Abstract
The influence of initial pH on growth and nutrient (total sugars, nitrogen, and phosphorous) consumption by Enterococcus faecium CECT 410 was studied during batch cultures in whey. With these data, two realkalized fed-batch fermentations were developed using different feeding substrates. The shift from homolactic to mixed acid fermentation, the biphasic kinetics observed for cell growth and nitrogen consumption and the increase in the concentrations of biomass and products (lactic acid, acetic acid, ethanol, and butane-2,3-diol) were the most noteworthy observations of these cultures. Modelling the fed-batch growth of Ent. faecium with the Logistic and bi-Logistic models was not satisfactory. However, biomass production was best mathematically described with the use of a double Monod model, which was expressed in terms of biomass, product accumulation, and nitrogen utilization. Product formation was successfully modelled with a modified form of the Luedeking and Piret model developed in this study.
1. Introduction
Different models, such as Logistic and modified Gompertz equations [1, 2] and the Monod model [3–6] have been widely used to describe the growth of different microorganisms in batch cultures. On the other hand, the classical Luedeking and Piret expression [7] has been used to describe the time course of the production of growth-associate (primary), nongrowth-associate (secondary), or mixed products.
However, these models have found to be inadequate to describe the productions of biomass and antimicrobial products in realkalized fed-batch cultures [8, 9]. This was basically due to the fact that the growth describes biphasic profiles in these cultures, and some metabolites (like acetic acid, ethanol and butane-2,3-diol) are produced only in the mixed acid fermentation phase. Therefore, in these cases, it could be more appropriate the use of models based on the sum of two simple growth pulses. With regard to this, a modified form of the double Monod model was developed to accurately describe the growth of Lactococcus lactis subsp. lactis CECT 539 and Pediococcus acidilactici NRRL B-5627 in realkalized fed-batch fermentations in culture media prepared with whey and mussel processing wastes [8, 9]. In the same way, a bi-Logistic model was successfully used to represent growth processes that experience two phases of logistic growth, either overlapping or sequentially [10].
In order to get a better understanding of biomass and antimicrobial product formation, to extend the results and to make large-scale productions, there are some important aspects that should be taken into account to model the growth and metabolite production curves. Firstly, a good model must include terms to take into account the influence of different factors (e.g., initial pH, substrate limitation, substrate inhibition, and product inhibition) on cell growth and product formation rate [8, 9, 11]. Secondly, significance of the estimated parameters, as well as the sensitivity and robustness of the fitted model should be evaluated by using statistically rigorous methods [8, 9, 12]. Thirdly, the scope of the model must be assessed by determining in which systems or situations it would be applicable [9].
Unfortunately, in some cases, it is difficult to know if a given model is the most appropriate to describe the trend observed in the experimental data. In these cases, it is more reasonable to compare different models by calculating the best-fit values of each model and determining if they are statistically significant and scientifically reasonable. In this way, a model must be rejected if the best-fit parameters of that model have no scientific sense. When the compared models fit the data with significant parameter values, it is necessary to compare the goodness of fit as quantified by sum of squares [12]. However, only a few studies [8] deal with the comparison between different models to describe the growth and product formation in cultures that exhibit a diauxic growth pattern.
Therefore, in the present study, the growth of Ent. faecium CECT 410 in whey was firstly followed in batch cultures at different initial pH values. After determining the most favourable initial pH, two realkalized fed-batch cultures were carried out in whey by using two different feeding media to produce high amounts of biomass and antimicrobial products. Subsequently, different modelling procedures were carried out to select the model capable of predicting biomass concentration and product formation in these realkalized fed-batch cultures. Finally, sensitivity and robustness of the models were discussed in relation to the values of both the determination coefficient and the mean relative percentage deviation modulus between the experimental data and the values predicted by the models.
2. Materials and Methods
2.1. Micro-Organisms and Media
Enterococcus faecium CECT 410 was obtained from the Spanish Type Culture Collection (CECT). Stock cultures were maintained at 4°C on Rothe (Cultimed Panreac Química S.A., Barcelona, Spain) agar slants. Working cultures were grown in Rothe broth at 30°C and 200 rpm.
Whey, which was obtained from a local dairy plant (Cooperativas Orensanas Sociedad Cooperativa Ltda, Spain) was used in two forms: as concentrated whey (CW: the liquid remaining after the first cheese pressing) and as diluted whey (DW: CW mixed with wash waters). The DW medium (2.7% dry weight after water evaporation) used as fermentation medium contained (in g/L): lactose, 22.0; total nitrogen, 0.45; total phosphorus, 0.25; proteins, 2.0; pH, 5.4. The CW medium (6.3% dry weight after water evaporation) used as feeding medium in the fed-batch fermentations, contained (in g/L): lactose, 48.1; total nitrogen, 1.05; total phosphorus, 0.43; proteins, 5.0; pH, 4.7. The preparation of these wastes to be used as culture media was performed as described previously [1].
2.2. Fermentation Conditions
The batch cultures of Ent. faecium CECT 410 on DW medium adjusted at initial pH values of 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, and 7.5 were performed in 250-mL Erlenmeyer flasks containing 50 mL of medium, on a rotary shaker (200 rpm) for 20 h at 30°C. The culture samples, which comprise an experimental unit (one flask), were withdrawn at regular intervals to perform analytical determinations.
The realkalized fed-batch cultures were carried out at 30°C in a 6 L bench top fermentor (New Brunswick Scientific, New Jersey) with an agitation of 200 rpm, an aeration flow rate of 0.5 L/h, and continuous-record of pH. These fermentations were initiated as batch processes with a working volume of 4 L of DW medium adjusted to an initial pH of 7.0. After 12 h, when the lower steady pH value was reached, the batch fermentations were converted into repeated fed-batch and realkalized mode by rapidly withdrawing a volume of 100 mL of the culture from the fermentor. After determining the total sugars concentration in the sample withdrawn, the medium was realkalized up to a set pH of 7.0 with 5 M NaOH. Then, the necessary volumes of feeding substrates to restore the initial total sugars concentration (22.0 g/L) in the fermentation medium were calculated by applying mass balance equations for the total sugars across the fermentor. In these equations, the volumes of NaOH added to the fermentor in each realkalization cycle were also taken into account, as indicated below.
In the first realkalized fed-batch culture (fed-batch fermentation I), the fermentor was fed with a 400 g/L concentrated lactose and sterile distilled water (if needed). In the second fed-batch culture (fed-batch fermentation II), the feeding substrate consisted in a mixture of a 400 g/L concentrated lactose and CW medium.
Samples were taken in each realkalization cycle to perform analytical determinations and to develop the corresponding mass balances for biomass, nutrients and products. The realkalization and feeding periods were maintained for as long as the Ent. faecium strain was able to bring about the decrease of pH.
Both batch and realkalized fed-batch cultures were started with a 2% (vol/vol) inoculum of a 12-h culture in DW medium.
2.3. Mass Balance Equations in the Realkalized Fed-Batch Fermentations
In this work, the volume of the fermentation medium (V) in the fed-batch fermentations was maintained constant ((dV/dt) = 0) by matching the volumes added to the fermentor (feeding volume (VF) plus the volume of 5M NaOH) with the sampling volume (VS).
