Abstract
In a variety of vertebrates, including turtle, many classical and contemporary studies of spinal cord neuronal networks generating rhythmic motor behaviors emphasize a Reciprocal Model with alternation of agonists and antagonists, alternation of excitatory and inhibitory postsynaptic potentials, and reciprocal inhibition. Some studies of spinal cord neuronal networks, including those in turtle during scratch motor rhythms, describe a Balanced Model with concurrent excitatory and inhibitory postsynaptic potentials. The present report reviews turtle spinal cord studies and concludes that there is support for a Combined Model with both alternating and concurrent excitation and inhibition, i.e., characteristics of both the Reciprocal and the Balanced Models, in the same spinal cord neuronal network for scratch reflex in turtle. Studies of spinal cord neuronal networks for locomotion in a variety of vertebrates also support a Combined Model.
Keywords: turtle, scratch reflex, spinal cord, Central Pattern Generator, reciprocal inhibition, balanced excitation and inhibition
Introduction
Agonist-antagonist alternation is a fundamental feature of the motor neuron patterns responsible for generating vertebrate rhythmic limb motor behaviors.1–19 These rhythmically alternating motor patterns are generated within the spinal cord by neuronal networks, termed central pattern generators (CPGs), without movement-related sensory inputs and without information from supraspinal structures.2–19 Hypotheses about the organization of these CPGs incorporate reciprocal inhibition between agonist and antagonist modules (also termed half-centers or unit-burst-generators) as one of the fundamental mechanisms responsible for the generation of agonist-antagonist alternation.1–19 This reciprocal inhibitory organization is also termed the Reciprocal Model.20
Concurrent excitation and inhibition is another important feature of neuronal networks.5–8, 10, 13, 20–28 Some of the experimental support for this feature in spinal cord utilizes intracellular recordings from turtle hindlimb motor neurons during fictive scratch motor rhythms.7, 20, 26, 27 During these rhythms, each motor neuron has an active phase during which the neuron fires action potentials and a quiet phase during which the motor neuron does not fire. For each motor neuron, there are concurrent excitatory and inhibitory synaptic potentials: this co-activation is termed Balanced Excitation and Inhibition, that is, the Balanced Model.20, 26, 27 The Reciprocal Model is contrasted with the Balanced Model in Fig. S1 of Berg, Alaburda, and Hounsgaard:20 one interpretation of this contrast is that these two Models represent divergent points of view with features that do not coexist in the same network.
The present review summarizes data obtained in studies of turtle scratch reflex7, 18, 29–66 that support the point of view that features of the Reciprocal Model characterize turtle spinal cord CPGs for scratch. The review further discusses studies that support the point of view that features of the Balanced Model also characterize these turtle scratch CPGs.7, 20, 26, 27 The review concludes with the point of view that features of both the Reciprocal and the Balanced Models are characteristics of the same neuronal network in the turtle spinal cord. The Combined Model is the term for a network with features of both the Reciprocal Model and the Balanced Model. The Combined Model is supported by experiments with turtle scratch neuronal networks that demonstrate in the same network that there is: some inhibition that alternates with excitation (Reciprocal Model); other inhibition that is concurrent with excitation during the active phase of that motor neuron’s motor pool (Balanced Model during Motor Pool’s Active Phase); and still other inhibition that is concurrent with excitation during the quiet phase of that motor neuron’s motor pool (Balanced Model during Motor Pool’s Quiet Phase).
Chance, Abbott, and Reyes23 and Abbott and Chance25 provide important perspectives in support of a neuronal network characterized by a Combined Model. They use the term “driving inputs” to describe a Reciprocal Model with excitation and inhibition acting in a push-pull manner and the term “modulatory inputs” to describe a Balanced Model with concurrent excitation and inhibition. They state “Sets of balanced inputs that have excitatory and inhibitory rates rising and falling together comprise modulatory inputs, and those for which excitation and inhibition vary in opposite directions act as driving inputs. This arrangement has the advantage that individual excitatory inputs can rapidly switch between driving and modulatory functions, depending upon whether they are varying in parallel with or in opposition to changes in inhibition.”23 Additional data supporting a Combined Model perspective that both alternating and concurrent excitation and inhibition are present in the same neuronal network are described in studies of the CPGs for Aplysia feeding,28 lamprey swimming,5, 21 zebrafish swimming,19, 24 tadpole swimming,6, 10 turtle scratching,7 and rodent locomotion.8, 13
The Reciprocal Model in Turtle
Agonist-Antagonist Rhythmic Alternation During Normal Scratch
