Abstract
Owing to its resemblance to the major inorganic constituent of bone and tooth, hydroxyapatite is recognized as one of the most biocompatible materials and is widely used in systems for bone replacement and regeneration. In this study the pulsed laser deposition technique was chosen to produce hydroxyapatite with different crystallographic orientations in order to investigate some of the material properties, including its in vitro dissolution behavior, as well as mechanical properties. The crystallographic orientations of hydroxyapatite coatings can be carefully controlled, mainly by varying the energy density of the KrF excimer laser (248 nm) used for deposition. Nanoindentation results showed that highly c-axis oriented hydroxyapatite coatings have higher hardness and Young's modulus values compared with the values of randomly oriented coatings. After 24 h immersion in simulated physiological solution the overall surface morphology of the highly oriented coatings was dramatically altered. The porosity was drastically increased and sub-micron pores were formed throughout the coatings, whereas the average size of the grains in the coatings was not significantly changed. The composition of the textured hydroxyapatite coatings remained essentially unchanged. Their c-axis texture, on the other hand, was rather enhanced with an increase in immersion time. The c-axis oriented hydroxyapatite surfaces are likely to promote preferentially oriented growth through a cyclic process of dissolution and reprecipitation, followed by homoepitaxial growth. The remarkable morphological and microstructural changes after dissolution suggest a capability of highly textured hydroxyapatite as a tissue engineering scaffold with an interconnecting porous network that may be beneficial for cellular activity.
Keywords: Hydroxyapatite, Preferred orientation, Pulsed laser deposition, Mechanical properties, Dissolution
1. Introduction
Calcium phosphates are highly biocompatible materials that have been extensively used in dentistry and orthopedics over the past decades due to their close chemical similarity to the mineral of natural teeth and bones [1–5]. Despite the outstanding biocompatibility of calcium phosphate bioceramics, their brittleness and low strength have commonly been cited as drawbacks that limit their usefulness in clinical applications requiring strong mechanical integrity and long-term durability [5–10]. Numerous efforts have been made to overcome their mechanical weakness via porosity/density control [11–14], ion beam implantation [15], and incorporation of a secondary phase, such as a metal [16,17], a polymer [18–24] or oxides [25–32]. Nevertheless, it is still challenging to achieve the desired mechanical properties. In addition, it is well known that calcium phosphate compounds with different Ca/P molar ratios exhibit different dissolution rates and hydroxyapatite (HA) [Ca10(PO4)6(OH)2, with Ca/P = 1.67] is the most stable phase in physiological solution [3,33–35]. Bioresorption of solidstate calcium phosphates has a substantial effect on bone remodeling and regeneration in the body [34,36]. However, synthetic calcium phosphate ceramics are often subject to inconsistent and unpredictable dissolution in physiological environments, which eventually impairs their reliability [37]. Their inconsistent and unpredictable dissolution behavior results from the inhomogeneity in composition, crystallinity and microstructure during materials processing [34]. Therefore, a significant need remains for an advanced level of control that enables the production of calcium phosphates with improved mechanical properties and controlled dissolution behavior.
Common factors influencing the dissolution behavior and mechanical properties of materials include their chemical composition, crystallinity, porosity, density and grain size. Another essential microstructural feature responsible for the properties of polycrystalline materials is crystallographic texture [38]. Naturally occurring apatite crystals frequently exhibit preferred orientations resulting from highly specific biological processes and these preferred orientations are believed to affect the biological and biomechanical performances of hard tissue [39,40]. Moreover, recent studies on the anisotropic behavior of protein adsorption on the different faces of hexagonal HA crystals suggest that calcium phosphates with surfaces exhibiting a tailored crystallographic texture may enable a new level of control of cellular behavior [41–45]. Despite these findings, the effect of crystallographic texture on calcium phosphate properties that are relevant for biomedical applications has not yet been systematically studied. Most references to the preferential orientation of calcium phosphate coatings in the literature are of serendipitous occurrences of texturing in coatings produced by various deposition techniques [46–49]. This is due in part to the experimental difficulty of controlling texture through conventional calcium phosphate fabrication methods. Previously we demonstrated a tailored approach to fabricate calcium phosphate systems with deliberate control of crystallographic texture using pulsed laser deposition (PLD) [50]. Such microstructural control targeted at preferentially oriented calcium phosphates may lead to better control over the dissolution and mechanical behavior of these micro-engineered calcium phosphates. In this paper we discuss the in vitro dissolution behavior and mechanical properties of c-axis preferentially oriented HA coatings produced by PLD in relation to those of randomly oriented ones, and predict the relevance of c-axis preferentially oriented HA in biomedical applications.
2. Materials and methods
2.1. Synthesis
We have obtained HA coatings of thickness 2–3 μm in an Ar/H2O atmosphere by PLD using a KrF excimer laser (248 nm) (Lambda Physik, USA) with an energy density of 5–8 J cm−2 and a pulse repetition rate of 30 Hz. The detailed experimental set-up has been described in previous reports [46,50]. For all depositions the base pressure in the deposition chamber was typically below 0.1 mPa and the chamber pressure was adjusted to 80 Pa by introducing an Ar/H2O gas mixture prior to deposition. A constant substrate temperature of 650 °C was used for deposition. The ablation targets, consisting of pure crystalline HA, were prepared by compressing a commercial HA powder (97.5% purity) (Plasma Biotal, UK) at a pressure of 17 MPa at room temperature and subsequent sintering at 1200 °C in an Ar/H2O atmosphere for 1.5 h. A titanium alloy (Ti–6Al–4V, Robin Materials, USA) was used as the substrate in the form of 1 cm diameter disks with a surface roughness of less than 250 nm achieved by fine polishing with SiC and diamond abrasives and successive cleaning with acetone, methanol and deionized water in an ultrasonic bath. The deposition rate of HA films in this protocol was typically in the range 1.11–1.67 nm s−1.