Thus, for the realkalized fed-batch fermentation I,
| (1) |
where VCW and VSDW are, respectively, the volumes (in L) of concentrated whey and sterile distilled water added to the fermentor at the beginning of each feeding cycle. VNaOH is the volume (in L) of 5 M NaOH added to the fermentor for re-alkalizing the medium up to the initial pH value of 7.0. For the second realkalized fed-batch fermentation, VSDW was substituted by the volume (in L) of concentrated solution of lactose (VCL).
The sum of the volumes of feeding substrates that must be added for restoring the initial total sugars (TS) in the fermentation medium are
| (2) |
from which it follows that
| (3) |
The reduction in the amounts (in grams) of TS in the medium due to the joint effect of the extraction of samples and the consumption of TS by the growing strain (TSC+E) can be calculated by applying a mass balance equation for the total sugars:
| (4) |
where [TStn-1] and [TStn] are the concentrations of total sugars (in g/L) at the beginning and at the end of each feeding cycle. The difference (Vtn−1 − VStn) represents the remaining volume (in L) in the fermentor after the extraction of samples.
Therefore, the amounts of TS that must be added to the fermentor to restore the initial TS concentration in the fermentation medium can be calculated by the following expressions:
| (5) |
where [TSCW] and [TSCL] are the concentrations of total sugars in the CW medium and in the concentrated solution of lactose, respectively.
Substituting (3) into equations (5) gives
| (6) |
Thus, the VSDW and VCL can be calculated as
| (7) |
Now, the VCW can be obtained by introducing the values of VSDW (in case of fed-batch fermentation I) or VCL (in case of fed-batch fermentation II) and VNaOH into (3).
2.4. Analytical Assays
Growth was monitored by absorbance at 700 nm and converted to dry cell weight from a standard curve. Cells were harvested by centrifugation (12,000 ×g for 15 min at 4°C) of culture samples and washed twice with saline (0.8% NaCl). The culture supernatants were used to determine total sugars, phosphorous, nitrogen, lactic acid, acetic acid, ethanol, and butane-2,3-diol by methods described in previous works [13].
2.5. Determination of Total Viable Counts
Total viable counts of Ent. faecium CECT 410 at the end of each experiment were determined by a pour plate method (in triplicate) using Rothe agar after serial 10-fold dilution in PBS. Plates were incubated at 30°C for 48 h and the results were expressed as colony forming units (CFU) per mL.
2.6. Statistical Analyses
Individual experiments were performed in triplicate and all data points are represented by the mean. Data sets were statistically analyzed by using the software package SPSS Statistics 17.0 for Windows (Release 17.0.1; SPSS Inc., Chicago, IL, 2008). A paired-samples t-test was conducted to determine whether significant differences (P < .05) existed between the mean concentrations of biomass and antimicrobial substances produced in the batch and the realkalized fed-batch cultures. The same statistical test was used to compare the values of the parameters obtained after modelling separately the first and the second growth pulses observed in the realkalized fed-batch fermentations.
2.7. Model Parameters Determination and Model Evaluation
The model parameters were obtained by using the nonlinear curve-fitting software of SigmaPlot (version 9.0, Systat Software, Inc., 2004), which minimised the deviations between model predictions and experimental data according to the sum of squares of errors (SSE) of the model fit:
| (8) |
where ∆i,j represents the difference between the model and the experimental value, n and m represent the number of experimental data points and the number of variables, respectively.
The coefficients of the models with P values lower than .05 were considered statistically significant. Parameters were removed from the models when their asymptotic interval of confidence included zero.
The criteria used to evaluate the goodness-of-fit of each model were the determination coefficient (R2) and the mean relative percentage deviation modulus (RPDM) [14]:
| (9) |
where Xi is the experimental value, Xpi is the calculated value, and N is the number of experimental data. The RPDM parameter is widely used to determine the quality of the fit, being a value of RPDM below 10% indicative of a good fit for practical purposes [8, 14, 15].
3. Results and Discussion
3.1. Batch Fermentation Kinetics of Ent. faecium CECT 410 on DW Medium at Different Initial pH Values
Since the initial pH of the culture can influence cell growth, nutrient consumption, and product formation [16], the use of a fermentation medium adjusted at the most favourable initial pH value is important for developing a realkalized fed-batch cultivation. For this reason, the growth of Ent. faecium CECT 410 was followed in seven series of batch cultures on DW medium adjusted at different initial pH values (4.5, 5.0, 5.5, 6.0, 6.5, 7.0, and 7.5).
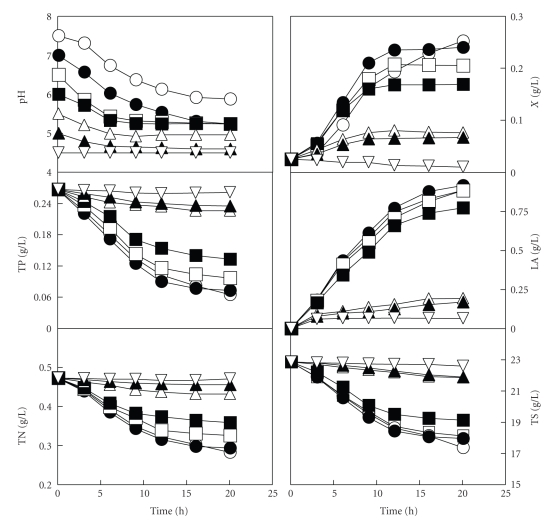
The kinetics of batch growth and product formation by Ent. faecium CECT 410 in these cultures are shown in Figure 1. A typically homolactic fermentation was observed in the seven cultures, since the lactic acid was the unique antimicrobial metabolite produced by the growing strain. The higher concentrations of biomass (0.24 g/L) and final viable cell concentrations (9 × 108 CFU/mL) were produced at an initial pH value of 7.0 after 12 h of incubation. After this time, the rates of biomass and lactic acid production and nutrient (total sugars, nitrogen, and phosphorous) consumption slowed down (Figure 1).
Figure 1.
Time course of batch cultures of Ent. faecium CECT 410 on DW medium adjusted to initial pH values of pH 7.5 (○), pH 7.0 (●), pH 6.5 (□), pH 6.0 (■), pH 5.5 (∆), pH 5.0 (▲), and 4.5 (∇). X, biomass; LA, lactic acid; TP, total phosphorous; TN, total nitrogen; TS, total sugars. Data reported are means ± standard deviations of three replicates.
With regard to this, the accumulation of lactic acid does not seem to be a cause for the cessation of growth, because the maximum amount produced (0.9 g/L at initial pH 7.0) was lower than that considered damaging for the Ent. faecium strains [17]. Then, the exhaustion of one or some micronutrients (vitamins, minerals and amino acids) in the medium or the limitation in the nutrients (total sugars, nitrogen, and phosphorous) or micronutrients transport when the cultures reached low pH values could be the possible causes for this fact [18–20].
Although the growth, nutrient consumption, and product formation were higher at initial pH 7.0, a high decrease in biomass production rate was observed when the culture reached a pH value of 5.4 (threshold pH value) after 12 h of fermentation, without nutrients exhaustion. The same trend was observed in the cultures adjusted at initial pH values of 6.0, 6.5 and 7.5. Interestingly, in the cultures adjusted at lower initial pH values (5.5, 5.0 and 4.5), which had the same initial media composition as the other cultures, the nutrient consumption was very low, falling off towards zero at initial pH 4.5.