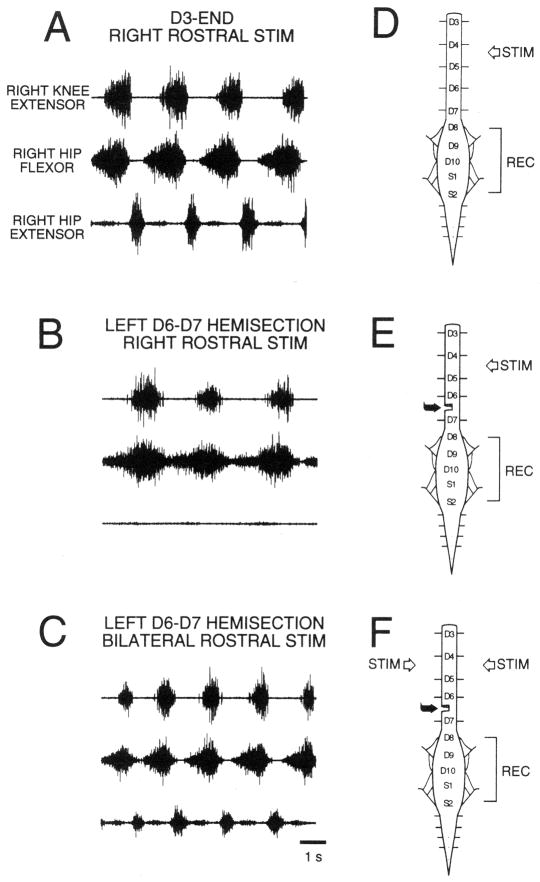
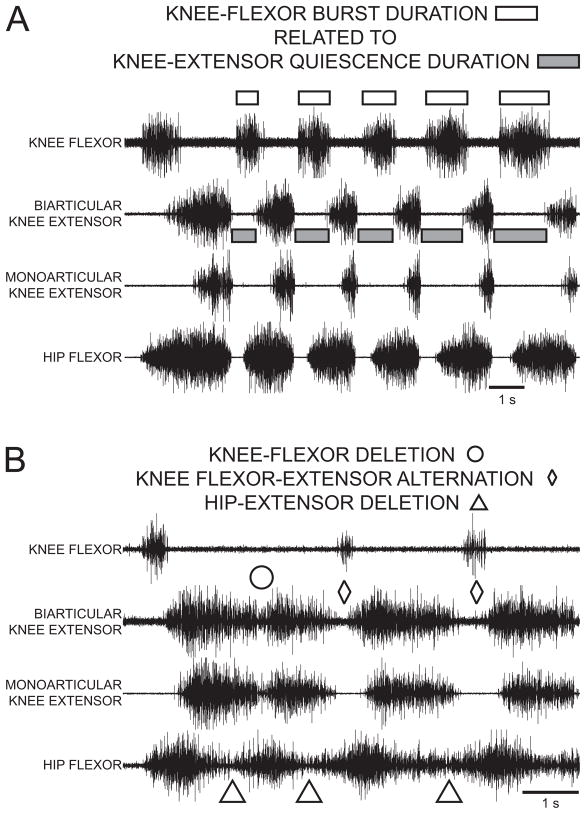
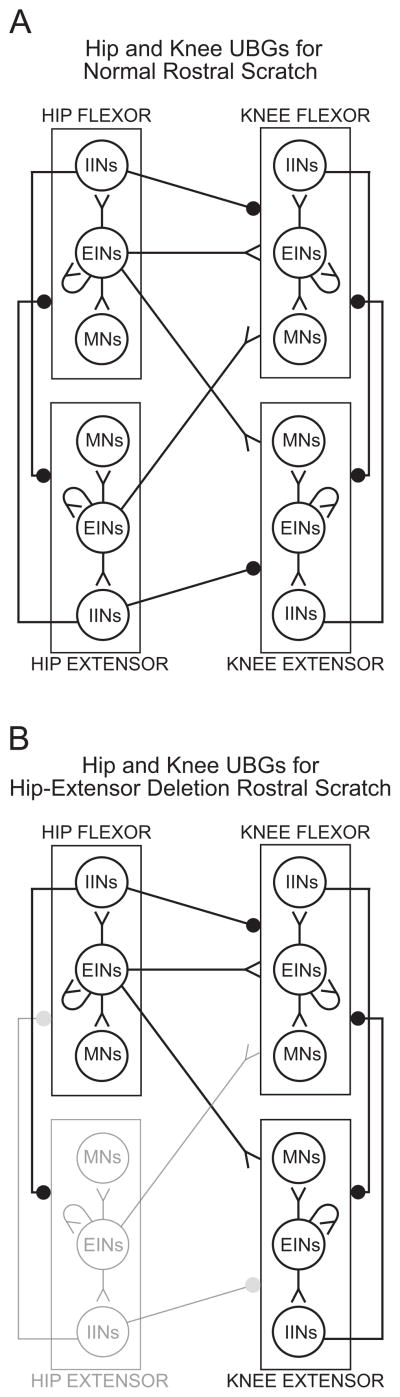
A fundamental feature of turtle normal hindlimb scratch is rhythmic alternation between hip-flexor motor activity and quiescence:7, 30, 34, 40, 47, 48, 54 hip-extensor motor activity occurs during hip flexor quiescence (Fig. 1A). Another feature of turtle normal scratch is rhythmic alternation between knee-extensor motor activity and quiescence. These features have been described during all 3 forms of scratch: rostral, pocket, and caudal.34 The most studied form of turtle scratch is the rostral scratch in which knee-extensor motor activity occurs during the latter portions of the hip-flexor motor neuron burst (Fig. 1A). In this form of scratch, knee-flexor motor activity occurs during knee-extensor quiescence30, 56, 57 (Fig. 2A) and the duration of knee-flexor motor activity is related to the duration of knee-extensor quiescence.57 Thus turtle normal scratch motor patterns demonstrate both hip and knee agonist-antagonist rhythmic alternations: these alternations are consistent with Reciprocal Models for hip modules as well as for knee modules. Fig. 3A is a schematic of some of the possible synaptic connections in the turtle rostral scratch CPG that includes reciprocal relationships between flexor and extensor neurons related to hip motor outputs and reciprocal relationships between flexor and extensor neurons related to knee motor outputs.
Figure 1.
Rostral scratch motor neuron activity patterns in the spinal immobilized turtle. Electroneurographic (ENG) recordings from the right biarticular knee-extensor nerve (top trace), the right hip-flexor nerve (middle trace), and the right hip-extensor nerve (bottom trace). (A) Right rostral scratch stimulation (STIM) in the D3-end preparation elicits normal rostral scratch with rhythmic right hip-flexor and hip-extensor alternation. (B) Right rostral scratch stimulation in the D3-end with left D6–D7 hemisection preparation elicits hip-extensor deletion rostral scratch with right hip-flexor rhythms. (C) Bilateral stimulation of mirror-image rostral scratch sites in the D3-end with left D6–D7 hemisection preparation elicits reconstructed normal rostral scratch with rhythmic right hip-flexor and hip-extensor alternation. (D) Sketch of the spinal cord in the D3-end preparation with a complete transection between the second and third postcervical spinal segments (D2–D3). (E and F) Sketches of the spinal cord in the D3-end with left D6–D7 hemisection preparation. All ENG recordings (REC) are from nerves on the right side. From48 and used with permission of and copyright 1998 by the New York Academy of Sciences and Wiley-Blackwell.
Figure 2.
Rostral scratch motor neuron activity patterns in response to stimulation of a site in the right rostral scratch receptive field in a D3-end preparation. ENG recordings from the right knee-flexor nerve, the right biarticular knee-extensor nerve, the right monoarticular knee-extensor nerve, and the right hip-flexor nerve. (A) Normal rostral scratch. Knee-flexor activity marked with unfilled rectangles; biarticular knee-extensor quiescence marked with gray-filled rectangles. (B) Rostral scratch with hip-extensor deletions. Hip-extensor deletions marked with unfilled triangles; knee-flexor deletion marked with unfilled circle; rhythmic knee-flexor bursts during knee-extensor quiescence marked with unfilled diamonds. From57 and used with permission of and copyright 2004 by the American Physiological Society.
Figure 3.
Schematic of a hypothesis describing a portion of the spinal neuronal network responsible for the production of the turtle rostral scratch as described in the modular Unit-Burst-Generator (UBG) hypothesis. Only ipsilateral hip and knee UBGs are shown; only a subset of the possible synaptic connections are included in the sketch. EINs: excitatory interneurons; IINs: inhibitory interneurons; MNs: motor neurons. Reciprocal inhibition between agonist and antagonist UBGs at each degree of freedom is a fundamental characteristic of organization of this network. Active neurons shown in black; quiet neurons shown in light gray. (A) During normal rostral scratch, all hip and knee UBGs are rhythmically active. (B) During hip-extensor deletion variation of rostral scratch, the hip-flexor, the knee-flexor, and the knee-extensor UBGs are rhythmically active. Neurons in the hip-extensor UBG are quiet. This schematic emphasizes that neurons in the hip-flexor UBG are rhythmic even when neurons in the hip-extensor UBG are quiet. From18 and used with permission of and copyright 2008 by Elsevier B.V.
Studies with many neuronal networks have demonstrated that reciprocal inhibitory networks are well suited to produce agonist-antagonist rhythmic alternation.4, 9, 11, 12, 14–18, 67 The sketch in Fig. 3A shares reciprocal inhibitory features with sketches for mammalian stepping CPGs presented by Grillner4, 11 in his unit-burst-generator hypothesis, by McCrea and Rybak17 in their two-layer CPG model, and by Endo and Kiehn14 in their CPG model. Agonist-antagonist rhythmic alternation when both agonists and antagonists are activated is a prediction of these Reciprocal Models.
