Abstract
Sodium butyrate (NaBu) has been used as a productivity enhancer for the synthesis of recombinant proteins in Chinese hamster ovary (CHO) cells. Thus, the influence of NaBu on the production of recombinant human prolactin (hPRL) from CHO cells was investigated for the first time. CHO cell cultures were submitted to a treatment with different concentrations of NaBu (0.25 to 4 mM). Quantitative and qualitative analyses by reverse-phase high-performance liquid chromatography (RP-HPLC) and Western blot or SDS-PAGE, carried out directly on CHO-conditioned medium, showed that the highest hPRL expression was obtained with 1 mM NaBu. In vitro biological assays based on noble rat lymphoma (Nb2) and mouse pro-B lymphoma (Ba/F3-LLP) cells were carried out on purified hPRL. Its bioactivity in the presence of NaBu was not apparently different from that of the First International Reference Reagent of recombinant hPRL (WHO 97/714). Our results show that NaBu increased the synthesis of recombinant hPRL in CHO cells, apparently without compromising either its structure or function.
1. Introduction
Human prolactin (hPRL), a 23 kDa polypeptide hormone with a single chain of 199 residues, is a member of the family of cytokines, which includes erythropoietin, interleukin-6, and many others, but is most closely related, both evolutionarily and functionally, to human growth hormone and placental lactogen [1]. This polypeptide is known to be involved in a variety of actions [2], more than all the other pituitary hormones combined, such as cell proliferation, growth and development, water and electrolyte balance, and several physiological and behavioral aspects of mammal, bird, and reptile reproduction [3]. In humans, prolactin is secreted by pituitary lactotrophs under hypothalamic regulation. It circulates in the bloodstream and acts distally as an endocrine hormone. Apart from the pituitary, many extrapituitary sites for hPRL synthesis have been documented, including breast, prostate, and female reproductive tract, where it appears to act locally to regulate cellular growth and differentiation [1, 4, 5]. The hPRL gene has been cloned and the authentic form of the hormone efficiently expressed in bacterial periplasm and in Chinese hamster ovary (CHO) cells in our laboratory [6–8]. Since hPRL is one of the hormones most frequently determined in routine clinical assays and several therapeutic applications are being considered, an increasing need for pure bioactive hPRL can be anticipated [9, 10].
Sodium butyrate (NaBu) is a short chain fatty acid, originally identified as a product of anaerobic bacterial fermentation, which has been shown to alter the structure of chromatin in the nucleus of mammalian cells by reducing the activity of histone deacetylase [11]. One of the possible causes for improved gene expression by NaBu cells is histone hyperacetylation, which facilitates the access of general transcription factors in eukaryotic cells [12, 13]. However, NaBu can also cause a cellular arrest, leading to increased apoptosis and resulting in an overall reduction in recombinant protein production over longer periods of time [14].
NaBu treatment has been shown, via immunoassay determination, to increase the expression levels of foreign proteins such as human thrombopoietin, interferon-β-1a, and chimeric IgG3 antibodies in CHO cell cultures [15–17] by factors of 2-, 2.5-, and 3.6-fold, respectively, relative to nontreated cells.This led us to investigate, for the first time, via physicochemical techniques, whether addition of NaBu would also increase hPRL production in these cell cultures. In order to characterize the product obtained, we took advantage of a specific RP-HPLC methodology previously developed in our laboratory [18], which permits qualitative and quantitative analysis of hPRL directly in CHO-conditioned medium. High-Performance Size-Exclusion Chromatography (HPSEC) methodology was used for both analytical and preparative purposes [19] and also allowed us to evaluated hPRL activity directly in two bioassays, after its purification from CHO-conditioned medium [9].
2. Materials and Methods
2.1. Cell Culture
The clone expressing hPRL, derived from CHO dhfr− cells (DUKX-B11) that had been transfected with the vector pEDdc-hPRL, was obtained in our laboratory [8]. Cells were thawed into T-flasks (75 cm2, from Corning Costar Corporation, Cambridge, MA) containing α-minimal essential medium (α-MEM, Gibco-Invitrogen Corporation, Grand Island, NY, USA) with L-glutamine and without ribonucleosides and deoxyribonucleosides, supplemented with 10% dialysed fetal bovine serum (dFBS), 40 μg/mL gentamycin, 4 g/L glucose, and 100 nM methotrexate. Cultures were maintained in a humidified incubator at 37°C in the presence of 5% CO2.
2.2. NaBu Treatment
100 mM NaBu was prepared in phosphate-buffered saline (PBS) (0.007 M Na2HPO4, 0.01 M NaH2PO4, pH 7.4 and 0.15 M NaCl), sterilized by passing through a 0.20 μm syringe filter (Corning Incorporated, Corning, NY, USA), and stored in aliquots at −20°C. To examine the effect of different concentrations of NaBu on hPRL production, 1 mL of CHO cell suspension (5 × 104 cells/mL) was seeded into each well of a 24-well plate to obtain a concentration of 2 × 105 cells/mL. Cultures were then rinsed twice in PBS to remove serum and medium. Fresh medium was then added containing NaBu to a final concentration between 0 and 4 mM. Cells were grown for 10 days and CHO cell-conditioned medium was collected daily and stored at −80°C for subsequent RP-HPLC or Western blot analysis.
For product purification, CHO cells were expanded into ten T-flasks, in α-MEM supplemented with dFBS, until they were confluent. Cultures also were rinsed twice in PBS to remove serum and medium. When the cell density was ~2 × 105 cells/mL, fresh medium was then added to the T-flasks: five contained no NaBu, while the chosen amounts of NaBu were added to the other five. The hPRL-secreting confluent cultures were then maintained for up to ten days by replacing the medium every 24 hours. The conditioned medium was collected and stored, as mentioned above, for SP-Sepharose Fast Flow and HPSEC purification.
