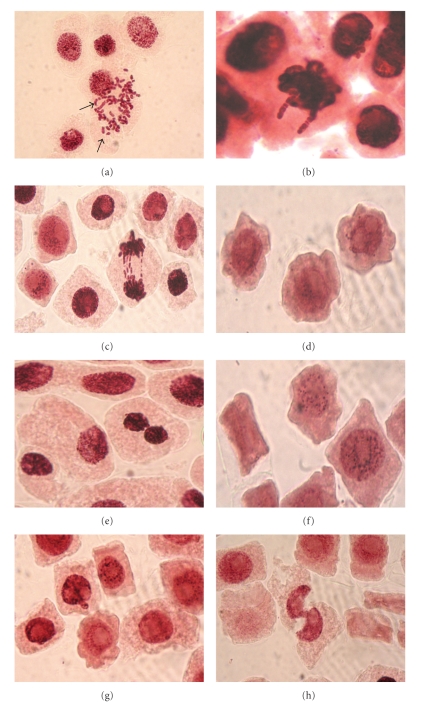
Figure 1.
Mitotic and chromosomal aberrations after the Inula viscosa leaf extract treatments in Allium cepa root tip meristem cells visualized with light microscopy. (a) Fragments; (b) stickiness; (c) polar deviation and chromatid bridges; (d) membrane damage; (e) binucleated cell; (f) apoptotic bodies; (g and h) cells with damaged nucleus.
In addition to the chromosome fragments, sticky metaphase and polar deviations (wrong directions of chromosome movement) were also observed (Figures 1(b) and 1(c)). In general, it was possible to observe an increase of different abnormalities as the I. viscosa leaf extracts concentration increased. In Allium test, a strong toxic effect of I. viscosa leaf extract was observed, supported by great occurrence of sticky metaphases, leading to cellular death (mitotic index decrease). A statistically significant increase in total aberrant cells (P < .05) (aberrant cells include chromosome breaks, stickiness and polar deviation) as compared with the negative control (Table 3), however, the highest value of aberrant cells is shown by the positive control.
Statistical analysis showed that the genotoxic activities of the I. viscosa leaf extracts induced micronuclei in the root tip meristem cells of A. cepa. Micronucleus formation in 1000 cells per slide (‰ MNC value) was also increased in extract concentrations compared with negative and positive control, which is statistically significant (P < .05) (Figure 3(a)). The increase occurred in the positive control, Inula1 and Inula2 with respect to the negative control, and not in Inula3 which is expected since MI is extremely low.
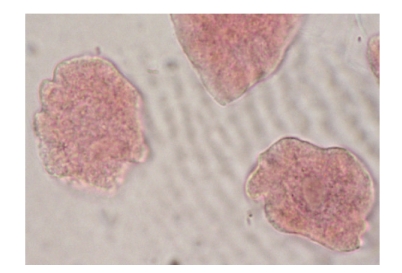
Figure 3.
Ghost cells.
In addition, cells with membrane damage (Figure 1(d)), binucleated cells (Figure 1(e)), and nucleus damage (Figures 1(g) and 1(h)) were found in various frequencies. Also, apoptotic cells (Figure 1(f)) were detected in the group treated with I. viscosa leaf extract. Moreover, ghost cells were detected in 10 mg/ml I. viscosa leaf extract treatment (Figure 2(a)).
Figure 2.
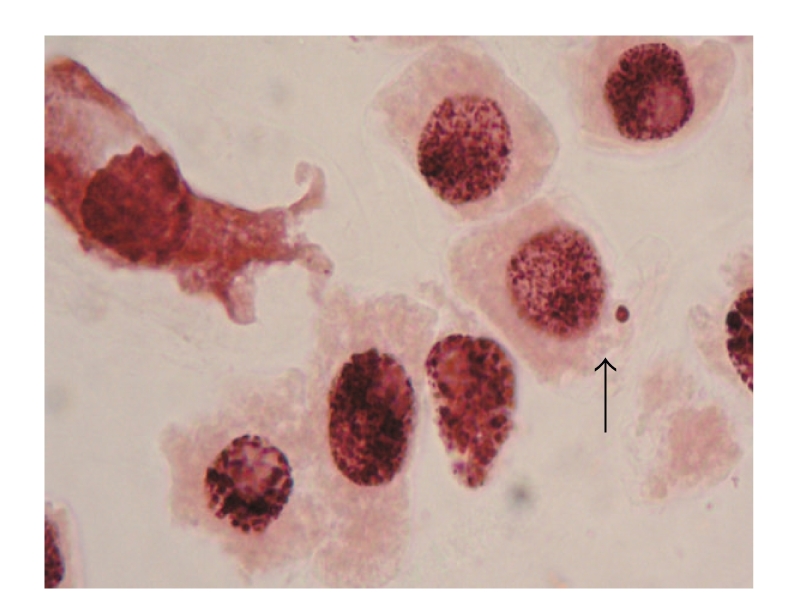
Micronucleus.