Agonist Quiescence Between Successive Agonist Bursts is Absent during Antagonist Deletions
Motor pattern variations of rostral scratch provide considerable insights into the underlying structure of the turtle scratch CPG. The most studied variation is hip-extensor deletion rostral scratch.7, 18, 29, 31, 40, 47, 48, 50, 54, 57, 60, 66 During a hip-extensor deletion rostral scratch, there is no activity in hip-extensor motor neurons and no quiescence between successive bursts of hip-flexor motor neuron activity (Fig. 1B, 2B). During a hip-extensor deletion rostral scratch, there is rhythmic alternation between knee-extensor and knee-flexor activity (last 2 cycles with unfilled diamonds in Fig. 2B). Hip-extensor deletions occur in a small percentage of rostral scratch cycles in preparations with a single complete transection of the spinal cord, e.g., D3-end preparations (Fig. 1D).40, 47 Preparations with additional removals of spinal circuitry (Fig. 1E) demonstrate higher percentages of hip-extensor deletions in response to one-site stimulation (Fig. 1B).36, 40, 47, 48, 50 A candidate neuronal network with quiescence of hip-extensor interneurons and motor neurons during hip-extensor deletions is sketched in Fig. 3B. The sketch emphasizes that knee-related flexor and extensor neurons are still alternately active along with each burst of hip-flexor neurons during a hip-extensor deletion. Data from hip-extensor deletion rostral scratch support the following Reciprocal Model concepts: certain subcomponents of a neuronal network, e.g., knee-flexor module and knee-extensor module, demonstrate rhythmic alternation that is a characteristic of many Reciprocal Models; other subcomponents of the same neuronal network, e.g., hip-flexor module and hip-extensor module, demonstrate continuous hip-flexor activity and no hip-extensor activity as predicted when one module in a Reciprocal Model completely inhibits its antagonist module (Fig. 3B).
More recently, two distinct knee-related deletions of rostral scratch motor patterns have been recognized.57 In a knee-flexor deletion, there is no activity in knee-flexor motor neurons and no quiescence between successive bursts of knee-extensor motor neuron activity (unfilled circle in Fig. 2B). In a knee-extensor deletion, there is no activity in knee-extensor motor neurons and no quiescence between successive bursts of knee-flexor motor neuron activity. In a deletion variation with no antagonist activity in a cycle, the Reciprocal Model predicts an absence of agonist quiescence between two successive agonist bursts due to the absence of reciprocal inhibition from the antagonist module during the deletion cycle. The knee-related deletion results therefore provide additional support for the Reciprocal Model.
Reconstruction of Normal Agonist-Antagonist Alternation Occurs with Two-Site Stimulation in Preparations that Show High Percentages of Hip-Extensor Deletions with One-Site Stimulation
In most studies of turtle rostral scratch hip-extensor deletions, the experimenter does not have direct control in a given episode over whether a variation is produced or whether a normal motor pattern is produced. In contrast, a preparation with a complete D2–D3 spinal cord transection just posterior to the second post-cervical segment with an additional transverse D6–D7 hemisection one segment anterior to the hindlimb enlargement (Fig. 1E) displays high percentages of hip-extensor deletions with one-site stimulation.47, 48 The side of the hemisection is termed lesion-side; the side opposite the hemisection is termed intact-side. Intact-side one-site stimulation in the rostral scratch receptive field results mainly in hip-extensor deletion rostral scratches in intact-side motor neurons (Fig. 1B, E). Reconstruction of normal rostral scratch motor patterns in intact-side motor neurons occurs with two-site bilateral stimulation of rostral scratch receptive fields (Fig. 1C, F). In this preparation, intact-side stimulation favors rhythmic activation of intact-side hip-flexor neurons and lesion-side stimulation favors activation of intact-side hip-extensor neurons and inhibition of intact-side hip-flexor neurons. Bilateral stimulation activates intact-side hip-flexor and hip-extensor neurons rhythmically in alternation. The rhythmic alternation between hip-flexor and hip-extensor motor activities in this preparation in response to 2-site stimulation is termed reconstruction.47, 48 There are several experimental situations in which the presence of hip-extensor activity is associated with quiescence of hip-flexor activity.47 This reconstruction of agonist-antagonist alternation when both hip-flexor and hip-extensor neurons are activated is consistent with reciprocal inhibitory connections between hip-flexor and hip-extensor interneurons as described by a Reciprocal Model (Fig. 3A).
Inhibitory Post-Synaptic Potentials During Motor Neuron Quiescence
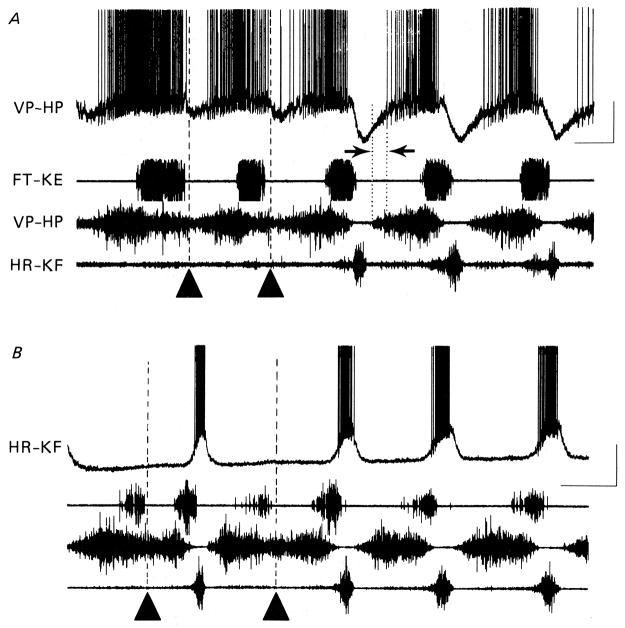
Additional experimental support for a Reciprocal Model comes from intracellular recordings from turtle motor neurons7, 31, 55, 58 during rhythmic scratch motor patterns. Intracellular recordings from a hip-flexor motor neuron (top trace marked VP-HP in Fig. 4A) and from a hip-extensor motor neuron (top trace marked HR-KF in Fig. 4B) are shown along with electoneurograms (ENGs) from three motor nerves: monoarticular knee-extensor (FT-KE), hip-flexor (VP-HP), and hip-extensor (HR-KF). In the last 3 cycles of Fig. 4A, there is rhythmic alternation between hip-flexor and hip-extensor motor activities characteristic of normal rostral scratch. Note the large negative-going inhibitory post-synaptic potentials (IPSPs) in the intracellular recording of the hip-flexor motor neuron during each burst of extracellular hip-extensor motor neuron activity. These large IPSPs during normal rostral scratch provide support for a Reciprocal Model (Fig. 3A).
Figure 4.
Comparison of voltage trajectories recorded intracellularly from hip-flexor (VP-HP) motor neuron (top trace in A) and hip-extensor (HR-KF) motor neuron (top trace in B) during normal rostral scratch and during hip-extensor deletion rostral scratch. In (A) and (B) ENG recordings from monoarticular knee-extensor nerve (FT-KE; second trace), hip-flexor nerve (VP-HP; third trace) and hip-extensor nerve (HR-KF; bottom trace). (A) Hip-extensor motor neuron activity is deleted in first two cycles (marked with filled triangles). Hip-flexor motor neuron intracellular recording shows corresponding deletion of hyperpolarization normally associated with hip-extensor activity (compare with last three cycles showing hip-extensor nerve activity). (B) Hip-extensor nerve activity is deleted in the first and third cycles (marked with filled triangles) and is associated with a complete absence of depolarization in the hip-extensor motor neuron intracellular recording (compare with cycles with hip-extensor nerve activity). Calibrations: 1 s, 20 mV. From7 and used with permission of and copyright 1988 by The Physiological Society and Wiley-Blackwell.