2.2. X-ray diffraction
The crystalline structure and the phase composition of the coatings were determined by thin film X-ray diffraction (XRD). XRD was carried out using an X-ray diffractor (X'Pert PW3050-MPD, Philips, The Netherlands) with Cukα radiation of 1.5418 Å wavelength at an incident angle of 3° and a scan speed of 0.008° s−1. To identify the HA phase in the coatings all the diffraction peaks obtained were compared with the data for HA from the Joint Committee on Powder Diffraction Standards (JCPDS No. 9-432). The Ti–6Al–4V substrate was polycrystalline with a random orientation, as indicated by the relative peak intensities of their 2θ XRD scans.
2.3. Scanning electron microscopy
The surface morphology and microstructure of the HA coatings were observed mainly by secondary electron imaging using a scanning electron microscope (SEM 515, Philips, The Netherlands) with an electron accelerating voltage of 15–20 keV. As preparation for scanning electron microscopy (SEM) the samples were sputtercoated with a very thin, electrically grounded layer of Au–Pd metallic alloy to minimize negative charge accumulation from the electron beam during scanning. A working distance ranging from 19 to 21 mm was used for the scans. The spot size of the focused electron beam used varied in the range 10–100 nm, depending on magnification. The thickness of the coatings was also estimated by cross-sectional scanning using SEM.
2.4. Atomic force microscopy
Atomic force microscopy (AFM) was used to examine the surface topography and measure the surface roughness and surface area of the HA coatings. The AFM investigation was conducted under ambient conditions with a commercial instrument (Nanoscope Dimension 5000, Digital Instruments, USA). The AFM probe was made out of a Si single crystal and the tip had a pyramidal geometry with an end radius of <10 nm. The cantilever had a spring constant of 25 N m−1 and a resonant frequency of 326 kHz. The topographic measurements were done in tapping mode at six different locations for each sample.
2.5. In vitro dissolution
In vitro dissolution studies were performed on our HA coatings under tissue culture conditions in a commercial simulated physiological solution. In order to observe their in vitro solubility the coatings were immersed in 1 ml of calcium- and magnesium-free Earle's balanced salt solution (EBSS) (Sigma, USA), composed of various inorganic salts (g1−1): 0.4 KCl, 2.2 NaHCO3, 6.8 NaCl and 0.122 NaH2PO4. The coatings placed in EBSS (pH 7.2–7.4) were kept in an incubator at 37 °C at 5% CO2 for 24 h. After a 24 h period the coatings were removed from the solution and their phase composition and crystal structure were identified using XRD. In addition, their surface morphology and microstructure were examined by SEM.
2.6. Nanoindentation
The mechanical properties of the HA coatings, such as hardness and Young's modulus, were evaluated by the nanoindentation technique with a Berkovich indenter of 50 nm radius (MTS Systems, Oak Ridge, TN). The diamond indenter tip was calibrated with a fused silica standard. Fifteen indents were made at 35 μm intervals for each sample. The maximum indentation depth was set to 350 nm, which is approximately equal to or slightly greater than 1/10 of the total coating thickness. The load–displacement data was continuously recorded during one complete cycle of loading and unloading. The Poisson's ratio of 0.30 reported for HA [51] was used to calculate the Young's modulus of the coatings.
3. Results and discussion
3.1. In vitro dissolution behavior
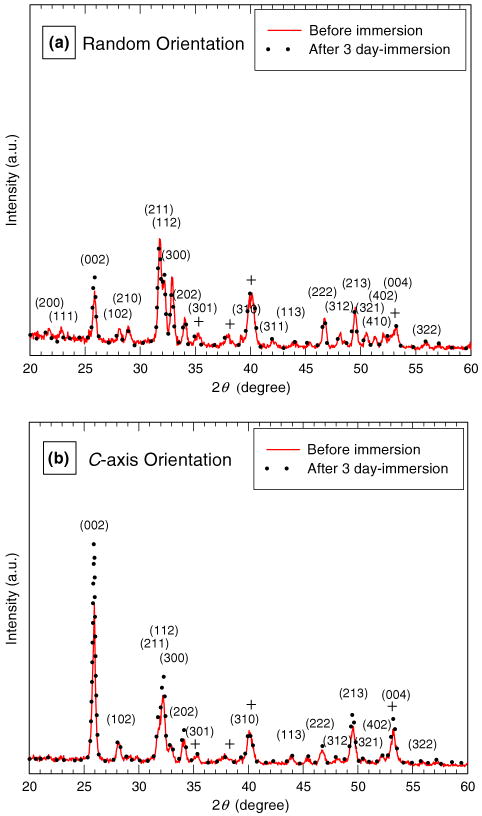
We have studied the dissolution behavior of HA coatings with a preferential orientation in comparison with that of HA coatings with a random orientation using XRD and SEM analysis. The HA coatings with random orientation and c-axis orientation were prepared using different laser fluences of 5 and 8 J cm−2 [50]. Dissolution studies were carried out on these coatings for various periods of immersion time in simulated body fluid (SBF) for 0, 24 and 72 h. The XRD scans shown in Fig. 1 revealed that the phase composition of both the randomly oriented and the c-axis oriented HA coatings was essentially unaltered after 72 h of immersion. All reflections are well indexed in terms of an HA hexagonal lattice with a = 9.424 Ǻ and c = 6.879 Ǻ (P63/m space group). No intermediate compounds, such as dicalcium phosphate dihydrate (DCPD) (CaH-PO4·2H2O) or octacalcium phosphate (OCP) (Ca8(HPO4)2(PO4)4·5H2O), were detected in those coatings with different orientations after immersion in SBF. No considerable change in crystal orientation was observed for the randomly oriented HA coatings before and after 24 h immersion. After 72 h immersion moderate c-axis texturing was revealed in the coatings. It could be expected that the steady-state dissolution behavior of the HA phase renders the coating stable without discernible thickness change [3,33–35]. The degree of c-axis texture in the c-axis oriented HA coatings, however, was significantly enhanced after immersion for 24–72 h, indicated by an increase in the relative peak intensity ratio of (002)/(211) and the high-k peak intensity. This enhanced c-axis texture in the c-axis oriented HA with an increase in the immersion time indicates that the textured HA surface promoted further preferentially oriented growth, compared with the randomly oriented HA.