2.3. Cell Counts
To examine the effect of different concentrations of NaBu on cell density and viability, 1 mL of CHO cell suspension (5 × 104 cells/mL) was seeded into each well of a 24-well plate to obtain a concentration of 2 × 105 cells/mL. NaBu was added to a final concentration between 0 and 4 mM and cells were grown for 10 days, as mentioned above. Every 24 hours a sample for the different concentrations of NaBu was detached from 24-well plate by treatment with 0.025% trypsin-EDTA in PBS. Total cell number and viability were determined by using the Trypan blue (Gibco-Invitrogen Corporation) exclusion method via haemocytometry and phase contrast microscopy, to distinguish viable and nonviable cells on the basis of dye uptake [20].
2.4. DNA Fragmentation Assay by Electrophoresis
5 × 104 cells/mL cells were seeded in 24-well plate, treated and detached at regular times, as mentioned above. 1 × 106 cells/mL per sample were lysed in 400 μL of lysis buffer (50 mM Tris, 10 mM EDTA pH 8.0, 1% SDS) and 500 μg/mL proteinase K (Fermentas, Life Sciences, São Paulo, Brazil) at 37°C for 16 hours. The lysates were extracted with phenol : clorophorm : isoamylalcohol (25 : 24 : 1). DNA was precipitated with 100% ice-cold ethanol. The resulting pellets of DNA were resuspended in TE buffer (pH 8.0) [14] and treated with 10 μg/mL RNase A (Fermentas, Life Sciences, Brazil) prior to electrophoresis on a 5% polyacrylamide gel at 70 V [21]. Intact and fragmented DNA bands were revealed by silver staining.
2.5. Gel Electrophoresis and Western Blot Analysis
Discontinuous SDS-PAGE, based on 15% polyacrylamide gels, was carried out under nonreducing conditions as described by Laemmli [22]. Proteins were revealed by silver or Coomassie brilliant blue G-250 (USB, Cleveland, OH) staining. The molecular mass markers were purchased from GE Healthcare Bio-Science Corp. (Piscataway, NJ, USA). Western blot analysis was performed according to Bannerman et al. [23], using 125I-labelled protein A [24] and anti-hPRL antiserum obtained in rabbit and validated in our laboratory against the NIDDK-anti-hPRL-3 from National Hormone and Pituitary Program, Torrance, CA, USA [7, 8].
2.6. Protein Determination
Total protein content was determinate by BCA protein assay, using bicinchoninic acid (Micro BCA protein assay kit, Pierce, Rockford, IL, USA). Pure bovine serum albumin (BSA, Sigma, São Paulo, Brazil) was used as standard.
2.7. Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
After the treatment with different NaBu concentrations, an aliquot of conditioned culture medium (~10 mL) was concentrated (~10 fold) by using a centrifugal membrane filter Ultra 4 (Amicon, Millipore Corporation, Billerica, MA, USA) and applied to a RP-HPLC column. A Shimadzu Model SCL-10A HPLC apparatus coupled to a SPD-10AV UV detector (Shimadzu, MD, USA) was used, employing the Class VP software, also from Shimadzu. The column was a C4 Vydac 214TP54 (25 cm × 4.6 mm id, pore diameter of 300 Å and particle diameter of 5 μm) with a silica precolumn packed with LiChrosorb Si-60, 7.9–12.4 μm (Merck, Darmstadt, Germany) located between the pump and the injector. All Vydac columns were purchased from Grace Vydac (Hesperia, CA, USA). The mobile phase consisted of 71% Tris-HCl buffer (50 mM, pH 7.5) and 29% n-propanol, as described by Dalmora et al. [19], with a flow-rate of 0.5 mL/min, a detector wavelength of 220 nm, a column temperature maintained at 45°C, and a sample volume of 25–500 μL [18]. This methodology was used for analytical purposes to examine the effect of NaBu on hPRL production.
2.8. High-Performance Size-Exclusion Chromatography (HPSEC)
The HPSEC is an entropically controlled separation technique in which molecules are separated on the basis of their hydrodynamic molecular volume or size [25, 26]. This methodology was used for both analytical and preparative purposes and allowed us to use purified hPRL directly in bioassays, the n-propanol interference being absent due to the RP-HPLC technique. HPSEC was carried out with the same Shimadzu apparatus, processing 25 to 500 μL of sample on a TosoHaas (Montgomeryville, PA, USA) G2000 SW column (60 cm × 7.5 mm i.d., particle size of 10 μm and pore size of 125 Å) coupled to a 7.5 cm × 7.5 mm i.d. SW guard column. The mobile phase was 0.025 M ammonium bicarbonate, pH 7.0, with a flow-rate of 1.0 mL/min [19].
2.9. hPRL Purification
The hPRL present in conditioned culture medium was purified as described by Soares et al. [9]. A two-step purification process was used: SP-Sepharose Fast Flow followed by a size exclusion chromatography employing HPSEC for preparative purposes. Briefly, conditioned medium (~500 mL) was adjusted to pH 5.0 using acetic acid. The material was then applied onto the SP-Sepharose Fast Flow column (GE Healthcare Bio-Science Corp., Piscataway, NJ, USA) equilibrated in 50 mM sodium acetate, pH 5.0. UV absorbance was monitored at 280 nm. After washing with the same buffer, the column was treated with 50 mM sodium acetate, pH 5.0, 90 mM NaCl buffer. The protein of interest was then eluted from the column with 25 mM Hepes, pH 8.0. Different fractions containing hPRL were analysed by HPSEC and the most concentrated fractions were purified on the same HPSEC column, working this time in a preparative mode. The maximum volume applied in each preparative HPSEC run was 500 μL. hPRL in the final pool obtained via the HPSEC purification was quantified for bioassay purposes by HPSEC and stored at −80°C.
2.10. In Vitro Bioassay
The biological activity of purified hPRL was determined via the Nb2 and Ba/F3-LLP lymphoma cell-proliferation assays, as previously described [27], against the First WHO Reference Reagent of recombinant hPRL, lyophilized in ampoules coded 97/714 [28].