In the first 2 cycles of Fig. 4A marked with filled triangles, there is a hip-extensor deletion rostral scratch. Note the absence of the large IPSPs in the hip-flexor motor neuron associated with the absence of hip-extensor motor activities. (See section below, however, on “Concurrent Excitation and Inhibition in a Motor Neuron during Activity of its Motor Pool” for a discussion that notes that some inhibition in the hip-flexor motor neuron still remains in the first 2 cycles of Fig. 4A.) Similarly, intracellular recordings from a hip-extensor motor neuron (Fig. 4B) display large excitatory post-synaptic potentials (EPSPs) during the extracellular hip-extensor motor neuron activity in normal rostral scratch cycles; these EPSPs are absent during rostral scratch cycles with a hip-extensor deletion shown with filled triangles in Fig. 4B. The absence of the large IPSPs in hip-flexor motor neurons and the absence of large EPSPs in hip-extensor motor neurons during hip-extensor deletion rostral scratch provides further support for a Reciprocal Model (Fig. 3B).
Robertson and Stein7 provided additional evidence for the presence of IPSPs in hip-flexor motor neurons during the phase of the cycle in which hip-flexor motor neurons are quiet. In their Fig. 3,7 they demonstrate a reversal of the IPSPs by DC hyperpolarization of the motor neuron. In their Fig. 4A,7 they demonstrate a high conductance during the quiet phase of the motor neuron’s cycle. This high-conductance state during the quiet phase of motor neuron rhythmic activity is also described by Alaburda et al.58 in their Figs. 1C and 2A. In addition, their Fig. 458 describes reduced excitability of the motor neuron during its quiet phase of the scratch cycle in response to depolarizing current pulses: this reduced excitability is consistent with inhibition due to IPSPs during this phase of the cycle. In Fig. 4B of Robertson and Stein,7 chloride injection into the motor neuron is used to demonstrate a depolarizing IPSP during the quiet phase of the motor neuron. Fig. 5 here is a summary sketch of the synaptic potentials recorded from motor neurons during turtle rostral scratch.7 There is a portion of the scratch cycle in which the motor neuron receives inhibition (designated “I”) and the motor neuron is not firing. These experimental results are consistent with a Reciprocal Model in which turtle motor neurons receive significant IPSPs during their quiescent phase of the scratch cycle.
Figure 5.
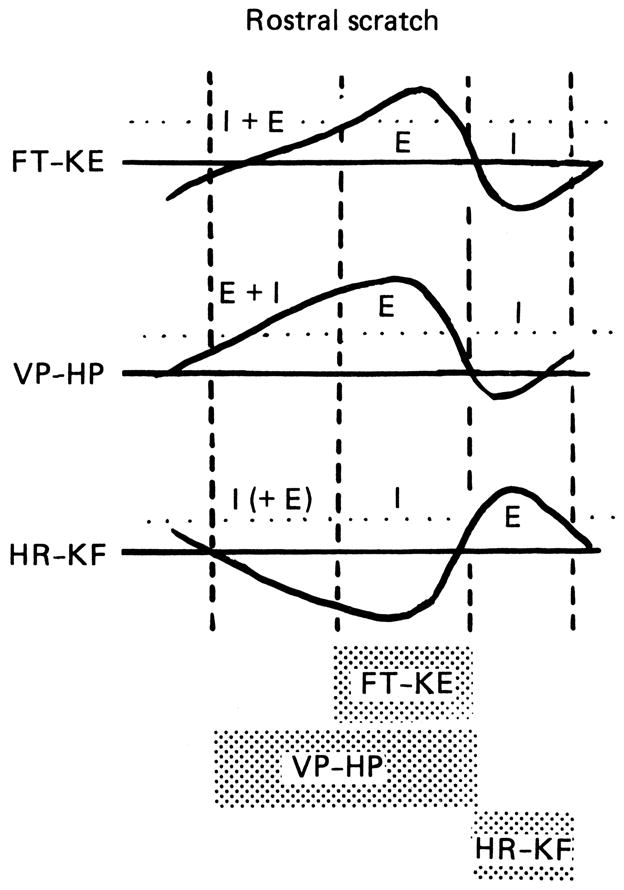
Diagram comparing intracellularly recorded voltage changes in monoarticular knee-extensor (FT-KE), hip-flexor (VP-HP), and hip-extensor (HR-KF) motor neurons during rostral scratch. ENG motor patterns depicting timing of corresponding motor pools shown at the bottom. Firing threshold shown as dotted line. I (IPSPs) and E (EPSPs) indicate synaptic inputs to motor neurons derived from data.7 Note there is at least one phase of reciprocal inhibition for each motor neuron and at least one phase of balanced inhibition for knee-extensor and hip-flexor motor neuron. The phase of balanced inhibition for the hip-extensor motor neuron is based upon preliminary observations. From7 and used with permission of and copyright 1988 by The Physiological Society and Wiley-Blackwell.
Antagonist Single-Unit Interneuron Activity During Normal and Deletion Rostral Scratch
In the Reciprocal Model, hip-extensor motor neurons and interneurons are active during the quiet phase of hip-flexor motor activity (Fig. 3A). A prediction of the Reciprocal Model is that hip-extensor interneurons will be quiet during hip-extensor deletion rostral scratch (Fig. 3B). The lack of IPSPs in hip-flexor motor neurons and the lack of EPSPs in hip-extensor motor neurons described in the prior section provide evidence in support of this prediction.
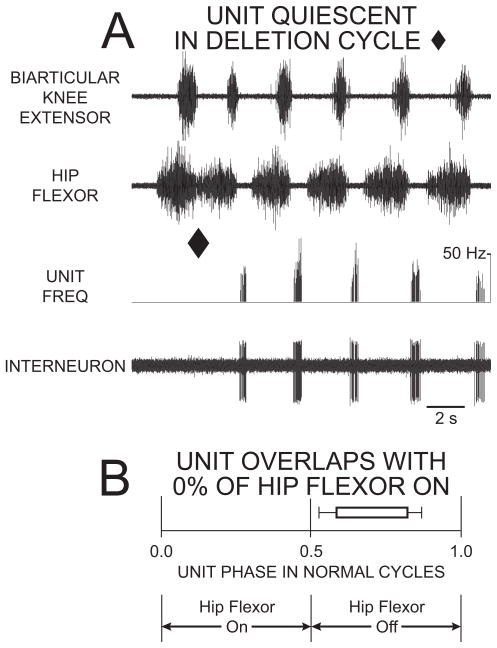
This prediction is tested further using single-unit extracellular axonal recordings from propriospinal interneurons with descending axons during normal rostral scratch and during hip-extensor deletion rostral scratch.54 Interneurons that fire during hip-flexor quiescence and/or hip-extensor activity during normal rostral scratch cycles are termed “hip-extensor interneurons.” Fig. 6A is an example of an hip-extensor interneuron active during hip-flexor quiescence of normal rostral scratch (last 5 cycles in Fig. 6A) and quiet during a hip-extensor deletion (cycle ending with filled diamond in Fig. 6A). Recordings54 from 18 hip-extensor interneurons produced a total of 9465 action potentials during 767 cycles of normal rostral scratch and a total of 24 action potentials during 167 cycles of hip-extensor deletion rostral scratch. These hip-extensor interneurons are mainly quiet during hip-extensor deletion rostral scratch: this provides further support for the Reciprocal Model hypothesis that neurons in the hip-extensor module (or unit-burst-generator or half-center) are mainly quiet during hip-extensor deletion rostral scratch (Fig. 3B).