Fig. 1.
X-ray diffraction scans for hydroxyapatite coatings deposited at 80 Pa of Ar/H2O and 650 °C with a laser energy density of (a) 5 J cm−2 and (b) 8 J cm−2 before and after immersion in simulated physiological solution for 72 h. +, Ti6Al4V.
Figs. 2 and 3 show SEM micrographs of the randomly oriented HA and the c-axis textured HA coatings at different magnifications as a function of time of immersion in SBF. As seen in Fig. 2a and b, the as deposited randomly oriented HA coatings contained irregular aggregates of grains ranging in size from a few hundred nanometers to sub-micron. Even the often present microscale grains were comprised of coalesced aggregates of nanoscale objects. Voids were also frequently observed in these coatings, even though the substrate temperature was high enough to activate the diffusion of atoms impinging on the substrate during the entire deposition process. The presence of these voids is likely a result of desorption of grains loosely bound to the film surface. The SEM images of the randomly oriented HA coatings in Fig. 2c and d reveal a minor change in morphology of the coatings during the 24 h dissolution experiment. After 24 h immersion in SBF the average size of the grains in the coatings was reduced and the grain edges became sharpened, but the overall topography was not greatly altered. In contrast, the overall coating surfaces after 72 h immersion became smoother and the grain edges became sharper, as revealed in Fig. 2e and f. These changes in individual grain morphologies are likely due to anisotropic dissolution and reprecipitation.
Fig. 2.
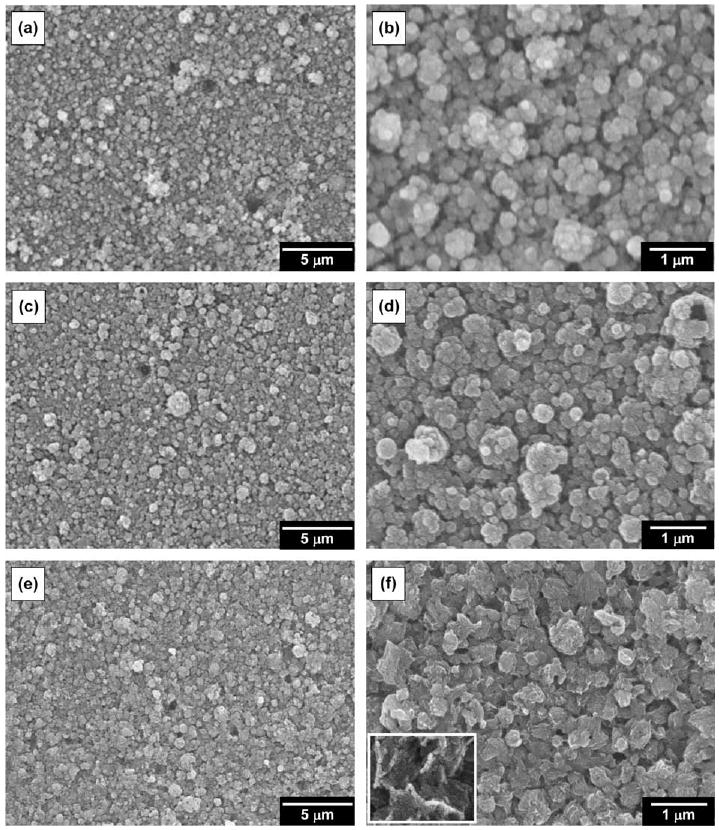
Typical scanning electron micrographs for randomly oriented hydroxyapatite coatings deposited at 80 Pa of Ar/H2O and 650 °C with a laser energy density of 5 J cm−2 (original magnification 5000× and 20,000×): as deposited (a and b) and after immersion in simulated physiological solution for 24 h (c and d) and 72 h (e and f). The inset represents a typical view of the surface after 72 h immersion at higher magnification (50,000×).
Fig. 3.

Typical scanning electron micrographs for c-axis oriented hydroxyapatite coatings deposited at 80 Pa of Ar/H2O and 650 °C with a laser energy density of 8 J cm−2 (original magnification: 5000× and 20,000×): as deposited (a and b) and after immersion in simulated physiological solution for 24 h (c and d) and 72 h (e and f). The inset represents a typical view of the surface after 72 h immersion at higher magnification (50,000×).
Compared with the images of the randomly oriented HA coatings shown in Fig. 2a–f the SEM images of the preferentially c-axis oriented HA coatings shown in Fig. 3a–f reveal a more significant change in the coating morphology before and after a 24 h dissolution experiment. In contrast to the randomly oriented coatings, the as deposited c-axis textured HA coatings had a highly heterogeneous structure containing aggregates of grains ranging in size from a few hundred nanometers to a few microns, as presented in Fig. 3a and b. When expanded at a higher magnification, as in Fig. 3b, the boundaries between grains revealed a tendency for hexagonal faceting caused by the c-axis texturing along the substrate normal. The preferentially oriented HA coatings did not contain the voids frequently observed in the randomly oriented HA coatings and exhibited a dense and compact appearance. Narrow crevices between tight grain aggregates were widespread in the preferentially oriented HA coatings. The pulsed laser with high energy employed to fabricate our textured coatings probably resulted in a reduction in porosity and densification of the films by the continual impact on the films of highly energetic species. The AFM results in Table 1 confirm that the c-axis oriented HA coatings showed an increase in average surface roughness due to the highly heterogeneous structure composed of a number of grain aggregates but a decrease in average surface area due to the dense and compact structure, compared with the randomly oriented HA. On the other hand, 24 h immersion of the c-axis oriented coatings in SBF dramatically altered the overall film morphology, as seen in Fig. 3c and d. The porosity increased dramatically with sub-micron pores distributed throughout the entire coatings, while the average size of the grains in the coatings appeared to be preserved. The grain aggregates consisted of a few tens of nanometers thick platelets arranged one above the other parallel to the substrate surface, indicating the in-plane alignment of the c-planes of HA crystallites. The HA hexagonal crystal facets became sharper and more clearly exposed in the textured HA coatings. Furthermore, after 72 h immersion significant morphological changes were observed. The porosity was greatly reduced and the neighboring grains were interconnected in the c-oriented HA coatings, compared with the coatings after 24 h immersion. The coating surfaces became smoother and more flattened during immersion for periods longer than 1 day, as revealed in Fig. 3e and f. Modest deformation of the edges of the thin platelets forming the flattened surfaces was often detected at higher magnification, probably resulting from surface tension at the solution–crystal interface or mechanical stress induced by a series of dissolution and reprecipitation.
