Abstract
Epidemiological research provides strong evidence for a link between repetitive work (RW) and the development of chronic trapezius myalgia (TM). The aims were to further elucidate if an accumulation of sensitising substances or impaired oxygenation is evident in painful muscles during RW. Females with TM (n = 14) were studied during rest, 30 minutes RW and 60 minutes recovery. Microdialysate samples were obtained to determine changes in intramuscular microdialysate (IMMD) [glutamate], [PGE2], [lactate], and [pyruvate] (i.e., [concentration]) relative to work. Muscle oxygenation (%StO2) was assessed using near-infrared spectroscopy. During work, all investigated substances, except PGE2, increased significantly: [glutamate] (54%, P < .0001), [lactate] (26%, P < .005), [pyruvate] (19%, P < .0001), while the %StO2 decreased (P < .05). During recovery [PGE2] decreased (P < .005), [lactate] remained increased (P < .001), [pyruvate] increased progressively (P < .0001), and %StO2 had returned to baseline. Changes in substance concentrations and oxygenation in response to work indicate normal increase in metabolism but no ongoing inflammation in subjects with TM.
1. Introduction
Epidemiological research provides strong evidence for a link between repetitive work and the development of chronic muscle pain [1–3], but to fully understand this relationship, the pathophysiological mechanisms behind it needs further elucidating. Several hypotheses that focus on hypoxia or other metabolic effects in the muscle have been suggested [4–6].
While the muscle biopsy technique have only been able to provide a “snap-shot” of the muscle chemistry [7, 8], the possibilities for real-time in vivo investigations have been greatly improved by combining microdialysis and near-infrared spectroscopy [9, 10].
Microdialysis (MD) permits in vivo measurements of changes in substance concentrations in different tissues in response to work, with minimal trauma [11]. MD is performed by implanting a probe with a semipermeable membrane in the tissue and slowly perfuses it with a physiological solution. Sample collection is based on passive diffusion of substances over the membrane, preferable during steady-state conditions.
We have previously reported significantly increased [lactate] and [glutamate] and unchanged [prostaglandin E2, PGE2] in the trapezius muscle of healthy females, in response to low-load repetitive work (RW) which we interpreted as normal responses to increased physical demands [9]. We have also reported similar absolute [glutamate] and [PGE2] in females with trapezius myalgia (TM) and asymptomatic controls during rest [12]. Few other studies have used MD to further elucidate the pathophysiology behind work-related trapezius myalgia, and to some extent the findings are conflicting. In a laboratory study Rosendal et al. [13] reported increased intramuscular [lactate] and [glutamate] in response to RW in TM, but not in healthy controls, findings which they were unable to verify in an occupational field study [14]. However, the pain subjects differed in severity of symptoms between studies. Ashina et al. [15] reported similar [glutamate] and [PGE2] in trapezius muscle tender points in response to low-load static work in subjects with chronic tension type headache (CTTH).
Near-infrared spectroscopy (NIRS) is a noninvasive method for measuring muscle oxygenation (% StO2), that is, the dynamic balance between oxygen delivery to and consumption within a tissue [16, 17]. The technique is based on the principle of differential absorption properties of oxygenated and deoxygenated forms of haemoglobin (and to a lesser extent, myoglobin) in the near infrared range (760–850 nm). NIRS is well suited to study the muscle microcirculation due to the minimal absorption of light in small vessels (i.e., arterioles, capillaries, and venules) compared to in veins and feed arteries [16, 17]. We have previously combined MD and NIRS to study the effects of RW of different duration [9] and RW with superimposed mental load [10] on % StO2 in the pain-free trapezius muscle. We found small changes in % StO2 and intramuscular lactate, indicative of a normal response to increased physical demands [9]. In one recent study no statistically significant differences for oxygenated haemoglobin during RW were reported between TM and healthy controls [18].
The aims of this study, which was purposely designed as a comparison to our previous study on asymptomatic females [9], were to investigate whether an interstitial accumulation of sensitising substances (glutamate, PGE2), or local metabolic changes indicative of an insufficient oxygen supply (e.g., greatly increased [lactate] and decreased % StO2) is evident during RW in subjects with TM (n = 14).We also wanted to investigate how % StO2, blood lactate, and intramuscular [lactate] relate during RW in TM.