4. Discussion
In this study, toxic effects of I. viscosa leaf extract was evaluated by analyzing root growth and root morphology. The higher I. viscosa extracts caused an inhibition of root growth and there was a statistically significant difference between control groups. In addition, the I. viscosa extracts induced slightly yellow, slightly brown and brownish in coloration in roots. Cyto- and genotoxicity were estimated by observing cytological parameters such as the mitotic index and number of chromosome abnormalities, including chromosome breaks, stickiness, and polar deviations. The mitotic index (MI) of A. cepa meristematic cells treated with the EMS was significantly decreased (1.924% in comparison to negative control). Significant inhibition in the onion roots treated with the I. viscosa extracts (3.200%, 1.984% and 0.088% compared to the negative control) (Table 2). A positive correlation was found between inhibition of root growth and decrease of MI. The decline of MI below 22% in comparison to negative control can have lethal impact on the organism [42], while a decrease below 50% usually has sublethal effects [43] and is called cytotoxic limit value [44]. MI measures the proportion of cells in the M-phase of the cell cycle and its inhibition could be interpreted as cellular death or a delay in the cell proliferation kinetics [45]. Reduction in the mitotic activity could be due to inhibition of DNA synthesis or a blocking in the G2 phase of the cell cycle, preventing the cell from entering mitosis [46]. Mitodepressive effects of some herbal extracts, including the ability to block the synthesis of DNA and nucleus-proteins, were reported earlier [47, 48]. Several other herbal extracts have been reported to inhibit mitosis [7, 49, 50]. The decreased MI in A. cepa roots treated with I. viscosa leaf extracts is probably due to either disturbances in the cell cycle or chromatin dysfunction induced by an external factor, in this case, I. viscosa extracts- DNA interactions. The results herein suggest that the tested I. viscosa leaf extracts concentrations have inhibitory, mito-depressive effects on root growth and cell division of A. cepa and it can prevent DNA synthesis and the reduction in number of the dividing cells in roots produced by the cytotoxic effects of compounds found in I. viscosa leaf extracts.
I. viscosa leaf extracts showed the strongest genotoxic effects in the root meristem cells. The observation of sticky metaphase reinforces the hypothesis of the toxic effect of I. viscosa leaf extracts. Metaphases with sticky chromosome, loses their normal appearance, and they are seen with a sticky “surface,” causing chromosome agglomeration [51]. Stickiness has been attributed to the effect of pollutants and chemical compounds on the physical-chemical properties of DNA, protein or both, on the formation of complexes with phosphate groups in DNA, on DNA condensation or on formation of inter- and intra chromatid cross links [52–56]. Chromosomal aberrations (CA) are changes in chromosome structure resulting from a break or exchange of chromosomal material. Most of the CA observed in cells are lethal, but there are many related aberrations that are viable and that can cause genetic effects, either somatic or inherited [57]. The presence of chromosome fragments is an indication of chromosome breaks, and can be a consequence of anaphase/telophase bridges [58, 59]. The induction of chromosome breaks, disturbances on microtubule assembly and cellular death can be related. Our results showed induction of chromosome type of aberration in the cells treated with the I. viscosa leaf extracts. Somehow the I. viscosa leaf extracts not only interfere with the cell cycle, but also affect chromatin organization or DNA replication, causing chromosome breaks. Frequencies of total chromosome aberrations increased significantly upon exposure to I. viscosa leaf extracts which indicate clastogenic activity (Table 3). These results are in line with the results of many research groups that examined the effects of different medicinal herbs [7, 60, 61].
