Abstract
Collagen V, a fibrillar collagen with important functions in tissues, assembles into distinct chain associations. The most abundant and ubiquitous molecular form is the heterotrimer [α1(V)]2α2(V). In the attempt to produce high levels of recombinant collagen V heterotrimer for biomedical device uses, and to identify key factors that drive heterotrimeric chain association, several cell expression systems (yeast, insect, and mammalian cells) have been assayed by cotransfecting the human proα1(V) and proα2(V) chain cDNAs. Suprisingly, in all recombinant expression systems, the formation of [α1(V)]3 homotrimers was considerably favored over the heterotrimer. In addition, pepsin-sensitive proα2(V) chains were found in HEK-293 cell media indicating that these cells lack quality control proteins preventing collagen monomer secretion. Additional transfection with Hsp47 cDNA, encoding the collagen-specific chaperone Hsp47, did not increase heterotrimer production. Double immunofluorescence with antibodies against collagen V α-chains showed that, contrary to fibroblasts, collagen V α-chains did not colocalized intracellularly in transfected cells. Monensin treatment had no effect on the heterotrimer production. The heterotrimer production seems to require specific machinery proteins, which are not endogenously expressed in the expression systems. The different constructs and transfected cells we have generated represent useful tools to further investigate the mechanisms of collagen trimer assembly.
1. Introduction
The extracellular matrix (ECM) consists for the most part of collagen proteins that form fibrils and other supramolecular structures from triple-helical collagen molecules. Collagen V is a quantitatively minor fibrillar collagen with a broad distribution in tissues such as dermis, tendons, bones, blood vessels, and cornea. Among the different members of the fibrillar collagens, collagen V has the particularity to assemble into different chain associations that can include hybrid molecules containing collagen XI chains [1, 2]. The most abundant and ubiquitous molecular form is the heterotrimer [α1(V)]2α2(V), which copolymerizes with collagen I to form heterotypic fibrils in tissues. The heterotrimers α1(V)α2(V)α3(V) and the homotrimer [α1(V)]3 represent minor isoforms that show a more restricted expression pattern [1, 3]. The physiological relevance of these three molecular minor forms is unclear. A wealth of evidence favours the role of the heterotrimer [α1(V)]2α2(V) in the control [4] and nucleation of fibril assembly [2, 5], a function that is not shared with the homotrimer [α1(V)]3 [6]. Many studies point to a role of collagen V heterotrimer in the control of collagen fibril diameter in cornea. Corneal injuries often result in cloudy or opacified scars which appears to develop from disruption of the organized collagen matrix and that require transplantation of donated or artificial corneas. The construction of a scaffold made of heterotypic collagen I/V collagen fibrils as in native stroma, would be an important asset in the development of bioengineered corneal implants. Recently, Torbet and coworkers [7] have reported that a stroma-like scaffold consisting of a stack of orthogonally disposed sheets of aligned collagen I fibrils can be built up using magnetic alignment. The recombinant technology represents a valuable alternative for collagen production to be used for tissue engineering.
Several attempts have been made to express collagens in different recombinant systems. Although recombinant expression of collagens was initially considered as a difficult task, these large, multimeric, and modular proteins have been successfully expressed in most of the existing expression systems. But the use of recombinant collagens has often been limited to their biochemical characterization in fundamental research because of the production yield that is so far not adapted to tissue engineering needs (for review see [8]). Another difficulty is the achievement of collagen heterotrimeric assembly in heterologous expression systems. Whereas the recombinant production of collagen homotrimeric molecular forms has been achieved in different expression systems, the production of collagen heterotrimers is still challenging.
Recombinant protein expression systems include a wide variety of organisms from bacteria to mammalian cells. However, production of recombinant collagen proteins presents various problems in part because the numerous posttranslational modifications required to achieve a correctly folded multimeric conformation are often crucial to preserve their functions. The choice of the expression cell system is therefore of fundamental importance to obtain a recombinant molecule in conformity with the natural protein to maintain its function (for a review, see [8]). One of the main structural properties of collagens is the thermostability of the triple helix domain, which is dependent on the hydroxylation of the proline residues in the Y-position of the collagenous Gly-X-Y triplet. The specific enzyme involved is prolyl 4-hydroxylase which is not present in prokaryotes and lower eukaryotes. Prokaryotic expression systems provide cheap maintenance and easy up-scaling properties of heterologous protein production. Human collagens have proved difficult to express in bacteria due to the triple helix folding and thermostability requirement. High-level expression of active human prolyl 4-hydroxylase (P4H) in E.coli has been the bottleneck until recently [9].
Yeast such as Pichia pastoris and Saccharomyces cerevisiae are frequently used as an expression system for the production of proteins. The advantages of yeast cells over bacteria are high-density fermentation, processing machinery similar to higher eukaryotes, and secretion to the culture media. Moreover, once the recombinant gene is in the genome of the yeast cell, a stable expression is achieved (for a review, see [10]). Thermostable fibrillar collagens I, II, and III have been successfully expressed after the lack of prolyl 4-hydroxylase was solved by stable transformation of Pichia pastoris with enzyme subunits [11–14].
Rod shaped baculoviruses capable of infecting only certain insects are efficient and safe expression vectors for large-scale production of recombinant proteins. Moreover, the recombinant proteins expressed in the baculovirus expression system are usually properly folded, disulfide-bonded, and localized to the correct subcellular compartment. Even though insect cells are higher eukaryotes than yeast cells, they do not have prolyl-4-hydroxylase activity. By expressing prolyl 4-hydroxylase subunits with collagens I, II, and III α-chains in insect cells, it was possible to achieve thermostable collagen I, II, and III trimeric molecules [15–18].
The advantages of mammalian expression systems include full range posttranslational modifications and secretion of the heterologous protein in the culture media. The HEK HEK-293 is the most commonly used cell line for large-scale matrix protein expression. Collagens recombinantly produced in HEK-293 cells are generally fully hydroxylated as we showed for the first time for collagen V homotrimer [19] and further proven to be true for other homotrimeric collagens [8]. Production of recombinant collagen V heterotrimer has already been reported in the HEK-293 cell line. However, when 15–20 μg/ml of homotrimer collagen V was produced in these cells [6, 19], only negligible amount of 0.8 μg/ml of heterotrimeric collagen V was obtained by cotransfecting the proα1(V) and proα2(V) chains [20].
In this paper, the three different eukaryotic expression systems have been used to monitor the expression of recombinant human collagen V heterotrimer, the most prevalent in vivo molecular form of collagen V: yeast, baculovirus-insect cell, and mammalian cell expression systems, in order to define the most suitable expression system to get substantial amounts of collagen V heterotrimer for biomedical device uses and to identify important factors that drive heterotrimeric chain association.
2. Materials and Methods
2.1. Preparation of Constructs
Human proα1(V) (Gene bank accession number P20908) and proα2(V) (Gene bank accession number P05997) cDNA sequences were used for this study.
2.1.1. Baculovirus Transfer Vectors
A pBlueScript KS clone, in which a NaeI-PstI digested fragment of proα1(V) cDNA (pBS51nae, a gift from D. Greenspan) was cloned, had an extra ATG nucleotide sequence near the original initiation codon in the 5′ UTR, and it was removed by site directed mutagenesis. A PCR primer containing a HindIII restriction site and an A to T mutation was designed (5′-CGATAAGCTTGTTGGCATGGACTGC-3′) and used to multiply a 912 nt fragment of proα1(V) cDNA together with a primer 5′AGCTTCCACGGGCTTCTTGCTG3′ corresponding to the nucleotides downstream of an AvrII restriction site in exon 6 of proα1(V) cDNA. The PCR products were ligated in pGEM-T easy-vector (Promega). The inserted cDNA was digested with HindIII-AvrII and ligated to pBS51nae digested with HindIII-AvrII. The whole proα1(V) cDNA was digested out from pBS51nae with HindIII-NotI, the protruding ends were filled using Taq polymerase and the cDNA was ligated to pGEM-T easy-vector. The resulting pGEMTe-C5A1 was digested with NotI-SpeI and the proα1(V) cDNA was ligated to pVL1392 digested with NotI-XbaI (pVLC5A1).
A XbaI restriction site was created six nucleotides upstream of the translation initiation codon to a full length proα2(V) cDNA (a gift from D. Greenspan) by PCR (Expand Long Template PCR Kit, Boehringer Mannheim) using expression vector pGGH31 as a template. The primers used for the PCR were 5′-AGTCTAATATCTAGACATGATGGCAAACTG-3′ corresponding to the 5′ end of the cDNA (the initiation codon of the cDNA is underlined) and 5′-TAAGGTTCCTTCGAAAAGATCGTC-3′ corresponding to the nucleotides next to the 3′-end of proα2(V) cDNA in the pGGH31. The PCR products were cloned in to pGEM-T easy-vector (Promega). The resulting pGEMTe-5A2 was digested with XbaI-NotI and proα2(V) cDNA was ligated to pVL1393 vector digested with XbaI-NotI (pVLC5A2).