2. Materials and Methods
2.1. Participants
Fourteen females with trapezius myalgia (TM) participated in the study. The group had a mean age of 40 (±8) years, height 167 (±4) cm, weight 66 (±9.5) kg, and BMI 23.0 (±2.3). The pain subjects were matched in age to a group of healthy asymptomatic females, who had participated in a previous study at our laboratory, and in which the same experimental protocol was used [9], to admit comparisons to be made.
Participants were required to be right-handed, nonsmokers, and not allergic to local anaesthesia. Further inclusion criteria were as follows: during the clinical examination subjects were required to (i) report pain of a duration of at least 3 months from the neck-shoulder region, (ii) verify pain in the upper part of the trapezius muscle with a pain drawing, (iii) have the most pronounced complaints on the side subjected to the greatest workload, and (iv) have reason to believe that the pain was caused by their work, that is, that they reported that the onset of their pain problems coincided with performance of static and/or repetitive work tasks. Also, that they reported less pain when being off work, and/or increased pain when coming back to work after a holiday.
All participants were examined by the same physiotherapist. Exclusion criteria were (a) previous trauma to the neck or shoulder, (b) signs of shoulder tendonitis or shoulder joint affection, (c) signs of nerve affection, (d) pronounced pain from more than three body regions, and (e) neurological or metabolic diseases, or (f) other diseases that demanded continuous medication.
The Nordic Ministry Council Questionnaire (NMCQ) [19] and the visual analogue scale (VAS) were used to survey pain during the last 12 months and at the time of participation. All participants reported pain from the neck-shoulder area during the last 7 days. The median (range) for the VAS-ratings of perceived pain in the right shoulder were 47 (12–79) for the last 12 months, 34 (14–63) for the last 7 days, and 27 (4–78) when arriving at the laboratory. The mean (±SD) duration of complaints from the neck-shoulder was 70.5 (±74.7) months. None of the subjects were on sick-leave at the time of the study.
The participants were recruited through contacts with local industries and through advertisement on the university hospital's intranet. The participants either worked at an assembly line (in a car factory) or at a VDU-station, thus performing work tasks of a repetitive and static character. All participants gave their informed and signed consent prior to inclusion in the study. The study conformed to the ethical standards laid down in the 1964 Declaration of Helsinki and was approved by the Ethical Committee of the Medical Faculty of the University of Umeå (Dnr 2004:M-150).
2.2. Methods
2.2.1. Experimental Protocol
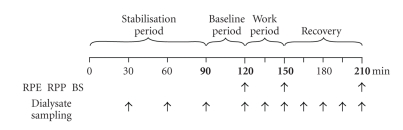
The experimental design is shown in Figure 1. The experimental conditions were exactly the same as in the previous study on asymptomatic control subjects [9] (same experimental protocol, same laboratory room and equipment, same controlled room temperature, all probe insertions were performed by the same medical doctor, and all analyses were performed by the same laboratory technicians). In addition to the previous protocol, the participants perceived pain intensity were also assessed. All experiments started at 7 a.m. The subjects were instructed not to perform any kind of heavy physical exercise 48 hours prior to the experiment, and to arrive fasting to the laboratory where they were given a standardised breakfast. They were also asked to refrain from using pain medication (NSAIDs) three days before the experiment to avoid interference with the pain substances under study, but also due to the increased risk of bleeding during the procedure (paracetamol was allowed). In the preparation period, before the MD-probe was inserted and the optical probe for oxygen saturation measurements was attached to the skin, measurements of skin-fat layer (SFL) and trapezius muscle thickness over the approximate site of the O2-probe were performed using ultrasonography [9, 10]. Subjects then rested comfortably seated for a total of 120 minutes (90 minutes stabilisation + 30 minutes baseline) before the 30 minutes work-period began.
Figure 1.
Experimental design. Schematic of the experimental design with 90 minutes stabilisation period, 30 minutes baseline, 30 minutes work, and a 60 minutes recovery period. Arrows indicate time points for sampling of dialysate, assessment of perceived exertion with the Borg CR-scale (RPE), assessment of perceived pain intensity with VAS (RPP), and capillary blood sampling (BS).
The repetitive low-load work, also used by Flodgren et al. [9, 10], was designed to simulate an occupational work task. It consisted in alternatively pushing in a piston and pressing down a button with the use of a hand-held manipulandum (130 grams), while seated at a table. The subjects maintained a pace of 30 work cycles per minute, with the aid of a metronome; a single piston push followed by a button press constituting one work cycle. The workstation was adjusted to each subject to provide optimal ergonomic conditions (Figure 2). In a pilot study (unpublished data) we used electromyography to assess the trapezius muscle activity during the same type of work. We then found the mean electrical activity to be 9.3% of maximal voluntary contraction, which is similar to muscle activity measured during low-load RW at a real work place [20].