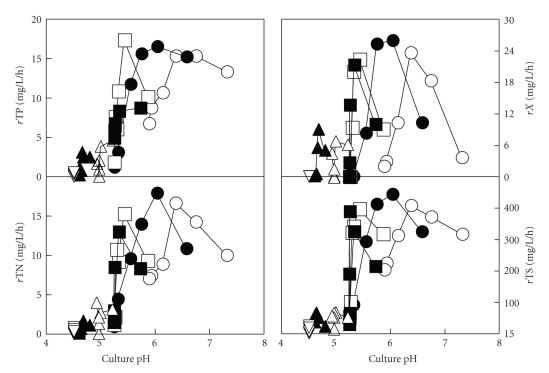
To check whether the nearness to the threshold pH value had some influence on nutrient consumption, the rates of growth and nutrient consumption for each sampling time were calculated and represented versus the culture pH (Figure 2). Although the biomass production and nutrient consumption decreased with the decrease in the initial pH value (Figure 1), both the growth and nutrient consumption rates in each culture also decreased as the cultures reached the above mentioned threshold pH value of 5.4 (Figure 2). Thus, the failure to grow at this acidic pH seems to be mainly caused by a limitation of cytoplasmic processes (acidification of the cytoplasm and the collapse of the motive force) that limits the nutrient transport [18–20].
Figure 2.
Changes in the absolute mean rates of growth (rX) and nutrient consumption (total phosphorous (rTP), total nitrogen (rTN), total sugars (rTS)) with respect to the culture pH in the batch cultures of Ent. faecium CECT 410 on DW media adjusted to initial pH values of 7.5 (○), pH 7.0 (●), pH 6.5 (□), pH 6.0 (■), pH 5.5 (∆), pH 5.0 (▲), and 4.5 (∇).
3.2. Realkalized Fed-Batch Fermentation Kinetics of Ent. faecium CECT 410 on DW Medium
An alternative way for increasing the productions of biomass and antimicrobial products by Ent. faecium in whey could be the increase in the period in which the cells are active. For this reason, a first realkalized fed-batch fermentation on DW medium at initial pH 7.0 was carried out with periods of realkalization and feeding of 12 h, when the culture reached the threshold pH value of 5.4 and the growth and nutrient consumption rates slowed down. In this culture, the fermentor was fed with a 400 g/L concentrated lactose to restore the initial concentration of total sugars in the fermentation medium.
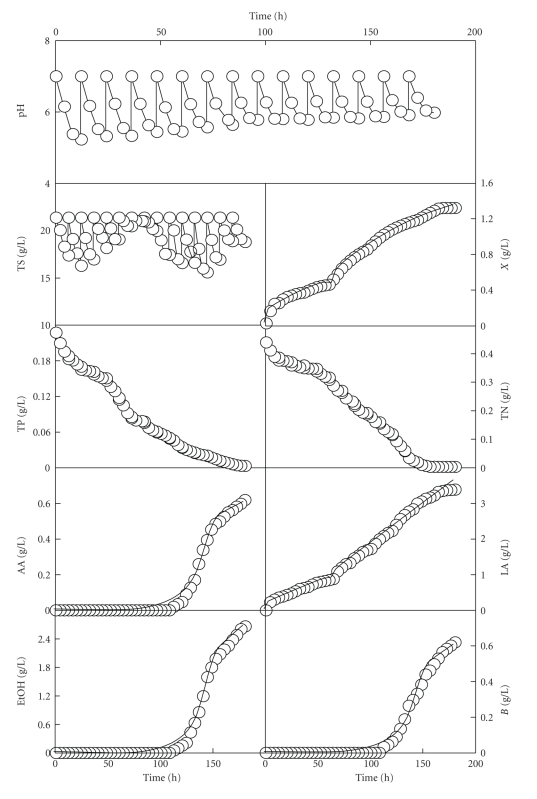
The results obtained in this fed-batch culture are shown in Figure 3. Compared with the batch cultures, the use of the fed-batch fermentation technique led to an increase in the metabolically active period of the cells. In addition, the concentrations of biomass, lactic acid, nitrogen and phosphorous described profiles with a wavy pattern, which were similar to that of a diauxic growth pattern.
Figure 3.
Time course of the realkalized fed-batch culture of Ent. faecium CECT 410 on whey with feeding with a 400 g/L concentrated lactose (fed-batch fermentation I). AA: acetic acid, EtOH: ethanol, B: butane-2,3-diol. Other notations are as in Figure 1. Data reported are means ± standard deviations of three replicates. The solid lines drawn through the experimental data of biomass and lactic acid were obtained according to the models (A.6) and (A.7), respectively. The solid lines drawn through the experimental data for AA, EtOH, and B were obtained according to the model (A.9).
The same trend was observed before in previous batch [21, 22] and realkalized fed-batch fermentations [8, 13, 23–26] with different strains and culture media. This diauxic growth was attributed to a biphasic nitrogen metabolism [21]. Thus, the free amino acids and utilizable oligopeptides originally present in milk are metabolized in the first growth phase, and the free amino acids produced by the hydrolysis of the proteins (like caseins in milk) by specific proteases are used in the second growth phase [22, 27–29]. This biphasic nitrogen consumption was also observed in batch cultures of Gibberella fujikuroi in a culture medium prepared with mussel processing wastes [30]. In these cultures, the growing strain consumed intensively both the inorganic nitrogen and the free amino acids during the first 48 h of incubation, but the nitrogen consumption rate slowed down afterwards.
Taking into account the production of metabolites, there were two distinct fermentation phases in the first realkalized fed-batch culture with Ent. faecium (Figure 3). The first was a homolactic phase (first 108 h of incubation) and the second was a mixed-acid fermentation phase (108 h to the end of the cultivation), which began with the accumulation of acetic acid, butane-2,3-diol, and ethanol in the culture medium.
The shift from homolactic to mixed-acid fermentation has been observed in cultures with other lactic acid bacteria at low growth rates under carbon or nitrogen limitation and under carbon-excess conditions with certain sugars such as lactose, maltose, or galactose [9, 13, 24–26]. This shift was associated to a modification of pyruvate metabolism with a decreased activity of lactate dehydrogenase and an increase in pyruvate dehydrogenase (aerobic conditions) or pyruvate formate lyase (anaerobic conditions) activity [31]. The decrease in lactate dehydrogenase activity was related to an overproduction of NADH oxidase by the cells, which produces the direct oxidation of the NADH necessary for pyruvate reduction [31, 32].
On the other hand, the final productions of biomass (1.3 g/L), lactic acid (3.4 g/L), acetic acid (0.6 g/L), ethanol (2.7 g/L), butane-2,3-diol (0.6 g/L), and the viable cell concentrations (4.0 × 109 CFU/mL) obtained at the end of this first fed-batch culture were higher (P < .05) than those obtained in the batch culture on DW medium at initial pH of 7.0. However, the final pH values at the end of each realkalization cycle increased progressively throughout the fermentation and after 168 h of incubation, the growth stopped probably due to the exhaustion of the nitrogen, and phosphorous sources. This decrease in the nitrogen concentration could lead to a low availability or the exhaustion of one or several amino acids or peptides essential for cell growth [33, 34]. Therefore, the growth cessation observed at the end of the realkalized fed-batch culture could be explained by nitrogen and phosphorous limitation and/or the accumulation of by-products with antibacterial activity.
For this reason, a second realkalized fed-batch fermentation on DW medium at initial pH 7.0 was carried out by using a mixture of a concentrated lactose (400 g/L) and CW medium as feeding substrates to replenish the lactose consumed and other nutrients (mainly nitrogen and phosphorous) in each feeding cycle.