Figure 6.
Extracellular unit recording of hip-extensor interneuron with 0% overlap with the hip-flexor burst was active in a burst during hip-flexor quiescence of normal rostral scratch and was quiet during rostral scratch with a hip-extensor deletion. (A) ENG recordings of the biarticular knee-extensor motor nerve (top trace); ENG recordings of the hip-flexor motor nerve (second trace); instantaneous frequency of unit (third trace); and interneuron unit activity (bottom trace). The first cycle is an example of rostral scratch with a hip-extensor deletion (end of cycle marked with filled diamond). The other cycles are examples of normal rostral scratch. (B) Start and end of bar represent mean ON-phase and mean OFF-phase, respectively, of unit firing during normal rostral scratch. Bar is unfilled to represent unit quiescence during hip-extensor deletion rostral scratch. From54 and used with permission of and copyright 2002 by the Society for Neuroscience.
ON-units are interneurons whose start-phases are positively correlated with the start-phases of knee-extensor motor neuron activity during normal rostral scratch.56 ON-units are candidate members of a knee-extensor module. OFF-units are interneurons whose end-phases are positively correlated with the start-phases of knee-extensor motor activity during normal rostral scratch.56 OFF-units are candidate members of a knee-flexor module. Both ON-units and OFF-units fire in bursts during hip-extensor deletions.56 ON-units are usually quiet during knee-extensor deletions and OFF-units are usually quiet during knee-flexor deletions.66 These data provide support for the Reciprocal Model hypotheses that: ON-units are members of the knee-extensor module; OFF-units are members of the knee-flexor module; and that there is reciprocal inhibition between the knee-extensor module and the knee-flexor module (Fig. 3).
Intracellular Interneuronal Recordings During Normal Scratch
Additional support for the Reciprocal Model has been obtained with direct intracellular recordings during fictive scratch from turtle interneurons.58, 59, 61, 62, 64 Berkowitz59, 61, 62, 64 recorded from interneurons during each of the 3 forms of scratch and characterized the morphology of each of the interneurons. He used averaging techniques to characterize the average membrane potential of each interneuron during each phase of the scratch cycle. The membrane voltage during each interneuron’s quiet phase displayed a significant trough. He also recorded intracellularly from turtle interneurons during fictive swim as well as during each of the 3 forms of fictive scratch.64 Most interestingly, he found that the phase of minimum of the membrane voltage during swim was strongly correlated with the phase of its minimum during scratch. This differed from measurements obtained with respect to the phases of maximum voltage during swim compared with scratch. Berkowitz64 suggested that this indicated the importance of reciprocal inhibition in interneurons not only during rhythmic scratch but also during rhythmic swim motor patterns. These data provide additional support for the Reciprocal Model hypothesis.
Reciprocal Model Conclusions
Evidence described above support Reciprocal Model concepts that agonist-antagonist alternation, alternation of excitatory and inhibitory postsynaptic potentials, and reciprocal inhibition are fundamental features of the spinal CPG for scratch in turtle spinal cord.7, 18, 29–66 Turtle scratch CPGs therefore share Reciprocal Model characteristics that are well established in other vertebrate CPGs.1–6, 8–17, 19
The next section summarizes support for the Balanced Model of Concurrent Excitation and Inhibition in these same turtle scratch CPGs. Some of the evidence for the Balanced Model is obtained in the same turtle experimental preparation that also provides support for the Reciprocal Model.7 The positive evidence for the Reciprocal Model presented in the above sections is therefore compatible with the positive evidence for the Balanced Model presented in the following sections.
The Balanced Model in Turtle
This section describes evidence for concurrent excitation and inhibition in turtle motor neurons during scratch motor rhythms in the work of Robertson and Stein7 and Berg et al.20 This evidence is gathered from adult turtles with intracellular recordings from motor neurons during scratch motor rhythms produced in response to natural mechanical stimulation of cutaneous sensory neurons.
Concurrent Excitation and Inhibition in a Motor Neuron during Activity of its Motor Pool
There is a distinct recruitment order68 observed in the extracellular recordings from the axons of the nerve innervating the hip-flexor muscle during each burst of a rostral scratch motor rhythm (third trace in Fig. 4A, labelled VP-HP). Axons with the smallest extracellular action potentials are recruited first, then those with medium-sized action potentials are recruited at intermediate phases, and finally those with the largest-sized action potentials are recruited during the highest amplitude part of the ENG burst. De-recruitment occurs in reverse order.
Robertson and Stein7 characterized the synaptic drive in medium-recruited hip-flexor motor neurons during rostral scratch (Fig. 4A). Using a variety of techniques, they established that medium-recruited hip-flexor motor neurons received concurrent excitation and inhibition during the early portion of the hip-flexor nerve burst when only small early-recruited hip-flexor motor axons are firing. This concurrent excitation and inhibition is present during normal rostral scratch (last 3 cycles of Fig. 4A) and also during hip-extensor deletion rostral scratch (first 2 cycles of Fig. 4A: note that reciprocal inhibition is absent during these cycles of hip-extensor deletion rostral scratch). The inhibition that is concurrent with excitation during normal rostral scratch is sensitive to strychnine.43 This supports the Balanced Model.
Concurrent excitation and inhibition in hip-flexor motor neurons (VP-HP) during normal rostral scratch is diagrammed as “E + I” in Fig. 5. Balanced excitation and inhibition may be a major contributor to the generation of motor neuron recruitment order: it may prevent medium- and late-recruited motor neurons from firing at the early phases of its motor pool’s activity. Importantly, positive data supporting both a Balanced Model and a Reciprocal Model are obtained during the same episode of rostral scratch.
Concurrent Excitation and Inhibition in a Motor Neuron During Quiescence of its Motor Pool
The monoarticular knee-extensor (FT-KE) nerve fires during the latter portion of the hip-flexor motor burst during normal rostral scratch. Intracellular recordings from monoarticular knee-extensor motor neurons during normal rostral scratch reveal concurrent excitation and inhibition during the early portion of the hip-flexor burst when the monoarticular knee-extensor motor pool is quiet.7 This is diagrammed as “I + E” for monoarticular knee-extensor motor neurons (FT-KE) during rostral scratch (Fig. 5). Thus balanced excitation and inhibition prevents knee-extensor motor neurons from firing during an inappropriate phase of the rostral scratch motor pattern. This may be especially important since a major difference between the motor pattern for rostral scratch and the motor patterns for other forms of scratch is the timing of knee-extensor motor neurons in the cycle of hip motor neuron activity. These observations support the Balanced Model and suggest that balanced excitation and inhibition may be an important contributor to the selection and production of the appropriate motor pattern for each form of scratch.
