Abstract
Liver cancer, especially hepatocellular carcinoma (HCC), is one of the most common tumors worldwide. The majority of people with HCC will die within 1 year of its detection. The high case–fatality rate can, in part, be attributed to a lack of diagnostic methods that enable the early detection of liver cancer. Hence, there is a need for further understanding of tumor biology and host response mechanisms so that new diagnostic and therapeutic tools can be developed. There has been a growing interest in using serum anti-tumor-associated antigen (TAA) antibodies as serological cancer biomarkers. This interest stems from the notion that these anti-TAA antibodies are ‘sensors’ or ‘reporters’ of molecular events associated with tumorigenesis. The persistence and stability of autoantibodies in the serum of cancer patients is an advantage over other potential markers, including the TAAs themselves, some of which are released by tumors but rapidly degrade or are cleared after circulating in the serum for a limited time. Furthermore, the widespread availability of methods and reagents to detect serum autoantibodies facilitates their characterization in cancer patients and assay development. The hypothesis is that ‘customized’ TAA arrays constitute promising and powerful tools for enhancing the serological anti-TAA antibody detection of cancer. The present review will focus on the recent advances in our studies primarily associated with the idea and possibility that autoantibodies to TAAs can be used as biomarkers in immunodiagnosis of HCC and other solid tumors.
Keywords: autoantibody, biomarker, hepatocellular carcinoma, immunodiagnosis, tumor-associated antigen
Antigenic changes in cancer cells can be recognized by the immune system of patients themselves, and present as immune responses to factors involved in malignant transformation. This is manifested in several ways, one of which is the appearance of circulating autoantibodies. We have called this type of autoantibody a ‘reporter’ from the immune system, which identifies the antigenic changes in cellular factors involved in the transformation process [1,2]. We have used circulating autoantibodies from cancer patients to isolate the cognate tissue antigens, many of which have been shown to be cellular factors participating in known tumorigenesis pathways. These cancer-related tissue antigens are generally known as tumor-associated antigens (TAAs). We have proposed that the information that the cancer patient’s immune system is conveying in the form of autoantibodies to TAAs should be utilized to a greater extent in identifying early signs of tumorigenesis.
Hepatocellular carcinoma (HCC) is one of the most common tumors worldwide, and is particularly prevalent in Africa and Asia. The majority of people with HCC will die within 1 year of its detection. This high case–fatality rate can, in part, be attributed to a lack of diagnostic methods that allow early detection. How to establish a methodology to identify high-risk individuals for HCC remains to be investigated. Although serum α-fetoprotein (AFP) is the most effective marker available to detect HCC, the sensitivity and specificity are not optimal, especially in patients with small tumors or in well-to-moderately differentiated HCC [3-5]. Therefore, there is a need for the development of more sensitive and specific methods that supplement AFP in the early detection of this cancer. The review will focus on recent advances, primarily associated with the idea and possibility that autoantibodies to TAAs can be used as biomarkers in immunodiagnosis of HCC and other solid tumors.
Paradigms in the humoral immune responses in cancer & rheumatic autoimmune diseases
Owing to experience with the humoral immune response in both cancer and systemic rheumatic autoimmune diseases, such as lupus, it was of interest to examine similarities and differences between these two apparently diverse conditions although they are characterized similarly by significant autoantibody production, with the anticipation that some insights might arise from such analysis. Unlike in lupus and other rheumatic diseases, where there is little information regarding how intracellular proteins acquire immunogenicity, in cancer there is substantial information on these issues. A study in lung cancer showed that point mutations of p53 in lung cancer leading to missense protein products were highly correlated to presence of autoantibodies, whereas stop, stop/splice and frameshift mutations were not [6]. The point mutations occurred in the hot-spot regions of p53 in exons 4–9 and increased its stability in contrast to the wild type which degraded protein more rapidly. Subsequent studies by other investigators were directed at the question of whether the gene mutations created new antigenic determinants or neoepitopes on p53 [7,8]. However, anti-p53 autoantibodies in cancer patients were all directed against antigenic determinants on the N- and C-termini of p53, away from the hot spots in the mid-region exons of the protein. Thus, it may not be an abnormal structure or composition of a mutated protein that is immunogenic but increased antigenic load (owing to resistance to normal degradation) to ectopic cellular localization, which has been observed with p53 in cancer cells and other factors. It is well-known that autoantibodies react with antigenic determinants in normal cells, since the methods for detecting autoantibodies do not require components or extracts from abnormal cells. These studies also showed that even if gene mutation was the principal factor in a particular tumorigenesis pathway, stop or frameshift mutations (where no expression product was present) would not lead to autoantibody production. In systemic autoimmune rheumatic illnesses, there is very little information concerning genetic alterations in cellular autoantigens.
In autoimmune rheumatic diseases, there are autoantibodies that are highly specific for one disease and rarely found in other diseases. These include anti-dsDNA and anti-Sm in lupus, anti-DNA topoisomerase I in systemic scleroderma and anti-transfer RNA synthetases in dermatomyositis/polymyositis [9]. The majority of autoantibodies in cancer do not appear to show restricted specificity. For example, the well-studied cancer autoantibody anti-p53 is found in almost all types of cancer, although there appear to be significant differences in frequency, with some cancers showing very low frequency. More extensive data are needed from larger cancer cohorts. However, this phenomenon, if substantiated, could be related to the fact that the p53 tumor-suppressor pathway is a dominant tumor surveillance pathway in many tissues.
What constitutes a TAA?