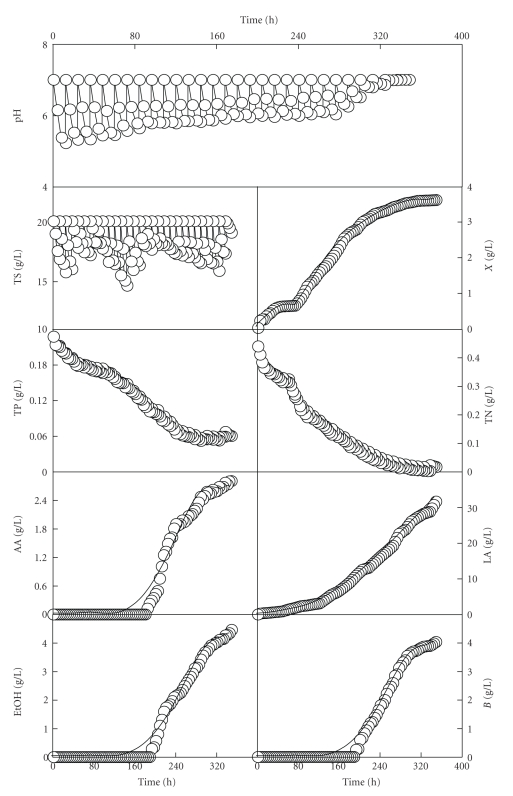
In this second fed-batch culture (Figure 4), again the concentrations of biomass, lactic acid, nitrogen and phosphorous described wavy profiles. In addition, after 324 h of incubation, the growth stopped coinciding again with the exhaustion of the nitrogen source and the growing strain was unable to bring about the decrease of pH (Figure 4). However, the phosphorous source was not completely consumed in this culture (Figure 4).
Figure 4.
Time course of the realkalized fed-batch culture of Ent. faecium CECT 410 on whey with feeding with concentrated whey and a 400 g/L concentrated lactose (fed-batch fermentation II). Notations are as in Figures 1 and 3. Data reported are means ± standard deviations of three replicates. The solid lines drawn through the experimental data of biomass and lactic acid were obtained according to the models (A.6) and (A.7), respectively. The solid lines drawn through the experimental data for AA, EtOH, and B were obtained according to the model (A.9).
From a comparative point of view, it can be noted that the final productions of biomass (3.6 g/L), lactic acid (31.7 g/L), acetic acid (2.8 g/L), ethanol (4.5 g/L), butane-2,3-diol (4.0 g/L), and the viable cell counts (1.1 × 1010 CFU/mL) obtained in this second fed-batch culture (Figure 4) were considerably higher (P < .05) than those obtained in the previous culture (Figure 3). In addition, the duration of the homolactic phase (192 h) in this second fed-batch culture was longer than that of the previous fed-batch culture (108 h), probably as a consequence of the additional supplements of nitrogen and phosphorous sources and other micronutrients added in each feeding with the CW medium. These results made evident that feeding the fermentor with a concentrated whey medium and lactose was an adequate alternative for obtaining further increases in biomass and product synthesis.
In the two realkalized fed-batch fermentations, the feeding substrates were added to bring the cultures up to the initial total sugars concentrations (22.0 g/L) in each feeding cycle. For this reason, there was no limitation by the carbon source during both fed-batch fermentations. However, due to the joint effect of consumption and dilution (which occurred due to the combined effect of sampling and nutrient feeding), the nitrogen concentration in the culture medium progressively decreased, thus becoming limiting for the growth of Ent. faecium at the end of the incubation. Thus, the progressive decrease in nitrogen concentration influenced the growth of Ent. faecium CECT 410, as it was observed before for other realkalized fed-batch cultures with other strains [8, 9]. In addition, biomass production stopped when the cultures reached a low nitrogen concentration, even though the concentrations of total sugars (in the two fed-batch cultures) and phosphorous (in the second fed-batch culture) were still sufficiently available (Figures 3 and 4). These observations suggest that the nitrogen source was the growth limiting substrate in these fermentations.
3.3. Mathematical Modelling
Three strategies were used to model the growth [X] of Ent. faecium CECT 410 in the two realkalized fed-batch cultures in DW medium. Firstly, the time-series data were split in two and each set was modelled by using a separate 3-parameter (K, c, μ) logistic model (see the appendix). Thus, models (A.1) and (A.2) were used to fit the experimental data corresponding to the first and second growth phase, respectively.
Since it is difficult to define when the first growth phase ends entirely before the second growth phase begins, the time-series data were split by changing sequentially the lengths of time of each growth phase and modelling them separately in each attempt. Therefore, three lengths of time were considered for the first (from 0 to 36 h, from 0 to 48 h, and from 0 to 60 h) and the second (from 36 to 180 h, 48 to 180 h, and 60 to 180 h) growth phases observed in the first realkalized fed-batch fermentation (see Figure 3). For the second fed-batch culture (see Figure 4), the lengths of time considered were from 0 to 48 h, from 0 to 60 h, and from 0 to 72 h (for the first growth phase), and from 48 to 348 h, from 60 to 348 h, and from 72 to 348 h (for the second growth phase).
By using this approach, it could be possible to determine whether the values of the three parameters of the logistic model are dependent on the duration of the first and second growth phases.
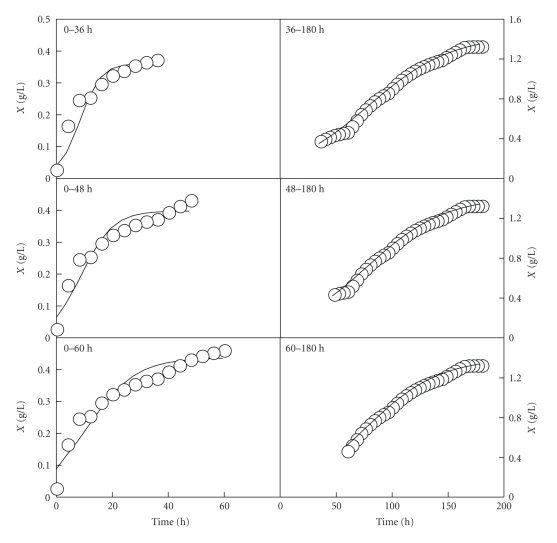
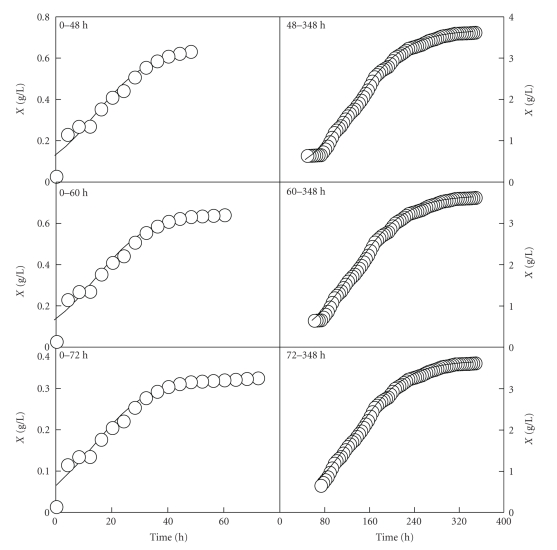
The results obtained are shown in Figures 5 and 6. After analysing the results, it can be noted that the use of models (A.1) and (A.2) to describe the growth of Ent. faecium in the realkalized fed-batch cultures in whey had some major drawbacks. On the one hand, the use of model (A.1) to describe the experimental data of the first growth pulses in both fed-batch cultures led to an overestimation of the initial biomass concentration (left parts of Figures 5 and 6). On the other hand, the values obtained for the three parameters (K, c and b) in the first realkalized fed-batch fermentation changed significantly (P < .05) depending on the period of time considered in each growth pulse (Table 1). In the second fed-batch culture, the values of K, c, and b did not changed significantly (P < .05) when different periods of time were considered for the first growth pulse (Table 2). However, for the second growth pulse, the values of the parameters K2, c2, and b2 were significantly different. These results indicate that modelling separately the two growth pulses is not always an adequate alternative to describe accurately the behaviour of systems exhibiting diauxic growth. In these conditions, it is difficult to define exactly where to split the growth data in the two realkalized fed-batch cultures of Ent. faecium CECT 410 in whey.