Figure 2.
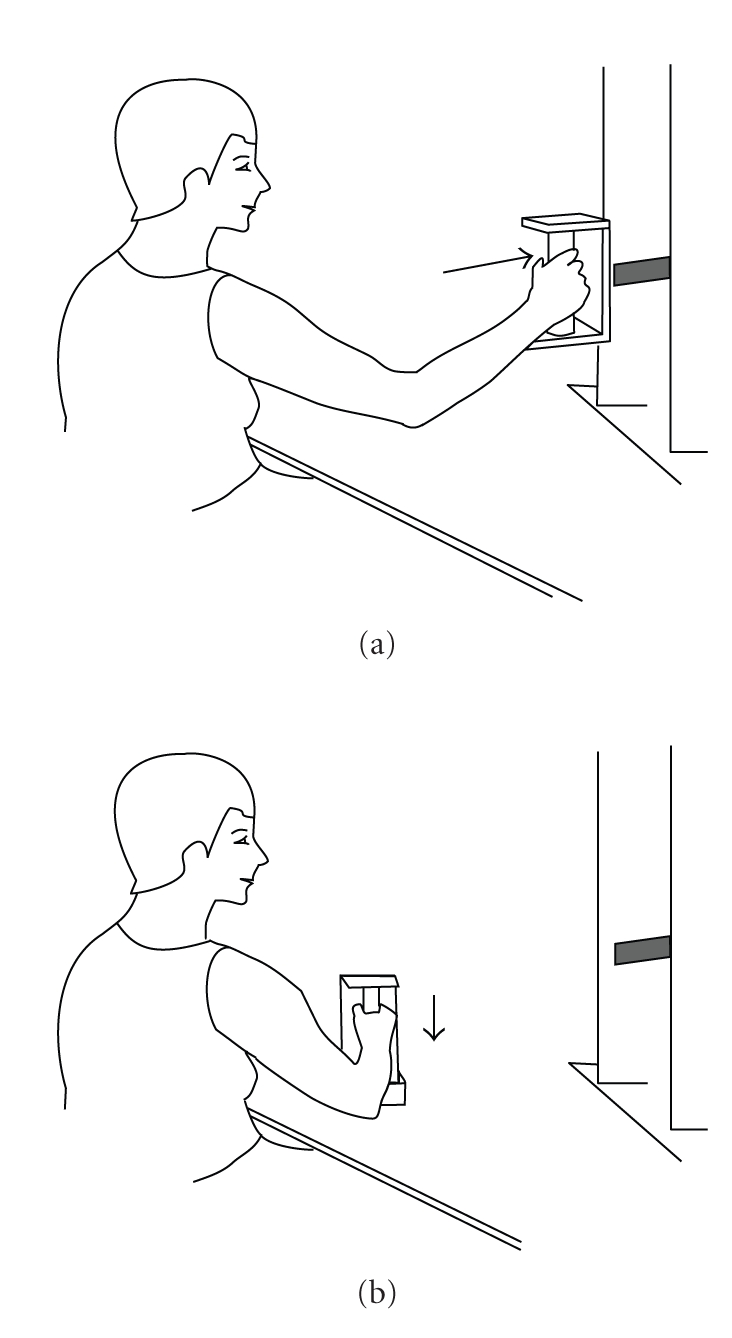
Repetitive work model. The low-load repetitive work consisted of (a) pushing in a piston and (b) pressing down a button on the table with a handheld manipulandum, at a pace of 30 work cycles per minute.
After the performance of the repetitive work subjects rested, comfortably seated, for yet another 60 minutes (see Figure 1). All subjects completed the work task. Throughout the experiment local % StO2 was recorded, microdialysate samples and capillary blood for lactate analyses were obtained, subjectively perceived exertion (Borg CR-10) and perceived pain intensity (VAS) were assessed, as shown in Figure 1. The room temperature was between 22 and 24 degrees Celsius during experiments.