Knee-extensor motor neurons are inhibited during flexion reflex produced by a brief mechanical stimulus to the dorsum of the foot.7 The hip-flexor ENG serves as a monitor of flexion reflex. There are strong chloride-dependent IPSPs in knee-extensor motor neurons during flexion reflex.7 The voltage trajectories of these IPSPs provide an index of the relative position of the chloride equilibrium potential relative to the resting membrane potential. Robertson and Stein7 presented synaptic potentials of knee-extensor motor neurons during both rostral scratch and flexion reflex in their Fig. 7A7 in 3 different situations: the chloride equilibrium potential was more negative than, equal to, and more positive than the resting potential. Based on these recordings, they concluded that, during rostral scratch, there was balanced excitation and inhibition (with inhibition dominating) during the early portion of the hip-flexor burst and reciprocal inhibition during the hip-extensor burst. These observations provide data that support both a Balanced Model and a Reciprocal Model in the same episode of rostral scratch.
Concurrent excitation and inhibition in knee-extensor motor neurons is present in the early portion of the hip-flexor burst during a hip-extensor deletion rostral scratch (Fig. 6A of ref.7). This balanced excitation and inhibition in the knee-extensor motor neuron is present even though the reciprocal inhibition normally present during the hip-extensor phase of a normal rostral scratch is absent during the hip-extensor deletion. This observation invites future work to examine the potential sources of inhibitory drives in motor neurons during Balanced Excitation and Inhibition and during Reciprocal Inhibition. An interesting hypothesis is that there may be one population of interneurons responsible for the inhibition characterized by the Balanced Model and a different population of interneurons responsible for the inhibition characterized by the Reciprocal Model.
Concurrent Excitation and Inhibition during Action Potential Firing in Motor Neurons during Scratch
Berg et al.20 measure the total excitatory conductance and the total inhibitory conductance in turtle motor neurons during scratch motor rhythms.69 This technique allows the evaluation of how much inhibitory conductance is present when there is a sufficient excitatory conductance to drive action potential firing during the scratch motor pattern. Interestingly, the peak of excitatory conductance occurs in-phase with the peak of inhibitory conductance. Additional support for their conclusion of concurrent excitation and inhibition is obtained by blocking inhibitory glycinergic receptors with strychnine and observing an increased depolarization during the motor neuron’s on-phase and a decreased variability of successive interspike intervals. These observations20 support the Balanced Model, establish that concurrent excitation and inhibition occur in turtle motor neurons in a much larger portion of the scratch cycle than described previously, and considerably extend the prior work.7
The observations of Berg et al.20 are obtained from motor neuron cell bodies in the D10 spinal segment, the third segment of the 5-segment turtle hindlimb enlargement.70 Peripheral axons of D10 spinal segment motor neurons run mainly in the hip-extensor nerve or the sciatic nerve. Berg et al.20 do not type-identify the specific muscle that each recorded motor neuron cell body innervates. Different motor neuron types have different patterns of synaptic inputs for rostral scratch (Fig. 5). Differences between these patterns are more pronounced when other forms of scratch, e.g., pocket and caudal, are also examined (see Figs. 1, 6–8 of ref.7). Future experiments that characterize concurrent excitation and inhibition69 in type-identified motor neurons during each of several forms of scratch will add additional information to our knowledge of turtle spinal cord neuronal networks.
Balanced Model Conclusions
Concurrent excitation and inhibition and support for the Balanced Model occur in turtle motor neurons during scratch motor patterns.7, 20 Observations of concurrent excitation and inhibition are obtained in the same preparations that also demonstrate alternation of agonists and antagonists, alternation of excitatory and inhibitory potentials, and reciprocal inhibition.7 Thus turtle scratch motor circuitry exhibits aspects of both the Reciprocal and the Balanced Models, i.e., the Combined Model.
Considerable work is now needed in turtles to characterize fully the extent of both concurrent and alternating excitation and inhibition. In particular, it will be important to see additional work in preparations that display: (1) strong agonist-antagonist rhythmic alternation, e.g., normal rostral scratch, and (2) antagonist deletion variations, e.g., hip-extensor deletion variations of rostral scratch. Such work can evaluate the relative contributions of both the Reciprocal Model and the Balanced Model under each of these conditions. We are only starting to appreciate the true complexity of the turtle spinal cord CPGs that generate these complex behaviors.
The Combined Model
This review describes evidence and concludes that the CPG neuronal circuitry that generates scratch motor patterns in turtle spinal cord has Combined Model features, that is, the same spinal circuit has features of both the Reciprocal Model (inhibition that alternates with excitation) and the Balanced Model (inhibition that is concurrent with excitation).7
The evidence described here for the Combined Model for the turtle scratch CPG adds to the considerable evidence for Combined Model circuitry with both Reciprocal and Balanced Model features in many vertebrate locomotion CPGs, e.g., lamprey,5, 21 zebrafish,19, 24 tadpole,6, 10 and rodent.8, 13 Thus concurrent and alternating excitation and inhibition are fundamental features of the spinal cord neuronal networks responsible for the production of rhythmic vertebrate behaviors.
Summary
The turtle spinal cord CPG that produces the motor patterns for scratch reflex is an excellent model system for the study of the mechanisms for spinal cord motor control. It displays many of the fundamental principles of organization of other vertebrate spinal CPGs. It offers the technical advantage that responses of an adult nervous system can be obtained with natural cutaneous stimulation: robust rhythms with complex coordination patterns are produced without the need for stimulation with electrical impulses or bath application of neuroactive agents. The network driving the scratch rhythms can be studied in a wide variety of preparations: (1) in vivo preparations with moving limbs;29, 30, 34, 44, 45, 51, 52, 60 (2) in vivo preparations without movement-related sensory input;7, 29, 34, 36, 38, 39, 54, 60, 61 and (3) in vitro preparations without muscles.20, 26, 27, 32, 41, 49, 55, 58 Considerable work is now required by future experimentalists to reveal all the molecular, synaptic, cellular, and systems mechanisms in the spinal cord responsible for orchestrating the elegant coordinated movements of the turtle hindlimb when it successfully rubs against a site on the turtle body surface that has received a gentle mechanical stimulus.
Acknowledgments
Studies on turtle spinal cord in the Stein Laboratory have been supported by grants to PSGS from the NIH (1979–1989, 1992–2010) and from the NSF (1972–1979, 1989–1992). Most recent support for the Stein Laboratory is provided by NIH Grant NS-30786. I thank Drs. Ari Berkowitz, Scott Currie, Ole Kiehn, and Gail Robertson for their editorial comments.