Figure 5.
Growth curves of Ent. faecium CECT 410 corresponding to the first (left part) and second (right part) growth phases observed in the realkalized fed-batch fermentation I. The growth data (symbols) represented in the left and right parts are the biomass data showed in Figure 3. The solid lines drawn through the experimental biomass data (left and right part) were obtained according to the Logistic models (A.1) and (A.2), respectively.
Figure 6.
Growth curves of Ent. faecium CECT 410 corresponding to the first (left part) and second (right part) growth phases observed in the realkalized fed-batch fermentation II. The growth data (symbols) represented in the left and right parts are the biomass data showed in Figure 4. The solid lines drawn through the experimental biomass data (left and right part) were obtained according to the Logistic models (A.1) and (A.2), respectively.
Table 1.
Parameter values (means ± SD) of the Logistic equations (A.1) and (A.2) obtained by considering different time intervals for the first and second growth phases in the first realkalized fed-batch culture of Ent. faecium CECT 410 in whey.
| Length of the first growth phase | |||
| Parameter | 0–36 h | 0–48 h | 0–60 h |
| K1 | 0.351 ± 0.0153 a | 0.392 ± 0.0168 b | 0.433 ± 0.0180 c |
| c1 | 1.328 ± 0.3491 a | 1.160 ± 0.2941 b | 1.037 ± 0.2360 b |
| b1 | 0.217 ± 0.0504 a | 0.145 ± 0.0320 b | 0.101 ± 0.0200 c |
| Length of the second growth phase | |||
| Parameter | 36–180 h | 48–180 h | 60–180 h |
| K2 | 1.420 ± 0.0174 a | 1.396 ± 0.0141 b | 1.390 ± 0.0147 c |
| c2 | 2.179 ± 0.0478 a | 2.310 ± 0.0540 b | 2.351 ± 0.0716 b |
| b2 | 0.027 ± 0.0008 a | 0.029 ± 0.0008 a | 0.030 ± 0.0010 b |
Statistically significant coefficients (P < .05) are expressed as means ± standard errors. The mean values within rows followed by the same letter are not significantly different (P > .05) from each other.
Table 2.
Parameter values (means ± SD) of the Logistic equations (A.1) and (A.2) obtained by considering different time intervals for the first and second growth phases in the second realkalized fed-batch culture of Ent. faecium CECT 410 in whey.
| Length of the first growth phase | |||
| Parameter | 0–36 h | 0–48 h | 0–60 h |
| K1 | 0.625 ± 0.0389 a | 0.649 ± 0.0207 a | 0.648 ± 0.0137 a |
| c1 | 1.458 ± 0.1972 a | 1.460 ± 0.1744 a | 1.460 ± 0.1560 a |
| b1 | 0.099 ± 0.0167 a | 0.100 ± 0.0123 a | 0.100 ± 0.0100 a |
| Length of the second growth phase | |||
| Parameter | 36–180 h | 48–180 h | 60–180 h |
| K2 | 3.642 ± 0.0116 a | 3.634 ± 0.0102 b | 3.635 ± 0.0105 c |
| c2 | 2.856 ± 0.0300 a | 2.906 ± 0.0291 b | 2.898 ± 0.0319 c |
| b2 | 0.021 ± 0.0002 a | 0.022 ± 0.0002 a, b | 0.022 ± 0.0003 b |
Statistically significant coefficients (P < .05) are expressed as means ± standard errors. The mean values within rows followed by the same letter are not significantly different (P > .05) from each other.
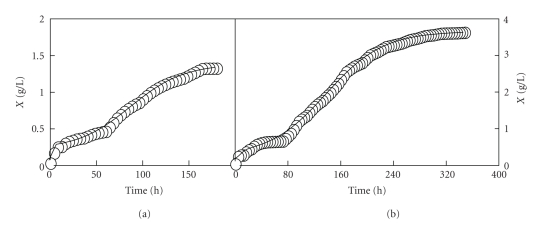
For this reason, the following alternative was focused on the analysis of the experimental growth data obtained from the two fed-batch cultures (Figures 3 and 4) by using a bi-Logistic model (A.3) comprised of the sum of two 3-parameter logistic growth pulses (see the appendix).
With this procedure, the results obtained (Figure 7 and Table 3) were not satisfactory. Thus, with the use of model (A.3), the initial biomass concentration was again overestimated in both cultures (Figure 7) and the values obtained for c3 and b3 were not statistically significant (Table 3). As it can be observed in both cultures (Figures 3, 4, and 7), the second growth pulse has a higher number of data points than the first growth pulse. In addition, the values of the data points corresponding to the second growth pulse are also higher than those of the first growth pulse. Thus, the least-squares technique used for fitting the model to the experimental data, that assumes constant error variance [10], weighs the data corresponding to the second growth pulse, more heavily than those corresponding to the first growth pulse. For this reason, statistically significant values for the parameters K4, c4, b4, and K3 and not statistically significant values for the c3 and b3 parameters were obtained. In this case, the bi-Logistic model takes the form of a simple Logistic model, which could not be used to fit a set of experimental data describing two growth pulses [8].
Figure 7.
Growth curves of Ent. faecium CECT 410 corresponding to the realkalized fed-batch fermentations I (a) and II (b). The growth data (symbols) represented in the left (a) and right (b) parts are, respectively, the experimental biomass data showed in Figures 3 and 4. The solid lines drawn through the experimental biomass data were obtained according to the bi-Logistic model (A.3).
Table 3.
Parameter values (means ± SD) of the bi-Logistic equation (A.3) used for predicting the biphasic growth data of Ent. faecium CECT 410 in the two realkalized fed-batch fermentations in whey.
| Parameter | Fed-batch fermentation I | Fed-batch fermentation II |
|---|---|---|
| K3 | 0.202 ± 0.0216 | 0.221 ± 0.0381 |
| c3 | NS | NS |
| b3 | NS | NS |
| K4 | 1.163 ± 0.0343 | 3.397 ± 0.0475 |
| c4 | 3.069 ± 0.1750 | 3.236 ± 0.1015 |
| b4 | 0.035 ± 0.0018 | 0.023 ± 0.0006 |
| R2 | 0.9827 | 0.9984 |
| RPDM | 4.974 | 8.840 |
Statistically significant coefficients (P < .05) are expressed as means ± standard errors, NS: no significant (P > .05).