Table 1.
Surface roughness and surface area data measured by atomic force microscopy over a fixed scan area of 20 × 20 μm for two different kinds of hydroxyapatite coatings.
| Surface roughness (nm) | Surface area (μm2) | ||
|---|---|---|---|
| Rq | Ra | ||
| Random oriented hydroxyapatite | 120 ±6 | 93 ±5 | 498 ±19 |
| c-axis oriented hydroxyapatite | 133 ±11 | 102 ±9 | 476 ±6 |
The measurements were performed on six different locations of each sample. Surface roughness was evaluated in terms of two different parameters, Rq, the root mean square roughness, and Ra, the arithmetic average roughness.
Combined with the XRD results, these morphological observations indicate that the c-axis textured surfaces undergo a surface restructuring without changing the composition and overall crystallographic orientation through a repeated cyclical process of dissolution and reprecipitation. They are more likely to promote c-axis preferentially oriented growth followed by homoepitaxial growth. Despite the dense and compact structure of the c-axis oriented HA and the slow dissolution rate of HA, this vigorous surface restructuring can be initiated by localized dissolution at energetically favorable sites inside or near the crevices in contact with the solution. In general, dissolution of crystal planes with higher surface energies will be more favorable to reduce the surface energy [42,45]. Rapid dissolution of the high surface energy planes will, therefore, precede dissolution of the c-planes because of the lowest surface energy of the c-plane, previously reported by several groups [42–45]. Subsequently, reprecipitation will take place preferentially in the c-planes aligned parallel to the substrate surface. Similar anisotropic dissolution and reprecipitation kinetics can be expected for randomly oriented HA surfaces, because they are also composed of HA crystalline phase. However, it is interesting that the random orientation of the grains with respect to the substrate surface changes the surface topography more slowly in comparison with the c-axis preferred orientation, although the relatively loose structure in the randomly oriented HA will allow easy penetration of the solution into the areas surrounding the grains, resulting in uniform dissolution and precipitation over the entire surface. Heterogeneous nucleation and recrystallization of HA during dissolution experiments is basically related to the existence of Ca2+ ions in the simulated physiological solution, which originally contained no Ca2+ ions, produced by initial dissolution at the HA surface. The increased pores present in the c-axis textured HA after immersion probably originated from extension of the crevices present in the as deposited coatings due to dissolution. Consequently, the surfaces with an oriented distribution of HA grains may promote better cell responses than surfaces with a random grain distribution, particularly during the initial stage, when inserted into the body. Indeed, our preliminary in vitro cell studies revealed that human mesenchymal stem cells attached in greater numbers to the c-axis textured HA coatings as compared with the randomly oriented HA coatings [52]. These findings suggest that highly textured HA coatings have the possibility of being used as tissue engineering scaffolds that require interconnecting porous networks aimed at promoting enhanced cellular activity, including cell migration, adhesion, differentiation and proliferation.
3.2. Mechanical properties
The mechanical properties of our HA coatings deposited at different laser energy densities were examined using nanoindentation testing with a Berkovich nanoindenter. The goal of the mechanical testing was to ensure that the coatings possessed sufficient mechanical integrity for load-bearing applications. As previously reported [50], laser energy density was employed as the main experimental parameter to control crystallographic texture in the HA coatings during deposition. Coatings deposited at a laser energy density of 5 J cm−2 exhibited a random orientation of the HA grains. As the laser energy density increased the c-axes of the HA grains in the coatings became more oriented in the direction perpendicular to the substrate. We investigated the effect of laser fluence (used to vary the crystallographic texture of the HA coatings) on the mechanical properties of the coatings. Fig. 4a and d shows the load–displacement curves generated during indentation on the HA coatings deposited at the two different laser energy densities of 5 and 7 J cm−2. Load–displacement curves allow the estimation of hardness and elastic modulus profiles from analysis of the unloading curves by the Oliver–Pharr method [53]. For the coatings deposited at higher energy densities the maximum load increased, indicating an increase in hardness. As seen in Fig. 4b and e, the hardness profiles as a function of indentation depth exhibited noticeably higher hardness values stabilized in the depth range 250–350 nm for the coatings deposited at higher laser energy densities. The slope of the initial portion of the unloading curve, i.e. initial unloading contact stiffness, in the load–displacement data also increased for the coatings deposited at higher energy densities. Since Young's modulus is deduced from this initial contact stiffness, it is anticipated that the Young's modulus of the coatings increased with laser energy density. Young's modulus profiles as a function of indentation depth are presented in Fig. 4c and f. Higher Young's modulus values stabilized in the depth range 250–350 nm were observed for the coatings deposited at higher laser energy densities.
Fig. 4.