2.1.2. Yeast Constructs (Table 1)
Table 1.
Yeast expression constructs, host cells, and recombinant strains.
| Constructs | Host cells | Recombinant yeast strains |
|---|---|---|
| pPICZα-C5A1N | P. pastoris X-33 | α1(V) chain with αMF signal, without C-propeptide |
| pPIC3.5K-C5A1M | P. pastoris yJC300 | full length prepro-α1(V) chain |
| pPICZ-C5A2 | P. pastoris yJC300 | full length prepro-α2(V) chain |
The proα1(V) cDNA was amplified by PCR using the forward primer 5′-AAGGTACCGCTCAGCCAGCAGATCTCC-3′ and the reverse primer 5′-AAGGTACCCTACGCGTAGTCCACGTAGTTCTC-3′ and pBS51nae as a template. A cDNA fragment was obtained without the native signal sequence and without the 3′ area coding for the C-propeptide, but including the C-telopeptide. Both primers contained a KpnI restriction site (underlined) for cloning the cDNA fragment into pPICZaA in frame with the Saccharomyces cerevisiae α-factor secretion signal (αMF). The vector pPICZaA and the PCR product were digested with KpnI and ligated and the resulting expression construct pPICZa-C5A1N was verified with sequencing. For the yeast expression of the full length collagen α1(V) chain the pGEMTe-C5A1 was digested with NotI and the proα1(V) cDNA was ligated to pPIC3.5K yeast vector (Invitrogen) digested with NotI (pPIC35-C5A1). To allow plasmid linearization with SalI restriction enzyme removal of a SalI site located in the exon 63 of proα1(V) cDNA was performed by site directed mutagenesis. A 2.7 kb fragment of pPIC35-C5A1 including the RrsII site in the exon 55 of proα1(V) cDNA and the HpaI site in the pPIC3.5-K vector was amplified and ligated to pGEMT easy-vector. The resulting plasmid was used as a template for introducing a null mutation according to the instructions of the QuikChange kit (Stratagene). After the point mutation was verified the plasmid was digested with RrsII-HpaI and the insert was ligated to pPIC35-C5A1 digested with the same enzymes (pPIC3.5K-C5A1M). To obtain the yeast expression construct for full length collagen α2(V) chain the pGEMTe-C5A2 was digested with SacII-NotI and ligated to pPICZ-A vector (Invitrogen) digested with SacII-NotI (pPICZ-C5A2).
2.1.3. Mammalian Expression Constructs
pcDNA3, pZeo-SV2, and Rc-CMV (Invitrogen) were used to optimize the stable production of both α1(V) and α2(V) collagen chains. For mammalian episomal and transient expression, two other vectors were used: pCEP4 and pCEP2 (Invitrogen). These vectors expressed different antibiotic resistance genes: hygromycin for pcDNA3-Hygro and pCEP4, puromycin for pCEP2, zeocin for pZeo-SV2, and neomycin for Rc-CMV. The full-length cDNA of the proα1(V) chain was excised from the Bluescript vector KS (as previously described in [19]) using KpnI and subcloned into the eukaryotic expression vectors pcDNA3-Hygro and pZeo-SV2, and into the mammalian episomal expression vectors pCEP4 and pCEP2. The full-length cDNA of proα2(V) chain was digested with NotI, from the Bluescript vector KS, and subcloned into the eukaryotic expression vector Rc-CMV, and into the mammalian episomal expression vector pCEP4. The cDNA of HSP47 was obtained from the cln9-33 plasmid (kindly provided by Dr T. Homma, Kyoto, Japan), after a HindIII/BamHI digestion and subcloned into the mammalian episomal expression vectors pCEP4 and pCEP2.
2.2. Cell Culture, Protein Production and Characterization
2.2.1. Generation of Recombinant α1(V) and α2(V) Baculoviruses and Protein Production in Insect Cells
The plasmids pVLC5A1 and pVLC5A2 were separately co-transfected with modified Autographa californica nuclear polyhedrosis virus DNA into Spodoptera frugiperda (Sf9) insect cells using the BaculoGold transfection kit (PharMingen). The resultant viral pools were collected, amplified, and purified using the Baculovirus expression system (BD Biosciences). Trichoplusia ni High Five (H5) insect cells were cultured in suspension in SF-900 SFM medium (Invitrogen) and were infected with baculoviruses coding for α1(V), α2(V) and a virus for both α and β subunit of human prolyl-4-hydroxylase at relative multiplicities of 3 : 1 : 1, respectively. Ascorbate was added to the culture medium daily at 80 μg/ml. The cells and culture medium were collected 72 h after infection and kept at −20°C until used.
2.2.2. Production of Recombinant Collagen V in Pichia Pastoris
The plasmids were linearized with SalI (pPIC3.5K-C5A1M) and PmeI (pPICZa-C5A1N, pPICZ-C5A2) and transformed either to a wild type P. pastoris X-33 or a yJC300 strain expressing α- and β-subunits of human prolyl-4-hydroxylase [21] by electroporation according to vector manufacturer's instructions (Invitrogen). Cells positive for genomic PCR for collagen V sequences were grown in a buffered glycerol complex medium (BMGY, pH 6.0) in 25 ml shaker flasks. Expression was induced in a buffered minimal methanol medium (BMM, pH 6.0) and methanol was added to a final concentration of 0.5% daily. Histidine was added to negative control up to 100 μg/l in the case of yJC300 strain. Cells were harvested after 75 h of methanol induction at 30°C, washed once with sterile H2O and divided into 100 mg aliquots and kept at −20°C until used.
2.2.3. Protein Production in Mammalian Cells
The human embryonic kidney cell line, HEK-293 that constitutively expresses the EBNA-1 protein from the Epstein-Barr virus, allowing the episomal replication of the expression vectors; the Chinese hamster ovary cell line, CHO-K1; the human rhabdomyosarcoma cell line, A204; the human fibrosarcoma cell line, HT1080; the human carcinoma cell line, HeLa and human dermal fibroblasts were all cultured at 37°C in DMEM (Dulbecco's Modified Eagle's Medium, Sigma), supplemented with 10% foetal calf serum (Sigma), 50 μg/ml gentamicin (Sigma) in 5% CO2. Media were changed every two days. The cells were transfected with expression plasmids, using the calcium-phosphate precipitation method (Invitrogen) or the FuGene 6 transfection reagent (Roche), according to the manufacturer's instructions. The transfected cells were selected during 15 days using hygromycin (250 or 300 μg/ml), puromycin (0.5 μg/ml), zeocin (1 μg/ml), neomycin (5 μg/ml) or, for co-transfected plasmids, using a mix of two different antibiotics according to the expression vector used. After selection, the resistant cells were cloned and tested for protein expression. For further protein production and characterization, cells were grown in serum-free medium in the presence or absence of sodium ascorbate (50 μg/ml).
2.3. SDS-PAGE and Western-Blot Analyses
Recombinant expression of the proα1(V) and proα2(V) chains in insect cells was analyzed as follows: the cells were homogenized with a glass homogenizer in 0.2 M NaCl, 0.1% Triton X-100, and 0.05 M Tris buffer, pH 7.4, and centrifuged at 10,000 g for 10 min. The media and the soluble fractions of the cells were analyzed by denaturing 4–6% SDS-PAGE under reducing conditions followed by Coomassie blue or staining and Western blotting with a monoclonal antibody 95D1A [15].
For recombinant protein expression analysis, the yeast cells were homogenized by vortexing with glass beads in neutral buffer (0.267 M NaCl, 60 mM Tris-HCl, pH 7.4, 0.2% Triton X-100), acidic (0.1 M HCl), or basic (50 mM NaOH) solutions. The lysate was centrifuged at 10000 × g for 15 min and aliquots of soluble fractions were analyzed by denaturing SDS-PAGE under reducing conditions followed by electrotransfer onto polyvinylidene difluoride membranes (Immobilon-P, Millipore) overnight in 10 mM CAPS (pH 11), 5% methanol. After saturation, membranes were then incubated with monoclonal antibody 95D1A [15] and a monoclonal antibody 18G5 [22]. The peroxidase conjugated secondary antibodies for the Western blots of the insect cell and yeast expression products were detected with an ECL Western Blotting Analysis System (Amersham Biosciences). Relative band intensities were determined after scanning the original membranes (or the dried gel) on optimized, nonedited images, using Quantity One image analysis software (version 4.6.5), after background substraction.