Ba/F3-LLP cells were routinely maintained in suspension in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 50 U/mL of penicillin, 50 μg/mL of streptomycin, 700 μg/mL of geneticin and 1 ng/mL of hPRL. Before carrying out the proliferation assay, cells were starved for 6 hours in the RPMI-1640 medium, as mentioned above, without hPRL. Cells were then distributed in flat bottom 96-well plates at a density of 5 × 104 cells/well in a final volume of 200 μL. After 72 hours at 37°C and 5% CO2, the presence of viable cells was assessed using MTS assay [29]. Briefly, 2 mg/mL MTS dye [3(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulf-ophenyl)-2H-tetrazolin (Promega Corp., Madison, WI,USA)] in PBS was mixed in a 20 : 1 ratio (vol/vol) with PMS (phenazine methosulphate) (Sigma, St. Louis, MO, USA), 0.92 mg/mL in PBS. Twenty microliters of mixture were then added to each well, and 2 hours after incubation at 37°C the absorbance at 490 nm was read in a microplate reader (Multiskan EX Thermo Electron Corporation, Vantaa, Finland).
Nb2 cells also routinely maintained in suspension in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 50 U/mL of penicillin, and 50 μg/mL of streptomycin mixed in a 50 : 1 ratio (vol/vol) with 5 mM 2-β mercaptoethanol in PBS. Before carrying out the proliferation assay, cells were maintained in the RPMI-1640, as mentioned above, containing 1% heat-inactivated FBS for 8 hours, constituting the preassay. Afterward, the cells were distributed in flat bottom 96-well plates at a density of 2 × 104 cells/well in a final volume of 200 μL, with no heat-inactivated FBS addition. After 72 hours at 37°C and 5% CO2, the presence of viable cells was assessed using MTS assay, as mentioned before.
3. Results
3.1. Cell Growth and Viability
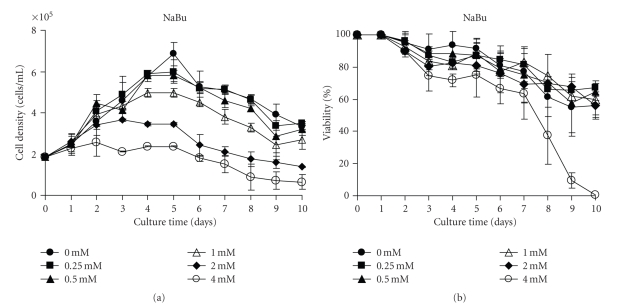
The influence of different concentrations of NaBu on hPRL-secreting CHO cellular growth and viability during a 10-day culture can be seen in Figure 1. The treatment with either 0.25 or 0.5 mM NaBu did not result in significant growth inhibition (P > 0.05) compared to the control without NaBu (Figure 1(a)). Nonetheless, a dose response-effect on cellular growth was observed in the concentration range of 1 to 4 mM NaBu, with a dramatic fall already occurring after day 2 at the concentrations of 2 mM and 4 mM NaBu. The treatment with 1 mM NaBu produced an apparent decline only 4 days after starting the treatment. With regard to cell viability, none of the concentrations except 4 mM NaBu (P < 0.05) showed any significant effect (Figure 1(b)).
Figure 1.
NaBu influence on cellular growth and viability of hPRL-secreting CHO cells. (a) Cellular growth and (b) cellular viability in a 10-day hPRL production period under different NaBu concentrations. The cell viability was determined by the trypan blue exclusion procedure, following trypsinization. Values are the mean of two independent determinations.
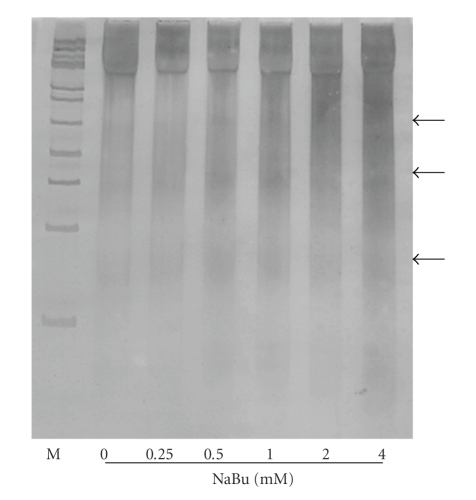
The rate of apoptosis in NaBu-treated and untreated CHO cells was examined by polyacrylamide gel electrophoresis and revealed by silver staining. Figure 2 shows that NaBu-treated and untreated CHO cells exhibit oligonucleosomal DNA ladder which is the hallmark of apoptosis, resulting from endonuclease activity at internucleosomal sites in an apoptotic cell. The intensity of the oligonucleosomal DNA ladder seems NaBu dose-dependent. On the other hand, the progressive removal of dFBS from the culture medium could also be associated with apoptotic cell death in NaBu-treated and untreated CHO cells, leading to a decrease of cellular growth after day 5. This can be due to the fact that serum contains growth factors that help the culture survival [30].
Figure 2.
Electrophoretic patterns of DNA isolated from NaBu-treated and untreated CHO cells. Isolated chromosomal DNA (2 μg) was electrophoresed on 5% polyacrylamide gel and intact and fragmented DNA bands were revealed by silver staining. Arrowheads designate DNA ladder formation. M: molecular weight marker, 100-bp DNA ladder. DNAs were isolated from day 8 cultures exposed to different concentrations of Nabu.
3.2. Effect of NaBu on hPRL Production
A RP-HPLC methodology, previously set up in our laboratory specifically for hPRL qualitative and quantitative analysis [18], was then employed to quantify hPRL production and detect the presence of possible alteration in hPRL structure or hydrophobicity due to the prolonged presence of NaBu. The data in Table 1 and Figure 3 show that 1 mM NaBu is capable of greatly increasing hPRL synthesis (P < 0.02), with no significant differences (P > 0.05) in the retention time (tR) of the resultant hPRL up to 4 mM NaBu.
Table 1.