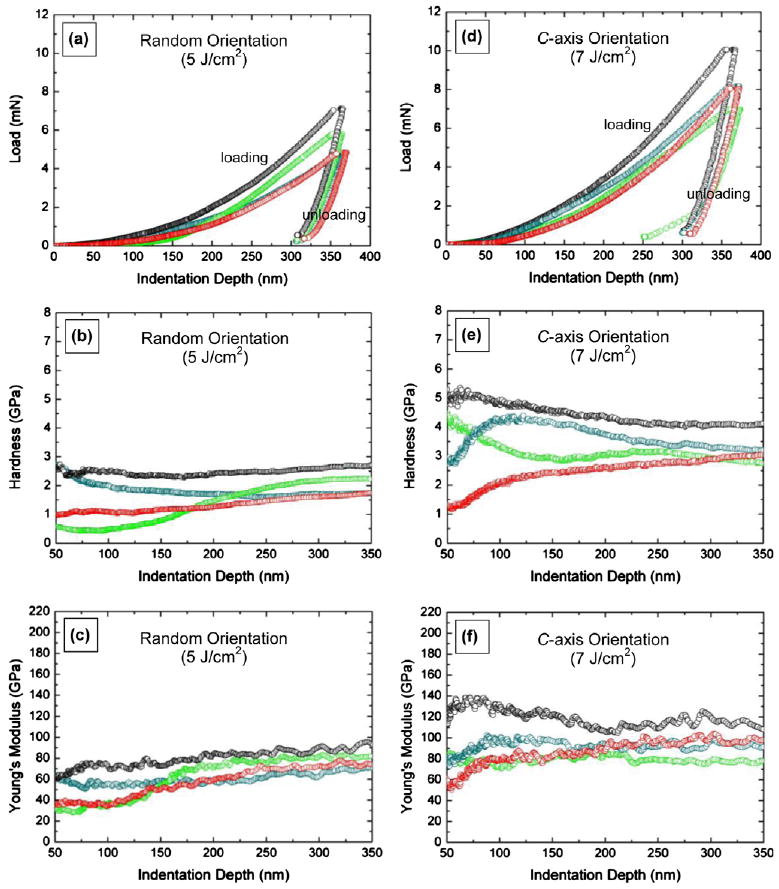
Representative load–displacement curves (a and d), hardness profiles (b and e) and Young's modulus profiles (c and f) for hydroxyapatite coatings deposited at 80 Pa of Ar/H2O and 650 °C with different laser energy densities: 5 and 7 J cm−2.
Fig. 5a and b represents the average hardness and Young's modulus of the HA coatings deposited at two different laser energy densities, respectively. The hardness was found to be 2.0 ± 0.4 GPa for coatings deposited at 5 J cm−2, and increased to 2.9 ±0.5 GPa for coatings deposited at 7 J cm−2. Young's modulus increased from 82 ± 11 GPa for samples prepared at 5 J cm−2 to 92 ± 11 GPa for coatings produced at 7 J cm−2. The hardness and the Young's modulus values are comparable with those reported in the literature (hardness 1.6–2.6 GPa, Young's modulus 90–120 GPa) [15,54,55]. Note that the Ti–6Al–4V substrate alone was reported to have a hardness of 4–5 GPa and a Young's modulus of 110–140 GPa [54,56] and bone was reported to have a hardness of 0.3–0.8 GPa and a Young's modulus of 14–27 GPa [57–59]. Compared with the increase in hardness ratio of 45%, Young's modulus increased by only 12% with increasing laser fluence from 5 to 7 J cm−2. A relatively small increase in Young's modulus probably indicates modest stiffening of the c-axis oriented HA, in spite of a remarkable increase in hardness. No significant stiffening of the c-axis oriented HA while hardening is potentially advantageous, because a reduction in modulus difference between the implant and the bone is desirable. A large modulus mismatch causes insufficient loading of bone adjacent to the implant and eventual failure of the implant [60]. Even though the standard deviations associated with the hardness and Young's modulus measurements were large, making it difficult to make a definite assessment, the trend of the data was reproducible. These large standard deviations may be due to the inherent inhomogeneity and rough surface morphology of the coatings. Consequently, it can be stated that the c-axis oriented coatings unambiguously exhibited higher hardness and Young's modulus values compared with the values of the randomly oriented coating. However, does the observed increase in hardness and Young's modulus result from the crystallographic texture of the coatings or from the densification of the coatings? Anisotropy in the mechanical properties of HA single crystals has recently been reported by several groups [61,62]. Nanoindentation studies on HA single crystals revealed a higher hardness and elastic modulus for the plane perpendicular to the c-axis than for the plane parallel to the c-axis. The mechanically stronger nature of the c-plane of HA is, therefore, likely to be responsible for the increased hardness and modulus of the HA coatings with c-planes aligned parallel to the substrate surface. The strengthening in the highly c-axis oriented HA coatings could also be a result of the reduced porosity of HA during deposition by means of bombardment of highly energetic plume species generated at high laser fluences. A similar finding that significant densification of pulsed laser deposited HA structures after implantation with Ar+ ions at high energy enhanced mechanical properties has been previously reported [15]. HA deposition at high laser fluences would influence adhesion strength at the coating–substrate interface. The adhesion strength of the randomly oriented HA coating deposited at a laser energy density of 5 J cm−2 has been previously reported [63], but comparison of its adhesion strength with that of the c-oriented coating deposited at a higher energy density has not been explored yet and might be an interesting subject for future research. It is expected that c-oriented coatings deposited at higher laser fluences would offer stronger bonding at the coating–substrate interface than randomly oriented coatings deposited at relatively lower laser fluences. Further studies are needed to elucidate the adhesion strength of HA coatings with different orientations. Control of the c-axis orientation in synthetic HA mimics bone-containing HA crystals with the c-axis aligned parallel to the long axis of bone imparting higher strength to the bone, and thus may be a promising strategy to improve the mechanical performance of HA in various load-bearing applications.