Recombinant protein expression (proα1(V), proα2(V) and HSP47) in mammalian cells was checked by 6% or 12% (SDS-PAGE) analysis of HEK-293 resistant cells media and lysates, followed by Coomassie Blue staining or by electrotransfer and immunostaining. In some cases, cell layers were scraped into phosphate-buffered saline (PBS) and centrifuged 5 minutes at 400 g. The pellet was resuspended in lysate buffer (100 mM NaCl, 20 mM Tris-HCl pH 7.6, 25 mM EDTA, 5 mM N-ethylmaleimide, 2 mM phenylmethanesulfonyl fluoride, 0.1% SDS, 1% Nonidet P-40). Cell lysates were centrifuged, and supernatants were analyzed by SDS-PAGE followed by Coomassie Blue staining or by Western blotting. The Western blot membranes of mammalian cell expression products were incubated with different antibodies as indicated: the monoclonal 18G5 antibody against the recombinant N-propeptide of the proα1(V) chain [22], the polyclonal collagen type V (G-15) antibody against the N-propeptide of the proα2(V) chain (Santa Cruz Biotechnology, Inc. California), polyclonal antibodies against the helical domain of the α1(V), α2(V) and α3(V) chains (Novotec, France), and the monoclonal anti-HSP47 (colligin) antibody (Stressgen Victoria, BC, Canada). Alkaline phosphatase conjugated secondary antibodies were detected with an AP Color kit (BioRad), and peroxydase conjugated secondary antibodies with a Fast 3-3′ DiAminoBenzidine Tablet Sets (Sigma) or with an ECL Western Blotting Analysis System (Amersham, Biosciences).
2.4. Protein Purification for Mammalian Cell Expression Products
For purification of a large amount of proteins, transfected cells were selected with antibiotics during 15 days, rinsed three times with PBS and placed in serum-free medium. Then, this medium was collected every 48 h and stored at −20°C. For separation of the different collagen molecular species when cells were transfected with proα1(V) and proα2(V) chains, media were dialyzed against 50 mM Tris-HCl pH 8.6 to precipitate the collagen V homotrimer as in [19]. After centrifugation, the pellet containing the homotrimer was resuspended in 50 mM Tris-HCl pH 7.5, 150 mM NaCl. The supernatant was passed over a DEAE-cellulose column (DE-52, Whatman) and subsequently eluted with a linear 0–0.6 M NaCl gradient. The different fractions obtained were analyzed by Western blotting.
For purification of the proα2(V) chains, transfected cell media were dialyzed against 50 mM Tris-HCl pH 8.6 and passed over a DEAE-cellulose column (DE-52, Whatman) and eluted with a linear gradient 0–0.6 M. The eluant was then dialyzed against 40 mM sodium acetate buffer pH 4.8. After centrifugation, the supernatant was passed over a HiTrap-SP column (Pharmacia-Biotech) and subsequently eluted with a linear 0–0.6 M NaCl gradient. The different fractions were analyzed by 6% SDS-PAGE. The proα2(V) containing fractions were pooled and stored at −80°C.
2.5. Proteolytic Digestion and Monensin Treatment
Yeast and insect cell expression products were digested with pepsin to attest for the triple helix formation. The pH of the sample was adjusted to around 2 with 2 M HCl and pepsin was added to a final concentration of 200–250 μg/ml. The reaction solution was incubated at room temperature for 1 h, neutralized with 2 M NaOH and an aliquot of the sample was analyzed by SDS-PAGE under reducing conditions followed by Coomassie blue staining and Western blotting with monoclonal antibody 95D1A.
For mammalian cell media, the different samples were digested with pepsin (Sigma) in 0.5 M acetic acid for 3 h at 20°C with an enzyme/substrate ratio of approximately 1 : 5. When indicated, 4·105 HEK-293 cells (C2 clone) were plated in 35 mm diameter Petri dishes and treated the day after with 50 μM monensin. After two hours incubation, cells were rinsed twice with PBS and incubated in culture medium for 24 h. Media were then collected and treated with pepsin in 0.5 M acetic acid for 2 h at 20°C with an enzyme/substrate ratio of 1 : 10. Samples were analyzed by Western blotting using polyclonal antibodies against collagen V α-chains. For bacterial collagenase digestions, serum-free medium was purified as described above. The sample was diluted in an optimal buffer: 25 mM Tris-HCl pH 7.5, 0.5 M NaCl, 6 mM CaCl2. Collagenase digestion was performed at 37°C for 3 h with an enzyme/substrate ratio of 1 : 7 (Advanced Biofacture).
The purified proα2(V) chain digestion by BMP-1 was performed essentially as previously described [23]. Briefly, the purified proα2(V) chain (100 ng) was incubated with BMP-1 (6 ng) in 25 μl of 50 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2, pH 7.5, for 6 h at 37°C. The reaction was stopped by heating at 60°C for 15 minutes in Laemmli SDS sample buffer containing 4% 2-mercaptoethanol. Digestion products were analyzed by 12% SDS-PAGE followed by silver staining.
2.6. Analytical Methods
For N-terminal sequencing of the proα2(V) chain, proteins were transferred to a polyvinylidene difluoride membrane and treated with pyroglutamate aminopeptidase (Boehringer-Mannheim) to remove the pyroglutamic acid blocking groups before performing automated Edman degradation. Amino acid sequencing was performed by automated Edman degradation using an Applied Biosystems 473A protein sequencer.
Amino acid compositions were determined after hydrolysis under vacuum (6 N HCl, 115°C, 24 h) in the presence of 2-mercaptoethanol in a Pico Tag system (Waters) with a Beckman amino acid analyzer.
2.7. Immunofluorescence
Human dermal fibroblasts, HeLa cells which have been transiently transfected with α1(V) and α2(V), and clone C2 (stably transfected with proα1(V), proα2(V) and HSP47 constructs as described above) were used for immunofluorescence double staining with the monoclonal antibody against collagen V proα1-chain (18G5 [22]) and the rabbit polyclonal antibodies raised against a synthetic peptide derived from the N-terminus of the proα2-chain (a generous gift from D.E. Birk, University of South Florida, USA).
For HeLa cell transient transfection, 2·105 cells were plated in 60 mm diameter Petri dishes and co-transfected the following day with the proα1(V) and proα2(V) expression plasmids, using the calcium-phosphate precipitation method according to the manufacturer's instructions (Invitrogen). 48 h after transfection, cells were washed three times with PBS and fixed for 20 min with 4% paraformaldehyde in PBS. After three washes with PBS, cells were permeabilized with 0.1% Triton in PBS and then blocked with 1% BSA. Efficiency of transfection was analyzed by transfecting the cells with a plasmid that expresses green fluorescent protein (GFP) as previously described [22]. The percentage of transfection was calculated 48 h after transfection by counting green cells with an inversed microscope equipped with fluorescence (Nikon). The values varied from 50% to 70% depending on the experiments.
For immunofluorescence, cells were grown for 24 h in medium supplemented with 50 μg/ml sodium ascorbate. After washing with PBS, cells were fixed for 20 min with 4% paraformaldehyde. After three washes with PBS, cells were permeabilized with Triton 0.1% in PBS and blocked with BSA 1% in PBS. Cells were first incubated for 1 hour with the monoclonal antibody 18G5 followed by incubation with secondary antibodies conjugated with Alexa 546 for 30 min. Then, cells were incubated with polyclonal antibodies against the proα2(V) chain followed by incubation with secondary antibodies conjugated with Alexa 488. As negative controls, primary antibodies were replaced by PBS. Coverslips were mounted in Dakocytomation Fluorescent Mounting Medium (Dako) and observed with a Zeiss Axioplan2 fluorescence microscope equipped with a high-resolution digital camera.
3. Results
3.1. Expression of Recombinant Human Collagen V Heterotrimer in Insect Cells
In order to obtain recombinant human collagen V heterotrimers in insect cells by baculovirus expression system, recombinant baculoviruses coding for proα1(V) and proα2(V) chains were generated and used to infect High Five insect cells together with a virus coding for both subunits of prolyl 4-hydroxylase. No collagen band was detectable in cell lysates and media of infected cells with Coomassie blue staining and Western blot analysis revealed that both collagen chains were faintly expressed in addition to protein degradation products (data not shown). A negligible amount of the collagen V molecules (heterotrimer and homotrimer) is produced in the baculovirus-insect cell expression system, indicating that the housekeeping and secretion machinery of insect cells are not adapted to the recombinant collagen V molecule production.
3.2. Expression of Recombinant Human Collagen V Heterotrimer in Yeast
The yeast P. pastoris, was selected based on previous data obtained on recombinant expression of the fibrillar collagens I, II, and III in P. pastoris [12–14]. Moreover, it has been shown that collagen I can efficiently be expressed in yeast as a heterotrimer [11].
