Abstract
BACKGROUND
Genetic determinants of blood pressure (BP) responses to the cold pressor test (CPT), a phenotype associated with risk of hypertension and cardiovascular disease has not been well studied.
METHODS
We examined the heritability of BP response to CPT in 1,994 subjects from 627 families in rural north China. BP was measured prior to and at 0, 1, 2, and 4 minutes after the participants immersed their hand in ice water for 1 minute. Heritabilities of baseline BP and responses at 0 minutes, maximum response, and area-under-the-curve during CPT were computed using a variance components method. Additionally, bivariate heritabilities were calculated to test the existence of shared genetic determinants between baseline BP and responses to CPT.
RESULTS
Heritabilities of baseline BP and responses to CPT were estimated from 14% to 35%, which all significantly differed from 0 (p≤0.002). Genetic correlations (standard error) due to the same genes between baseline BP and responses to CPT ranged from −0.07 (0.14) to 0.21 (0.15), which were not significantly different from 0. Genetic correlations between reactivity and recovery were 0.67 (0.10) and 0.59 (0.10) for systolic and diastolic BP, respectively, which were significantly different from 0.
CONCLUSIONS
We concluded: 1) baseline BP and BP responses to CPT had strong genetic determinants; 2) baseline BP and BP response to CPT did not share the same genetic components; and 3) BP reactivity and recovery shared the same genetic components. These findings may lead to a better understanding of the genetic mechanism of BP responses to CPT.
Keywords: blood pressure, heritability, cold pressor test, genetics, hypertension
Introduction
It is well documented that genes, environment, and gene-environment interactions determine an individuals’ blood pressure (BP) level and risk of hypertension1. Genetic epidemiology studies reported that the heritability of BP, the proportion of variance explained by genetic factors, ranged from 20% to 50% in human populations 2. These studies also indicated that genetic influences on BP varied by race, age, and environmental factors 2-5.
The cold pressor test (CPT), which measures the response of BP to the stimulus of external cold, has long been a standard test for characterization of sympathetic function and has been documented to predict the subsequent risk of hypertension in normotensive persons6-9. Twin and pedigree studies reported that BP reactivity to CPT was significantly influenced by genetic effects10-14. For example, significant heritabilities were found for systolic BP (SBP) responses (0.12-0.37) but not for diastolic BP (DBP) among 419 individuals distributed across four large European American families13. A recent study reported a significant genetic influence on BP reactivity and recovery during CPT among 835 participants from 18 extended Amish families14. It was also found that both shared and unshared genetic factors influence BP reactivity and recovery. Shared genetic factors contribute to the total correlation of the two phenotypes, and this genetic correlation can be inferred by bivariate analysis. The heritability of BP responses to CPT and the genetic correlation between BP reactivity and recovery has not been reported in Asian populations.
In the Genetic Epidemiology Network of Salt Sensitivity (GenSalt) study, we conducted CPT among 1,994 subjects from 627 families living in rural north China. In this analysis, we report the heritabilities of BP responses to CPT as well as the genetic correlations between baseline, reactivity, and recovery BP phenotypes.
Methods
Study Participants
The GenSalt study participants were recruited from 6 sites in rural areas of north China. These sites were selected based on homogeneity of the population with respect to ethnicity, lifestyle and nutritional risk factors15. A community-based BP screening was conducted among persons aged 18-60 years in the study villages to identify potential probands and their families for the study. Those with mean systolic BP between 130-160 mm Hg and/or diastolic BP between 85-100 mm Hg and no use of antihypertensive medications and their siblings, offspring, parents, and spouses were recruited for the CPT study. The detailed eligibility criteria for the probands and siblings/offspring/parents/spouses are presented elsewhere 15. In general, individuals who had stage-2 hypertension, secondary hypertension, use of antihypertensive medications, history of clinical cardiovascular disease, diabetes, or chronic kidney disease, pregnancy, or heavy alcohol use were excluded from the GenSalt study. Overall, 2,000 subjects took part in the CPT and 1,994 participants from 627 families completed the entire test and were included in this analysis. Family relationships were verified using genome-wide microsatellite data.
Institutional Review Boards at all participating institutes approved the GenSalt study. Written informed consent was obtained from each participant.