In these conditions, it could be advantageous to use a regression method that assumes constant relative error [10], such as the mean relative percentage deviation modulus. Thus, the data corresponding to the first growth pulse are more heavily weighted than the latter data when fitting. However, with this procedure, again significant values for K4, c4, b4, and K3, and nonsignificant values for c3 and b3 were obtained. In addition, the new values obtained for these four parameters were very similar to those obtained when the least squares technique was used to fit the bi-Logistic model to the experimental growth data.
Therefore, in the third alternative, the biphasic growth of Ent. faecium CECT 410 in the two realkalized fed-batch cultures was described by using a diauxic Monod-type equation [8, 9], that relates the growth with the concentration of the growth controlling (limiting) substrate. Taking into account that nitrogen was the growth limiting substrate and it seemed to be consumed in two phases (Figures 3 and 4), the specific growth rate (μ in h−1) was expressed as the sum of the specific growth rates (μ1 and μ2) on each nitrogen consumption phase multiplied by a dimensionless inhibition function (δi) for accounting the inhibition by product formation [4, 8, 9] (see equations (A.4) and (A.5) in the appendix).
In order to take into account the effect of dilution, which occurred due to the sample extraction and the feeding of nutrients, mass balance was carried out across the fermentor and the biomass accumulation rate (rX) by using the expression (A.6) (in the appendix).
Since lactic acid was produced in both the homolactic and the mixed acid fermentation phase, its accumulation rate (rLA) in the fed-batch model was represented to contain growth (αLA) and nongrowth (βLA) associated constants (model (A.7) in the appendix).
Modelling the production of mixed acid products such as acetic acid, ethanol, and butane-2,3-diol in other realkalized fed-batch cultures using model (A.7) was unsatisfactory [8, 9]. This was probably due to the fact that these products were not produced from the beginning of the cultures. With regard to this, the appearance of mixed acid products has been usually related with a nutrient (carbon or nitrogen source) limitation [35–37]. Thus, it is reasonable to suppose that the production of acetic acid, ethanol, and butane-2,3-diol started when the culture medium reached a nitrogen concentration that limits the growth of Ent. faecium CECT 410. Then, it could be considered that the productions of these three mixed acid products are inhibited in the homolactic fermentation phase, by nitrogen concentrations higher than the limiting nitrogen concentration (substrate inhibition). In the same way, it could be also considered, that nitrogen concentrations lower than the limiting concentration favoured the production of these metabolites. These considerations were taken into account to develop a mathematical model that describes the production of these mixed acid products.
Then, the total cumulative amounts of mixed acid products (Ptotal) in these cultures could be calculated as the sum of the amounts produced in the homolactic fermentation phase (PHM) and in the mixed acid fermentation phase (PMA), as indicated in the model (A.8) of the appendix. Therefore, the accumulation rates for these three mixed acid products (rPMA) were calculated according to the model (A.9).
The results obtained with this last modelling strategy are shown in Figures 3 and 4 and in Tables 4 and 5 for the first and second fed-batch cultures. With the use of models (A.6), (A.7), and (A.9), excellent agreement was found between model predictions and experimental results for biomass and product formation (Figures 3 and 4). In addition, high R2 values (higher than 0.98) and low RPDM values (lower than 10) for biomass and products were obtained with the use of models (A.6), (A.7) and (A.9). These facts have strengthened the usefulness of these models for describing growth and product formation by Ent. faecium CECT 410 in the realkalized fed-batch fermentations in whey.
Table 4.
Parameter values (means ± SD) of the models (A.6) and (A.7) used for predicting the biphasic growth of Ent. faecium CECT 410 and lactic acid production in the two realkalized fed-batch fermentations.
| Parameter | Fed-batch fermentation I | Fed-batch fermentation II |
|---|---|---|
| μmax1 (h−1) | 0.130 ± 0.0341 | 0.365 ± 0.0653 |
| KN1 (g/L) | 0.050 ± 0.0165 | 0.029 ± 0.0046 |
| Nmin1 (g/L) | 0.346 ± 0.0180 | 0.331 ± 0.0038 |
| μmax2 (h−1) | 0.300 ± 0.0215 | 0.118 ± 0.0108 |
| KN2 (g/L) | 0.0012 ± 0.00001 | 0.020 ± 0.0053 |
| Nmin2 (g/L) | 0.022 ± 0.0018 | 0.035 ± 0.0159 |
| KiLA (g/L) | 0.029 ± 0.0043 | 0.055 ± 0.0064 |
| KiAA (g/L) | NS | NS |
| KiEtOH (g/L) | NS | NS |
| KiB (g/L) | NS | NS |
| RX2 | 0.9977 | 0.9980 |
| RPDMX | 2.5779 | 3.8516 |
| αLA (dimensionless) | 1.371 ± 0.2090 | 0.075 ± 0.0027 |
| βLA (h−1) | 0.013 ± 0.0092 | 0.040 ± 0.0014 |
| RLA2 | 0.9953 | 0.9978 |
| RPDMLA | 3.049 | 8.661 |
Statistically significant coefficients (P < .05) are expressed as means ± standard errors, NS: no significant (P > .05).
Table 5.
Parameter values (means ± SD) of the model (A.9) developed for describing the production of mixed acid metabolites by Ent. faecium CECT 410.
| Fed-batch fermentation I | |||
|---|---|---|---|
| Parameters | Acetic acid | Ethanol | Butane-2,3-diol |
| αMA (dimensionless) | NS | NS | NS |
| βMA (h−1) | −0.003 ± 0.0003 | −0.012 ± 0.0010 | −0.002 ± 0.0003 |
| αHM (dimensionless) | NS | NS | NS |
| βHM (h−1) | 0.405 ± 0.0119 | 1.307 ± 0.0312 | 0.260 ± 0.0102 |
| KN (g/L) | 0.232 ± 0.0140 | 0.163 ± 0.0073 | 0.159 ± 0.0115 |
| KIN (L/g) | 388.734 ± 19.7455 | 334.610 ± 13.6935 | 356.484 ± 23.1473 |
| R2 | 0.9945 | 0.9908 | 0.9951 |
| RPDM | 2.986 | 5.600 | 2.866 |
| Fed-batch fermentation II | |||
| αMA (dimensionless) | NS | NS | NS |
| βMA (h−1) | −0.009 ± 0.0010 | −0.010 ± 0.0012 | −0.010 ± 0.0005 |
| αHM (dimensionless) | NS | NS | NS |
| βHM (h−1) | 7.892 ± 0.1269 | 4.706 ± 0.1716 | 3.985 ± 0.0411 |
| KN (g/L) | 14.424 ± 0.2671 | 5.766 ± 0.2749 | 5.067 ± 0.1056 |
| KIN (L/g) | 958.354 ± 98.8409 | 553.908 ± 63.835 | 466.274 ± 44.0840 |
| R2 | 0.9844 | 0.9903 | 0.9941 |
| RPDM | 9.092 | 4.918 | 3.071 |
Statistically significant coefficients (P < .05) are expressed as means ± standard errors, NS: no significant (P > .05).
With regard to the model (A.6), it can be noted that the calculated values for KiAA, KiEtOH, and KiB were found to be not significant (P > .05) for both realkalized fed-batch fermentations (Table 4). This indicates that the increase in the concentrations of these mixed acid products did not produce an important influence on the growth of Ent. faecium CECT 410. Therefore, the biomass production in each realkalized fed-batch culture was remodelled by suppressing the terms δAA, δEt, and δB from model (A.4). In these conditions, the values of Nmin (P < .05) predicted by the model (A.6) are in perfect agreement with the experimental values observed in the two growth phases in each fed-batch culture (Table 4 and Figures 3 and 4).