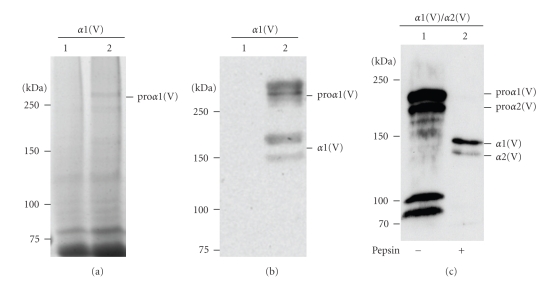
Several expression constructs for producing human collagen V in P. pastoris were generated. The first expression construct encoding the proα1(V) chain without the C-propeptide and the native signal sequence replaced with the S. cerevisiae α-factor secretion signal (pPICZa-C5A1N, Table 1) was transformed by electroporation into a wild type P. pastoris strain X-33. The positive strain was cultured in buffered glycerol complex medium and the expression was induced in buffered minimal methanol medium. Medium and aliquots of soluble fraction of acidic extraction of the yeast cells were analyzed by SDS-PAGE under reducing conditions followed by Coomassie blue staining or Western blotting with the monoclonal antibody 18G5 to the collagen V α1 N-propeptide and with the monoclonal antibody 95D1A. No high molecular weight band was detectable either with Coomassie blue staining or Western blotting in the medium suggesting that the proα1(V) chain was not secreted despite the presence of S. cerevisiae α-factor secretion signal in the N-terminus of the proα1(V) chain. After Coomassie blue staining a faint band with high-molecular weight was detected in the cell sample (Figure 1(a), lane 2) that is absent in untransformed cells (Figure 1(a), lane 1). Nevertheless, this indicates that a higher expression level can be obtained in yeast when compared to insect cells for which no band was readily detectable in Coomassie blue stained gels.
Figure 1.
Expression of α1(V) without the native signal sequence and the C-propeptide (a, b) and of α1(V)/α2(V) chains (c) in P. pastoris. (a) 5% SDS-PAGE analysis under reducing conditions of the soluble fraction of acidic extraction from untransformed yeast cells (lane 1) and from yeast cells transformed with the construct pPICZα-C5A1N (lane 2). (b) Western blot analysis of the same samples as in panel (a) probed with monoclonal pan-collagen antibody 95D1A. (c) Western blot analysis of the soluble fraction of neutral extraction of yeast cells transformed with α1(V) and α2(V) chains and induced with 0.5% methanol induction. Samples were incubated without (lane 1) or with (lane 2) pepsin before analysis. Left, molecular mass standards expressed in kDa.
Lysates of cells transfected with the construct containing the proα1(V) chain devoid of the native signal sequence and the C-propeptide were analyzed by Western blotting using both 95D1A (Figure 1(b), lane 2) and 18G5 antibodies (data not shown). Both antibodies recognized high molecular weight bands, corresponding to the pNα1(V) chain which is absent in untransformed cells (Figure 1(b), lane 1). Surprisingly, the proα1(V) chain expressed with native signal sequence and C-propeptide had unexpectedly low abundance detectable only by Western blotting (data not shown). To further obtain recombinant collagen V molecules from association of the intact pro-chain cDNAs, the yeast constructs coding for prepro-α1(V) and prepro-α2(V) chains (pPIC3.5K-C5A1M and pPICZ-C5A2, Table 1) were transformed by electroporation into P. pastoris yJC300 strain expressing active human prolyl 4-hydroxylase. The strains were cultured and the protein production was induced as above. Two high-molecular weight bands were detected by western blot probed with 95D1A in the neutral extract (Figure 1(c), lane 1). The recombinant collagen V yield in yeast is mostly underestimated since a large amount of recombinant protein remains in the insoluble material (data not shown). After pepsin treatment, two bands were detected with the correct position of the pepsinized α1 and α2 chains of the native collagen V heterotrimer indicating that the heterotrimer is correctly formed in yeast (Figure 1(c), lane 2). However, the α1/α2 chain ratio (4 : 1 as estimated by gel densitometry) indicated that a mixture of collagen V homotrimer and heterotrimer molecules are produced.
3.3. Production and Characterization of the Proα2(V) Chain in Mammalian Cells
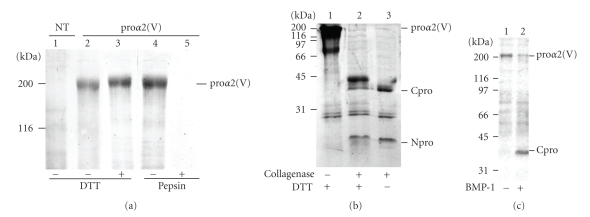
Prior to expressing the collagen V heterotrimer, the production and characterization of the human proα2(V) chains in HEK-293 cells singly transfected with the mammalian episomal expression vector pCEP4-α2(V) were analyzed by biochemical approaches. Electrophoresis analysis of serum-free medium from proα2(V)-transfected cells demonstrated an additional 200 kDa protein band, which was absent in non-transfected cells medium (Figure 2(a)). Moreover, this protein band corresponded to the expected molecular mass for the full proα2(V) chain. N-terminal amino-acid sequencing confirmed that this band is the proα2(V) chain. The N-terminal sequence obtained after pyroglutamate aminopeptidase treatment, Glu-Glu-Asp-Glu-Asp-Glu-Gly, identified this band as the human proα2(V) chain and indicated that the peptide bond Ala26-Glu27 is the signal peptide cleavage site. In nonreduced conditions, the proα2(V) chain migrated only slightly faster than the proα2(V) chain in reduced conditions likely due to the presence of intrachain disulfide bonds within the N-terminal noncollagenous cystein rich domain. The secretion in the medium of the proα2(V) chains was unexpected for two reasons. First, the proα2(V) chains were shown to be unable to form homotrimers [24, 25]. In tissues, the proα2(V) chains form a heterotrimer with two proα1(V) chains. Second, it is acknowledged that fibrillar collagens that are not properly folded are degraded intracellularly. To check whether in our experimental conditions, the proα2(V) chains can fold into a triple helix, proα2(V)-transfected cell medium was digested with pepsin. The results showed that the 200 kDa protein band disappeared after pepsin treatment (Figure 2(a)), indicating that the proα2(V) chains did not fold into a stable triple helix but can be secreted. The best yield obtained for the recombinant proα2(V) chain production was 15 μg/ml.
Figure 2.
Biochemical analysis of the recombinant proα2(V) chains produced in HEK-293 cells. (a) 6% SDS-PAGE analysis of cell media from untransfected cells (lane 1) and from cells transfected with human proα2(V) construct (lanes 2–5) under unreduced (lanes 1, 2) or reduced (lanes 3, 4, 5) conditions. Transfected cells media were incubated without (lane 4) or with (lane 5) pepsin before SDS-PAGE analysis. (b) Digestion with bacterial collagenase: transfected cell media were incubated without (lane 1) or with (lanes 2, 3) collagenase before 12% SDS-PAGE analysis under reduced (lanes 1, 2) or unreduced (lane 3) conditions. (c) Digestion with BMP-1: 12% SDS-PAGE patterns of transfected cells media digested (lane 2) or not (lane 1) with BMP-1. Gels (a) and (b) were stained with Coomassie blue whereas gel (c) was silver stained. Left, molecular mass standards expressed in kDa. NT: not transfected, proα2(V): transfected with proα2(V) construct, DTT: dithiothreitol, Cpro: C-propeptide, Npro: N-propeptide, BMP-1: Bone Morphogenetic Protein 1.
The presence of the N- and C-propeptides was verified by bacterial collagenase treatment of medium from α2(V)-transfected cells (Figure 2(b)). The 200 kDa protein band, which corresponded to the full proα2(V), disappeared in the presence of collagenase and two bands with lower molecular weights were observed. The lower protein band corresponded to the N-terminal cystein rich domain. In reduced conditions, this band migrated slightly slower compared to the nonreduced conditions (Figure 2(b)). In reduced conditions, the protein band observed at 33 kDa corresponded to the C-propeptide. In unreduced conditions, the protein migrated slower likely due to intrachain disulfide bonds. This result showed that interchain disulfide bonds did not form as was the case for the proα1(V) chains produced in the same cell system [19]. It also confirmed that the proα2(V) chains did not form a triple helix. The C-propeptide of the proα2(V) chains when incorporated into a heterotrimer was shown to be processed by BMP-1 [26]. Digestion of purified recombinant proα2(V) chains with BMP-1 showed the presence of a band corresponding to the C-propeptide (Figure 2(c)). This result is not surprising since BMP-1 activity is not dependent on triple helix formation. These results all together indicate that the proα2(V) chains can be efficiently produced in HEK-293 cells and that the recombinant chains exhibited the same features as the native chains.