Expression levels and RP-HPLC retention times (tR) of hPRL obtained from CHO cells treated with different concentrations of NaBu for 10 days. The tR were compared with those of the Internal Reference Preparation of periplasmic E. coli-derived hPRL (tR : 26.00 ± 0.72 minutes).
| NaBu (mM) | Expression levels of hPRLa (μg/mL) | tRa (min) |
|---|---|---|
| 0 | 0.92 ± 0.22 | 25.81 ± 0.52 |
| 0.25 | 1.30 ± 0.06 | 25.68 ± 0.47 |
| 0.5 | 1.67 ± 0.00 | 25.90 ± 0.77 |
| 1 | 2.00 ± 0.03 | 25.77 ± 0.73 |
| 2 | 1.48 ± 0.05 | 25.66 ± 0.87 |
| 4 | 1.23 ± 0.11 | 25.68 ± 0.82 |
| Average tR = 25.75 ± 0.09 | ||
| RSD = 0.4% |
aEach value is the mean ± SD of duplicate experiments.
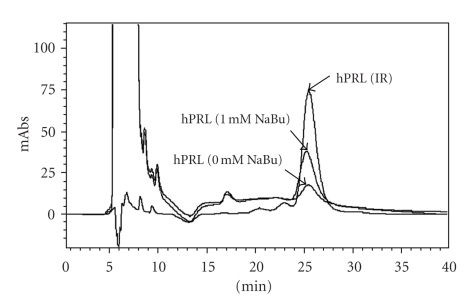
Figure 3.
Overlay of RP-HPLC qualitative and quantitative analyses of hPRL secreted by CHO cells treated with different concentrations of NaBu. IR: Internal Reference Preparation of periplasmic E. coli-derived hPRL (7.0 μg). mAbs: milliabsorbance.
Human prolactin quantification carried out by RP-HPLC in CHO cell-conditioned medium (Table 1) indicated that the highest production was obtained with 1 mM NaBu, which increased the secretion ~2-fold relative to control cultivation without NaBu addition. Despite an increase in protein expression, the concentrations of 0.5 mM and 2 mM NaBu provided lower hPRL volumetric yields than 1 mM NaBu. The greatest inhibition of cell growth was produced by 4 mM NaBu leading to lower hPRL synthesis (Figure 1(a) and Table 1). Clearly, it will be important to determine the optimal NaBu concentration in long-term cell cultures.
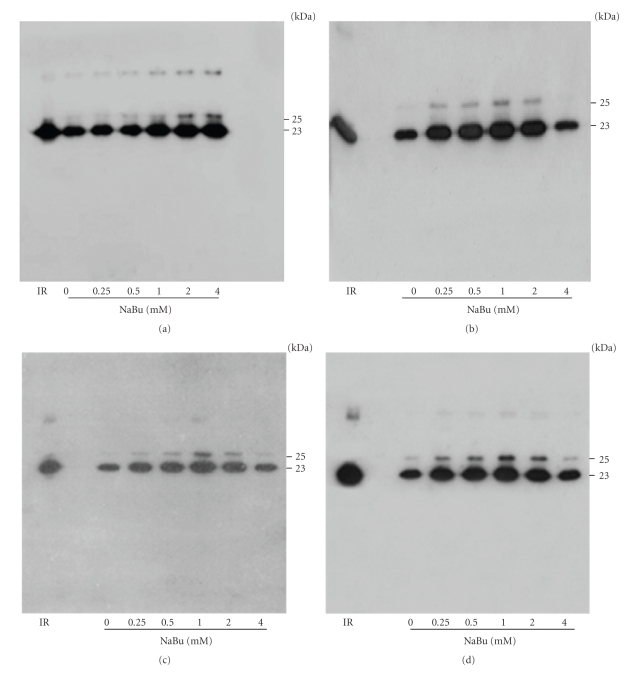
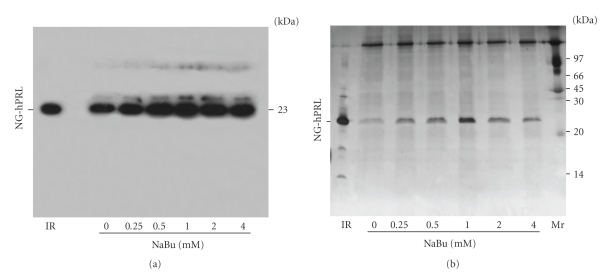
To confirm the expression and purity level of hPRL, equal volumes of CHO-conditioned medium, collected on days 1, 4, 5, and 6, were analyzed by Western blot (Figure 4); the same was done with pools obtained after 10 days of cultivation (Figure 5(a)). These pools were also analyzed via SDS-PAGE followed by AgNO3 staining (Figure 5(b)), which demonstrated the predominance, with 1 mM NaBu, of hPRL relative to other proteins that might be present in CHO cell-conditioned medium. Taken together, these RP-HPLC, Western blot, and SDS-PAGE analyses indicate that, under our conditions, 1 mM NaBu was the ideal concentration, providing the highest hPRL expression level, while still maintaining good cell viability and only a limited decrease in cell growth.
Figure 4.
Western blot analysis of hPRL production in CHO cells treated with different concentrations of NaBu on days 1 (a), 4 (b), 5 (c), and 6 (d). Molecular weights G-hPRL (25 kDa) and NG-hPRL (23 kDa) are indicated. IR: Internal Reference of periplasmic E. coli-derived hPRL.
Figure 5.
(a) Western blot analysis and (b) SDS-PAGE stained with AgNO3 of 10-day production pools of hPRL secreted by CHO cells treated with different NaBu concentrations. IR: Internal Reference of periplasmic E. coli-derived hPRL. Mr: molecular mass marker.