Fig. 5.
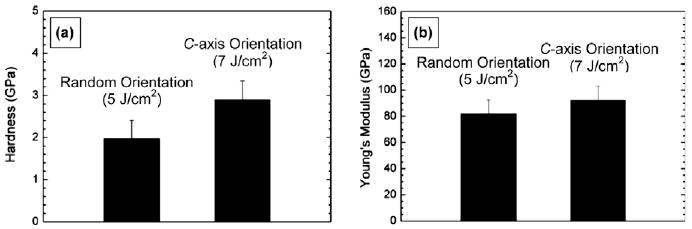
(a) Hardness and (b) Young's modulus of hydroxyapatite coatings deposited at 80 Pa of Ar/H2O and 650 °C with different laser energy densities: 5 and 7 J cm −2.
4. Conclusions
We have investigated the in vitro dissolution and mechanical characteristics of c-axis textured HA in comparison with randomly oriented HA. The PLD technique was used to produce HA polycrystalline coatings with different orientations, e.g. c-axis preferred orientation and random orientation. A high laser energy density and normal incidence of the plasma plume during PLD led to HA coatings whose grains had the c-axis preferentially aligned perpendicular to the substrate. In contrast to the randomly oriented HA, SEM identified a dramatic change in overall surface morphology of the c-axis textured HA after 24 h immersion of the coatings in SBF. The porosity was dramatically increased and sub-micron pores were found throughout the coatings, while the average size of the grains in the coatings was not significantly changed. The composition of these textured HA coatings remained essentially unaltered through dissolution. The crystallographic texture, on the other hand, was further enhanced after 24 h immersion, probably due to reprecipitation effects. Highly oriented surfaces probably stimulate surface restructuring through a process of dissolution and reprecipitation, leading to homoepitaxial growth. Moreover, a nanoindentation study revealed that the highly c-axis oriented coatings exhibited mechanical properties, such as hardness and Young's modulus, superior to those of the randomly oriented coatings. These findings indicate that c-axis textured HA with improved mechanical properties and a controlled dissolution behavior potentially offers an affordable route to biomedical applications requiring both mechanical reliability and bioactivity.
Acknowledgments
This research was supported by the National Institute of Dental and Craniofacial Research (NIDCR) under Grant No. R01 DE013952-07 and by a Major Research Instrumentation Award from the National Science Foundation (NSF) under Grant No. DMR-0116098.
Appendix A. Figures with essential colour discrimination
Certain figures in this article, particularly Figs. 1 and 4, are difficult to interpret in black and white. The full colour images can be found in the on-line version, at doi: 10.1016/j.actbio.2010.02.031.
References
- 1.Kay MI, Young RA, Posner AS. Crystal structure of hydroxyapatite. Nature. 1964;204:1050. doi: 10.1038/2041050a0. [DOI] [PubMed] [Google Scholar]
- 2.LeGeros RZ. Calcium phosphates in oral biology and medicine. Monogr Oral Sci. 1991;15:1. [PubMed] [Google Scholar]
- 3.Hench LL, Wilson J. An introduction to bioceramics. Singapore: World Scientific; 1993. [Google Scholar]
- 4.De Groot K. Application of porous bioceramics in surgery. Mater Technol. 1993;8:12. [Google Scholar]
- 5.Suchanek W, Yoshimura M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J Mater Res. 1998;13:94. [Google Scholar]
- 6.Sun L, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasm-sprayed hydroxyapatite coatings. J Biomed Mater Res. 2001;58:570. doi: 10.1002/jbm.1056. [DOI] [PubMed] [Google Scholar]
- 7.Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop. 1981;157:259. [PubMed] [Google Scholar]
- 8.Yamamoto H, et al. Mechanical strength of calcium phosphate cement in vivo and in vitro. Biomaterials. 1998;19:1587. doi: 10.1016/s0142-9612(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 9.Kweh SW, Khor KA, Cheang P. Plasma-sprayed hydroxyapatite (HA) coatings with flame-spheroidized feedstock: microstructure and mechanical properties. Biomaterials. 2000;21:1223. doi: 10.1016/s0142-9612(99)00275-6. [DOI] [PubMed] [Google Scholar]
- 10.Xu HHK, Takagi S, Quinn JB, Chow LC. Fast-setting calcium phosphate scaffolds with tailored macropore formation rates for bone regeneration. J Biomed Mater Res A. 2004;68:725. doi: 10.1002/jbm.a.20093. [DOI] [PubMed] [Google Scholar]
- 11.Werner J, Linner-Krcamar B, Friess W, Greil P. Mechanical properties and in vitro cell compatibility of hydroxyapatite ceramics with graded pore structure. Biomaterials. 2002;23:4285. doi: 10.1016/s0142-9612(02)00191-6. [DOI] [PubMed] [Google Scholar]
- 12.Metsger DS, Rieger MR, Foreman DW. Mechanical properties of sintered hydroxyapatite and tricalcium phosphate ceramic. J Mater Sci Mater Med. 1999;10:9. doi: 10.1023/a:1008883809160. [DOI] [PubMed] [Google Scholar]
- 13.Dinda GP, Shin J, Mazumder J. Pulsed laser deposition of hydroxyapatite thin films on Ti–6Al–4V: effect of heat treatment on structure and properties. Acta Biomater. 2009;5:1821. doi: 10.1016/j.actbio.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Otsuka M, Matsuda Y, Suwa Y, Fox JL, Higuchi WI. Effect of particle size of metastable calcium phosphates on mechanical strength of a novel self-setting bioactive calcium phosphate cement. J Biomed Mater Res. 1995;29:25. doi: 10.1002/jbm.820290105. [DOI] [PubMed] [Google Scholar]