A major issue in the field of cancer immunodiagnosis is the definition of what constitutes a TAA. It is erroneous to include all cellular antigens identified by autoantibodies in cancer sera as TAAs, since some autoantibodies may exist in conditions that pre-date malignancy. Many antibodies to autologous cellular proteins can be detected in the sera of patients with cancer, and the reactive antigens have been called TAAs without considering the possibility that the autoantibodies might have existed in the patient before cancer. We became aware of this situation when studying HCC patients in whom serial samples of stored sera had been collected at stages when these patients had liver cirrhosis or chronic viral hepatitis [10-13]. Immunofluorescent and western blotting studies showed that, in many patients, two types of autoantibodies could be detected – the first type were autoantibodies appearing de novo with cancer and the second type, autoantibodies present before and persisting into the cancer phase of illness (see later for further description). It became obvious that autoantibodies from a cancer patient obtained at only one time point would not necessarily represent immune responses to a TAA. Failing to recognize the likelihood of premalignancy circulating antibodies would result in the inclusion of many antigens erroneously as TAAs, since this might include both cancer-related and unrelated antigens. Some reviews have reported more than 2800 tumor-related antigens that were identified with sera from cancer patients [14]. In many studies, further work would be needed to establish true tumor-related antigens and to exclude those that might have been present prior to malignancy.
Many putative TAAs can be isolated on the basis of reactivity with antibodies from cancer patients and the question has been one of selection among many putative antigens to validate its tumor relatedness. Our approach has been to select those cellular proteins with possible functions in tumorigenesis pathways, proteins associated with oncogenes or tumor-suppressor gene products, proteins involved in the cell cycle, such as cyclins or cyclin-dependent kinases, proteins involved in cell division or in cell growth or in cell functions where dysregulation could contribute to tumorigenesis. However, at this stage of our understanding of mechanisms leading to tumorigenesis, the notion of what constitutes a TAA does not exclude other cellular proteins whose functions do not appear to be involved in tumorigenesis. In the final analysis, an anti-TAA autoantibody can be useful as a diagnostic or early-detection biomarker only after it has undergone extensive trials with many cancer sera and with many control noncancer sera.
Changes & de novo appearance of autoantibodies during transition from chronic liver disease to HCC
A feature of HCC is that antecedent liver cirrhosis and chronic hepatitis are common precursor conditions and, during transition to malignancy, some patients develop autoantibodies that were not present during the preceding chronic liver-disease phase [10-13]. A hypothesis that has been proposed is that these antibody responses may be stimulated by cellular proteins that are involved in carcinogenesis. The de novo appearance of autoantibodies with cancer was best observed with serial samples of HCC sera going back many years before liver cancer was detected [10,11]. One of our previous studies showed three patients with cirrhosis and one with chronic viral hepatitis where serial serum samples collected over 5–10 years were available for immunological studies [11]. In immunofluorescence histochemistry using HEp-2 tissue culture cells as substrate, one patient was positive for antinuclear antibodies (ANAs) at 1/40 titer, whereas three other patients were ANA negative. In these patients, ANA rose in titer or converted from negative to positive before, or at the same time, HCC was detected (chronic liver disease patients were followed in the clinic every 6 months in a standard surveillance program). In one of the four patients (patient ‘OM’ with cirrhosis), abnormal increase of AFP could be detected but not in the others, showing that the autoimmune responses and increase in AFP were independent of each other. In general, it appeared that the antigenic stimulus provoking autoantibody response could be detected 1 year before cancer detection, suggesting that autoantibodies could be early detection biomarkers.
A study to identify the cellular antigens driving the autoantibody response in patient ‘NK’ is shown in Figure 1 [10]. The sera collected at time points marked with short vertical bars were used in western blotting against extracts of HEp-2 cell nuclei. Note that in lanes 1 and 2, prior to cancer, antibodies to nuclear antigens of approximately 97 kDa were already present and persisted when the patient converted to malignancy, as shown in lanes 3 and 4. Importantly, novel antibodies were detected during cancer conversion. Not only was there increased intensity of nuclear staining but also the appearance of nucleolar staining. These patterns of changes in autoantibody profiles, increases in titer, new antibodies or both, were observed in all the four patients. Also of great interest was that there were several different autoantibodies induced with malignant transformation in all four patients. This characteristic of multiple autoantibody responses in cancer is reflected in the frequent finding of multiple autoantibodies when cancer sera are screened with panels or arrays of TAAs (see later).
Figure 1. Serial study of ANA during a 4-year period in patient ‘NK’.
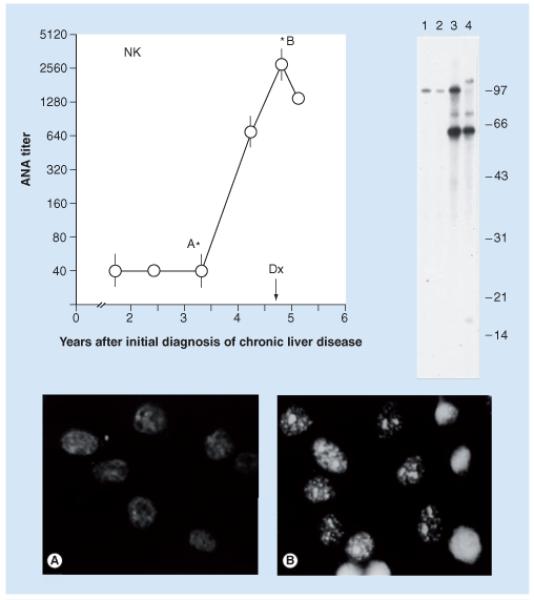
Western blotting was performed on sera at time points indicated by short vertical bars. Immunofluorescence studies were performed at points indicated with asterisks. The arrow indicates the point in time when the diagnosis of hepatocellular carcinoma was made. The new appearance of antibodies detected by immunofluorescence was probably associated with new reactive bands in western blots in lanes 3 and 4.