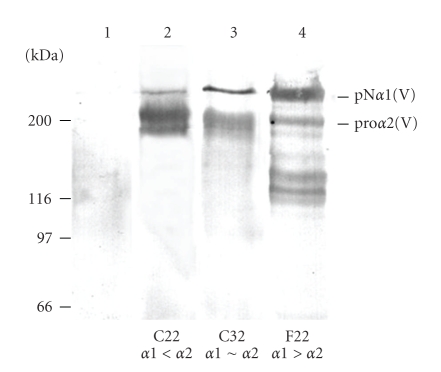
To optimize the proα1(V) and the proα2(V) chains production in HEK-293 cells in order to favor heterotrimer formation, different constructs were prepared and different clones were selected after cotransfection with the different constructs for their high expression of the two pro-chains. The cDNA of the proα1(V) chain was cloned in two eukaryotic expression vectors, pZeo-SV2 and pcDNA-Hygro, containing the SV40 and the CMV promoters, respectively. The cDNA of the proα2(V) chain was cloned in the Rc-CMV eukaryotic expression vector containing the CMV promoter. The construct α2(V)-Rc-CMV was co-transfected with either α1(V)-pZeo-SV2 or α1(V)-pcDNA-Hygro, in HEK-293 cells. After selection with the appropriate antibiotics, the serum-free culture media were analyzed by Western blotting with antibodies to collagen V (Figure 3). In order to modulate the expression ratio between proα1(V) and proα2(V) chains, three clones were selected: (i) clone F22 which expressed more efficiently the proα1(V) chain than the proα2(V) chain; (ii) clone C22 which expressed higher levels of proα2(V) chain than proα1(V) chain and (iii) clone C32 which equally expressed the two chains (Figure 3). The results suggested that the CMV promoter is more efficient for the fibrillar collagen chain expression than the SV40 promoter.
Figure 3.
Selection of clones expressing α1(V) and α2(V) chains. Western blot analysis with antibody to collagen V of recombinant α1(V) and α2(V) chains synthesized and secreted in the culture medium of co-transfected HEK-293 cells. The constructs α1(V)-pZeo-SV2 (lanes 2, 3) or α1(V)-pcDNA-Hygro (lane 4) and the construct α2(V)-Rc-CMV (lanes 2, 3, 4) were co-transfected into the HEK-293 cells. These cells do not naturally express these chains (lane 1). Three clones have been selected to express different ratios of α1(V)/α2(V): clone C22, which expressed more of the α2(V) chain than the α1(V) chain, clone C32, which expressed both chains at approximately the same amount, and clone F22, which expressed more of the α1(V) chain than the α2(V) chain. Left, molecular mass standards expressed in kDa.
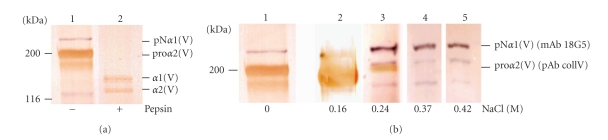
The analysis of the recombinant procollagen V molecular forms from the three selected clones C22, C32, and F22 gave similar results. As a representative example, the results presented here were obtained with the clone C22, in which expression level of the proα2(V) chain was higher than the proα1(V) chain (Figure 4). The transfected cell medium was dialyzed against Tris-HCl 50 mM pH 8.6 to eliminate most of the formed homotrimer (data not shown) [19]. After centrifugation, a fraction of the supernatant which contained both proα1(V) and proα2(V) chains was digested with pepsin prior to Western blot analysis (Figure 4(a)). After digestion with pepsin, the protein bands migrated at the pepsinised α1(V) and α2(V) chain positions. The intensity of the proα1(V) band was identical before and after pepsin digestion, contrary to the intensity of the proα2(V) band, which was weaker after pepsin digestion (Figure 4(a)). This result indicated that a large fraction of the proα2(V) chains did not fold into a heterotrimer and were produced as free pro-chains. To separate the different collagen V molecular species, the supernatant was applied to a cation exchange chromatography. The eluted fractions were analyzed to detect both pro-chains by Western blotting using two different collagen V antibodies and detection systems as described in Section 2 to discriminate the α1(V) and α2(V) chains (Figure 4(b)). Three populations of molecular forms were identified. The free proα2(V) chain containing fraction, was first eluted at 0.16 M NaCl. Then, another peak eluted at 0.24 M was shown to contain proα1(V) and proα2(V) chains with an estimated ratio of 2 : 1 and likely corresponded to the heterotrimer. Finally, fractions eluted at 0.37 M and 0.42 M NaCl were shown to contain only the proα1(V) chains and could correspond to the homotrimer. Thus, only a small amount of proα2(V) chains were found associated to the proα1(V) chains to form the heterotrimer. Contrary to the in vivo situation, the heterotrimer was not the major molecular form produced by the HEK-293 mammalian cells.
Figure 4.
Characterization of the recombinant procollagen V molecular forms produced in HEK-293 transfected cells. (a) Western blot analysis of the supernatant of transfected cell media dialysed against Tris-HCl 50 mM, pH 8.6, digested (lane 2) or not (lane1) with pepsin. Transfected cell media were centrifuged and the supernatant was applied to a DEAE column. (b) Separation of the different collagen V molecular species from transfected cell media by cation exchange chromatography. Western blot analysis of the loaded sample (lane 1) and of the fractions eluted at 0.16 M (lane 2), 0.24 M (lane 3), 0.37 M (lane 4), and 0.42 M (lane 5) NaCl. Membranes were double probed with mAb 18G5 followed by alkaline phosphatase antimouse secondary antibodies and with pAb CollV followed by peroxydase antirabbit secondary antibodies. Left, molecular mass standards expressed in kDa.
3.4. Effect of HSP47, a Collagen-Specific Chaperone Protein, in Collagen V Heterotrimer Expression in HEK-293 Cells
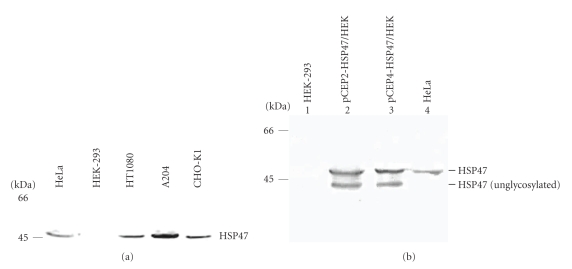
The HEK-293 cells were shown to contain the cell machinery necessary for correct biosynthesis of procollagens [19, 27]. To understand the failure to favor heterotrimer [α1(V)]2α2(V) in these cells, we hypothesize that one of the enzyme or/and chaperone proteins crucial for chain assembly may lack. The heat shock protein HSP47, described as a collagen-specific molecular chaperone, was a potential good candidate. The level of HSP47 expression was undetectable in the HEK-293 cell lysates (Figure 5(a)). In contrast, the presence of HSP47 was detected in several other cells lines (Figure 5(a)) such as HT1080, HeLa, A204, and CHO-K1 cells for which endogenous collagen V expression was shown by Western blotting (data not shown). Cell lysates were all positive for HSP47 expression except the HEK-293 cells. This result suggested that HSP47 could be necessary for the efficient heterotrimer [α1(V)]2α2(V) production.
Figure 5.
(a) HSP47 expression in different cell lines. Western blot analysis of conditioned cell lysates from different cell lines probed with monoclonal antibody to HSP47. Left, molecular mass standards expressed in kDa. (b) Western blot analysis of HSP47 expression in untransfected HEK-293 cells (lane 1), HEK-293 cells transfected with pCEP2-HSP47 (lane 2) or with pCEP4-HSP47 (lane 3) constructs and in HeLa cell lysate as positive control (lane 4). Two bands were detected in transfected HEK-293 cells lysates corresponding, respectively, to the glycosylated and unglycosylated forms. Left, molecular mass standards expressed in kDa.
Two constructs, pCEP4-Hsp47 and pCEP2-Hsp47, were prepared. HEK-293 cells were then transiently transfected and the cells lysates were analyzed by Western blotting (Figure 5(b)). The HeLa cell line was used as a positive control. Contrary to untransfected cells, in the HEK-293 cells transfected with the pCEP4-Hsp47 or pCEP2-Hsp47 construct, two bands were detected by immunodetection with antibodies to HSP47. The upper protein band migrated at the expected position and likely corresponds to the mature form of HSP47. The faster band probably corresponds to the unglycosylated form of HSP47 as described in [28].