Data Collection
A standardized questionnaire was administered by a trained staff member to collect information on demographic characteristics, personal and family medical history, and lifestyle risk factors. BP measurements were obtained at each clinical visit by trained and certified observers according to a common protocol adapted from procedures recommended by the American Heart Association16. Participants were advised to avoid alcohol, cigarette smoking, coffee/tea, and exercise for at least 30 minutes prior to their BP measurement. After the participant had remained sitting for 20 minutes and before the ice water immersion, 3 BP measurements were obtained using a standard mercury sphygmomanometer. The mean of these three BP measurements was considered the baseline BP. Then, the participant immersed their left hand in the ice water bath (3°C to 5°C) to just above the wrist for 1 minute. BP measurements at 0, 1, 2, and 4 minutes were obtained using a standard mercury sphygmomanometer after the left hand had been removed from the ice water bath. The CPT was well-tolerated in all subjects and no side effects were reported.
Statistical Analyses
Heritability was analyzed for baseline BP and BP responses to CPT, including the maximum BP response, BP response at time 0, and area-under-curve (AUC) above baseline BP. Response at time 0 is the difference between BP at time 0 and baseline BP; and maximum response is the largest BP difference between BP at any of the 4 CPT tested time-points and baseline BP. In addition, the AUC of BP response to CPT was further divided into BP reactivity (from baseline to time 0) and recovery (from time 0 to time 4).
Pearson’s correlations between BP responses and age, sex, generation, field center, baseline BP, BMI, water temperature, room temperature and arm circumference are −0.133~0.146. The BP responses were adjusted for the effects of age, sex, generation, and field center groups. BP responses were regressed on the covariates in a stepwise manner, and only those with significant p-values (less than 0.05) were retained. Next, the squared residual from the first regression was regressed on the same covariates in a stepwise manner to control for heteroscedasticity. The final adjusted phenotype was computed as the residual from the first regression, divided by the square root of the predicted score from the second regression. A final standardization step was taken to ensure a mean of zero and a standard deviation of one.
Heritability analysis was performed using a maximum likelihood variance components method that was implemented in the program SOLAR17. For a phenotype variable y, its variance is decomposed into the part from the additive genetic effect and the remaining part from the unexplained effect , i.e. . The heritability (h2) of phenotype y is computed by the ratio of variance attributable to additive genetic effects versus the total phenotypic variance . The hypothesis test of no heritability (H0: h2=0) versus having heritability (H1: h2≠0) is conducted by likelihood ratio test.
We also performed a bivariate analysis to estimate the correlations of genetic components using SOLAR17. For a pair of traits, y1 and y2, with heritabilities h12 and h22 respectively, their correlation (ρ) can be decomposed into genetic correlation (ρg) and environment correlation (ρe) according to the following mathematical formula: . We tested the null hypothesis ρG=0 versus H1: ρG≠0 to infer correlated or shared genetic effects (reject H0), and tested the null hypothesis of ρG=1 (or −1) versus H1: ρG≠1 (and −1) to infer independent or non-shared genetic effects (reject H0). We also tested H0: ρE=0 versus H1: ρE≠0 for correlated or shared environment effects.
BMI, water temperature, room temperature and arm circumference were used as covariates in the heritability and bivariate anlaysis by SOLAR17.
Results
The 1,994 participants who completed CPT consisted of 638 probands, 921 siblings, 61 spouses, 182 parents and 192 offspring from 627 two and three generation families. The characteristics of study participants are summarized in table 1. As expected, the probands had the highest mean baseline levels of body mass index (BMI), SBP and DBP among all groups.
Table 1. Characteristics of 1,994 Study Participants.
| Probands | Siblings | Offspring | Spouses | Parents | |
|---|---|---|---|---|---|
| No. of subjects | 638 | 921 | 192 | 61 | 182 |
| Age, years | 40.7 ± 8.2 | 39.5 ± 7.7 | 23.5 ± 6.7 | 48.7 ± 6.5 | 55.5 ± 3.7 |
| Male, % | 61.1 | 50.6 | 46.4 | 32.8 | 44.8 |
| BMI, kg/m2 | 24.2 ± 3.3 | 23.1 ± 2.9 | 21.5 ± 3.3 | 23.7 ± 3.7 | 23.6 ± 3.5 |
| SBP, mm Hg | 129.3 ± 12.1 | 114.3 ± 12.7 | 110.6 ± 12.3 | 117.0 ± 17.1 | 128.3 ± 21.4 |
| DBP, mm Hg | 80.6 ± 9.7 | 72.2 ± 9.8 | 66.6 ± 9.4 | 74.2 ± 11.2 | 75.6 ± 12.0 |
Data presented are mean ± standard deviation values, unless otherwise stated.