I. viscosa leaf extracts significantly induced the formation of MNC in A. cepa root cells at 2.5–10 mg/ml concentrations. Frequencies of MNC increased in 2.5 mg/ml and 5 mg/ml I. viscosa leaf extract. However, MNC frequency decreased in A. cepa roots treatment at the highest I. viscosa leaf extract concentration (10 mg/ml), due to high cytotoxicity. The frequency of cells with micronuclei is a good indicator of the cytogenetic effects of tested chemicals. Micronuclei (MN) often results from the acentric fragments or lagging chromosomes that fail to incorporate into the daughter nuclei during telophase of the mitotic cells and can cause cellular death due to the deletion of primary genes [62, 63]. Recent studies have suggested MNC-induced effect of various plant extracts. In our recent study, we report MNC-induced effect of Lavandula stoechas aqueous extract and Ecballium elaterium fruit juices on A. cepa root tip meristematic cells [7, 64]. Furthermore, Soliman [60] reported MNC formation by Azadirachta indica A. Juss. aqueous extract treatment on A. cepa root tip meristematic cells.Akinboro and Bakare [50] reported MNC formation by treatment of some Psychotria species extracts on A. cepa root tip meristematic cells.
In this study, membrane damage cells was observed in groups treated with 5 mg/ml and 10 mg/ml I. viscosa leaf extracts. These results show that I. viscosa leaf extracts over certain concentrations may cause cytotoxicity as they cause membrane damage. Maoz and Neeman [29] evaluated effects of I. viscosa extract on chitin synthesis in dermatophytes and Candida albicans. They demonstrated that I. viscosa extract inhibited the growth of dermatophytes and C. albicans and caused a significant decline in chitin content. Chemically speaking, chitin is closely related to chitosan (a more water soluble derivative of chitin). It is also closely related to cellulose in that it is a long unbranched chain of glucose derivates which replaces chitin in plants. In addition, it is needed to clarify whether the decline of cellulose is due to direct inhibition of the membrane enzyme, cellulose synthase, or due to damage of the whole membrane. Binucleated cells have been observed in 5 mg/ml extract treatment group. The occurrence of binucleated cells is the result of prevention of cytokinesis or cell plate formation. Microtubules have been implicated in cell plate formation and I. viscosa leaf extracts can be one of the involved factors, resulting in inhibition of cytokinesis. These results are in accordance with the literature data. Similar inhibition of cytokinesis cells were also reported by Kaushik [65], Borah and Talukdar [66], and Gömürgen et al. [67].
On the other hand, ghost cells have been observed in various frequencies in this study for the first time (Figure 2). The ghost cell induced effect of I. viscosa was not reported by other authors. Ghost cell is a dead cell in which the outline remains visible, but whose nucleus and cytoplasmic structures are not stainable [68]. It is a possibility that substances in the high concentrations (10 mg/ml) of I. viscosa leaf extract leading to nucleus damage and prevention of cytoplasmic structures resulted in ghost cells.
In addition, I. viscosa leaf extracts induced DNA damage and cell death and/or apoptosis in various frequencies in this study. This study shows for the first time the induction of cell death and/or apoptosis caused by high concentrations (5 mg/ml and 10 mg/ml). Cell death is a basic biological process of living organism. The cell death was induced by high concentrations of such as toxin, stress, heavy metals, chemicals and other. The authors suggested that cells undergo death after moderate stress. In this way, in this paper demonstrated that I. viscosa leaf extract induced cytogenetic alterations (cytoplasmic shrinkage, nuclear condensation, DNA fragmentation, membrane blebbing, cytoskeleton alterations and appearance of apoptotic bodies) aid mainly cell death in root tips of A. cepa (Figures 1(d), 1(f), 1(g), and 1(h)). A. cepa demonstrated to be more sensitive as expected. Considering this, the aim of this study is to determine the features of cell death in root tips of A. cepa induced by I. viscosa leaf extracts.