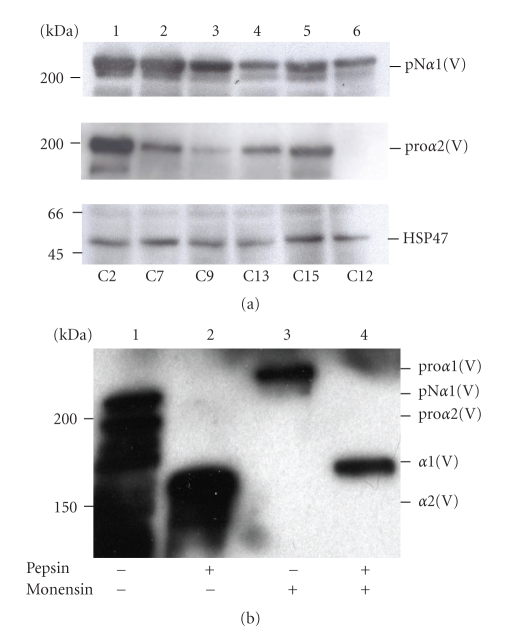
The HEK-293 cells were then transfected with three different constructs: pCEP4-α2(V) and pCEP4-α1(V) or pCEP2-α1(V) together with pCEP4-Hsp47 or pCEP2-Hsp47. Different transfection combinations were tested: (1) pCEP4-α1(V), pCEP4-α2(V) and pCEP4-HSP47, (2) pCEP4-α1(V), pCEP4-α2(V) and pCEP2-Hsp47, (3) pCEP2-α1(V), pCEP4-α2(V) and pCEP4-Hsp47, and (4) pCEP2-α1(V), pCEP4-α2(V) and pCEP2-Hsp47. Hygromycin or/and puromycin were used for positive cell selection depending on the constructs used for cell transfection. From all these tested combinations, we failed to get substantial expression of the three partners together. Therefore, HEK-293 cells were transfected with the pCEP2-α1(V), pCEP4-α2(V) and pCEP2-Hsp47 constructs, stable clones were obtained and tested for the expression of the three partners. Lysates and media of twenty-one different clones were analyzed by Western blotting. The three partners, the recombinant proα1(V), proα2(V) chains and HSP47 (Figure 6(a)), were expressed together in only five clones named C2, C7, C9, C13, and C15. These clones expressed the recombinant proα1(V) and proα2(V) chains with different ratios (Figure 6(a)). The clones C2 (lane1), C13 (lane 4) and C15 (lane 5) expressed similarly the recombinant proα1(V) and proα2(V). The proα1(V) chain was expressed at a higher level than the proα2(V) chain in clones C7 (lane 2) and C9 (lane 3). As an example, the clone C12 (Figure 6(a), lane 6) only expressed the proα1(V) chain and HSP47. Unfortunately, pepsin digestion of culture media from the five selected clones revealed that even in the presence of HSP47, the homotrimer was again the major molecular form produced by the cells. As illustrated for clone C2 (Figure 6(b)), Western blot analysis of conditioned media treated with or without pepsin revealed the presence of two major pepsin-resistant bands migrating in the position expected for the α1(V) and α2(V) chains, respectively. The ratio between the α1(V) and the α2(V) bands showed that, expression of HSP47 in HEK-293 cells did not increase the heterotrimer production. The heterotrimer [α1(V)]2α2(V) production by other mammalian cell lines, CHO and Hela cells, which both endogenously produce low amounts of collagen V, was analyzed after cotransfection of the 2 proα-chains of collagen V. The analysis of the collected media did not show any significant increased of collagen V heterotrimer production (data not shown). The next question was whether monensin treatment, which blocks the vesicle transport within the Golgi complex, can improve the procollagen V heterotrimer production. We argue that the transient retention of the newly synthesized proα-chains in the endoplasmic reticulum (ER) can favor the procollagen V heterotrimer formation and its subsequent secretion after monensin removing. Conditioned media of clone C2 treated with monensin for 2 h were collected and digested with pepsin before Western blot analysis (Figure 6(b)). Surprisingly, only one band was revealed by the polyclonal antibodies to collagen V which migrated in the position of the proα1(V) chain. This band is converted to a faster migrating product after pepsin treatment which corresponded to the position of the α1(V) triple helix domain (Figure 6(b)). The lack of proα2(V) monomer secretion after monensin treatment indicates that the retention of the proα2(V) in the ER did not improve hetrotrimer formation but caused its intracellular degradation.
Figure 6.
(a) Selection of clones expressing proα1(V), proα2(V) chains and HSP47. Western blot analysis of transfected cell media (for proα1(V) and proα2(V) chain detection) or cell lysates (for HSP47 detection) probed with (first panel) 6A7 monoclonal antibody, which recognizes the N-propeptide of the human proα1(V) chain, (second panel) with collagen type V (G-15) antibodies, which recognize the human proα2(V) chain, (third panel) with antibody to HSP47. Of twenty-one clones, only five clones expressed the three partners with different expression levels: C2 (lane 1), C7 (lane 2), C9 (lane3), C13 (lane 4), and C15 (lane 5). The other clones expressed only two of the three partners. For example, clone C12 (lane 6) expressed only the proα1(V) chain and HSP47. (b) Western blot analysis of media from clone C2 treated with (lanes 3, 4) or without monensin (lanes 1, 2) using polyclonal collagen V antibodies. Media were incubated with (lanes 2, 4) or without pepsin (lanes 1, 3). Left, molecular mass standards expressed in kDa.
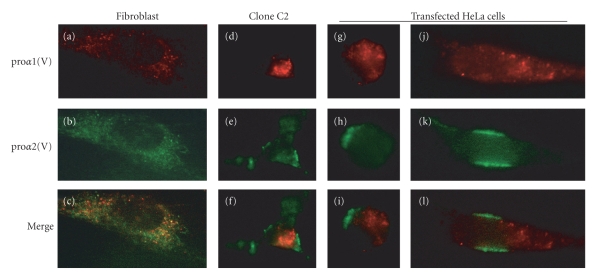
To determine the intracellular localization of the collagen V proα-chains in transfected mammalian cells, we performed immunofluorescence staining using specific antibodies to the proα1(V) and the proα2(V) chains in transfected HEK-293 and HeLa cells compared to cultured human dermal fibroblasts which endogenously express collagen V heterotrimer. For these experiments, we used the clone C2 which stably express high levels of the proα1(V) and proα2(V) chains and HSP47 (Figure 6(a)). The proα1(V) and the proα2(V) chains were transiently expressed in HeLa cells which endogenously express HSP47 (Figure 5(a)). In fibroblasts, the proα1(V) and proα2(V) chains mainly colocalized in ER and secretory vacuoles (Figure 7). Surprisingly, the proα1(V) and proα2(V) chains showed distinct intracellular distribution in stably transfected HEK-293 cells and in transiently transfected HeLa cells (Figure 7).
Figure 7.
Double immunofluorescence staining was performed using monoclonal antibody against human proα1(V) chain (18G5) (a, d, g, j) and rabbit polyclonal proα2(V) chain (b, e, h, m), and merge (c, f, i, l). (a, b, c) human dermal fibroblasts; (d, e, f) clone 22 cells; (g)–(l) HeLa cells transiently transfected with human proα1(V) and proα2(V) constructs. Double immunofluorescence staining shows distinct intracellular distribution of the proα(V) chains in round (g)–(i) and spreading (j, k, l) HeLa cells. Magnification: x1000.
4. Discussion
Whereas the recombinant production of collagen homotrimers has been achieved in different expression systems, the production of collagen heterotrimers is still challenging. In a previous study, we have shown that collagen V homotrimer [α1(V)]3 can be produced in substantial amounts in HEK-293 cells (about 20 mg/L) [19]. In the present report, the different expression systems used so far to express recombinant collagens have been tested in parallel for their capacity to produce high amount of collagen V heterotrimer [α1(V)]2α2(V) which is the most prevalent molecular form encountered in tissues.
Here we show that insect cells infected with recombinant baculovirus carrying full-length proα1(V) and proα2(V) expressed both chains, but at very low levels, which were undetectable with Coomassie blue staining. Moreover, cell infection with both chains led to the prevalent formation of the homotrimer [α1(V)]3 (data not shown). More intriguing is the result we obtained with the yeast expression system. The yeast system has been described as an ideal system for the high-level production of various recombinant collagens for numerous scientific and medical purposes. Yields in excess of 1 g/L have been achieved for collagen types I-III [12]. We show that collagen V heterotrimer was efficiently produced in the yeast system but in much lower level than the collagen I heterotrimer [12]. Whereas the procollagen α1(V) chain was weakly expressed in P. pastoris transformed with α1(V) chain alone, pepsin-resistant collagen V heterotrimer was obtained in yeasts cotransformed by the two collagen V α chains. However, the collagen V heterotrimer production level is far from reaching the yields obtained for collagen I heterotrimers.