ANA: Antinuclear antibodies.
Isolation & identification of a TAA (HCC1.4/CAPER-α)
Serum from patient NK (Figure 1) at the time cancer was diagnosed was used to immunoscreen a HepG2 (a hepatoma cell line) λ-zap cDNA expression library in attempt to clone genes encoding any of the protein antigens. Six cDNA clones were isolated, which were all different-length isolates of a full-length sequence, known as HCC1.4, encoding a protein of 530 amino acids and migrating at 64 kDa in SDS-PAGE. The nucleotides and protein sequences of HCC1.4 were not found at that time in GenBank and EMBL and SwissProt databanks and, therefore, was a novel gene with unknown function. Analysis of the 64-kDa protein revealed several interesting polypeptide motifs. HCC1.4 contained a RS (arginine–serine) motif in the NH-terminus and three RNA-binding domains, RNP-CS (ribonucleoprotein consensus sequence, which has paired RNP1 and RNP2 regions) in the rest of the molecule. The structural peptide motifs of HCC1.4 were most similar to U2AF65, a smaller protein of 475 amino acids whose function was already characterized and shown to be an alternative splicing factor involved in pre-mRNA processing. Other proteins with somewhat similar domain structure, such as SC35 and SF2/ASF and SRp55, were also splicing factors involved in mRNA processing. HCC1.4 also co-localized with SC35 in nuclear speckles, where pre-mRNA processing takes place, giving further evidence of its possible function. However, at this time, there was no indication as to how it might be involved in tumorigenesis [12].
Several years later, an investigator in Seoul, South Korea, who was studying transcriptional co-activation asked for a HCC1.4 plasmid. He had screened a yeast two-hybrid mouse liver cDNA library using a transcriptional co-activator (ASC-2) as bait and isolated cDNA encoding HCC1.4 [15]. He showed that HCC 1.4 was a co-activator of activating protein-1 and estrogen receptors and proposed naming it CAPER. Approximately 3 years later, O’Malley and his colleagues at Baylor reported that CAPER/HCC1.4 was also an alternative splicing factor involved in the regulation of calcitonin and VEGF gene expression [16], confirming function that was expected of its peptide motifs. Of more interest and pertinent to tumorigenesis was a more recent report that CAPER/HCC1.4 has an important role in modulating the oncogenic activity of Rel/NF-κB [17]. Reticuloendotheliosis-like (Rel) oncoprotein transforms primary splenic lymphocytes and causes lymphoma in chickens and mice. Also using the yeast two-hybrid system, Gelinas and co-workers captured CAPER/HCC1.4 with Rel-transactivating domain as ‘bait’. Their studies showed that a dominant-negative mutant of CAPER/HCC1.4 enhanced vRel-mediated transformation. Thus, 15 years after HCC1.4 had been isolated and identified as a candidate TAA, its role in tumorigenesis has been reported.
IGF-2 mRNA binding proteins-1, -2 & -3: a family of TAAs
In our laboratory, analysis of sera from Chinese HCC patients identified a 62-kDa autoantigen, p62, which contained two types of RNA binding motifs, the RNP-consensus motif (described previously) in the N-terminal region and four repeats of heterogeneous nuclear ribonucleoprotein K (hnRNP-K) homology motif in the C-terminal two-thirds of the protein [18]. While this article was in press, Nielsen et al. reported on a family of three proteins that bind to the 5′-untranslated region of leader 3 IGF-2 mRNA [19]. These IGF-2 mRNA binding proteins (IMPs), known as IMP-1, -2 and -3, all contained the same RNP consensus sequence and KH motifs. The p62 we isolated as a TAA is a smaller, alternatively spliced form of IMP-2. An interesting feature about this family of proteins is that IMP-3 was found to be overexpressed in pancreatic and other types of cancer in a large-scale screen for genes that were differentially expressed in cancer specimens [20]. In addition, IMP-1 was reported to be overexpressed in breast cancer [21]. Although the latter two studies did not look for autoantibodies to IMP-1 or IMP-3, we have found that autoantibodies to all three members of the IMP family can be detected in a variety of cancers [22, 23] and that they bind to different determinants on these proteins [23] in spite of their high degree of similarity and identity in protein and nucleotide sequence. The credibility of the IMP proteins as TAAs can be assigned to their binding partner, IGF-2 mRNA. The protein product, IGF-2 is a fetal growth factor that is structurally and functionally related to insulin and IGF-1 [24]. Increased levels of IGF-2 in humans are associated with cancer, disproportionate overgrowth of the fetus and malformations [25]. IGF-2 transgenic mice have increased frequency of diverse malignancies [26] and, in SV40 oncogene-induced tumorigenesis, IGF-2 serves as an accessory factor or second signal in transformation [27].