The solid lines drawn through the experimental biomass data (Figures 3 and 4) are the trajectories predicted by the model (A.6) after calculating the values of μ with the model (A.4). As it can be observed, model (A.6) offered a more accurate description of the diauxic growth of Ent. faecium CECT 410 in relation with the biphasic nitrogen consumption than the bi-Logistic model (A.3).
In the same way, the production of lactic acid in both fed-batch cultures (Figures 3 and 4) was successfully described (R2> 0.99 and RPDM < 9) with the Luedeking and Piret model (A.7). The calculated parameters (α, β) from this model revealed that lactic acid was produced as a primary metabolite (α ≫ β) in the first fed-batch culture and as a mixed metabolite (α and β ≠ 0) in the second fed-batch culture (Table 4).
However, to model the production of mixed acid metabolites, the Luedeking and Piret model (A.7) was modified by introducing a term for the above-mentioned effect of the nitrogen concentration. From these results, it can be pointed out that the model (A.9) developed in this work (solid lines in Figures 3 and 4) successfully described the formation of mixed acid products by Ent. faecium CECT 410 in the two realkalized fed-batch cultures in whey. Therefore, these products showed a pure secondary character (αHM and αMA = 0; βHM and βMA ≠ 0), as it was expected, in the two realkalized fed-batch cultures (Table 5). The negative values for βMA are explained by the fact that the biomass production rate (rX) decreased until reaching a value of zero in the last sampling times, meanwhile the rates of production of acetic acid, ethanol, and butane-2,3-diol are maintained in constant positive values (Figures 3 and 4).
4. Conclusion
The use of the models (A.6), (A.7), and (A.9) may lead to the development of better strategies for the optimization of the fermentation process to ensure its economic viability. Thus, the double Monod model developed in this study, resulted in improved predictions of the experimental growth data over the previously mentioned Logistic and bi-Logistic models. Therefore, the double Monod model could constitute a viable alternative to pilot plant or industrial scale trial-and-error experiments, with economic and safety advantages. Model (A.7) could be used as a tool that has potential in optimization studies of industrial reactors and in the development of new bioproductions with other lactic acid bacteria. In addition, the proposed model (A.9) for the production of acetic acid, ethanol, and butane-2,3-diol could also be used to describe the synthesis of products that are produced with a time delay, which are usually difficult to describe [8, 9].
Acknowledgments
The research presented in this paper was financially supported by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA), Spain (project CAL01-045-C2-2) and The Xunta de Galicia, Spain (project PGIDT00BIO1E), and the Ministerio de Educación y Ciencia (projects MAT2005-05393-C03-03 and MAT2006-11662-C03-03). The authors COREN, S.C.L. for their collaboration in the elaboration of this work.
Symbolic Notation Used
- [X0]:
initial biomass concentration (g/L)
- K1:
maximum biomass concentration (g/L) in the first growth phase
- K2:
maximum biomass concentration (g/L) in the second growth phase
- b1 and b2:
logistic rate parameters (h−1)
- t:
time (h)
- K3 and K4:
maximum biomass concentration (g/L) in the first and second growth phases
- c3, c4, b3, and b4:
logistic rate parameters (h−1)
- μ:
specific growth rate (h−1)
- μ1 and μ2:
specific growth rates (h−1) in the first and second nitrogen consumption phase
- μmax 1:
maximum specific growth rates (h−1) in the first exponential growth phase
- μmax 2:
maximum specific growth rates (h−1) in the second exponential growth phase
- [N]:
nitrogen concentration (g/L) in each realkalization cycle just after feeding the fermentor
- KN1 and KN2:
nitrogen affinity constants (g/L) in the first and second consumption phases
- [N]min 1:
minimum nitrogen concentration (g/L) that supports the growth in the first growth phase
- [N]min 2:
minimum nitrogen concentration (g/L) that supports the growth in the second growth phase
- δLA, δAA, δEtOH, and δB:
individual inhibition functions which account for the inhibition of cell growth by the increase in the concentrations (g/L) of lactic acid [LA], acetic acid [AA], ethanol [EtOH], and butane-2,3-diol [B]
- KiLA, KiAA, KiEtOH, and KiB:
inhibition constants (g/L) for lactic acid, acetic acid, ethanol, and butane-2,3-diol, respectively
- [X](tn) and [X](tn−1):
biomass concentrations (g/L) inside the fermentor at the times (in h) t = tn and t = tn−1, respectively
- rLA:
lactic acid production rate (g/L/h)
- αLA:
growth-associated constant. Dimensionless
- βLA:
nongrowth-associated constant (h−1)
- [LA](tn) and [LA](tn−1):
lactic acid concentrations (g/L) inside the fermentor at the times (in h) t = tn and t = tn−1, respectively
- tHM and tf:
duration (in h) of the homolactic fermentation phase and the total time of fermentation (in h), respectively
- [PMA](tn) and [PMA](tn−1):
product concentrations (g/L) inside the fermentor at the times (in h) t = tn and t = tn−1, respectively
- αHM:
growth-associated constant for product formation during the homolactic fermentation phase. Dimensionless
- βHM:
nongrowth-associated constants (in h−1) for product formation during the homolactic fermentation phase
- KN:
nitrogen affinity constants (g/L) for the nitrogen source in the homolactic fermentation phase
- KIN:
inhibition constant (L/g) for the nitrogen source in the homolactic fermentation phase
- αMA
growth-associated constant for product formation during the mixed acid fermentation phase. Dimensionless
- βMA:
nongrowth-associated constants (in h−1) for product formation during the mixed acid fermentation phase.