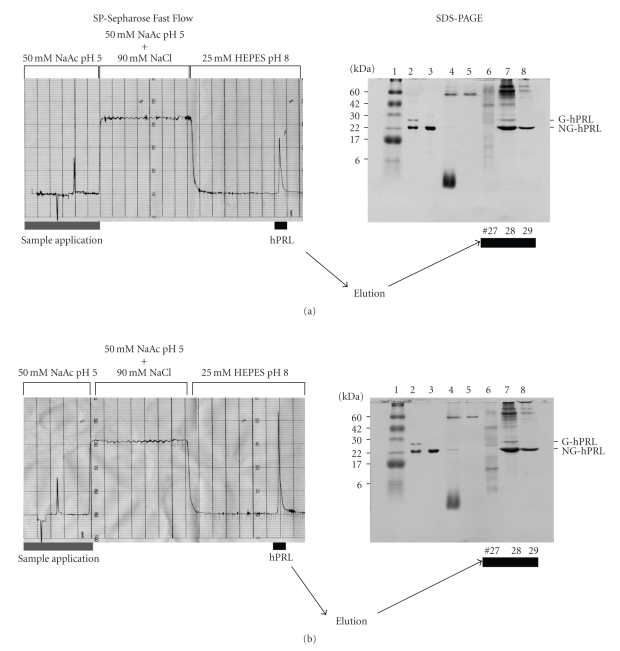
SP-Sepharose Fast Flow chromatographic purification of NaBu-treated and untreated cells, together with SDS-PAGE analysis of applied and eluted fractions, is shown in Figure 6. This confirmed the presence of about twice as much hPRL in the medium treated with 1 mM NaBu and the efficient adsorption of both the 25 kDa glycosylated (G-hPRL) and the 23 kDa nonglycosylated hPRL (NG-hPRL) isoforms by the cationic exchanger [31].
Figure 6.
Example of chromatographic purification of hPRL on SP-Sepharose Fast Flow and SDS-PAGE analysis of CHO cell-conditioned medium of (a) nontreated cells and (b) cells treated with 1 mM NaBu. Lane 1: molecular mass marker. Lane 2: Chemical Reference Standard of G-hPRL and NG-hPRL (WHO-CRS). Lane 3: Internal Reference of periplasmic E. coli-derived hPRL. Lane 4: CHO-conditioned medium. Lane 5: unretained fraction. Lanes 6–8: fractions eluted with 25 mM HEPES, pH 8.0. NaAc: sodium acetate.
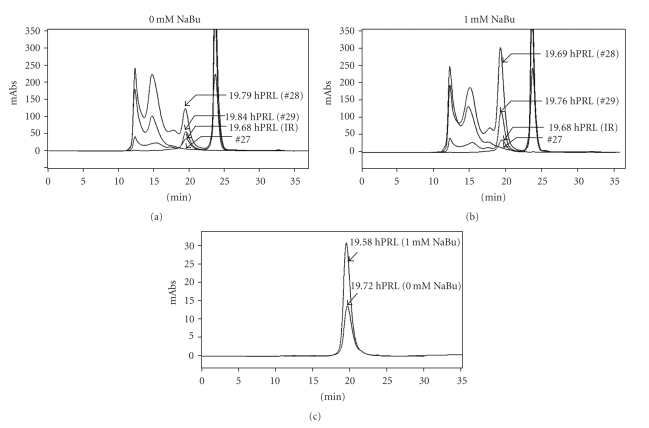
The same partially purified products were also analyzed by HPSEC before and after using this same column for preparative purposes (Figures 7(a), 7(b), and 7(c)). The purity obtained for both products after this second chromatographic step is noteworthy (>97%) (Figure 7(c) and Table 2). From Table 2, on the basis of mass fractions, it is possible to calculate an overall purification factor of ~55 and 122-fold for NaBu-treated and untreated CHO cells, respectively. The ~2-fold increase in hPRL production obtained in the presence of 1mM NaBu was also confirmed after purification. Also remarkable are the perfectly coincident tR values of the two CHO-derived hPRL preparations and of the Internal Reference of E. coli-derived hPRL (19.58 minutes <tR < 19.72 minutes), confirming their identical hydrodynamic properties in this long (60 cm) HPSEC column (Figure 7(c)). It has been reported that these properties can be altered even by subtle modifications in the secondary and tertiary structure of the molecule [32, 33].
Figure 7.
(a) and (b) Qualitative and quantitative HPSEC analysis of NaBu-treated and untreated CHO-derived hPRL after SP-Sepharose Fast Flow and (c) after preparative HPSEC purification, comparing the product obtained from tube #28 of nontreated (a) and NaBu-treated cells (b). IR: Internal Reference of periplasmic E. coli-derived hPRL (7.0 μg). mAbs: milliabsorbance. Tube numbers (#27, #28, and #29) are from SP-Sepharose chromatography. Retention Times (tR) are indicated.
Table 2.
Recombinant hPRL purification starting from 500 mL of NaBu-treated or untreated CHO-conditioned medium.
| Purification step | Total protein (mg)a | hPRL (mg) | Mass fraction | |||
|---|---|---|---|---|---|---|
| 0 mM NaBu | 1 mM NaBu | 0 mM NaBu | 1 mM NaBu | 0 mM NaBu | 1 mM NaBu | |
| Conditioned medium | 64.7 | 66.2 | 0.54 | 1.20b | 0.008 | 0.018 |
| SP-Sepharose FF | 2.3 | 3.0 | 0.51 | 1.22c | 0.219 | 0.403 |
| Preparative HPSEC | 0.14 | 0.25 | 0.13 | 0.25c | 0.978 | 1.000 |
aEstimated by BCA assay. bEstimated by RP-HPLC. cEstimated by HPSEC.