- 15.Nelea V, et al. Mechanical properties improvement of pulsed laser-deposited hydroxyapatite thin films by high energy ion-beam implantation. Appl Surf Sci. 2002;186:483. [Google Scholar]
- 16.Khor KA, Yip CS, Cheang P. Ti–6Al–4V/hydroxyapatite composite coatings prepared by thermal spray techniques. J Therm Spray Technol. 1997;6:109. [Google Scholar]
- 17.Ozeki K, Yuhta T, Fukui Y. A functionally graded titanium/hydroxyapatite film obtained by sputtering. J Mater Sci Mater Med. 2002;13:253. doi: 10.1023/a:1014002732373. [DOI] [PubMed] [Google Scholar]
- 18.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 19.Bonfield W, Grynpas MD, Tully AE, Bowman J, Abram J. Hydroxyapatite-reinforced polyethylene–a mechanically compatible implant material for bone replacement. Biomaterials. 1981;2:185. doi: 10.1016/0142-9612(81)90050-8. [DOI] [PubMed] [Google Scholar]
- 20.Deng X, Hao J, Wang C. Preparation and mechanical properties of nanocomposites poly(d,l-lactide) with Ca deficient hydroxyapatite nanocrystals. Biomaterials. 2001;22:2867. doi: 10.1016/s0142-9612(01)00031-x. [DOI] [PubMed] [Google Scholar]
- 21.Bakar MSA, Cheang P, Khor KA. Tensile properties and microstructural analysis of spheroidized hydroxyapatite–poly(etheretherketone) biocomposites. Mater Sci Eng A. 2003;345:55. [Google Scholar]
- 22.Doi Y, Horiguchi T, Moriwaki Y, Kitago H, Kajimoto T, Iwayama Y. Formation of apatite–collagen complexes. J Biomed Mater Res. 1996;31:43. doi: 10.1002/(SICI)1097-4636(199605)31:1<43::AID-JBM6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Itoh S, Kikuchi M, Koyama Y, Takakuda K, Shinomiya K, Tanaka J. Development of an artificial vertebral body using a novel biomaterial, hydroxyapatite/collagen composite. Biomaterials. 2002;23:3919. doi: 10.1016/s0142-9612(02)00126-6. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi I, et al. Preparation and microstructure analysis of chitosan/hydroxyapatite nanocomposites. J Biomed Mater Res. 2001;55:20. doi: 10.1002/1097-4636(200104)55:1<20::aid-jbm30>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Lin JHC, Liu ML, Ju CP. Structure and properties of hydroxyapatite–bioactive glass composites plasma sprayed on Ti6Al4V. J Mater Sci Mater Med. 1994;5:279. [Google Scholar]
- 26.Georgiou G, Knowles JC. Glass reinforced hydroxyapatite for hard tissue surgery–part I: mechanical properties. Biomaterials. 2001;22:2811. doi: 10.1016/s0142-9612(01)00025-4. [DOI] [PubMed] [Google Scholar]
- 27.Milella E, Cosentino F, Licciulli A, Massaro C. Preparation and characterization of titania/hydroxyapatite composite coatings obtained by sol–gel process. Biomaterials. 2001;22:1425. doi: 10.1016/s0142-9612(00)00300-8. [DOI] [PubMed] [Google Scholar]
- 28.Diaz-Estrada JR, Camps E, Escobar-Alarcon L, Ascencio JA. Mechanical improvement of hydroxyapatite by TiOxnanoparticles deposition. J Mater Sci. 2007;42:1360. [Google Scholar]
- 29.Li H, Khor KA, Cheang P. Titanium dioxide reinforced hydroxyapatite coatings deposited by high velocity oxy-fuel(HVOF) spray. Biomaterials. 2002;23:85. doi: 10.1016/s0142-9612(01)00082-5. [DOI] [PubMed] [Google Scholar]
- 30.Chang E, Chang WJ, Wang BC, Yang CY. Plasma spraying of zirconia-reinforced hydroxyapatite composite coatings on titanium: part 1. Phase, microstructure and bonding strength. J Mater Sci Mater Med. 1997;8:193. doi: 10.1023/a:1018583522322. [DOI] [PubMed] [Google Scholar]
- 31.Kim HW, Noh YJ, Koh YH, Kim HE, Kim HM. Effect of CaF2 on densification and properties of hydroxyapatite–zirconia composites for biomedical applications. Biomaterials. 2002;23:4113. doi: 10.1016/s0142-9612(02)00150-3. [DOI] [PubMed] [Google Scholar]
- 32.Ning CY, Wang YJ, Lu WW, Chen XF, Wu G, Zhao NR. Microstructure and mechanical performances of plasma-sprayed functionally gradient HA-ZrO2–bioglass coatings. Key Eng Mater. 2005;280–283:1893. [Google Scholar]
- 33.Klein CPAT. Studies of the solubility of the different calcium phosphates and other ceramic particles in vitro. Biomaterials. 1990;11:509. doi: 10.1016/0142-9612(90)90067-z. [DOI] [PubMed] [Google Scholar]
- 34.LeGeros RZ. Biodegradation and bioresorption of calcium phosphate ceramics. Clin Mater. 1993;14:65. doi: 10.1016/0267-6605(93)90049-d. [DOI] [PubMed] [Google Scholar]
- 35.Ducheyne P, Radin S, King L. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. I. Dissolution. J Biomed Mater Res. 1993;27:25. doi: 10.1002/jbm.820270105. [DOI] [PubMed] [Google Scholar]
- 36.El-Ghannam A, Ning CQ. Effect of bioactive ceramic dissolution on the mechanism of bone mineralization and guided tissue growth in vitro. J Biomed Mater Res A. 2006;76:386. doi: 10.1002/jbm.a.30517. [DOI] [PubMed] [Google Scholar]
- 37.Zeng H, Lacefield WR. XPS, EDX and FTIR analysis of pulsed laser deposited calcium phosphate bioceramic coatings: the effects of various process parameters. Biomaterials. 2000;21:23. doi: 10.1016/s0142-9612(99)00128-3. [DOI] [PubMed] [Google Scholar]