We have looked for the occurrence of autoantibodies to IMP-2 (p62) and IMP-3 (koc) in a total of 777 cancer patients [23]. Within this group, there were ten different types of solid tumors, and sera were tested in ELISA using full-length recombinant proteins as antigens. The frequency of autoantibodies to p62 or Koc fell within a narrow range that did not exceed an upper range of approximately 18%. In several subsequent studies with other TAAs, this upper limit of frequency was up to approximately 25%. The reason why the frequency rate of autoantibodies was in this limited range is not currently understood. Another observation was that autoimmune response to p62 and Koc sometimes were present simultaneously in the same serum but, in others, they were independent of each other. In every type of tumor (except for ovarian cancer) the likelihood of detecting anti-TAA antibody was higher with two TAAs in the detection system. This study gave rise to the notion that an array or panel of multiple TAAs would offer increased sensitivity if anti-TAAs were to be used as cancer detection biomarkers. In this study, sera from patients with prototypic systemic autoimmune disorders (lupus, rheumatoid arthritis and scleroderma) had the lower frequency of anti-TAAs than those seen in normal adults.
Cloning of TAA p90/CIP2A
During studies that identified p62/IMP-2, it was noticed that an antigen of approximately 90 kDa was detected with similar frequency (Figure 2). Sera with anti-p90 also localized to the cytoplasm, and were detected by immuno staining in fetal mouse liver but not in adult liver. Full-length cDNA encoding p90 was successfully isolated from a T24 expression library and comprised a sequence coding for a protein of 905 amino acids with predicted molecular mass of 102 kDa [28]. There were no distinct polypeptide motifs, and search of GenBank databases at that time (2002) did not generate any significant alignment to established gene products. Limited analysis showed that anti-p90 was present in 21 out of 160 samples (13.1%) in HCC, three out of 91 (3.3%) in gastric cancer and one out of 20 (5.0%) in esophageal cancer. Gastric cancer tissue confirmed cytoplasmic expression of p90.
Figure 2. Reactivity of three hepatocellular carcinoma sera in western blotting against whole cell extracts from MOLT-4, T24, and HepG2 cell lines.
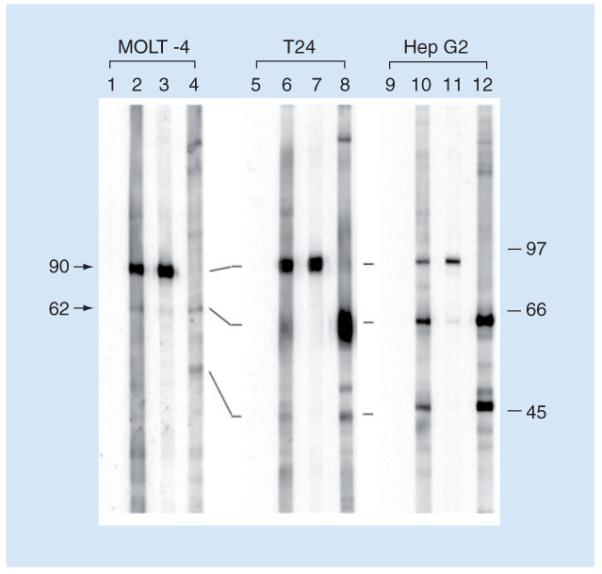
Lanes 1, 5 and 9 were normal human sera. Serum YZ (lanes 2, 6 and 10) showed strong reactivity with a 90-kDa protein in MOLT-4 cells and reacted weakly with a 62-kDa protein in MOLT-4 cells and T24 cell extracts. A strong reaction with the 62-kDa protein was detected with HepG2 cell extracts, together with a strong reaction with a 50-kD protein. Sera YL (lanes 3, 7 and 11) and CH (lanes 4, 8 and 12) demonstrate other types of reactions (see text). These representative data demonstrate that HCC sera are heterogeneous in their antibody repertoires and that different cell lines apparently have different expressions of 90-, 62- and 50-kDa proteins.
Reproduced from [18].
Protein phosphatase 2A (PP2A) is a widely conserved serine/threonine phosphatase and recent studies have identified target molecules for which dephosphorylation is critical for tumor-suppressor activity of this protein [29,30]. Dephosphorylation of oncogenic transcription factor c-myc is a critical pathway for PP2A tumor-suppressor activity. PP2A is a trimeric holoenzyme, with a scaffold subunit (A-subunit), a catalytic subunit (C-subunit) and several different regulatory B-subunits. The B-subunits determine the substrate specificity of the PP2A holoenzyme. The mechanism whereby PP2A exerts its tumor-suppressor function on c-myc is by dephosphorylation at serine 62 of myc. Juntilla et al. looked for endogenous cellular proteins, which could be co-purified with the A-subunit and found a protein, which turned out to be p90 [31]. They showed that p90 was an inhibitor of the tumor-suppressor function of PP2A and that this was related to p90 binding c-myc and inhibiting dephosphorylation of S62 by PP2A. This and other studies showed that p90 overexpression enhanced or induced transformation of several tumorigenesis models. They proposed naming p90, cancerous inhibitor of PP2A (CIP2A). They also found that p90/CIP2A was overexpressed in low-passaged cell lines from head and neck squamous cell carcinoma. Subsequent studies also demonstrated that CIP2A is associated with human breast cancer aggressivity [32], and CIP2A immunopositivity can be a predictor of survival for some subgroups of gastric cancer patients [33].
Related to the report by Janssens et al., who proposed in their review that PP2A should be an expected tumor suppressor [30], in 1994 and 1995 we published the finding of a cell cycle nuclear autoantigen expressed mainly in the S- and G2-phases of the cell cycle, which we named S- and G2-phase nuclear autoantigen (SG2NA) [34,35]. This cellular autoantigen was isolated with serum from a patient with bladder and lung cancer. Among other features, the cellular protein contained a WD40 motif, which is present in a large family of proteins with diverse functions. When we isolated the cDNA clone by immunoscreening, we only surmised that it could be a candidate TAA because it was isolated from the serum of a patient with cancer. Most recently, the possible function of the protein was further elucidated by observations that SG2NA was a B-subunit of PP2A [36,37]. The B-subunits are the regulatory components of the trimolecular holoenzyme PP2A and other B-subunits play important roles in the tumor-suppressor function of the holoenzyme.