Finally, we conclude that when applied in high doses, I. viscosa leaf extract shows cytotoxic and genotoxic activity. We used in this study crude extracts of I. viscosa leaves. Studying with crude extracts is appropriate because traditional medicinal herbs are generally used as crude extracts. However, working with crude extracts also means working with complex mixtures of biologically active compounds. Some of these compounds can be cytotoxic and/or genotoxic; others can be cytoprotective and/or antigenotoxic. The results of this study suggest that, although I. viscosa has beneficial effects as a medicinal herb, it can cause serious problems and damage on cells when used improperly.
References
- 1.Zink T, Chaffin J. Herbal health products: what family physicians need to know. American Family Physician. 1998;58(5):1133–1140. [PubMed] [Google Scholar]
- 2.Basaran AA, Yu T-W, Plewa MJ, Anderson D. An investigation of some Turkish herbal medicines in Salmonella typhimurium and in the COMET assay in human lymphocytes. Teratogenesis Carcinogenesis and Mutagenesis. 1996;16(2):125–138. doi: 10.1002/(SICI)1520-6866(1996)16:2<125::AID-TCM6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Plewa MJ, Wagner ED. Activation of promutagens by green plants. Annual Review of Genetics. 1993;27:93–113. doi: 10.1146/annurev.ge.27.120193.000521. [DOI] [PubMed] [Google Scholar]
- 4.Higashimoto M, Purintrapiban J, Kataoka K, et al. Mutagenicity and antimutagenicity of extracts of three spices and a medicinal plant in Thailand. Mutation Research. 1993;303(3):135–142. doi: 10.1016/0165-7992(93)90026-r. [DOI] [PubMed] [Google Scholar]
- 5.Schimmer O, Kruger A, Paulini H, Haefele F. An evaluation of 55 commercial plant extracts in the Ames mutagenicity test. Pharmazie. 1994;49(6):448–451. [PubMed] [Google Scholar]
- 6.Kassie F, Parzefall W, Musk S, et al. Genotoxic effects of crude juices from Brassica vegetables and juices and extracts from phytopharmaceutical preparations and spices of cruciferous plants origin in bacterial and mammalian cells. Chemico-Biological Interactions. 1996;102(1):1–16. doi: 10.1016/0009-2797(96)03728-3. [DOI] [PubMed] [Google Scholar]
- 7.Aşkın Çelik T, Aslantürk ÖS. Cytotoxic and genotoxic effects of Lavandula stoechas aqueous extracts. Biologia. 2007;62(3):292–296. [Google Scholar]
- 8.Ames BN. Food constituents as a source of mutagens, carcinogens and anticarcinogens. In: Knudsen I, editor. Genetic Toxicology of the Diet. New York, NY, USA: Alan R. Liss; 1986. pp. 55–62. [PubMed] [Google Scholar]
- 9.Fernandes De Sá Ferreira IC, Ferrão Vargas VM. Mutagenicity of medicinal plant extracts in Salmonella/microsome assay. Phytotherapy Research. 1999;13(5):397–400. doi: 10.1002/(sici)1099-1573(199908/09)13:5<397::aid-ptr473>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Wynder EL, Hall NEL, Polansky M. Epidemiology of coffee and pancreatic cancer. Cancer Research. 1983;43(8):3900–3906. [PubMed] [Google Scholar]
- 11.Nagao M, Wakabayashi K, Fujita Y, Tahira T, Ochiaia T, Sugimura T. Mutagenic compounds in soy sauce, Chinese cabbage, coffee and herbal teas. In: Knudsen I, editor. Genetic Toxicology of the Diet. New York, NY, USA: Alan R. Liss; 1986. pp. 55–62. [PubMed] [Google Scholar]
- 12.Nguyen T, Fluss L, Hodej R, Ginther G, Leighton T. The distribution of mutagenic activity in red rose and white wines. Mutation Research. 1989;223:205–212. doi: 10.1016/0165-1218(89)90048-7. [DOI] [PubMed] [Google Scholar]
- 13.Brito MT, Martinez A, Cadavid NFC. Mutagenic activity in regional foods and beverages from the Venezuelan Andean region. Mutation Research. 1990;243(2):115–120. doi: 10.1016/0165-7992(90)90032-f. [DOI] [PubMed] [Google Scholar]