2.2.2. Microdialysis
After local anaesthesia of the skin and subcutaneous tissue by injection of 1.5 mL Xylocain (10 mg ml−1), a microdialysis probe (CMA 60, CMA/Microdialysis AB, Sweden, 20 kDa molecular cut-off, membrane length 30 mm, 0.5 mm outer diameter) was implanted in the middle third of the upper part of the trapezius muscle in the direction lateral to medial. The same investigator performed all probe insertions, and ultrasonography was used to confirm the placement of the probe (Aloka SSD-2000, Aloka Co., Ltd., Japan). The catheter was secured to the skin with adhesives, connected to a portable syringe pump (CMA 107, CMA/Microdialysis, AB, Sweden), and perfused with solution containing 147 mM L−1Na+, 4 mM L−1K+, 2.3 mM L−1Ca2+, and 156 mM L−1 Cl− (perfusion fluid T1, CMA/Microdialysis AB, Stockholm, Sweden), at a flow rate of 2 μL min−1. The pump was secured in level with the probe. After insertion of the probe in the muscle, the subjects rested for 90 minutes to allow the tissue to stabilise after the initial trauma of probe insertion [11, 12, 21]. Samples were obtained every 30th minute during the initial two hours of rest, and every 15th minute during work and recovery. The last sample obtained during rest will be referred to as baseline. All probes kept functioning throughout the experiment. The samples were immediately frozen to −70° until analyses were performed. All samples were coded by the authors and analysed blindly by an independent laboratory technician. Lactate, glutamate and pyruvate were analysed with the CMA 600 Microdialysis Analyser (CMA Microdialysis, Solna, Sweden), and PGE2 with a radioimmunoassay kit (NEN, Du Pont, Boston, Mass, USA). The detection limits are 0.1 mmol × L−1 for lactate, 10 μmol × L−1 for pyruvate and 1 μmol × L−1 for glutamate. The detection limit for the PGE2 assay is 0.5 pg mL−1.
2.2.3. Oxygen Saturation (% StO2)
Measurements of trapezius muscle % StO2 were performed during the experiment using a near infrared spectrometer, NIRS (INSPECTRA Tissue Spectrometer—model 325, Hutchinson Technology Inc, Netherlands).
A self-adhesive O2-shield was placed on the skin overlying the upper part of the trapezius muscle, medial of the MD-probe insertion site. Before connecting the optical cable to the shield, the system was calibrated according to instructions from the manufacturers, that is, inserted in a light scattering calibrator for capturing reference light intensities of all wavelengths. All tissue measurements were related to the reference measurement, thereby converting light intensity measurements to optical absorbance. Optical absorbance values were further processed into a scaled second derivative absorbance spectrum, whereby a measure of percent oxygen saturation was obtained. The software supplied with the In Spectra device allows for absolute values of % StO2. The distance between the light transmitter and the detector was 12 mm, and the sampling frequency 0.3 Hz.
2.2.4. Blood Sampling
In order to assess possible systemic effects in response to work, that is, changes in blood lactate concentration, capillary blood samples were obtained from a right hand finger at baseline, directly after work and after the recovery period (Figure 1). The coded samples were immediately put on ice, and were later the same day analysed with a lactate analysis device YSI 2300 STAT plus (Clandon Scientific, Famborough, UK) by an independent laboratory technician.
2.2.5. Rating of Perceived Exertion (RPE) and Perceived Pain Intensity (RPP)
At baseline, and at the end of both the work and the recovery period (see Figure 1), subjects were required to rate their (i) perceived exertion of the right shoulder in accordance to the Borg CR-10 scale [22], with 0 = no fatigue and 10 = severe fatigue, and (ii) their perceived pain intensity in the right shoulder, using the nonhatched VAS, marked at one end as “no pain at all” and at the other “worst pain imaginable” [23].
2.2.6. Estimation of Skin-Fat Layer and Trapezius Muscle Thickness
Measurements of skin-fat layer (SFL) and trapezius muscle thickness were performed using ultrasonography, with a 75 mm probe (Aloka SSD-2000, Aloka Co., Ltd., Japan). Measurements were performed at three measuring-points over the upper trapezius muscle medially of the MD-probe insertion point. (1, 3.75 and 7.5 cm)
2.2.7. Data Analyses and Statistics
SPSS statistical software, version 13.0 (Chicago, III, USA) was used for all analyses. The level of significance was set to P < .05. The Kolmogorov-Smirnov test was used to test for normal distribution, and data for all variables was normally distributed.
The change in substance concentration relative to work was calculated as the baseline values subtracted from the values obtained after work (work-baseline = change).