Appendix
Equations Used for the Model Development
| (A.1) |
| (A.2) |
| (A.3) |
| (A.4) |
| (A.5) |
| (A.6) |
| (A.7) |
| (A.8) |
| (A.9) |
References
- 1.Guerra NP, Pastrana L. Modelling the influence of pH on the kinetics of both nisin and pediocin production and characterization of their functional properties. Process Biochemistry. 2002;37(9):1005–1015. [Google Scholar]
- 2.Chowdhury BR, Chakraborty R, Chaudhuri UR. Validity of modified Gompertz and Logistic models in predicting cell growth of Pediococcus acidilactici H during the production of bacteriocin pediocin AcH. Journal of Food Engineering. 2007;80(4):1171–1175. [Google Scholar]
- 3.Reardon KF, Mosteller DC, Rogers JDB. Biodegradation kinetics of benzene, toluene, and phenol as single and mixed substrates for Pseudomonas putida F1. Biotechnology and Bioengineering. 2000;69(4):385–400. doi: 10.1002/1097-0290(20000820)69:4<385::aid-bit5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Callewaert R, De Vuyst L. Bacteriocin production with Lactobacillus amylovorus DCE 471 is improved and stabilized by fed-batch fermentation. Applied and Environmental Microbiology. 2000;66(2):606–613. doi: 10.1128/aem.66.2.606-613.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biazar J, Tango M, Babolian E, et al. Solution of the kinetic modeling of lactic acid fermentation using Adomian decomposition method. Applied Mathematics and Computation. 2003;144(2-3):433–439. [Google Scholar]
- 6.Ghaly AE, Kamal M, Correia LR. Kinetic modelling of continuous submerged fermentation of cheese whey for single cell protein production. Bioresource Technology. 2005;96(10):1143–1152. doi: 10.1016/j.biortech.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Luedeking R, Piret EL. A kinetic study of the lactic acid fermentation. Batch process at controlled pH. Journal of Biochemical and Microbiological Technology Engineering. 1959;1(4):393–412. doi: 10.1002/(sici)1097-0290(20000320)67:6<636::aid-bit3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Guerra NP, Agrasar AT, Macías CL, et al. Dynamic mathematical models to describe the growth and nisin production by Lactococcus lactis subsp. lactis CECT 539 in both batch and re-alkalized fed-batch cultures. Journal of Food Engineering. 2007;82(2):103–113. [Google Scholar]
- 9.Guerra NP, Bernárdez PF, Castro LP. Modelling the stress inducing biphasic growth and pediocin production by Pediococcus acidilactici NRRL B-5627 in re-alkalized fed-batch cultures. Biochemical Engineering Journal. 2008;40(3):465–472. [Google Scholar]
- 10.Meyer P. Bi-logistic growth. Technological Forecasting and Social Change. 1994;47(1):89–102. doi: 10.1016/0040-1625(96)00001-7. [DOI] [PubMed] [Google Scholar]
- 11.Stanescu D, Chen-Charpentier BM. Random coefficient differential equation models for bacterial growth. Mathematical and Computer Modelling. 2009;50(5-6):885–895. [Google Scholar]
- 12.Taylor KB. Methods for model evaluation. In: Taylor KB, editor. Enzyme Kinetics and Mechanisms. chapter 3. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2002. pp. 14–24. [Google Scholar]
- 13.Guerra NP, Castro LP. Enhancement of nisin production by Lactococcus lactis in periodically re-alkalized cultures. Biotechnology and Applied Biochemistry. 2003;38(2):157–167. doi: 10.1042/BA20030059. [DOI] [PubMed] [Google Scholar]
- 14.Lomauro CJ, Bakshi AS, Labuza TP. Evaluation of food moisture sorption isotherm equations part I: fruit, vegetable and meat products. LWT—Food Science and Technology. 1985;18(2):111–117. [Google Scholar]
- 15.Aguerre RJ, Suarez C, Viollaz PE. New BET type multilayer sorption isotherms. Part II: modelling water sorption in foods. LWT—Food Science and Technology. 1989;22(4):192–195. [Google Scholar]
- 16.Åkerberg C, Hofvendahl K, Hahn-Hägerdal B, et al. Modelling the influence of pH, temperature, glucose and lactic acid concentrations on the kinetics of lactic acid production by Lactococcus lactis ssp. lactis ATCC 19435 in whole-wheat flour. Applied Microbiology and Biotechnology. 1998;49(6):682–690. [Google Scholar]
- 17.Herranz C, Martínez JM, Rodríguez JM, et al. Optimization of enterocin P production by batch fermentation of Enterococcus faecium P13 at constant pH. Applied Microbiology and Biotechnology. 2001;56(3-4):378–383. doi: 10.1007/s002530100656. [DOI] [PubMed] [Google Scholar]
- 18.Poolman B, Konings WN. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. Journal of Bacteriology. 1988;170(2):700–707. doi: 10.1128/jb.170.2.700-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bibal B, Goma G, Vayssier Y, et al. Influence of pH, lactose and lactic acid on the growth of Streptococcus cremoris: a kinetic study. Applied Microbiology and Biotechnology. 1988;28(4-5):340–344. [Google Scholar]
- 20.Gonçalves LMD, Ramos A, Almeida JS, et al. Elucidation of the mechanism of lactic acid growth inhibition and production in batch cultures of Lactobacillus rhamnosus. Applied Microbiology and Biotechnology. 1997;48(3):346–350. [Google Scholar]
- 21.Niven GW, Knight DJ, Mulholland F. Changes in the concentrations of free amino acids in milk during growth of Lactococcus lactis indicate biphasic nitrogen metabolism. Journal of Dairy Research. 1998;65(1):101–107. doi: 10.1017/s002202999700263x. [DOI] [PubMed] [Google Scholar]
- 22.Letort C, Nardi M, Garault P, et al. Casein utilization by Streptococcus thermophilus results in a diauxic growth in milk. Applied and Environmental Microbiology. 2002;68(6):3162–3165. doi: 10.1128/AEM.68.6.3162-3165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabo ML, Murado MA, González MAP, et al. Effects of aeration and pH gradient on nisin production. A mathematical model. Enzyme and Microbial Technology. 2001;29(4-5):264–273. [Google Scholar]
- 24.Vázquez JA, Cabo ML, González MP, et al. The role of amino acids in nisin and pediocin production by two lactic acid bacteria: a factorial study. Enzyme and Microbial Technology. 2004;34(3-4):319–325. [Google Scholar]
- 25.Guerra NP, Agrasar AT, Macías CL, et al. Modelling the fed-batch production of pediocin using mussel processing wastes. Process Biochemistry. 2005;40(3-4):1071–1083. [Google Scholar]
- 26.Guerra NP, Bernárdez PF, Agrasar AT, et al. Fed-batch pediocin production by Pediococcus acidilactici NRRL B-5627 on whey. Biotechnology and Applied Biochemistry. 2005;42(1):17–23. doi: 10.1042/BA20040146. [DOI] [PubMed] [Google Scholar]
- 27.Flambard B, Richard J, Juillard V. Interaction between proteolytic strains of Lactococcus lactis influenced by different types of proteinase during growth in milk. Applied and Environmental Microbiology. 1997;63(6):2131–2135. doi: 10.1128/aem.63.6.2131-2135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juillard V, Helinck S, Flambard B, et al. Amino acid supply of Lactococcus lactis during growth in milk. Recent Research and Development in Microbiology. 1998;2:233–252. [Google Scholar]
- 29.Juillard V, Le Bars D, Kunji ERS, et al. Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Applied and Environmental Microbiology. 1995;61(8):3024–3030. doi: 10.1128/aem.61.8.3024-3030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastrana LM, Gonzalez MP, Murado MA. Production of gibberellic acid from mussel processing wastes in submerged batch culture. Bioresource Technology. 1993;45(3):213–221. [Google Scholar]
- 31.Garrigues C, Loubiere P, Lindley ND, et al. Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: predominant role of the NADH/NAD+ ratio. Journal of Bacteriology. 1997;179(17):5282–5287. doi: 10.1128/jb.179.17.5282-5287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Felipe FL, Kleerebezem M, De Vos WM, et al. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. Journal of Bacteriology. 1998;180(15):3804–3808. doi: 10.1128/jb.180.15.3804-3808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Vuyst L. Nutritional factors affecting nisin production by Lactococcus lactis subsp. lactis NIZO 22186 in a synthetic medium. Journal of Applied Bacteriology. 1995;78(1):28–33. [Google Scholar]
- 34.Kim WS, Hall RJ, Dunn NW. The effect of nisin concentration and nntrient depletion on nisin production of Lactococcus lactis. Applied Microbiology and Biotechnology. 1997;48(4):449–453. doi: 10.1007/s002530051078. [DOI] [PubMed] [Google Scholar]
- 35.Thomas TD, Ellwood DC, Longyear VMC. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. Journal of Bacteriology. 1979;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cocaign-Bousquet M, Garrigues C, Loubiere P, et al. Physiology of pyruvate metabolism in Lactococcus lactis. Antonie van Leeuwenhoek. 1996;70(2-4):253–267. doi: 10.1007/BF00395936. [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. Journal of Bacteriology. 1975;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]