Autoantibodies to TAAs as biomarkers in cancer immunodiagnosis
As described previously, many studies have demonstrated that cancer sera contain antibodies that react with a unique group of autologous cellular antigens (TAAs) [1,2]. The types of cellular proteins that induce these autoantibody responses are quite varied and include the tumor-suppressor p53 [38,39], oncogene products, such as HER-2/neu [40], proteins that protect mRNAs from degradation, such as p62 [18] and CRD-BP [21], onconeural antigens [41], differentiation antigens, such as tyrosinase, cancer/testis antigens [42], and antiapoptotic proteins, such as survivin [43] and LEDGF [44] and, undoubtedly, others to be identified. The different factors leading to the production of such autoantibodies are not completely understood. However, available data show that many of the target antigens are cellular proteins, such as p53, whose aberrant regulation or overexpression could lead to tumorigenesis [38,39]. In the case of the mRNA binding protein p62, a fetal protein absent in adult tissues, the presence of autoantibodies relates to abnormal expression of p62 in tumor cells [45]. The immune systems of certain cancer patients appear to sense these aberrant tumor-associated proteins as foreign antigens and have the capability to respond by producing autoantibodies [1,2,46]. Thus, cancer-associated autoantibodies might be regarded as reporters identifying aberrant de novo or dysregulated cellular mechanisms in tumorigenesis. In recent years, the potential utility of TAA–autoantibody systems as early cancer biomarker tools to monitor therapeutic outcomes or as indicators of disease prognosis has been pursued.
Interest in the use of anti-TAA antibodies as serological markers for cancer diagnosis derives from the recognition that these antibodies are generally absent, or present in low frequency in normal individuals and in noncancer conditions [1,2,47]. Their persistence and stability in the serum of cancer patients is an advantage over other potential markers, including the TAAs themselves, some of which are released by tumors but rapidly degrade or are cleared after circulating in the serum for a limited time [46]. Furthermore, the widespread availability of methods and reagents to detect serum autoantibodies facilitates their characterization in cancer patients and assay development. However, in contrast to autoimmune diseases, where the presence of an individual autoantibody may have diagnostic value, cancer-associated autoantibodies, when evaluated individually, have little diagnostic value, primarily because of their low frequency. We have observed that this drawback can be overcome by using miniarrays of carefully selected TAAs, and that different types of cancer may require different TAA arrays to achieve the sensitivity and specificity required to make immunodiagnosis a feasible adjunct to tumor diagnosis [48].
Miniarray of multiple TAAs in detection of HCC & other types of cancer
Following up on observations of one of our previous studies [23], we have evaluated several anti-TAA/TAA systems as biomarkers in several type of cancer, such as liver, lung, breast, prostate and ovarian cancer [22,48]. We did not limit our analysis only to TAAs identified in our laboratories, but also included TAAs identified and validated by other groups. Our studies demonstrated that detection of cancer anti-TAA antibodies can be enhanced by using a miniarray of multiple TAAs as target antigens [22,48]. For example, a miniarray that was used in a newly published study for detection of HCC [49], comprised full-length recombinant proteins expressed from cDNAs encoding oncoprotein c-myc [50], tumor-suppressor protein p53 [6], cancer-associated cell-cycle protein cyclin B1 [51,52], IGF-II mRNA binding proteins p62 [18,23], Koc [20,23] and IMP-1 [19], apoptosis-inhibiting protein survivin [43], a cyclin-dependent kinase p16 [53,54], Sui1 and RalA. Sui1 was also a novel gene associated with apoptosis, and was shown to be a tumor suppressor in hepatocarcinogenesis [55,56]. RalA was a Ras-related oncogene [57,58]. The antibody frequency to any of these individual TAAs was variable, ranging from 10 to 20% in HCC. However, with the successive addition of TAAs (in our case, to a total of ten antigens), there is a stepwise increase of positive antibody reactions up to 66.2% in HCC. The specificity for HCC compared with liver cirrhosis, chronic hepatitis and normal individuals, was 66.7, 80.0 and 87.8%, respectively. When anti-TAAs were added to abnormal serum AFP as combined diagnostic markers, the diagnostic sensitivity increased from 66.2 to 88.7%. This study shows that addition of cancer biomarkers, other than autoantibodies is clearly an approach that should be considered. Positive results were also confirmed by slot blot and western blot. A study from Wang et al. demonstrated that, with a phage-display library derived from prostate-cancer tissue, a phage protein microarray was developed to analyze autoantibodies in serum samples from patients with prostate cancer and controls [59]. Approaches from Wang et al., as well as our panel of TAAs, do not justify adoption in a cancer screening program, and could be improved by the inclusion of additional tumor antigens. Our data indicate that the combination of multiple TAAs might yield higher sensitivity for serological diagnosis of cancer and, in addition, that some of the TAAs may turn out to be more specific for certain types or stages of cancer. Furthermore, the sensitivity of 66.2% in HCC using this array might be expected to be further increased by addition of other TAAs discovered by ourselves and others in future investigations.