3.3. Biological Assays
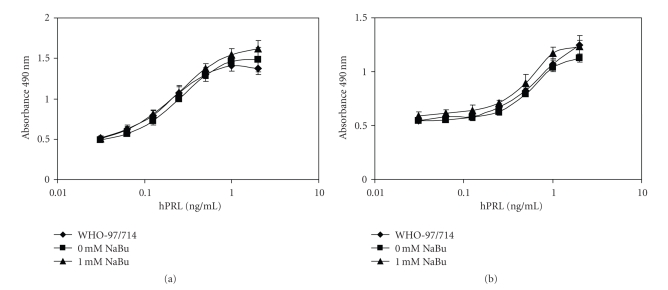
Finally, the two purified products were tested for their biological activity against the Reference Reagent for recombinant hPRL (WHO 97/714) in two different in vitro bioassays based on Nb2 and Ba/F3-LLP cells. The data illustrated in Figure 8 and reported in Table 3 show that apparently there were no differences between the potencies of the purified products and that of the WHO Reference Reagent.
Figure 8.
Bioactivity of purified hPRL obtained from CHO cells treated or not with NaBu, determined via (a) Nb2 or (b) Ba/F3-LLP cell assays against the International Standard of Recombinant hPRL (WHO 97/714). Values are the mean of two independent determinations.
Table 3.
Bioactivity of purified hPRL obtained from CHO cells treated or not with NaBu, determined via Nb2 or Ba/F3-LLP cells against the First International Standard for recombinant hPRL (WHO 97/714) with an activity of 57.2 ± 11.4 IU/mg.
| IU/mg hPRLa | ||
|---|---|---|
| Cells | 0 mM NaBu | 1 mM NaBu |
| Nb2 | 50.88 ± 5.33 | 61.21 ± 4.66 |
| Ba/F3-LLP | 50.07 ± 5.64 | 71.47 ± 4.33 |
aValues are means ± SD of two independent assays, each run with intra-assay quadruplicates.
4. Discussion
Previous studies have examined the production of recombinant proteins by NaBu-treated CHO cells. Several of these have discussed the benefits or warned against the problems caused by the presence of NaBu [14–16]. NaBu has, in fact, been used for increasing the expression of foreign proteins in animal cell cultures. It is recognized that NaBu alters primarily chromatin structure through inhibition of histone deacetylases, resulting in hyperacetylation of histones and, consequently, alterations in DNA transcription. Therefore, these alterations might be correlated with the modulation of gene expression [11]. On the other hand, hyperacetylation of histones in NaBu-treated mammalian cancer cell lines has been shown to induce apoptosis mediated by activation of caspase-3 activity, leading to the translation of putative cell death proteins [34] and resulting in an increased possibility of DNA cleavage [35].
In this study, we examined the expression level and quality of hPRL directly in CHO cell-conditioned medium, after adding different amounts of NaBu. The different NaBu concentrations (0.25 to 2 mM) remarkably increased hPRL expression.
Addition of 1 mM NaBu to the culture medium resulted in a twofold increase in hPRL expression, with only a relatively small decrease in cell growth compared with the untreated control; this was confirmed by RP-HPLC and by Western blot and SDS-PAGE analysis. Although 2 mM and 4 mM NaBu apparently induced a higher synthesis of hPRL in the first 24 hours of treatment, this synthesis exhibited a decrease in the following days of culture. A higher NaBu concentration (2 or 4 mM) may be more effective in inhibiting the activity of histone deacetylases and, consequently, increase the initial expression of proteins. However, a greater modification of the chromatin structure could make the DNA more susceptible to apoptotic action of endonucleases, leading to decreased productivity of hPRL.
The addition of different NaBu concentrations apparently did not affect the hydrophobicity or the hydrodynamic proprieties of the hPRL molecule. This was inferred from the fact that the tR values determined by RP-HPLC and HPSEC were not significantly altered by the NaBu treatment relative to the untreated control or the internal reference standard of periplasmic E. coli-derived hPRL. Our analytical determinations, as a difference from other authors that studied NaBu influence on heterologous protein production with basis on immunological techniques [15–17], have been mostly based on physicochemical methodologies.
The cell viability for different concentrations of NaBu was similar to that of the untreated control except in the experiments with 4 mM added NaBu. Therefore, the arrest of cell growth after day 5 and similar decrease in cell viability between 0–2 mM NaBu was probably caused by removal of dFBS from the culture medium and NaBu treatment during the production process. This could make the CHO cell more susceptible to apoptosis, leading to decreased cellular density and viability.
Finally, two different in vitro bioassays for purified hPRL, based on Nb2 and Ba/F3-LLP cells, were carried out. The first bioassay is the classical heterologous Nb2 rat lymphoma cell proliferation assay [36], while the second is the recently developed homologous Ba/F3-LLP assay [37], based on murine pro-B lymphoma cells containing the sequence encoding the human PRL receptor and previously utilized in our laboratory [9, 27]. As described by Crowell et al. [38], NaBu treatment can potentially modify the oligosaccharide content of glycoproteins in various expression systems. Although NaBu apparently has no effect on the protein structure of hPRL, there might be modifications of the oligosaccharide structure of G-hPRL. In this regard, it is known that the oligosaccharide nature and content can influence the in vivo bioactivities of a glycoprotein [39–42]. It is therefore important to note that the potency of hPRL obtained in the presence of NaBu was comparable to that of the Reference Reagent for recombinant hPRL (WHO 97/714). In conclusion, for the first time recombinant hPRL production from genetically modified CHO cells has been significant increased (~2-fold) via NaBu addition, apparently without compromising either its structure or function; the quantitative and qualitative effects of this addition have been determined via accurate physicochemical techniques. The same treatment can potentially be applied to other hormones, for example, human thyrotropin (hTSH), which is also produced in our laboratory [43]. The qualitative and quantitative influence of this treatment on the carbohydrate moieties of G-hPRL and possibly of other glycohormones requires further investigation.
Acknowledgments
The authors are grateful to José Maria de Sousa and João Ezequiel de Oliveira for valuable and skilled technical assistance. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo, Brazil (Projects 05/60826-3, 06/52973-9, 07/59709-8, 07/59540-3, 07/54493-7), by Conselho Nacional de Pesquisa (CNPq), Brasília, Brazil (Project PQ300473/2009-2), and by FK Biotecnologia/Proteogenética (Porto Alegre, RS, Brazil).