Many studies have enlightened the critical role of endoplasmic reticulum-resident enzymes and molecular chaperones in the collagen assembly pathway, from the folding and assembly, to the quality control and secretion [29, 30]. The roles of prolyl 4-hydroxylase and HSP47, a collagen-specific binding protein, are the most documented. As discussed above, yeast and insects cells show no or insufficient P4H activity and cotransfection with P4H subunits cDNA has improved triple helical folding and stability [13]. Along the line, mammalian cells appeared to be the most adapted expression system to properly assemble collagen chains. The HEK-293 cells are by far the most frequently used because they proved to be efficient for the production of numerous recombinant extracellular matrix proteins. Several collagens, all produced as homotrimers, have been efficiently expressed in these cells [8]. Collagen V homotrimer was shown to be fully hydroxylated indicating that HEK-293 cells express endogenous P4H that is fully active [19]. However, in the present study, only negligible amounts of heterotrimeric collagen V were obtained by cotransfecting the proα1(V) and proα2(V) chains and the proα1(V) homotrimeric association was always prevalent, indicating that these cells do not contain the full protein machinery necessary for controlling collagen chain selection and assembly. Collagen VIII was described as heterotrimers in tissues but has been expressed as homotrimers in HEK-293 cells [31]. Also, procollagen quality control and secretion appear to be deficient in HEK-293 cells. We show that pepsin-sensitive proα2(V) monomers were secreted in substantial amounts in HEK-293 cell media. Fibrillar collagen molecules assemble through their C-propeptides that interact with each other and form interchain disulfide bonds. In the absence of reducing agent, the recombinant proα2(V) chain did not migrate as a trimer though the presence of the C-propeptide was attested. It migrated at the expected size (200 kDa) and after collagenase treatment, the digestion products migrated at the position of the N- and C-propeptides. These results show that the proα2(V) chains cannot assemble into trimers as previously shown for the proα2(I) chains [16]. Most importantly, our results showed that these cells lack quality control proteins that can prevent collagen monomer secretion. HSP47 is responsible for preventing aggregation and secretion of partially folded and misfolded molecules rather than for triple helix stabilization [32] and binds similarly to collagens I to V in vitro [33]. Moreover, procollagens secreted from Hsp47−/− mouse cells did not form correctly aligned triple helices [34]. In earlier studies, mouse Hsp47 cDNA was introduced into insect cells to improve collagen I heterotrimer secretion [35]. Cotransfection of HEK-293 cells with HSP47 and proα1(III) constructs prevents for collagen III overmodification [36]. HSP47 may thus play critical role in collagen V biosynthesis. Our results reveal that there is a good correlation between the cell line capacity to express endogenous collagen heterotrimers and HSP47. The A204 cells [37] and as we show in the present report, the CHO-K1 and HeLa cells express low levels of endogenous collagen V heterotrimer whereas the HT1080 cells express collagen IV heterotrimer [38]. The CHO-K1 cells were used to produce recombinant collagen IV heterotrimer [α1(IV)]2α2(IV), though only negligible amounts were obtained [39]. We show that they all express HSP47. Conversely, HSP47 and collagens were expressed at an undetectable level in the HEK-293 cells. In order to test whether HSP47 can favor collagen V heterotrimer expression in HEK-293 cells, we have stably transfected cells with human proα1(V), proα2(V) chains and HSP47 constructs. However, after a triple cell transfection with Hsp47 and both collagen V proα-chains cDNAs, none of the selected clones expressed collagen V heterotrimer as the major molecular form. The results indicate that neither P4H nor HSP47 are key factors controlling the heterotrimer assembly of fibrillar collagens. Uncontrolled secretion of proα2(V) monomers by HEK-293 cells may be unfavorable for heterotrimer formation. However, the use of monensin to retain the new synthesized α-chains in the ER and subsequently improve heterotrimer folding had no effect on the level of collagen V heterotrimer expression. The data even suggested that the retention of the proα2(V) monomers in the ER increased its intracellular degradation and limited its uncontrolled secretion as monomers. This is in agreement with the role of the ER-resident HSP47 in preventing aggregation and secretion of unfolded collagen molecules [32].
Fibrillar collagens share similar structure and thus one can expect that they share similar synthesis and folding mechanisms. However, whatever the expression system used, the preferred molecular form was the homotrimer whereas fibroblasts, the in vivo collagen-producing cells, predominantly express collagen V heterotrimer. The HEK-293 mammalian cells that likely contain the appropriate environment to produce the collagen V heterotrimer, failed to produce high amounts of collagen V heterotrimer whereas they produce considerable amounts of the homotrimer [19]. The HEK-293 cells are an epithelial cell line originally derived from human embryonic kidney. Embryonic epithelial cells can produce low amount of collagen V, the molecular form they produced has not been characterized (for review [19, 40]). It is possible that collagen V isoform assembly is critically dependent upon the cell type. The lack of coordinated and controlled synthesis of the two proα-chains in transfected cells may also prevent heterotrimer formation. Our double immunofluorescence staining with antibodies against the proα1(V) and proα2(V) chains showed that the two proα-chains mainly colocalized in fibroblasts whereas they displayed distinct localization in transfected HEK-293 and HeLa cells. Another likely explanation is that collagen V homotrimers may have intrinsic ability to form the triple helical structure more easily than collagen V heterotrimer and that the formation of the heterotrimeric molecular form may require specific protein chaperones. HSP47 is not the only molecule that has this function. SPARC family proteins may have a similar chaperone activity. Collagens I and V are generally coexpressed in tissues and produced by the same cells, fibroblasts and osteoblasts. Our results strongly suggest that collagen V biosynthesis presents type-specific pathway that at least partially differs from collagen I. The expression systems described here provide valuable model systems to study key factors in collagen V heterotrimer chain association.
Acknowledgments
The authors thank Dr. S. Nagata for kindly providing the human Hsp47 cDNA to FR and Dr. DS Greenspan for the generous gift of proα1(V) and pro proα2(V) cDNAs to E-R H. They are grateful to Dr. D. E. Birk for supplying FR with rabbit polyclonal antibodies raised against the N-terminus of the proα2(V) chain. They also thank Marc Dziasko for his excellent technical assistance. This paper was supported by grants from the “Association pour la Recherche contre le Cancer” (no. 3652), the Emergence Research Program (Région Rhône-Alpes), GIS Maladies Rares INSERM (A04115SP), the European Commission (Contract NMP2-CT-2003-504017), and in part by Grants 736/01 and 1360/07 from the Israel Science Foundation (to EK). M.Roulet and M. Välkkilä contributed equally to the article.
References
- 1.Fichard A, Kleman JP, Ruggiero F. Another look at collagen V and XI molecules. Matrix Biology. 1995;14(7):515–531. doi: 10.1016/s0945-053x(05)80001-0. [DOI] [PubMed] [Google Scholar]
- 2.Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. Journal of Biological Chemistry. 2004;279(51):53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 3.Ricard-Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathologie Biologie. 2005;53(7):430–442. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32(3):223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 5.Chanut-Delalande H, Bonod-Bidaud C, Cogne S, et al. Development of a functional skin matrix requires deposition of collagen V heterotrimers. Molecular and Cellular Biology. 2004;24(13):6049–6057. doi: 10.1128/MCB.24.13.6049-6057.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanut-Delalande H, Fichard A, Bernocco S, Garrone R, Hulmes DJS, Ruggiero F. Control of heterotypic fibril formation by collagen V is determined by chain stoichiometry. Journal of Biological Chemistry. 2001;276(26):24352–24359. doi: 10.1074/jbc.m101182200. [DOI] [PubMed] [Google Scholar]