- 14.Al-Eisawi D. Field Fuide to Wild Flowers in Jordan and Neighboring Countries. Amman, Jordan: Jordan Foundation Press; 1998. [Google Scholar]
- 15.Baytop T. Therapy with Medicinal Plants in Turkey. İstanbul, Turkey: Nobel Medical Publication; 1999. [Google Scholar]
- 16.Al-Dissi NM, Salhab AS, Al-Hajj HA. Effects of Inula viscosa leaf extracts on abortion and implantation in rats. Journal of Ethnopharmacology. 2001;77(1):117–121. doi: 10.1016/s0378-8741(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 17.Barbetti P, Chiappini I, Fardella G, Menghini A. A new eudesmane acid from Dittrichia (Inula) viscosa. Planta Medica. 1985;51:p. 471. doi: 10.1055/s-2007-969564. [DOI] [PubMed] [Google Scholar]
- 18.Lauro L, Rolih C. Observations and research on an extract of Inula viscosa. Bollettino della Societa Italiana di Biologia Sperimentale. 1990;66(9):829–834. [PubMed] [Google Scholar]
- 19.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. Journal of Ethnopharmacology. 2000;72(1-2):191–205. doi: 10.1016/s0378-8741(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 20.Yaniv Z, Dafni A, Friedman J, Palevitch D. Plants used for the treatment of diabetes in Israel. Journal of Ethnopharmacology. 1987;19(2):145–151. doi: 10.1016/0378-8741(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 21.Maoz M, Kashman Y, Neeman I. Isolation and identification of a new antifungal sesquiterpene lactone from Inula viscosa. Planta Medica. 1999;65(3):281–282. doi: 10.1055/s-2006-960780. [DOI] [PubMed] [Google Scholar]
- 22.Qasem JR, Al-Abed AS, Abu-Blan MA. Antifungal activity of clammy inula (Inula viscosa) on Helminthrosporium sativum and Fusarium oxysporum f. sp. lycopersici. Phytopathologia Mediterranea. 1995;34:7–14. [Google Scholar]
- 23.Debat J. Inula extract, its method of preparation and its use as pharmaceutical. US patent no. 4254112, 1991.
- 24.Cohen Y, Baider A, Ben-Daniel BH, Ben-Daniel Y. Fungicidal preparations from Inula viscosa. Plant Protection Science. 2002;38:629–630. [Google Scholar]
- 25.Lastra C, Lopez A, Motilva V. Gastroprotection and prostaglandin E2 generation in rats by flavonoids of Dittrichia viscosa. Planta Medica. 1993;59(6):497–501. doi: 10.1055/s-2006-959747. [DOI] [PubMed] [Google Scholar]
- 26.Alkofahi A, Atta AH. Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. Journal of Ethnopharmacology. 1999;67(3):341–345. doi: 10.1016/s0378-8741(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 27.Farnsworth NR, Bingel AS, Cordell GA, Crane FA, Fong HS. Potential value of plants as sources of new antifertility agents II. Journal of Pharmaceutical Sciences. 1975;64(5):717–754. doi: 10.1002/jps.2600640504. [DOI] [PubMed] [Google Scholar]
- 28.Karim F, Al-Okleh A, Suleiman S, Quraan S. Poisonous Plants in Jordan. Irbid, Jordan: Jordan Natural History Museum; 1990. [Google Scholar]
- 29.Maoz M, Neeman I. Effect of Inula viscosa extract on chitin synthesis in dermatophytes and Candida albicans. Journal of Ethnopharmacology. 2000;71(3):479–482. doi: 10.1016/s0378-8741(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 30.Schinella GR, Tournier HA, Prieto JM, Mordujovich P, Rios JL. Antioxidant activity of anti-inflammatory plant extracts. Life Sciences. 2002;70(9):1023–1033. doi: 10.1016/s0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- 31.Oka Y, Ben-Daniel B-H, Cohen Y. Nematicidal activity of powder and extracts of Inula viscosa. Nematology. 2001;3(8):735–742. [Google Scholar]
- 32.Ulubelen A, Öksüz S, Gören N. Sesquiterpene acids from Inula viscosa. Phytochemistry. 1987;26(4):1223–1224. [Google Scholar]
- 33.Wollenweber E, Mayer K, Roitman JN. Exudate flavonoids of Inula viscosa. Phytochemistry. 1991;30(7):2445–2446. [Google Scholar]