- 38.Kocks UF, Tome CN, Wenk HR. Texture and anisotropy. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- 39.Wenk HR, Heidelbach F. Crystal alignment of carbonated apatite in bone and calcified tendon: results from quantitative texture analysis. Bone. 1999;24:361. doi: 10.1016/s8756-3282(98)00192-6. [DOI] [PubMed] [Google Scholar]
- 40.Nakano T, et al. Unique alignment and texture of biological apatite crystallites in typical calcified tissues analyzed by microbeam X-ray diffractometer system. Bone. 2002;31:479. doi: 10.1016/s8756-3282(02)00850-5. [DOI] [PubMed] [Google Scholar]
- 41.Kawasaki T. Hydroxyapatite as a liquid-chromatographic packing. J Chromatogr. 1991;544:147. [Google Scholar]
- 42.Filgueiras MRT, Mkhonto D, De Leeuw NH. Computer simulations of the adsorption of citric acid at hydroxyapatite surfaces. J Cryst Growth. 2006;294:60. [Google Scholar]
- 43.De Leeuw NH, Rabone JAL. Molecular dynamics simulations of the interaction of citric acid with the hydroxyapatite (0001) and (011̄0) surfaces in an aqueous environment. Cryst Eng Comm. 2007;9:1178. [Google Scholar]
- 44.Dong XL, Zhou HL, Wu T, Wang Q. Behavior regulation of adsorbed proteins via hydroxyapatite surface texture control. J Phys Chem B. 2008;112:4751. doi: 10.1021/jp0768672. [DOI] [PubMed] [Google Scholar]
- 45.Almora-Barrios N, Austen KF, De Leeuw NH. Density functional theory study of the binding of glycine, proline, and hydroxyproline to the hydroxyapatite (0001) and (011̄0) surfaces. Langmuir. 2009;25:5018. doi: 10.1021/la803842g. [DOI] [PubMed] [Google Scholar]
- 46.Cotell CM, Chrisey DB, Grabowski KS, Sprague JA, Gossett CR. Pulsed laser deposition of hydroxyapatite thin films on Ti–6Al–4V. J Appl Biomater. 1992;3:87. [Google Scholar]
- 47.Tong W, et al. Preferred orientation of plasma sprayed hydroxyapatite coatings. J Mater Sci. 1996;31:3739. [Google Scholar]
- 48.Roome CM, Adam CD. Crystallite orientation and anisotropic strains in thermally sprayed hydroxyapatite coatings. Biomaterials. 1995;16:691. doi: 10.1016/0142-9612(95)99696-j. [DOI] [PubMed] [Google Scholar]
- 49.Van Dijk K, et al. Influence of discharge power level on the properties of hydroxyapatite films deposited on Ti6A14V with RF magnetron sputtering. J Biomed Mater Res. 1995;29:269. doi: 10.1002/jbm.820290218. [DOI] [PubMed] [Google Scholar]
- 50.Kim H, Camata RP, Lee S, Rohrer GS, Rollett AD, Vohra YK. Crystallographic texture in pulsed laser deposited hydroxyapatite bioceramic coatings. Acta Mater. 2007;55:131. doi: 10.1016/j.actamat.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park JB, Lakes RS. Biomaterials: an introduction. New York: Plenum Press; 1992. [Google Scholar]
- 52.Kim H, et al. Calcium phosphate bioceramics with tailored crystallographic texture for controlling cell adhesion. Mater Res Soc Sym Proc E. 2006;925:0925. [Google Scholar]
- 53.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:564. [Google Scholar]
- 54.Arias JL, Mayor MB, Pou J, Leng Y, Leon B, Perez-Amor M. Micro- and nano-testing of calcium phosphate coatings produced by pulsed laser deposition. Biomaterials. 2003;24:3403. doi: 10.1016/s0142-9612(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 55.Nelea V, Morosanu C, Iliescu M, Mihailescu IN. Hydroxyapatite thin films grown by pulsed laser deposition and radio-frequency magnetron sputtering: comparative study. Appl Surf Sci. 2004;228:346. [Google Scholar]
- 56.Barbieri FC, Otani C, Lepienski CM, Urruchi WI, Maciel HS, Petraconi G. Nanoindentation study of Ti6Al4V alloy nitrided by low intensity plasma jet process. Vacuum. 2002;67:457. [Google Scholar]
- 57.Oyen ML. Nanoindentation hardness of mineralized tissues. J Biomech. 2006;39:2699. doi: 10.1016/j.jbiomech.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 58.He LH, Swain MV. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J Mech Behav Biomed. 2008;1:18. doi: 10.1016/j.jmbbm.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Norman J, Shapter JG, Short K, Smith LJ, Fazzalari NL. Micromechanical properties of human trabecular bone: a hierarchical investigation using nanoindentation. J Biomed Mater Res A. 2008;87:196. doi: 10.1002/jbm.a.31766. [DOI] [PubMed] [Google Scholar]
- 60.Long M, Rack HJ. Titanium alloys in total joint replacement–a materials science perspective. Biomaterials. 1998;19:1621. doi: 10.1016/s0142-9612(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 61.Viswanath B, Raghavan R, Ramamurty U, Ravishankar N. Mechanical properties and anisotropy in hydroxyapatite single crystals. Scr Mater. 2007;57:361. [Google Scholar]
- 62.Saber-Samandari S, Gross KA. Micromechanical properties of single crystal hydroxyapatite by nanoindentation. Acta Biomater. 2009;5:2206. doi: 10.1016/j.actbio.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Vasanthan A, Kim H, Drukteinis S, Lacefield W. Implant surface modification using laser guided coatings: in vitro comparison of mechanical properties. J Prosthodont. 2008;17:357. doi: 10.1111/j.1532-849X.2008.00307.x. [DOI] [PubMed] [Google Scholar]