References
- 1.Tettamanzi MC, Keeler C, Meshack S, Hodsdon ME. Analysis of site-specific histidine protonation in human prolactin. Biochemistry. 2008;47(33):8638–8647. doi: 10.1021/bi800444t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiological Reviews. 2000;80(4):1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 3.Goodman G, Bercovich D. Prolactin does not cause breast cancer and may prevent it or be therapeutic in some conditions. Medical Hypotheses. 2008;70(2):244–251. doi: 10.1016/j.mehy.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocrine Reviews. 1996;17(6):639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Jonathan N, Liby K, McFarland M, Zinger M. Prolactin as an autocrine/paracrine growth factor in human cancer. Trends in Endocrinology and Metabolism. 2002;13(6):245–250. doi: 10.1016/s1043-2760(02)00603-3. [DOI] [PubMed] [Google Scholar]
- 6.Morganti L, Huyer M, Gout PW, Bartolini P. Production and characterization of biologically active Ala-Ser-(His)6-lle-Glu-Gly-Arg-human prolactin (tag-hPRL) secreted in the periplasmic space of Escherichia coli. Biotechnology and Applied Biochemistry. 1996;23(1):67–75. [PubMed] [Google Scholar]
- 7.Morganti L, Scares CRJ, Affonso R, Gout PW, Bartolini P. Synthesis and characterization of recombinant, authentic human prolactin secreted into the periplasmic space of Escherichia coli. Biotechnology and Applied Biochemistry. 1998;27, part 1:63–70. doi: 10.1111/j.1470-8744.1998.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 8.Soares CRJ, Morganti L, Miloux B, Lupker JH, Ferrara P, Bartolini P. High-level synthesis of human prolactin in Chinese-hamster ovary cells. Biotechnology and Applied Biochemistry. 2000;32(2):127–135. doi: 10.1042/ba20000047. [DOI] [PubMed] [Google Scholar]
- 9.Soares CRJ, Glezer A, Okazaki K, et al. Physico-chemical and biological characterizations of two human prolactin analogs exhibiting controversial bioactivity, synthesized in Chinese hamster ovary (CHO) cells. Protein Expression and Purification. 2006;48(2):182–194. doi: 10.1016/j.pep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Soares CRJ, Ueda EKM, Oliveira TL, Gomide FIC, Heller SR, Bartolini P. Distinct human prolactin (hPRL) and growth hormone (hGH) behavior under bacteriophage lambda P promoter control: temperature plays a major role in protein yields. Journal of Biotechnology. 2008;133(1):27–35. doi: 10.1016/j.jbiotec.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 11.Davie JR. Inhibition of histone deacetylase activity by butyrate. Journal of Nutrition. 2003;133(7, supplement):2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 12.Leibovitch M-P, Leibovitch S-A, Harel J, Kruh J. Effect of sodium butyrate on messenger RNA populations in myogenic cells in culture. Differentiation. 1982;22(2):106–112. doi: 10.1111/j.1432-0436.1982.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee DY, Hayes JJ, Pruss D, Wolffe AP. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72(1):73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- 14.Kim NS, Lee GM. Overexpression of bcl-2 inhibits sodium butyrate-induced apoptosis in Chinese hamster ovary cells resulting in enhanced humanized antibody production. Biotechnology and Bioengineering. 2000;71(3):184–193. doi: 10.1002/1097-0290(2000)71:3<184::aid-bit1008>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Mimura Y, Lund J, Church S, et al. Butyrate increases production of human chimeric IgG in CHO-K1 cells whilst maintaining function and glycoform profile. Journal of Immunological Methods. 2001;247(1-2):205–216. doi: 10.1016/s0022-1759(00)00308-2. [DOI] [PubMed] [Google Scholar]
- 16.Han KO, Moon KS, Yang J, et al. Effect of N-Acetylcystein on butyrate-treated Chinese hamster ovary cells to improve the production of recombinant human interferon-beta-1a. Biotechnology Progress. 2005;21(4):1154–1164. doi: 10.1021/bp050057v. [DOI] [PubMed] [Google Scholar]
- 17.Yun HS, Gyun ML. Enhanced human thrombopoietin production by sodium butyrate addition to serum-free suspension culture of Bcl-2-overexpressing CHO cells. Biotechnology Progress. 2005;21(1):50–57. doi: 10.1021/bp049892n. [DOI] [PubMed] [Google Scholar]
- 18.Soares CRJ, Camargo IMC, Morganti L, et al. Reversed-phase high-performance liquid chromatography method for the determination of prolactin in bacterial extracts and in its purified form. Journal of Chromatography A. 2002;955(2):229–236. doi: 10.1016/s0021-9673(02)00229-7. [DOI] [PubMed] [Google Scholar]
- 19.Dalmora S, Oliveira JE, Affonso R, Gimbo E, Ribela MTCP, Bartolini P. Analysis of recombinant human growth hormone directly in osmotic shock fluids. Journal of Chromatography A. 1997;782(2):199–210. doi: 10.1016/s0021-9673(97)00493-7. [DOI] [PubMed] [Google Scholar]
- 20.Tennant JR. Evaluation of the trypan blue technique for determination of cell viability. Transplantation. 1964;2:685–694. doi: 10.1097/00007890-196411000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Bassam BJ, Caetano-Anolles G, Gresshoff PM. Fast and sensitive silver staining of DNA in polyacrylamide gels. Analytical Biochemistry. 1991;196(1):80–83. doi: 10.1016/0003-2697(91)90120-i. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Bannerman DD, Tupper JC, Erwert RD, Winn RK, Harlan JM. Divergence of bacterial lipopolysaccharide pro-apoptotic signaling downstream of IRAK-1. Journal of Biological Chemistry. 2002;277(10):8048–8053. doi: 10.1074/jbc.M111249200. [DOI] [PubMed] [Google Scholar]