- 34.Marongiu B, Piras A, Pani F, Porcedda S, Ballero M. Extraction, separation and isolation of essential oils from natural matrices by supercritical CO2. Flavour and Fragrance Journal. 2003;18(6):505–509. [Google Scholar]
- 35.Fiskesjo G. A 2-3 day plant test for toxicity assessment by measuring the mean root growth of onions (Allium cepa L.) Environmental Toxicology and Water Quality. 1993;8(4):461–470. [Google Scholar]
- 36.Fiskesjö G. Allium test for screening chemicals; evaluation of cytological parameters. In: Wang W, Gorsuch JW, Hughes JS, editors. Plants for Environmental Studies. New York, NY, USA: Lewis; 1997. pp. 308–333. [Google Scholar]
- 37.Sega GA. A review of the genetic effects of ethyl methanesulfonate. Mutation Research. 1984;134(2-3):113–142. doi: 10.1016/0165-1110(84)90007-1. [DOI] [PubMed] [Google Scholar]
- 38.Platzek T, Bochert G, Meister R. Embryotoxicity induced by alkylating agents: 9. Low dose prenatal-toxic risk estimation of ethylmethanesulfonate based on no-observed-adverse-effect-level risk factor approach, dose-response relationships, and molecular dosimetry. Teratogenesis Carcinogenesis and Mutagenesis. 1995;15(2):81–92. doi: 10.1002/tcm.1770150205. [DOI] [PubMed] [Google Scholar]
- 39.Bökel C. EMS screens: from mutagenesis to screening and mapping. Methods in Molecular Biology. 2008;420:119–138. doi: 10.1007/978-1-59745-583-1_7. [DOI] [PubMed] [Google Scholar]
- 40.Müller L, Gocke E, Lavé T, Pfister T. Ethyl methanesulfonate toxicity in viracept-a comprehensive human risk assessment based on threshold data for genotoxicity. Toxicology Letters. 2009;190(3):317–329. doi: 10.1016/j.toxlet.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Pavlica M, Besendorfer V, Roša J, Papěs D. The cytotoxic effect of wastewater from the phosphoric gypsum depot on common oak (Quercus robur L.) and shallot (Allium cepa var. ascalonicum) Chemosphere. 2000;41(10):1519–1527. doi: 10.1016/s0045-6535(00)00106-5. [DOI] [PubMed] [Google Scholar]
- 42.Antonsie-wiez D. Analysis of the cell cycle in the root meristem of Allium cepa under the influence of Leda krin. Folia Histochemica et Cytobiologica. 1990;26:79–96. [PubMed] [Google Scholar]
- 43.Panda BB, Sahu UK. Induction of abnormal spindle function and cytokinesis inhibition in mitotic cells of Allium cepa by the organophosphorus insecticide fensulfothion. Cytobios. 1985;42(167-168):147–155. [Google Scholar]
- 44.Sharma CBSR. Plant meristems as monitors of genetic toxicity of environmental chemicals. Current Science. 1983;52:1000–1002. [Google Scholar]
- 45.Rojas E, Herrera LA, Sordo M, et al. Mitotic index and cell proliferation kinetics for identification of antineoplastic activity. Anti-Cancer Drugs. 1993;4(6):637–640. doi: 10.1097/00001813-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Sudhakar R, Ninge Gowda KN, Venu G. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia. 2001;66(3):235–239. [Google Scholar]
- 47.Mercykutty VC, Stephen J. Adriamycin induced genetic toxicity as demonstrated by Allium cepa test. Cytologia. 1980;45(4):769–777. [Google Scholar]
- 48.Schulze E, Kirschner M. Microtubule dynamics in interphase cells. Journal of Cell Biology. 1986;102(3):1020–1031. doi: 10.1083/jcb.102.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aşkin Çelik T, Aslantürk ÖS. Anti-mitotic and anti-genotoxic effects of Plantago lanceolata aqueous extract on Allium cepa root tip meristem cells. Biologia. 2006;61(6):693–697. [Google Scholar]
- 50.Akinboro A, Bakare AA. Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants. Journal of Ethnopharmacology. 2007;112(3):470–475. doi: 10.1016/j.jep.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Babich H, Segall MA, Fox KD. The Allium test—a simple, eukaryote genotoxicity assay. American Biology Teacher. 1997;59(9):580–583. [Google Scholar]