Expert commentary
Early detection is essential for the optimal management of cancer. Thus, extensive studies are being conducted to identify and validate new biomarkers that would add to current markers and increase the sensitivity and specificity of cancer detection. Given that the presence of serum anti-TAA antibodies might signal molecular events associated with tumorigenesis, we could use highly sensitive and specific TAA chips for screening populations at high risk of developing HCC and other solid tumors, which may lead to early preventive or therapeutic interventions aimed at suppressing or slowing the appearance of a tumor. The use of TAA arrays to profile the anti-TAA antibody response in a particular cancer patient could also provide key information on disease progression, which, combined with other available clinical information, could guide physicians and patients in making important decisions regarding individualized treatment options.
Five-year view
In the past few years, the potential utility of autoantibody to TAAs as cancer biomarker for early detection or as indicators of disease prognosis has been explored. We predict that, in the next 5 years, the research on the cancer TAA autoantibody biomarkers will become a challenging field. We anticipate that cancers of different types will have different profiles of anti-TAAs and once these are identified and validated, TAA array chips for immunodiagnosis of different cancers will be developed. The sensitivity and specificity of the system might, to a large extent, depend on the selection of TAAs to be included in one array versus another. With the data at hand, we know that many TAAs are not unique to any particular type of solid tumor and this should not be unexpected since tumorigenesis pathways are common to many tumors. Waiting for the generation of TAA arrays for immunodiagnosis of specific cancers, however, should not preclude taking advantage of the sensitivity of the anti-TAA/TAA systems in the initial screening process for early detection of cancer.
Key issues.
The high case–fatality rate in hepatocellular carcinoma (HCC) and other cancers can in part be attributed to lack of diagnostic methods that allow early detection. Although serum α-fetoprotein (AFP) is the most common marker available to detect HCC, the sensitivity and specificity are not optimal, especially in patients with small tumors or in well-to-moderately differentiated HCC. Therefore, there is a need for the development of more sensitive and specific methods that supplement AFP in the early detection of this cancer.
Antigenic changes in cancer cells can be recognized by the immune system of patients themselves and presented as immune responses to factors involved in malignant transformation. This is manifested in several ways, one of which is the appearance of circulating autoantibodies against tumor-associated antigens (TAAs). Therefore, serum anti-TAA antibodies can be used as serological cancer biomarkers for early detection.
A feature of HCC is that antecedent liver cirrhosis and chronic hepatitis are common precursor conditions and, during transition to malignancy, some patients develop autoantibodies that were not present during the preceding chronic liver disease phase. A hypothesis is that these antibody responses may be stimulated by cellular proteins involved in carcinogenesis.
‘Customized’ TAA arrays constitute promising and powerful tools for enhancing the serological anti-TAA antibody detection in HCC and other cancers.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH grants 2S06GM008012, 5G12RR08124 and CA56956. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Jian-Ying Zhang, Department of Biological Sciences, The University of Texas at El Paso, 500 West University Avenue, El Paso, TX 79968, USA, Tel.: +1 915 747 6995, Fax: +1 915 747 5808, jzhang@utep.edu.
Eng M Tan, Department of Molecular and Experimental Medicine, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Tel.: +1 858 784 8686, Fax: +1 858 784 2131, emtan@scripps.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J. Clin. Invest. 2001;108(10):1411–1415. doi: 10.1172/JCI14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol. Rev. 2008;222:328–340. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo F, Wada K, Nagato Y, et al. Biopsy diagnosis of well-differentiated hepatocellular carcinoma based on new morphologic criteria. Hepatology. 1989;9(5):751–755. doi: 10.1002/hep.1840090516. [DOI] [PubMed] [Google Scholar]
- 4.Gebo KA, Chander G, Jenckes MW, et al. Screening tests for hepatocellular carcinoma in patients with chronic hepatitis C: a systematic review. Hepatology. 2002;36(5 Suppl. 1):S84–S92. doi: 10.1053/jhep.2002.36817. [DOI] [PubMed] [Google Scholar]
- 5.Daniele B, Bencivenga A, Megna AS, Tinessa V. α-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl. 1):S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdar AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52(15):4168–4174. [PubMed] [Google Scholar]
- 7.Labrecque S, Naor N, Thomson D, Matlashewski G. Analysis of the anti-p53 antibody response in cancer patients. Cancer Res. 1993;53(15):3468–3471. [PubMed] [Google Scholar]
- 8.Lubin R, Schlichtholz B, Bengoufa D, et al. Analysis of p53 antibodies in patients with various cancers define B-cell epitopes of human p53: distribution on primary structure and exposure on protein surface. Cancer Res. 1993;53(24):5872–5876. [PubMed] [Google Scholar]
- 9.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv. Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 10.Imai H, Nakano Y, Kiyosawa K, Tan EM. Increasing titers and changing specificities of antinuclear antibodies in patients with chronic liver disease who develop hepatocellular carcinoma. Cancer. 1993;71(1):26–35. doi: 10.1002/1097-0142(19930101)71:1<26::aid-cncr2820710106>3.0.co;2-n. • First article from our group that described the development of antinuclear antibodies in patients with chronic liver disease who develop hepatocellular carcinoma (HCC).
- 11.Imai H, Kiyosawa K, Chan EKL, Tan EM. Autoantibodies in viral hepatitis related hepatocellular carcinoma. Intervirology. 1993;35(1–4):73–85. doi: 10.1159/000150297. [DOI] [PubMed] [Google Scholar]
- 12.Imai H, Chan EKL, Kiyosawa K, Fu X, Tan EM. Novel nuclear autoantigen with splicing factor motifs identified with antibody from hepatocellular carcinoma. J. Clin. Invest. 1993;92(5):2419–2426. doi: 10.1172/JCI116848. • First article from our group reported the isolation and identification of a novel tumor-associated antigens (TAA) (HCC1.4/CAPER-α) in HCC.