- 24.Ribela MTCP, Bianco AC, Bartolini P. The use of recombinant human thyrotropin produced by Chinese hamster ovary cells for the preparation of immunoassay reagents. Journal of Clinical Endocrinology and Metabolism. 1996;81(1):249–256. doi: 10.1210/jcem.81.1.8550760. [DOI] [PubMed] [Google Scholar]
- 25.Ribela MTCP, Gout PW, Oliveira JE, Bartolini P. HPLC analysis of human pituitary hormones for pharmaceutical applications. Current Pharmaceutical Analysis. 2006;2(2):103–126. [Google Scholar]
- 26.White GW, Katona T, Zodda JP. The use of high-performance size exclusion chromatography (HPSEC) as a molecular weight screening technique for polygalacturonic acid for use in pharmaceutical applications. Journal of Pharmaceutical and Biomedical Analysis. 1999;20(6):905–912. doi: 10.1016/s0731-7085(99)00083-7. [DOI] [PubMed] [Google Scholar]
- 27.Glezer A, Soares CRJ, Vieira JG, et al. Human macroprolactin displays low biological activity via its homologous receptor in a new sensitive bioassay. Journal of Clinical Endocrinology and Metabolism. 2006;91(3):1048–1055. doi: 10.1210/jc.2005-1831. [DOI] [PubMed] [Google Scholar]
- 28.Rafferty B, Rigsby P, Gaines-Das RE, et al. Draft report of an international collaborative study of proposed WHO reference reagents for rDNA-derived prolactin and its glycosylated and non-glycosylated componentes. WHO Technical Report Series. 2001;52(924) [Google Scholar]
- 29.Chen T-J, Kuo CB, Tsai KF, Liu JO-W, Chen D-Y, Walker AM. Development of recombinant human prolactin receptor antagonists by molecular mimicry of the phosphorylated hormone. Endocrinology. 1998;139(2):609–616. doi: 10.1210/endo.139.2.5758. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y-S, Nakajima H, Chang Y-C, et al. Alleviation of apoptosis by serum in Chinese hamster ovary cells ectopically expressing human Fas antigen. Molecules and Cells. 1998;8(3):272–279. [PubMed] [Google Scholar]
- 31.Heller SR, Rodrigues Goulart H, Arthuso FS, Oliveira TL, Bartolini P, Soares CRJ. Synthesis, purification and characterization of recombinant glycosylated human prolactin (G-hPRL) secreted by cycloheximide-treated CHO cells. Journal of Biotechnology. 2010;145(4):334–340. doi: 10.1016/j.jbiotec.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Ribella MTCP, Bartolini P. Stokes radius determination of radioiodinated polypeptide hormones by gel filtration. Analytical Biochemistry. 1988;174(2):693–697. doi: 10.1016/0003-2697(88)90075-9. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho CM, Oliveira JE, Almeida BE, et al. Efficient isolation of the subunits of recombinant and pituitary glycoprotein hormones. Journal of Chromatography A. 2009;1216(9):1431–1438. doi: 10.1016/j.chroma.2008.12.096. [DOI] [PubMed] [Google Scholar]
- 34.Medina V, Edmonds B, Young GP, James R, Appleton S, Zalewski PD. Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin a (inhibitors of histone deacetylase): dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Research. 1997;57(17):3697–3707. [PubMed] [Google Scholar]
- 35.Smith PJ. n-Butyrate alters chromatin accessibility to DNA repair enzymes. Carcinogenesis. 1986;7(3):423–429. doi: 10.1093/carcin/7.3.423. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T, Shiu RPC, Gout PW. A new sensitive and specific bioassay for lactogenic hormones: measurement of prolactin and growth hormone in human serum. Journal of Clinical Endocrinology & Metabolism. 1980;51(5):1058–1063. doi: 10.1210/jcem-51-5-1058. [DOI] [PubMed] [Google Scholar]
- 37.Bernichtein S, Jeay S, Vaudry R, Kelly PA, Goffin V. New homologous bioassays for human lactogens show that agonism or antagonism of various analogs is a function of assay sensitivity. Endocrine. 2003;20(1-2):177–190. doi: 10.1385/ENDO:20:1-2:177. [DOI] [PubMed] [Google Scholar]
- 38.Crowell CK, Qin Q, Grampp GE, Radcliffe RA, Rogers GN, Scheinman RI. Sodium butyrate alters erythropoietin glycosylation via multiple mechanisms. Biotechnology and Bioengineering. 2008;99(1):201–213. doi: 10.1002/bit.21539. [DOI] [PubMed] [Google Scholar]
- 39.Damiani R, Oliveira JE, Vorauer-Uhl K, et al. Stable expression of a human-like sialylated recombinant thyrotropin in a Chinese hamster ovary cell line expressing alpha2,6-sialyltransferase. Protein Expression and Purification. 2009;67(1):7–14. doi: 10.1016/j.pep.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Goochee CF, Gramer MJ, Andersen DC, Bahr JB, Rasmussen JR. The oligosaccharides of glycoproteins: bioprocess factors affecting oligosaccharide structure and their effect on glycoprotein properties. Biotechnology. 1991;9(12):1347–1355. doi: 10.1038/nbt1291-1347. [DOI] [PubMed] [Google Scholar]
- 41.Mendonça F, Oliveira JE, Bartolini P, Ribela MTCP. Two-step chromatographic purification of recombinant human thyrotrophin and its immunological, biological, physico-chemical and mass spectral characterization. Journal of Chromatography A. 2005;1062(1):103–112. doi: 10.1016/j.chroma.2004.10.084. [DOI] [PubMed] [Google Scholar]
- 42.Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiological Reviews. 2002;82(2):473–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- 43.Peroni CN, Soares CRJ, Gimbo E, Morganti L, Ribela MTCP, Bartolini P. High-level expression of human thyroid-stimulating hormone in Chinese hamster ovary cells by co-transfection of dicistronic expression vectors followed by a dual-marker amplification strategy. Biotechnology and Applied Biochemistry. 2002;35, part 1:19–26. doi: 10.1042/ba20010061. [DOI] [PubMed] [Google Scholar]