- 52.Shahin SA, El-Amoodi KHH. Induction of numerical chromosomal aberrations during DNA synthesis using the fungicides nimrod and rubigan-4 in root tips of Vicia faba L. Mutation Research. 1991;261(3):169–176. doi: 10.1016/0165-1218(91)90064-s. [DOI] [PubMed] [Google Scholar]
- 53.Rencüzoğulları E, İla HB, Kayraldiz A, Topaktaş M. Chromosome aberrations and sister chromatid exchanges in cultured human lymphocytes treated with sodium metabisulfite, a food preservative. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2001;490(2):107–112. doi: 10.1016/s1383-5718(00)00142-x. [DOI] [PubMed] [Google Scholar]
- 54.El-Ghamery AA, El-Kholy MA, El-Yousser MAA. Evaluation of cytological effects of Zn2+ in relation to germination and root growth of Nigella sativa L. and Triticum aestivum L. Mutation Research. 2003;537:29–41. doi: 10.1016/s1383-5718(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 55.Gömürgen AN. Cytological effect of the potassium metabisulphite and potassium nitrate food preservative on root tips of Allium cepa L. Cytologia. 2005;70(2):119–128. [Google Scholar]
- 56.Türkoglu Ş. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2007;626(1-2):4–14. doi: 10.1016/j.mrgentox.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Swierenga SHH, Heddle JA, Sigal EA, et al. Recommended protocols based on a survey of current practice in genotoxicity testing laboratories, IV. Chromosome aberration and sister-chromatid exchange in Chinese hamster ovary, V79 Chinese hamster lung and human lymphocyte cultures. Mutation Research. 1991;246(2):301–322. doi: 10.1016/0027-5107(91)90050-x. [DOI] [PubMed] [Google Scholar]
- 58.Sharma A, Sen S. Chromosome Botany. Enfield, NH, USA: Science; 2002. [Google Scholar]
- 59.Singh RJ. Plant Cytogenetics. Boca Raton, Fla, USA: CRC Press; 2003. [Google Scholar]
- 60.Soliman MI. Genotoxicity testing of neem plant (Azadirachta indica A. Juss.) using the Allium cepa chromosome aberration assay. Journal of Biological Sciences. 2001;1(11):1021–1027. [Google Scholar]
- 61.Bidau CJ, Amat AG, Yajia M, Marti DA, Riglos AG, Silvestroni A. Evaluation of the genotoxicity of aqueous extracts of Ilex paraguariensis St. Hil. (Aquifoliaceae) using the Allium test. Cytologia. 2004;69(2):109–117. [Google Scholar]
- 62.Albertini RJ, Anderson D, Douglas GR, et al. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutation Research. 2000;463(2):111–172. doi: 10.1016/s1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- 63.Krishna G, Hayashi M. In vivo rodent micronucleus assay: protocol, conduct and data interpretation. Mutation Research. 2000;455(1-2):155–166. doi: 10.1016/s0027-5107(00)00117-2. [DOI] [PubMed] [Google Scholar]
- 64.Aşkin Çelik TA, Aslantürk ÖS. Investigation of cytotoxic and genotoxic effects of Ecballium elaterium juice based on Allium test. Methods and Findings in Experimental and Clinical Pharmacology. 2009;31(9):591–596. doi: 10.1358/mf.2009.31.9.1434629. [DOI] [PubMed] [Google Scholar]
- 65.Kaushik GC. Cytological effects of Lantana camara L. leaves extract on Vicia faba root tip cells. Advanced Plant Science. 1996;9:159–164. [Google Scholar]
- 66.Borah SP, Talukdar J. Studies on the cytotoxic effects of extract of castor seed (Ricinus communis L.) Cytologia. 2002;67(3):235–243. [Google Scholar]
- 67.Gömürgen AN, Mutlu F, Bozcuk S. Effects of polyamines (Putrescine, spermidine and spermine) on root tip mitosis and chromosomes in Allium cepa L. Cytologia. 2005;70(2):217–224. [Google Scholar]
- 68.Medical dictionary. http://medical-dictionary.thefreedictionary.com/ghost+cell.