- 13.Zhang JY, Zhu W, Imai H, Kiyosawa K, Chan EKL, Tan EM. De-novo humoral immune responses to cancer-associated autoantigens during transition from chronic liver disease to hepatocellular carcinoma. Clin. Exp. Immunol. 2001;125(1):3–9. doi: 10.1046/j.1365-2249.2001.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jongeneel V. Towards a cancer immunome database. Cancer Immun. 2001;1:3. [PubMed] [Google Scholar]
- 15.Jung DJ, Na SY, Na DS, Lee JW. Molecular cloning and characterization of CAPER, a novel co-activator of activating protein-1 and estrogen receptors. J. Biol. Chem. 2002;277(2):1229–1234. doi: 10.1074/jbc.M110417200. [DOI] [PubMed] [Google Scholar]
- 16.Dowhan DH, Hong EP, Auboeuf D, et al. Steroid hormone receptor coactivation and alternative RNA splicing by U2AF65-related proteins CAPER α and CAPER β. Mol. Cell. 2005;17(3):429–439. doi: 10.1016/j.molcel.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Dutta J, Fan G, Gelinas C. CAPER α is a novel Rel-TAD interacting factor that inhibits lymphocyte transformation by the potent Rel/NF-κB oncoprotein v-Rel. J. Virol. 2008;82(21):10792–10802. doi: 10.1128/JVI.00903-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JY, Chan EK, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J. Exp. Med. 1999;189(7):1101–1110. doi: 10.1084/jem.189.7.1101. 4• First article published from our group that described the identification and characterization of a novel TAA p62, also known as IGF-2 mRNA binding protein 2 (IMP2) in HCC.
- 19.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol. Cell Biol. 1999;19(2):1262–1270. doi: 10.1128/mcb.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller-Pillasch F, Lacher U, Wallrap C, et al. Cloning of a gene highly expressed in cancer coding for a novel KH-domain containing protein. Oncogene. 1997;14(22):2729–2733. doi: 10.1038/sj.onc.1201110. [DOI] [PubMed] [Google Scholar]
- 21.Doyle GA, Bourdeau-Heller JM, Coulthard S, Meisner F, Ross J. Amplification in human breast cancer of a gene encoding a c-myc mRNA binding protein. Cancer Res. 2000;60(11):2756–2759. [PubMed] [Google Scholar]
- 22.Zhang JY, Casiano C, Peng XX, Koziol J, Chan EKL, Tan EM. Enhancement of antibody detection in cancer using a panel of recombinant tumor-associated antigens. Cancer Epidemiol. Biomarkers Prev. 2003;12(2):136–143. • One of the pioneering works in using miniarray of multiple TAAs in detection of HCC and other types of cancer.
- 23.Zhang JY, Chan EK, Peng XX, et al. Autoimmune responses to mRNA binding proteins p62 and Koc in diverse malignancies. Clin. Immunol. 2001;100(2):149–156. doi: 10.1006/clim.2001.5048. [DOI] [PubMed] [Google Scholar]
- 24.Westley BR, May FE. Insulin-like growth factors: the unrecognized oncogenes. Br. J. Cancer. 1995;72(5):1065–1066. doi: 10.1038/bjc.1995.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cariani E, Lasserre C, Seurin D, et al. Differential expression of insulin-like growth factor II mRNA in human primary liver cancers, benign liver tumors and liver cirrhosis. Cancer Res. 1988;48(23):6844–6849. [PubMed] [Google Scholar]
- 26.Rogler CE, Yang D, Rosetti L, et al. Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor II transgenic mice. J. Biol. Chem. 1994;269(19):13779–13784. [PubMed] [Google Scholar]
- 27.Christofori G, Naik P, Hanahan D. A second signal supplied by insulin-like growth factor II in oncogenes-induced tumorigenesis. Nature. 1994;369(6479):414–417. doi: 10.1038/369414a0. [DOI] [PubMed] [Google Scholar]
- 28.Soo Hoo L, Zhang JY, Chan EKL. Characterization of a novel 90 kDa ‘companion’ auto-antigen of p62 overexpressed in cancer. Oncogene. 2002;21(32):5006–5015. doi: 10.1038/sj.onc.1205625. • First article from our group that reported the identification and characterization of a novel TAA p90, also known as cancerous inhibitor of PP2A (CIP2A) in cancer.