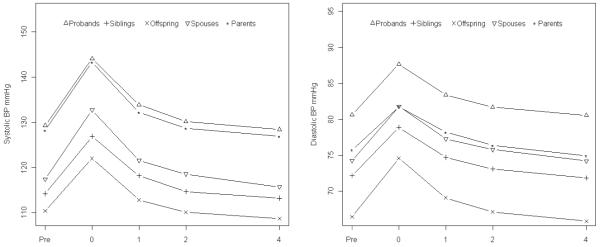
The mean SBP and DBP responses to CPT are presented in figure 1. Both mean SBP and DBP achieved maximum levels at time 0 after ice water immersion for one minute among all groups. Thereafter, mean BP levels gradually recovered back to the baseline level 4 minutes after cold stimulus. Ninety-one point five percent of probands, 95.6% of parents, 90.2% of siblings, 100% of spouses and 96.4% of offspring achieved maximum SBP at time 0 while 80.4% of probands, 80.8% of parents, 81.4% of siblings, 83.6% of spouses and 82.8% of offspring achieved maximum DBP at time 0. Table 2 shows the maximum BP responses, BP responses at time 0 and AUC of BP responses for both SBP and DBP. The parents had the largest SBP responses but smallest DBP responses while offspring had the smallest SBP responses but largest DBP responses. This pattern was similarly observed for absolute BP responses as well as reactivity AUC. On the other hand, probands had the largest and offspring had the smallest recovery AUC of SBP while spouses had the largest and parents had the smallest recovery AUC of DBP.
Figure 1.
Mean systolic blood pressure (left panel) and mean diastolic blood pressure (right panel) during the cold pressor test among probands, siblings, offspring, spouses, and parents. Pre, 0, 1, 2 and 4 on the x-axis indicate cold pressor test time points: prior to ice water immersion, immediately after end of ice water immersion, and 1, 2 and 4 minutes after end of ice water immersion.
Table 2. Mean Blood Pressure Response to Cold Pressor Test.
| Probands | Siblings | Offspring | Spouses | Parents | |
|---|---|---|---|---|---|
| Maximum BP Response | |||||
| SBP | 15.1 ± 10.3 | 13.1 ± 9.5 | 11.8 ± 8.2 | 15.0 ± 11.3 | 15.5 ± 12.1 |
| DBP | 7.8 ± 5.9 | 7.5 ± 6.1 | 8.8 ± 6.7 | 8.1 ± 6.3 | 6.9 ± 5.2 |
| BP Response at time 0 | |||||
| SBP | 14.8 ± 10.6 | 12.6 ± 10.1 | 11.6 ± 8.3 | 15.0 ± 11.3 | 15.3 ± 12.2 |
| DBP | 7.1 ± 6.5 | 6.7 ± 6.8 | 8.0 ± 7.3 | 7.4 ± 6.7 | 6.1 ± 5.7 |
| Reactivity area-under-curve | |||||
| SBP | 7.4 ± 5.3 | 6.3 ± 5.0 | 5.8 ± 4.2 | 7.5 ± 5.6 | 7.7 ± 6.1 |
| DBP | 3.5 ± 3.3 | 3.4 ± 3.4 | 4.0 ± 3.6 | 3.7 ± 3.4 | 3.1 ± 2.9 |
| Recovery area-under-curve | |||||
| SBP | 12.4 ± 19.0 | 9.5 ± 18.1 | 5.6 ± 16.3 | 10.6 ± 22.0 | 11.3 ± 21.2 |
| DBP | 7.8 ± 13.7 | 7.0 ± 14.2 | 7.0 ± 14.8 | 8.1 ± 15.3 | 6.2 ± 14.3 |
| Total area-under-curve | |||||
| SBP | 19.7 ± 22.7 | 15.8 ± 21.7 | 11.4 ± 19.1 | 18.1 ± 26.7 | 18.9 ± 25.9 |
| DBP | 11.3 ± 15.9 | 10.3 ± 16.6 | 11.0 ± 17.2 | 11.8 ± 17.6 | 9.3 ± 16.4 |
Data presented are mean ± standard deviation values.
Table 3 presents the heritabilities (standard error, SE) of baseline BP and BP responses to CPT. All heritability estimates are significantly different from zero (all p≤0.002). The heritabilities of baseline BP were 0.20 for SBP and 0.35 for DBP. The heritabilities of SBP responses to CPT varied from 0.28 to 0.30 while the heritabilities of DBP responses to CPT varied from 0.25 to 0.28. The heritability of SBP recovery AUC was 0.14 and DBP recovery AUC was 0.20. The overall AUC was 0.16 for SBP and 0.21 for DBP.