References
- 1.Sherrington CS. The Integrative Action of the Nervous System. Yale University Press; New Haven, Connecticut: 1906. [Google Scholar]
- 2.Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond Biol. 1911;84:308–319. [Google Scholar]
- 3.Brown TG. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of evolution of function in the nervous system. J Physiol (London) 1914;48:18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB, editor. Handbook of Physiology, Sect. 1, The Nervous System, Vol. 2, Motor Control. American Physiological Society; Bethesda, Maryland: 1981. pp. 1179–1236. [Google Scholar]
- 5.Kahn JA. Patterns of synaptic inhibition in motoneurons and interneurons during fictive swimming in the lamprey, as revealed by Cl injections. J Comp Physiol A. 1982;147:189–194. [Google Scholar]
- 6.Dale N. Reciprocal inhibitory interneurones in the Xenopus embryo spinal cord. J Physiol (London) 1985;363:61–70. doi: 10.1113/jphysiol.1985.sp015695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson GA, Stein PSG. Synaptic control of hindlimb motoneurones during three forms of the fictive scratch reflex in the turtle. J Physiol (London) 1988;404:101–128. doi: 10.1113/jphysiol.1988.sp017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raastad M, Johnson BR, Kiehn O. Analysis of EPSCs and IPSCs carrying rhythmic, locomotor-related information in the isolated spinal cord of the neonatal rat. J Neurophysiol. 1997;78:1851–1859. doi: 10.1152/jn.1997.78.4.1851. [DOI] [PubMed] [Google Scholar]
- 9.Stein PSG, Grillner S, Selverston AI, et al., editors. Neurons, Networks, and Motor Behavior. MIT Press; Cambridge, MA: 1997. [Google Scholar]
- 10.Li WC, Higashijima S, Parry DM, et al. Primitive roles for inhibitory interneurons in developing frog spinal cord. J Neurosci. 2004;24:5840–5848. doi: 10.1523/JNEUROSCI.1633-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 13.Nishimaru H, Restrepo CE, Kiehn O. Activity of Renshaw cells during locomotor-like rhythmic activity in the isolated spinal cord of neonatal mice. J Neurosci. 2006;26:5320–5328. doi: 10.1523/JNEUROSCI.5127-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endo T, Kiehn O. Asymmetric operation of the locomotor central pattern generator in the neonatal mouse spinal cord. J Neurophysiol. 2008;100:3043–3054. doi: 10.1152/jn.90729.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grillner S, El Manira A, Kiehn O, et al., editors. Brain Res Rev. Vol. 57. 2008. Networks in Motion; pp. 1–270. [Google Scholar]
- 16.Kiehn O, Quinlan KA, Restrepo CE, et al. Excitatory components of the mammalian locomotor CPG. Brain Res Rev. 2008;57:56–63. doi: 10.1016/j.brainresrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 17.McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein PSG. Motor pattern deletions and modular organization of turtle spinal cord. Brain Res Rev. 2008;57:118–124. doi: 10.1016/j.brainresrev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabriel JP, Mahmood R, Kyriakatos A, et al. Serotonergic modulation of locomotion in zebrafish: endogenous release and synaptic mechanisms. J Neurosci. 2009;29:10387–10395. doi: 10.1523/JNEUROSCI.1978-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science. 2007;315:390–393. doi: 10.1126/science.1134960. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan JT, Grillner S. A new class of small inhibitory interneurones in the lamprey spinal cord. Brain Res. 1988;438:404–407. doi: 10.1016/0006-8993(88)91373-x. [DOI] [PubMed] [Google Scholar]
- 22.Parkis MA, Dong X, Feldman JL, et al. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: phase-specific control of excitability. J Neurosci. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- 24.Higashijima S, Masino MA, Mandel G, et al. Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J Neurosci. 2004;24:5827–5839. doi: 10.1523/JNEUROSCI.5342-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott LF, Chance FS. Drivers and modulators from push-pull and balanced synaptic input. Prog Brain Res. 2005;149:147–155. doi: 10.1016/S0079-6123(05)49011-1. [DOI] [PubMed] [Google Scholar]
- 26.Berg RW, Ditlevsen S, Hounsgaard J. Intense synaptic activity enhances temporal resolution in spinal motoneurons. PLoS One. 2008;3:e3218. doi: 10.1371/journal.pone.0003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg RW, Hounsgaard J. Signaling in large-scale neural networks. Cogn Process. 2009;10(Suppl 1):S9–S15. doi: 10.1007/s10339-008-0238-7. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki K, Brezina V, Weiss KR, et al. Distinct inhibitory neurons exert temporally specific control over activity of a motoneuron receiving concurrent excitation and inhibition. J Neurosci. 2009;29:11732–11744. doi: 10.1523/JNEUROSCI.3051-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein PSG, Grossman ML. Central program for scratch reflex in turtle. J Comp Physiol A. 1980;140:287–294. [Google Scholar]
- 30.Bakker JGM, Crowe A. Multicyclic scratch reflex movements in the terrapin Pseudemys scripta elegans. J Comp Physiol A. 1982;145:477–484. [Google Scholar]
- 31.Stein PSG, Robertson GA, Keifer J, et al. Motor neuron synaptic potentials during fictive scratch reflex in turtle. J Comp Physiol A. 1982;146:401–409. [Google Scholar]
- 32.Keifer J, Stein PSG. In vitro motor program for the rostral scratch reflex generated by the turtle spinal cord. Brain Res. 1983;266:148–151. doi: 10.1016/0006-8993(83)91318-5. [DOI] [PubMed] [Google Scholar]
- 33.Mortin LI, Keifer J, Stein PSG. Three forms of the scratch reflex in the spinal turtle: movement analyses. J Neurophysiol. 1985;53:1501–1516. doi: 10.1152/jn.1985.53.6.1501. [DOI] [PubMed] [Google Scholar]
- 34.Robertson GA, Mortin LI, Keifer J, et al. Three forms of the scratch reflex in the spinal turtle: central generation of motor patterns. J Neurophysiol. 1985;53:1517–1534. doi: 10.1152/jn.1985.53.6.1517. [DOI] [PubMed] [Google Scholar]
- 35.Currie SN, Stein PSG. Interruptions of fictive scratch motor rhythms by activation of cutaneous flexion reflex afferents in the turtle. J Neurosci. 1989;9:488–496. doi: 10.1523/JNEUROSCI.09-02-00488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mortin LI, Stein PSG. Spinal cord segments containing key elements of the central pattern generators for three forms of scratch reflex in the turtle. J Neurosci. 1989;9:2285–2296. doi: 10.1523/JNEUROSCI.09-07-02285.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mortin LI, Stein PSG. Cutaneous dermatomes for the initiation of three forms of the scratch reflex in the spinal turtle. J Comp Neurol. 1990;295:515–529. doi: 10.1002/cne.902950402. [DOI] [PubMed] [Google Scholar]