- 29.Arroyo JD, Hahn H. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24(52):7746–7755. doi: 10.1038/sj.onc.1209038. [DOI] [PubMed] [Google Scholar]
- 30.Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Curr. Opin Genet. Dev. 2005;15(1):34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Juntilla MR, Puustinen P, Niemela M, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130(1):51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 32.Côme C, Laine A, Chanrion M, et al. CIP2A is associated with human breast cancer aggressivity. Clin. Cancer Res. 2009;15(16):5092–5100. doi: 10.1158/1078-0432.CCR-08-3283. [DOI] [PubMed] [Google Scholar]
- 33.Khanna A, Böckelman C, Hemmes A, et al. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J. Natl Cancer Inst. 2009;101(11):793–805. doi: 10.1093/jnci/djp103. [DOI] [PubMed] [Google Scholar]
- 34.Landberg G, Tan EM. Characterization of a DNA-binding nuclear autoantigen mainly associated with S phase and G2 cells. Exp. Cell Res. 1994;212(2):255–261. doi: 10.1006/excr.1994.1141. [DOI] [PubMed] [Google Scholar]
- 35.Muro Y, Chan EK, Landberg G, Tan EM. A cell-cycle nuclear autoantigen containing WD40 motifs expressed mainly in S and G2 phase cells. Biochem. Biophys. Res. Comm. 1995;207(3):1029–1037. doi: 10.1006/bbrc.1995.1288. [DOI] [PubMed] [Google Scholar]
- 36.Moreno CS, Park S, Nelson K, et al. WD40 repeat proteins striatin and S/G2 nuclear autoantigen are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A. J. Biol. Chem. 2000;275(8):5257–5263. doi: 10.1074/jbc.275.8.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta. 2009;1795(1):1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Soussi T. p53 antibodies in the sera of patients with various types of cancer. A review. Cancer Res. 2000;60(7):1777–1788. [PubMed] [Google Scholar]
- 39.Crawford LV, Pim DC, Bulbrook RD. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int. J. Cancer. 1982;30(4):403–408. doi: 10.1002/ijc.2910300404. [DOI] [PubMed] [Google Scholar]
- 40.Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J. Clin. Oncol. 1997;15(11):3363–3367. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 41.Keene JD. Why is Hu where? Shuttling of early response gene messenger RNA subsets. Proc. Natl Acad. Sci. USA. 1999;96(1):5–7. doi: 10.1073/pnas.96.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockert E, Jager E, Chen YT, et al. A survey of humoral immune response of cancer patients to a panel of human tumor antigens. J. Exp. Med. 1998;187(8):1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997;3(8):917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 44.Daniels T, Zhang J, Gutierrez I, et al. Antinuclear autoantibodies in PCa: Immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate. 2005;62(1):14–26. doi: 10.1002/pros.20112. [DOI] [PubMed] [Google Scholar]
- 45.Lu M, Nakamura RM, Dent ED, et al. Aberrant expression of fetal RNA-binding protein p62 in liver cancer and liver cirrhosis. Am. J. Pathol. 2001;159(3):945–953. doi: 10.1016/S0002-9440(10)61770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J. Proteome Res. 2005;4(4):1123–1133. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Old LJ, Chen YT. New paths in human cancer serology. J. Exp. Med. 1998;187(8):1163–1167. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang JY, Megliorino R, Peng XX, Tan EM, Chen Y, Chan EKL. Antibody detection using tumor-associated antigen mini-array in immunodiagnosing human hepatocellular carcinoma. J. Hepatol. 2007;46(1):107–114. doi: 10.1016/j.jhep.2006.08.010. •• Highlights new avenues for HCC biomarker identification.
- 49.Chen Y, Zhou Y, Qiu S, et al. Autoantibodies to tumor-associated antigens combined with abnormal α-fetoprotein enhance immunodiagnosis of hepatocellular carcinoma. Cancer Lett. 2010;289(1):32–39. doi: 10.1016/j.canlet.2009.07.016. •• Demonstrated that autoantibodies to multiple TAAs combined with abnormal α-fetoprotein can enhance immunodiagnosis of HCC.
- 50.Yamamoto A, Shimizu E, Takeuchi E, et al. Infrequent presence of anti-c-Myc antibodies and absence of c-Myc oncoprotein in sera from lung cancer patients. Oncology. 1999;56(2):129–133. doi: 10.1159/000011953. [DOI] [PubMed] [Google Scholar]
- 51.Covini G, Chan EK, Nishioka M, Morshed SA, Reed SI, Tan EM. Immune response to cyclin B1 in hepatocellular carcinoma. Hepatology. 1997;25(1):75–80. doi: 10.1002/hep.510250114. [DOI] [PubMed] [Google Scholar]
- 52.Kao H, Marto JA, Hoffmann TK, et al. Identification of cyclin B1 as a shared human epithelial tumor-associated antigen recognized by T cells. J. Exp. Med. 2001;194(9):1313–1323. doi: 10.1084/jem.194.9.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl Acad. Sci. USA. 1996;93(24):13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Looi K, Megliorino R, Shi FD, Peng VV, Chen Y, Zhang JY. Humoral immune response to p16, a cyclin-dependent kinase inhibitor in human malignancies. Oncology Rep. 2006;16(5):1105–1110. [PubMed] [Google Scholar]
- 55.Yoon HJ, Donahue TF. The sui1 suppressor locus in Saccharomyces cerevisiae encodes a translation factor that functions during tRNAiMet recognition of the start codon. Mol. Cell. Biol. 1992;12(1):248–260. doi: 10.1128/mcb.12.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lian Z, Pan J, Liu J, et al. The translation initiation factor, hu-Sui1 may be a target of hepatitis B X antigen in hepatocarcinogenesis. Oncogene. 1999;18(9):1677–1687. doi: 10.1038/sj.onc.1202470. [DOI] [PubMed] [Google Scholar]
- 57.Gildea JJ, Harding MA, Seraj MJ, Gulding KM, Theodorescu D. The role of Ral A in epidermal growth factor receptor-regulated cell motility. Cancer Res. 2002;62(4):982–985. [PubMed] [Google Scholar]
- 58.Lim KH, Baines AT, Fiordalisi JJ, et al. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell. 2005;7(6):533–545. doi: 10.1016/j.ccr.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Yu J, Sreekumar A, et al. Autoantibody signatures in prostate cancer. N. Engl. J. Med. 2005;353(12):1224–1235. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]