Pearson test was used to investigate possible correlations between (i) pain substances (glutamate and PGE2) and pain intensity, (ii) IMMD lactate and local muscle oxygenation, (iii) local (IMMD lactate) and systemic (blood lactate) changes, and (iv) key metabolites (IMMD lactate and glutamate).
Repeated measures ANOVA (RM ANOVA) were used for the within group comparisons of data for the different variables in response to work and recovery (except for the RPE- and RPP-data). If the assumption of sphericity of variance was violated in the RM ANOVA, the Huyn-Feldt correction was used. The sequential Bonferroni [24] was used to compare specific pairs of means when the RM ANOVA revealed a significant difference.
To relate the oxygenation and the MD-data in time, a mean value of the oxygenation data recorded during a 5-min period just before each sampling of dialysate was calculated. Data are presented as means ± SD in both text and graphs, except for the RPE and RPP which are presented as median and range. Wilcoxon nonparametric test was used for the within group comparisons of the subjective ratings.
3. Results
3.1. Biochemical Alterations during Work
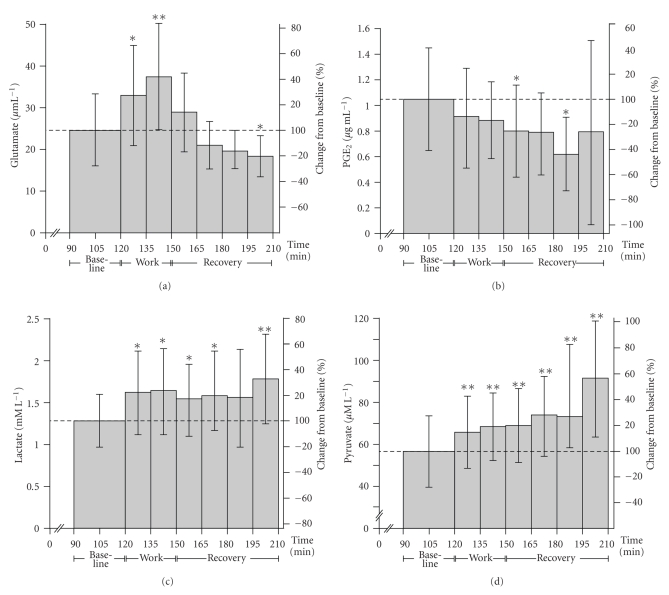
During work [glutamate] increased (54%) (P < .0001, F = 16.957), and decreased to 26% below baseline during the recovery period (P < .0001, F = 14.293) (see Figure 3(a)).
Figure 3.
(a) IMMD glutamate concentrations. Mean (±SD) intramuscular microdialysate [glutamate] (μM L−1) at baseline, in response to work and recovery. Percent difference in comparison to baseline is depicted on the right hand y-axis. Significant differences with respect to baseline concentrations are indicated with * for a P-value <.05 and ** for a P-value <.001. (b) IMMD PGE2 concentrations. Mean (±SD) intramuscular microdialysate [PGE2] (μg mL−1) at baseline, in response to work and recovery. Percent difference in comparison to baseline is depicted on the right hand y-axis. Significant differences with respect to baseline concentrations are indicated with * for a P-value <.05 and ** for a P-value <.001. (c) IMMD lactate concentrations. Mean (±SD) intramuscular microdialysate [lactate] (mM L−1) at baseline, in response to work and recovery. Percent difference in comparison to baseline is depicted on the right hand y-axis. Significant differences with respect to baseline concentrations are indicated with * for a P-value <.05 and ** for a P-value <.001. (d) IMMD pyruvate concentrations. Mean (±SD) interstitial microdialysate [pyruvate] (mM L−1) at baseline, during work and recovery. Percent difference in comparison to baseline is depicted on the right hand y-axis. Significant differences with respect to baseline concentrations are indicated with * for a P-value <.05 and ** for a P-value <.001.
The mean IMMD [PGE2] remained unchanged during work (P = .300), but showed a significant overall decrease (40% lower than baseline) during recovery (P = .004, F = 5.224) (see Figure 3(b)).
An overall significant increase (28%) in mean IMMD [lactate] was found in response to work (P = .003, F = 10.813), and recovery (P > .001, F = 7.392) as compared to baseline (see Figure 3(c)).