- 38.Berkowitz A, Stein PSG. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: broad tuning to regions of the body surface. J Neurosci. 1994;14:5089–5104. doi: 10.1523/JNEUROSCI.14-08-05089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkowitz A, Stein PSG. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: phase analyses. J Neurosci. 1994;14:5105–5119. doi: 10.1523/JNEUROSCI.14-08-05105.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein PSG, Victor JC, Field EC, et al. Bilateral control of hindlimb scratching in the spinal turtle: contralateral spinal circuitry contributes to the normal ipsilateral motor pattern of fictive rostral scratching. J Neurosci. 1995;15:4343–4355. doi: 10.1523/JNEUROSCI.15-06-04343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Currie SN, Lee S. Sensory-evoked pocket scratch motor patterns in the in vitro turtle spinal cord: reduction of excitability by an N-Methyl-D-Aspartate antagonist. J Neurophysiol. 1996;76:81–92. doi: 10.1152/jn.1996.76.1.81. [DOI] [PubMed] [Google Scholar]
- 42.Currie SN, Gonsalves GG. Right-left interactions between rostral scratch networks generate rhythmicity in the preenlargement spinal cord of the turtle. J Neurophysiol. 1997;78:3479–3483. doi: 10.1152/jn.1997.78.6.3479. [DOI] [PubMed] [Google Scholar]
- 43.Currie SN, Lee S. Glycinergic inhibition contributes to the generation of rostral scratch motor patterns in the turtle spinal cord. J Neurosci. 1997;17:3322–3333. doi: 10.1523/JNEUROSCI.17-09-03322.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Field EC, Stein PSG. Spinal cord coordination of hindlimb movements in the turtle: intralimb temporal relationships during scratching and swimming. J Neurophysiol. 1997;78:1394–1403. doi: 10.1152/jn.1997.78.3.1394. [DOI] [PubMed] [Google Scholar]
- 45.Field EC, Stein PSG. Spinal cord coordination of hindlimb movements in the turtle: interlimb temporal relationships during bilateral scratching and swimming. J Neurophysiol. 1997;78:1404–1413. doi: 10.1152/jn.1997.78.3.1404. [DOI] [PubMed] [Google Scholar]
- 46.Stein PSG, Smith JL. Neural and biomechanical control strategies for different forms of vertebrate hindlimb motor tasks. In: Stein PSG, Grillner S, Selverston AI, et al., editors. Neurons, Networks, and Motor Behavior. MIT Press; Cambridge, MA: 1997. pp. 61–73. [Google Scholar]
- 47.Stein PSG, McCullough ML, Currie SN. Reconstruction of flexor/extensor alternation during fictive rostral scratching by two-site stimulation in the spinal turtle with a transverse spinal hemisection. J Neurosci. 1998;18:467–479. doi: 10.1523/JNEUROSCI.18-01-00467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein PSG, McCullough ML, Currie SN. Spinal motor patterns in the turtle. Ann NY Acad Sci. 1998;860:142–154. doi: 10.1111/j.1749-6632.1998.tb09045.x. [DOI] [PubMed] [Google Scholar]
- 49.Currie SN. Fictive hindlimb motor patterns evoked by AMPA and NMDA in turtle spinal cord-hindlimb nerve preparations. J Physiol (Paris) 1999;93:199–211. doi: 10.1016/s0928-4257(99)80152-1. [DOI] [PubMed] [Google Scholar]
- 50.Currie SN, Gonsalves GG. Reciprocal interactions in the turtle hindlimb enlargement contribute to scratch rhythmogenesis. J Neurophysiol. 1999;81:2977–2987. doi: 10.1152/jn.1999.81.6.2977. [DOI] [PubMed] [Google Scholar]
- 51.Earhart GM, Stein PSG. Scratch-swim hybrids in the spinal turtle: blending of rostral scratch and forward swim. J Neurophysiol. 2000;83:156–165. doi: 10.1152/jn.2000.83.1.156. [DOI] [PubMed] [Google Scholar]
- 52.Earhart GM, Stein PSG. Step, swim, and scratch motor patterns in the turtle. J Neurophysiol. 2000;84:2181–2190. doi: 10.1152/jn.2000.84.5.2181. [DOI] [PubMed] [Google Scholar]
- 53.Juranek J, Currie SN. Electrically evoked fictive swimming in the low-spinal immobilized turtle. J Neurophysiol. 2000;83:146–155. doi: 10.1152/jn.2000.83.1.146. [DOI] [PubMed] [Google Scholar]
- 54.Stein PSG, Daniels-McQueen S. Modular organization of turtle spinal interneurons during normal and deletion fictive rostral scratching. J Neurosci. 2002;22:6800–6809. doi: 10.1523/JNEUROSCI.22-15-06800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alaburda A, Hounsgaard J. Metabotropic modulation of motoneurons by scratch-like spinal network activity. J Neurosci. 2003;23:8625–8629. doi: 10.1523/JNEUROSCI.23-25-08625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stein PSG, Daniels-McQueen S. Timing of knee-related spinal neurons during fictive rostral scratching in the turtle. J Neurophysiol. 2003;90:3585–3593. doi: 10.1152/jn.00762.2003. [DOI] [PubMed] [Google Scholar]
- 57.Stein PSG, Daniels-McQueen S. Variations in motor patterns during fictive rostral scratching in the turtle: knee-related deletions. J Neurophysiol. 2004;91:2380–2384. doi: 10.1152/jn.01184.2003. [DOI] [PubMed] [Google Scholar]
- 58.Alaburda A, Russo R, MacAulay N, et al. Periodic high-conductance states in spinal neurons during scratch-like network activity in adult turtles. J Neurosci. 2005;25:6316–6321. doi: 10.1523/JNEUROSCI.0843-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berkowitz A. Physiology and morphology indicate that individual spinal interneurons contribute to diverse limb movements. J Neurophysiol. 2005;94:4455–4470. doi: 10.1152/jn.00229.2005. [DOI] [PubMed] [Google Scholar]
- 60.Stein PSG. Neuronal control of turtle hindlimb motor rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:213–229. doi: 10.1007/s00359-004-0568-6. [DOI] [PubMed] [Google Scholar]
- 61.Berkowitz A, Yosten GL, Ballard RM. Somato-dendritic morphology predicts physiology for neurons that contribute to several kinds of limb movements. J Neurophysiol. 2006;95:2821–2831. doi: 10.1152/jn.01246.2005. [DOI] [PubMed] [Google Scholar]
- 62.Berkowitz A. Spinal interneurons that are selectively activated during fictive flexion reflex. J Neurosci. 2007;27:4634–4641. doi: 10.1523/JNEUROSCI.5602-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samara RF, Currie SN. Crossed commissural pathways in the spinal hindlimb enlargement are not necessary for right left hindlimb alternation during turtle swimming. J Neurophysiol. 2007;98:2223–2231. doi: 10.1152/jn.00722.2007. [DOI] [PubMed] [Google Scholar]
- 64.Berkowitz A. Physiology and morphology of shared and specialized spinal interneurons for locomotion and scratching. J Neurophysiol. 2008;99:2887–2901. doi: 10.1152/jn.90235.2008. [DOI] [PubMed] [Google Scholar]
- 65.Samara RF, Currie SN. Electrically evoked locomotor activity in the turtle spinal cord hemi-enlargement preparation. Neurosci Lett. 2008;441:105–109. doi: 10.1016/j.neulet.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Stein PSG. Modules for rostral scratch pattern generation in the turtle spinal cord. Proceedings of the Conference on Cellular and Network Functions in the Spinal Cord; Madison, WI. 2009. p. 4. [Google Scholar]
- 67.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 68.Stuart DG. The segmental motor system--advances, issues, and possibilities. Prog Brain Res. 1999;123:3–28. doi: 10.1016/s0079-6123(08)62840-x. [DOI] [PubMed] [Google Scholar]
- 69.Borg-Graham LJ, Monier C, Fregnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature. 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- 70.Ruigrok TJH, Crowe A. The organization of motoneurons in the turtle lumbar spinal cord. J Comp Neurol. 1984;228:24–37. doi: 10.1002/cne.902280105. [DOI] [PubMed] [Google Scholar]