Table 3. Heritability of Blood Pressure at Baseline and Blood Pressure Response to Cold Pressor Test.
| SBP |
DBP |
|||
|---|---|---|---|---|
| h2 ± SE | p-value | h2 ± SE | p-value | |
| Baseline BP | 0.20 ± 0.05 | <0.0001 | 0.35 ± 0.05 | <0.0001 |
| Maximum BP response | 0.29 ± 0.05 | <0.0001 | 0.28 ± 0.05 | <0.0001 |
| BP response at time 0 | 0.28 ± 0.05 | <0.0001 | 0.25 ± 0.05 | <0.0001 |
| Reactivity AUC | 0.30 ± 0.05 | <0.0001 | 0.26 ± 0.05 | <0.0001 |
| Recovery AUC | 0.14 ± 0.05 | 0.002 | 0.20 ± 0.05 | <0.0001 |
| Overall AUC | 0.16 ± 0.05 | 0.0002 | 0.21 ± 0.05 | <0.0001 |
Response at time 0 is the difference between BP at time 0 and baseline BP while maximum response is the largest BP difference between BP at any of the 4 CPT tested time-points and baseline BP. h2 = heritability; SE = standard error.
Table 4 shows that genetic correlations (ρG) between baseline SBP and SBP responses to CPT (maximum response, response at time 0, and reactivity, recovery and overall AUC) varied from 0.16 to 0.21, which were not significantly different from 0 but differed from 1. On the other hand, the genetic correlation between SBP reactivity and recovery AUC was 0.67, which significantly differed from both 0 and 1. The environmental correlation was not significant between baseline SBP and maximum SBP response and SBP response at time 0, but significant between baseline SBP and SBP recovery AUC and overall AUC. Both genetic and environmental correlations between SBP recovery and reactivity AUC significantly differed from 0. A similar pattern of genetic correlations was observed between baseline DBP and DBP responses to CPT. Environmental correlation, however, was significant between baseline DBP and all DBP responses to CPT.
Table 4. Genetic (ρG) and Environmental (ρE) Correlation Between Baseline Blood Pressure and Blood Pressure Response to the Cold Pressor Test.
| P value |
P value |
|||
|---|---|---|---|---|
| Pairs of traits | ρG (SE) | H0: ρG =0 | ρE (SE) | H0: ρE =0 |
| Systolic blood pressure | ||||
| Baseline-maximum response |
0.21 (0.15) | 0.14 | −0.02 (0.05) | 0.61 |
| Baseline-response at time 0 |
0.18 (0.15) | 0.21 | −0.005 (0.05) | 0.91 |
| Baseline-reactivity AUC | 0.18 (0.15) | 0.21 | −0.005 (0.05) | 0.91 |
| Baseline-recovery AUC | 0.16 (0.21) | 0.46 | −0.13 (0.04) | 0.004 |
| Baseline-overall AUC | 0.16 (0.19) | 0.41 | −0.11 (0.04) | 0.02 |
| Recovery AUC-reactivity AUC |
0.67 (0.10) | 0.0007 | 0.66 (0.03) | <0.0001 |
| Diastolic blood pressure | ||||
| Baseline-maximum response |
0.10 (0.12) | 0.42 | −0.19 (0.05) | 0.0003 |
| Baseline-response at time 0 |
0.08 (0.12) | 0.53 | −0.14 (0.05) | 0.005 |
| Baseline-reactivity AUC | 0.08 (0.12) | 0.48 | −0.15 (0.05) | 0.004 |
| Baseline-recovery AUC | −0.07 (0.14) | 0.63 | −0.22 (0.05) | <0.0001 |
| Baseline-overall AUC | −0.03 (0.13) | 0.83 | −0.22 (0.05) | <0.0001 |
| Recovery AUC-reactivity AUC |
0.59 (0.10) | 0.0006 | 0.67 (0.03) | <0.0001 |
ρ is the Pearson correlation between pairs of traits, and it is split into two components, genetic correlation (ρG) and environment correlation (ρE). For H0: ρG =1 (or −1), the p-value is ≤0.005 and results are not shown here.