- 7.Torbet J, Malbouyres M, Builles N, et al. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials. 2007;28(29):4268–4276. doi: 10.1016/j.biomaterials.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Ruggiero F, Koch M. Making recombinant extracellular matrix proteins. Methods. 2008;45(1):75–85. doi: 10.1016/j.ymeth.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Neubauer A, Neubauer P, Myllyharju J. High-level production of human collagen prolyl 4-hydroxylase in Escherichia coli. Matrix Biology. 2005;24(1):59–68. doi: 10.1016/j.matbio.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiology Reviews. 2000;24(1):45–66. doi: 10.1111/j.1574-6976.2000.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 11.Nokelainen M, Tu H, Vuorela A, Notbohm H, Kivirikko KI, Myllyharju J. High-level production of human type I collagen in the yeast Pichia pastoris. Yeast. 2001;18(9):797–806. doi: 10.1002/yea.730. [DOI] [PubMed] [Google Scholar]
- 12.Olsen D, Yang C, Bodo M, et al. Recombinant collagen and gelatin for drug delivery. Advanced Drug Delivery Reviews. 2003;55(12):1547–1567. doi: 10.1016/j.addr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Myllyharju J, Nokelainen M, Vuorela A, Kivirikko KI. Expression of recombinant human type I-III collagens in the yeast Pichia pastoris. Biochemical Society Transactions. 2000;28(4):353–357. [PubMed] [Google Scholar]
- 14.Vuorela A, Myllyharju J, Nissi R, Pihlajaniemi T, Kivirikko KI. Assembly of human prolyl 4-hydroxylase and type III collagen in the yeast Pichia pastoris: formation of a stable enzyme tetramer requires coexpression with collagen and assembly of a stable collagen requires coexpression with prolyl 4-hydroxylase. EMBO Journal. 1997;16(22):6702–6712. doi: 10.1093/emboj/16.22.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vuori K, Pihlajaniemi T, Marttila M, Kivirikko KI. Characterization of the human prolyl 4-hydroxylase tetramer and its multifunctional protein disulfide-isomerase subunit synthesized in a baculovirus expression system. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(16):7467–7470. doi: 10.1073/pnas.89.16.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myllyharju J, Lamberg A, Notbohm H, Fietzek PP, Pihlajaniemi T, Kivirikko KI. Expression of wild-type and modified proα chains of human type I procollagen in insect cells leads to the formation of stable [α1(I)]2α2(I) collagen heterotrimers and [α1(I)]3 homotrimers but not [α2(I)]3 homotrimers. Journal of Biological Chemistry. 1997;272(35):21824–21830. doi: 10.1074/jbc.272.35.21824. [DOI] [PubMed] [Google Scholar]
- 17.Lamberg A, Helaakoski T, Myllyharju J, et al. Characterization of human type III collagen expressed in a baculovirus systemml: production of a protein with a stable triple helix requires coexpression with the two types of recombinant prolyl 4-hydroxylase subunit. Journal of Biological Chemistry. 1996;271(20):11988–11995. doi: 10.1074/jbc.271.20.11988. [DOI] [PubMed] [Google Scholar]
- 18.Nokelainen M, Helaakoski T, Myllyharju J, et al. Expression and characterization of recombinant human type II collagens with low and high contents of hydroxylysine and its glycosylated forms. Matrix Biology. 1998;16(6):329–338. doi: 10.1016/s0945-053x(98)90004-x. [DOI] [PubMed] [Google Scholar]
- 19.Fichard A, Tillet E, Delacoux F, Garrone R, Ruggiero F. Human recombinant α1(V) collagen chain. Homotrimeric assembly and subsequent processing. Journal of Biological Chemistry. 1997;272(48):30083–30087. doi: 10.1074/jbc.272.48.30083. [DOI] [PubMed] [Google Scholar]
- 20.Unsold C, Pappano WN, Imamura Y, Steiglitz BM, Greenspan DS. Biosynthetic processing of the pro-α1(V)2pro-α2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases. Journal of Biological Chemistry. 2002;277(7):5596–5602. doi: 10.1074/jbc.M110003200. [DOI] [PubMed] [Google Scholar]
- 21.Pakkanen O, Hamalainen E-R, Kivirikko KI, Myllyharju J. Assembly of stable human type I and III collagen molecules from hydroxylated recombinant chains in the yeast Pichia pastoris. Effect of an engineered C-terminal oligomerization domain foldon. Journal of Biological Chemistry. 2003;278(34):32478–32483. doi: 10.1074/jbc.M304405200. [DOI] [PubMed] [Google Scholar]
- 22.Bonod-Bidaud C, Beraud M, Vaganay E, et al. Enzymatic cleavage specificity of the proα1(V) chain processing analysed by site-directed mutagenesis. Biochemical Journal. 2007;405(2):299–306. doi: 10.1042/BJ20070051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessler E, Fichard A, Chanut-Delalande H, Bruselt M, Ruggiero F. Bone morphogenetic protein-1 (BMP-1) mediates C-terminal processing of procollagen V homotrimer. Journal of Biological Chemistry. 2001;276(29):27051–27057. doi: 10.1074/jbc.M102921200. [DOI] [PubMed] [Google Scholar]
- 24.Dion AS, Myers JC. COOH-terminal propeptides of the major human procollagens. Structural, functional and genetic comparisons. Journal of Molecular Biology. 1987;193(1):127–143. doi: 10.1016/0022-2836(87)90632-2. [DOI] [PubMed] [Google Scholar]
- 25.Mclaughlin SH, Bulleid NJ. Molecular recognition in procollagen chain assembly. Matrix Biology. 1998;16(7):369–377. doi: 10.1016/s0945-053x(98)90010-5. [DOI] [PubMed] [Google Scholar]
- 26.Imamura Y, Steiglitz BM, Greenspan DS. Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-α1(V) collagen. Journal of Biological Chemistry. 1998;273(42):27511–27517. doi: 10.1074/jbc.273.42.27511. [DOI] [PubMed] [Google Scholar]
- 27.Frischholz S, Beier F, Girkontaite I, et al. Characterization of human type X procollagen and its NC-1 domain expressed as recombinant proteins in HEK293 cells. Journal of Biological Chemistry. 1998;273(8):4547–4555. doi: 10.1074/jbc.273.8.4547. [DOI] [PubMed] [Google Scholar]
- 28.Nagata K, Saga S, Yamada KM. Characterization of a novel transformation-sensitive heat-shock protein (HSP47) that binds to collagen. Biochemical and Biophysical Research Communications. 1988;153(1):428–434. doi: 10.1016/s0006-291x(88)81242-7. [DOI] [PubMed] [Google Scholar]
- 29.Lamande SR, Bateman JF. Procollagen folding and assembly: the role of endoplasmic reticulum enzymes and molecular chaperones. Seminars in Cell and Developmental Biology. 1999;10(5):455–464. doi: 10.1006/scdb.1999.0317. [DOI] [PubMed] [Google Scholar]
- 30.Tasab M, Batten MR, Bulleid NJ. Hsp47: a molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO Journal. 2000;19(10):2204–2211. doi: 10.1093/emboj/19.10.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephan S, Sherratt MJ, Hodson N, Shuttleworth CA, Kielty CM. Expression and supramolecular assembly of recombinant α1(VIII) and α2(VIII) collagen homotrimers. Journal of Biological Chemistry. 2004;279(20):21469–21477. doi: 10.1074/jbc.M305805200. [DOI] [PubMed] [Google Scholar]
- 32.Ishida Y, Kubota H, Yamamoto A, Kitamura A, Bachinger HP, Nagata K. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Molecular Biology of the Cell. 2006;17(5):2346–2355. doi: 10.1091/mbc.E05-11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natsume T, Koide T, Yokota S-I, Hirayoshi K, Nagata K. Interactions between collagen-binding stress protein HSP47 and collagen. Analysis of kinetic parameters by surface plasmon resonance biosensor. Journal of Biological Chemistry. 1994;269(49):31224–31228. [PubMed] [Google Scholar]
- 34.Nagai N, Hosokawa M, Itohara S, et al. Embryonic lethality of molecular chaperone Hsp47 knockout mice is associated with defects in collagen biosynthesis. Journal of Cell Biology. 2000;150(6):1499–1505. doi: 10.1083/jcb.150.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomita M, Yoshizato K, Nagata K, Kitajima T. Enhancement of secretion of human procollagen I in mouse HSP47- expressing insect cells. Journal of Biochemistry. 1999;126(6):1118–1126. doi: 10.1093/oxfordjournals.jbchem.a022557. [DOI] [PubMed] [Google Scholar]
- 36.Hosokawa N, Hohenadl C, Satoh M, Kuhn K, Nagata K. HSP47, a collagen-specific molecular chaperone, delays the secretion of type III procollagen transfected in human embryonic kidney cell line 293: a possible role for HSP47 in collagen modification. Journal of Biochemistry. 1998;124(3):654–662. doi: 10.1093/oxfordjournals.jbchem.a022162. [DOI] [PubMed] [Google Scholar]
- 37.Alitalo K, Myllyla R, Sage H, Pritzl P, Vaheri A, Bornstein P. Biosynthesis of type V procollagen by A204, a human rhabdomyosarcoma cell line. Journal of Biological Chemistry. 1982;257(15):9016–9024. [PubMed] [Google Scholar]
- 38.Toth M, Sado Y, Ninomiya Y, Fridman R. Biosynthesis of α2(IV) and α1(IV) chains of collagen IV and interactions with matrix metalloproteinase-9. Journal of Cellular Physiology. 1999;180(1):131–139. doi: 10.1002/(SICI)1097-4652(199907)180:1<131::AID-JCP15>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda K, Hori H, Utani A, Burbelo PD, Yamada Y. Formation of recombinant triple-helical [α1(IV)]2α2(IV) collagen molecules in CHO cells. Biochemical and Biophysical Research Communications. 1997;231(1):178–182. doi: 10.1006/bbrc.1997.6069. [DOI] [PubMed] [Google Scholar]
- 40.Roulet M, Ruggiero F, Karsenty G, LeGuellec D. A comprehensive study of the spatial and temporal expression of the col5a1 gene in mouse embryos: a clue for understanding collagen V function in developing connective tissues. Cell and Tissue Research. 2007;327(2):323–332. doi: 10.1007/s00441-006-0294-1. [DOI] [PubMed] [Google Scholar]