Also, [pyruvate] increased significantly during work (P < .0001, F = 36.4), and continued to increase progressively (up to 60% > baseline) during the recovery period (P < .0001, F = 25.3) (see Figure 3(d)).
3.2. Oxygen Saturation (% StO2)
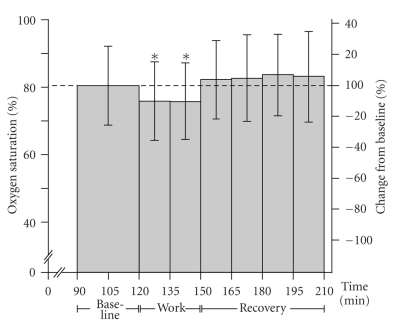
A small, but significant, decrease in % StO2 in response to work was found (P = .016, F = 7.816), and directly after the cessation of work baseline was re-established (see Figure 4).
Figure 4.
Muscle oxygen saturation. Mean (±SD) local trapezius muscle oxygen saturation (% StO2) at baseline, during work and recovery. Significant differences with respect to baseline concentrations are indicated with * for a P-value <.05.
3.3. Blood Lactate
Plasma [lactate] decreased significantly in response to work (P = .005; F = 8.109), from 1.1 mmol × L−1 (SD ± 0.3) at baseline to 0.9 mmol × L−1 (SD ± 0.2) after work and baseline was re-established at the end of the recovery period (1.3 ± 0.6).
3.4. Rating of Perceived Exertion (RPE) and Pain Intensity (RPP)
The median (range) RPE in the right shoulder (Borg CR-10), increased significantly from 1.5 (0–3.5) at baseline to 7.0 (3.0–7.0) after work, and remained increased also after recovery 2.25 (0–4.5) The median (range) RPP in the right shoulder (VAS) increased (P < .001) from 17.5 (2–52) at baseline, to 69 (28–82) after work and tended to be significantly increased also at the end of recovery 26.5 (3–69) (P = .054).
3.5. Skin-Fat Layer and Trapezius Muscle Thickness
The mean SFL (fascia included) and the mean trapezius muscle thickness for the three measuring-points medial to the MD-probe insertion point (1, 3.5 and 7 cm) were 4.6 ± 0.5, 4.7 ± 0.3 and 4.5 ± 0.3 mm and 12.3 ± 0.4 mm, and 12.2 ± 0.5 and 10.0 ± 0.6 mm, respectively. Mean values for the SFL (4.6 ± 0.4 mm) and the trapezius muscle thickness (11.5 ± 0.5 mm) were calculated.
3.6. Correlations
A significant negative correlation was found between changes in IMMD [lactate] and plasma [lactate] during work (P < .05), and a tendency to a correlation between IMMD lactate and % StO2 (P = .073). The pain intensity was uncorrelated to IMMD [glutamate] (P = .276) and [PGE2]. (P = .492), but significantly correlated to the perceived exertion in the shoulder (P < .0001). A significant correlation was also found between changes in IMMD [glutamate] and IMMD [lactate] during work (P < .05).
4. Discussion
The main results of the present study were: significantly increased IMMD [glutamate] and [lactate], unchanged [PGE2], and decreased % StO2 in the trapezius muscle during work in females with TM. Furthermore, that glutamate and PGE2 were uncorrelated to pain intensity.
4.1. Glutamate and PGE2
Glutamate is a well-known pain-mediator in the CNS [25, 26], and suggested to contribute to localised pain and peripheral sensitisation in certain pain conditions [13, 27, 28]. It is also a key metabolite in cellular metabolism [29]. In our study we found a significant increase in IMMD [glutamate] in response to work (54%), which was in accordance with our previous results for healthy controls [9]. However, at all time points [glutamate] was lower in TM, and uncorrelated to pain intensity [9]. While we found a moderate increase in [glutamate], the suggested concentration needed to excite and sensitize nociceptors is 2-3-fold greater than physiological concentrations [30]. These findings taken together do not support an involvement of glutamate in peripheral pain conditions. They are best interpreted as signs of normally increased metabolism in response to work.
The increase in [glutamate] in TM in response to work was also in accordance with results reported by others [13]. In contrast, Rosendal et al. found overall higher [glutamate] in TM, and a correlation between pain ratings and [glutamate] [13]. A recent study reported higher [glutamate] in TM, but no definite baseline was used as comparison [14]. It should be noted that the increased [glutamate] reported [13, 14] was similar to absolute resting concentrations reported in the trapezius of pain subjects and controls [12].