Discussion
This study contributes to our understanding on the genetic mechanisms of BP responses to CPT in several ways. To our knowledge, this is the largest family study and the first one in an Asian population to examine the genetic contribution to BP responses to CPT, which adds to findings from other populations 13, 14. Our study also comprehensively examined the heritabilities of absolute BP responses, AUC of BP responses, as well as AUC of BP reactivity and recovery. Furthermore, genetic correlations among baseline BP and BP responses to CPT were investigated.
Additional strengths of our study include that all study participants were living in north rural China with homogeneous lifestyles for many generations and were all of Han Chinese ethnicity. A stringent quality control process was implemented during data collection. We controlled for potential confounding effects due to age, sex, generation, field center, BMI, water temperature, room temperature and arm circumference in our analysis.
Our study documents a genetic influence on baseline BP with heritability of 20%-35% which is consistent with prior reports in Western populations 3, 4, 13, 14, 18-20. Our study further indicates that both SBP and DBP responses to CPT are significantly inheritable. A few twin studies similarly suggested a genetic basis for both SBP and DBP responses to CPT. 11, 12, 21 In addition, two previous family studies in Caucasians reported a significant heritability of BP responses to CPT, although one of them did not show significant heritability for DBP 13.
The CPT method in our study differs from one conducted in Amish families14. In the present study, subjects immersed their hand in ice water for 1 minute and BP measurements were taken after the cold stimulation. In contrast, in the Amish families, subjects immersed their hand in ice water for 2.5 minutes and BP measurements were taken both during and after the stimulation. Due to these methodological differences, the highest BP responses occurred at different times and altered the estimation of BP reactivity and recovery. In spite of this variation, both studies concluded that BP response is inheritable, and that both shared and unshared genetic components underlie BP reactivity and recovery during CPT.
Our bivariate analysis of genetic correlation indicates independent genetic effects between baseline BP and BP responses to CPT. This is consistent with previous twin studies that showed no evidence for common genetic effects on baseline BP and BP responses to CPT11, 12, 21, and is also consistent with a previous family study that showed no genetic correlation between baseline BP and BP responses to CPT 13. Our findings of an independent genetic effect may suggest different physiologic pathways involved in the regulation of baseline BP and BP response to CPT.
Our study showed that BP recovery had a strong positive genetic correlation with BP reactivity during CPT. This finding suggests that the same genes might influence both BP recovery and reactivity. Nevertheless, our findings also suggest that BP recovery and reactivity are not the result of complete pleiotropy and unique genes are responsible for BP recovery and reactivity, given the fact that the genetic correlation significantly differs from 1 (p≤0.005). The shared but incomplete pleiotropic effects were also consistently found in a recent family study conducted by Roy-Gagnon et al14.
In summary, our findings of genetic influences on baseline BP and BP responses to CPT in Chinese participants are in strong agreement with previous family and twin studies in Western populations. Our results contribute new evidence that different genetic factors are responsible for the regulation of baseline BP and BP responses to CPT, and both different and common genetic factors controlled BP reactivity and recovery during CPT. These findings could be used to guide the search for specific genes underlying baseline BP and BP responses to CPT. Not only can the finding of genetically correlated traits increase the power of linkage mapping, but also differentiate pleiotropic effects from coincidence linkage22.
Acknowledgments
We express our sincere appreciation to the Genetic Epidemiology Network of Salt Sensitivity Study participants for their participation and cooperation in this project.
Source of Funding
The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) is supported by research grants (U01HL072507, R01HL087263, and R01HL090682) from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland.
Footnotes
Disclosures
None
References
- 1.Gerber LM, Halberstein RA. Blood pressure: genetic and environmental influences. Hum Biol. 1999;71(4):467–73. [PubMed] [Google Scholar]
- 2.Barlassina C, Lanzani C, Manunta P, Bianchi G. Genetics of essential hypertension: from families to genes. J Am Soc Nephrol. 2002;13(Suppl 3):S155–64. doi: 10.1097/01.asn.0000032524.13069.88. [DOI] [PubMed] [Google Scholar]
- 3.Gu C, Borecki I, Gagnon J, Bouchard C, Leon AS, Skinner JS, Wilmore JH, Rao DC. Familial resemblance for resting blood pressure with particular reference to racial differences: preliminary analyses from the HERITAGE Family Study. Hum Biol. 1998;70(1):77–90. [PubMed] [Google Scholar]
- 4.Perusse L, Rice T, Bouchard C, Vogler GP, Rao DC. Cardiovascular risk factors in a French-Canadian population: resolution of genetic and familial environmental effects on blood pressure by using extensive information on environmental correlates. Am J Hum Genet. 1989;45(2):240–51. [PMC free article] [PubMed] [Google Scholar]
- 5.Livshits G, Ginsburg E, Kobyliansky E. Heterogeneity of genetic control of blood pressure in ethnically different populations. Hum Biol. 1999;71(4):685–708. [PubMed] [Google Scholar]
- 6.Victor RG, Leimbach WN, Jr., Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9(5):429–36. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- 7.Wood DL, Sheps SG, Elveback LR, Schirger A. Cold pressor test as a predictor of hypertension. Hypertension. 1984;6(3):301–6. doi: 10.1161/01.hyp.6.3.301. [DOI] [PubMed] [Google Scholar]
- 8.Menkes MS, Matthews KA, Krantz DS, Lundberg U, Mead LA, Qaqish B, Liang KY, Thomas CB, Pearson TA. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension. 1989;14(5):524–30. doi: 10.1161/01.hyp.14.5.524. [DOI] [PubMed] [Google Scholar]
- 9.Kasagi F, Akahoshi M, Shimaoka K. Relation between cold pressor test and development of hypertension based on 28-year follow-up. Hypertension. 1995;25(1):71–6. doi: 10.1161/01.hyp.25.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Miller JZ, Weinberger MH, Christian JC, Daugherty SA. Familial resemblance in the blood pressure response to sodium restriction. Am J Epidemiol. 1987;126(5):822–30. doi: 10.1093/oxfordjournals.aje.a114719. [DOI] [PubMed] [Google Scholar]
- 11.McIlhany ML, Shaffer JW, Hines EA., Jr The heritability of blood pressure: an investigation of 200 pairs of twins using the cold pressor test. Johns Hopkins Med J. 1975;136(2):57–64. [PubMed] [Google Scholar]
- 12.Busjahn A, Faulhaber HD, Viken RJ, Rose RJ, Luft FC. Genetic influences on blood pressure with the cold-pressor test: a twin study. J Hypertens. 1996;14(10):1195–9. doi: 10.1097/00004872-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Choh AC, Czerwinski SA, Lee M, Demerath EW, Wilson AF, Towne B, Siervogel RM. Quantitative genetic analysis of blood pressure response during the cold pressor test. Am J Hypertens. 2005;18(9 Pt 1):1211–7. doi: 10.1016/j.amjhyper.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Roy-Gagnon MH, Weir MR, Sorkin JD, Ryan KA, Sack PA, Hines S, Bielak LF, Peyser PA, Post W, Mitchell BD, Shuldiner AR, Douglas JA. Genetic influences on blood pressure response to the cold pressor test: results from the Heredity and Phenotype Intervention Heart Study. J Hypertens. 2008;26(4):729–36. doi: 10.1097/HJH.0b013e3282f524b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The.GenSalt.Collaborative.Research.Group GenSalt: rationale, design, methods and baseline characteristics of study participants. J Hum Hypertens. 2007;21(8):639–46. doi: 10.1038/sj.jhh.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5 Pt 1):2460–70. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 17.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, Cupples LA, Myers RH. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension. 2000;36(4):477–83. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 19.Rotimi CN, Cooper RS, Cao G, Ogunbiyi O, Ladipo M, Owoaje E, Ward R. Maximum-likelihood generalized heritability estimate for blood pressure in Nigerian families. Hypertension. 1999;33(3):874–8. doi: 10.1161/01.hyp.33.3.874. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell BD, Kammerer CM, Blangero J, Mahaney MC, Rainwater DL, Dyke B, Hixson JE, Henkel RD, Sharp RM, Comuzzie AG, VandeBerg JL, Stern MP, MacCluer JW. Genetic and environmental contributions to cardiovascular risk factors in Mexican Americans. The San Antonio Family Heart Study. Circulation. 1996;94(9):2159–70. doi: 10.1161/01.cir.94.9.2159. [DOI] [PubMed] [Google Scholar]
- 21.Ditto B. Familial influences on heart rate, blood pressure, and self-report anxiety responses to stress: results from 100 twin pairs. Psychophysiology. 1993;30(6):635–45. doi: 10.1111/j.1469-8986.1993.tb02089.x. [DOI] [PubMed] [Google Scholar]
- 22.Almasy L, Dyer TD, Blangero J. Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet Epidemiol. 1997;14(6):953–8. doi: 10.1002/(SICI)1098-2272(1997)14:6<953::AID-GEPI65>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]