PGE2 is known to act directly on nociceptors, and for its ability to sensitise nociceptors to other substances [31]. PGE2 is also an important regulator of blood flow [16, 32], but its effect is highly dependent on intensity and mode of exercise, that is, during high intensity dynamic contractions, [PGE2] is reported to increase significantly, but to remain unchanged during low intensity static contractions [33].
In our study IMMD [PGE2] did not increase in response to work, which is in agreement with our previous results on healthy subjects [9]. Furthermore, [PGE2] was uncorrelated to pain intensity. These findings suggest that there is no ongoing inflammation in the chronic phase of TM. Our findings and conclusions are in general agreement with the results for [PGE2] reported by Ashina et al. [15].
However, tissue damage and inflammation may still be initiating factors for the development of muscle pain [34], and a shift from pain mechanisms in the periphery to the CNS may occur at a later stage in the disease process [35].
4.2. Lactate, Pyruvate, and % StO2
Lactate is an important fuel source and a glukoneogenetic precursor [36, 37]. Muscles produce lactate to yield energy during anaerobic conditions, but there is also a significant lactate production in the fully oxygenated contracting muscle [38]. In our study, we found significantly increased [lactate] in response to work TM, which was in accordance with our previous results for healthy females [9]. Ashina et al. [39] also found similar [lactate] in pain subjects and controls in response to static work. However, higher [lactate] in TM in response to RW has been reported [13, 18], findings which the same authors were unable to verify in an occupational study [14]. However, many of the subjects in [13] were on sick-leave, while in the latter participants were occupationally active [18].
During prolonged low-intensity work oxidation of lactate into pyruvate is enhanced, and muscles revert from net lactate release to net uptake [40]. We found a significant increase in [pyruvate] during work, and a progressive increase during recovery, which differed from our results on healthy controls [9]. Larsson et al., reported unchanged [pyruvate] during work in both myalgic and healthy workers, but this study had no real baseline for comparison [14]. It may be speculated that the higher [pyruvate] may be due to a greater activation of fatigable type II fibres in subjects with TM, in comparison to asymptomatic subjects, which would be in accordance with muscle activation patterns reported for chronic pain cases [41].
As far as we know, we are first to combine MD and NIRS to investigate biochemical alterations in the trapezius muscle in response to RW [9, 10]. In the present study, we found a significant decrease in local muscle % StO2 during work, and a tendency to a significant correlation between % StO2 and IMMD [lactate]. Our findings are in general agreement with the results reported by Sjøgaard et al. [18]. The decrease in % StO2 and the increase in IMMD [lactate] did not differ from our previous results on healthy controls, that is why these findings must be interpreted as a normal response to increased metabolic demands.
Blood [lactate] decreased during work and was negatively correlated with IMMD [lactate], which may be explained by an enhanced uptake and use of lactate as a fuel by active muscles during prolonged low-load work [40]. During high-intensity work, [lactate] in muscle and blood is correlated [42, 43],while low-intensity work is suggested not to cause systemic effects [16, 44].
4.3. Methodological Considerations
4.3.1. Microdialysis
After the trauma of probe insertion, interstitial substance concentrations are abnormal and the influx of substances random. To avoid biased results sufficient time for the establishment of a new steady-state must be allowed [11]. We applied a 90 minutes stabilisation time, which is ample time for stabilisation of lactate, pyruvate [21], glutamate [12], but maybe not for PGE2, which decreased below baseline during recovery.
Refraining from calibrating the probe in vivo is suggested to introduce bias since the relative recovery of substances (RR) is reported to increase with high intensity exercise in the absence of true interstitial changes [11, 44]. However, most studies of low-load work have reported no change [13, 14, 39, 45] or a small change [13] in RR of lactate in the trapezius muscle during work. This lack of change in RR may be explained by that intramuscular pressure does not increase in the trapezius during low-load work [46], and that the changes in blood flow are small [39],which are suggested not to affect RR of substances in vivo [47, 48]. We did not assess RR, and cannot therefore exclude the presence of a bias, although we find it unlikely that this bias is significant.
4.3.2. Near-Infrared Spectroscopy (NIRS)
Ideally, we also should have measured blood-flow (BF), since metabolic insufficiencies may be the result of impaired BF [49]. Existing results are conflicting and provide no convincing evidence for impaired BF in TM as compared to healthy controls [14, 18, 39, 45].
NIRS may provide information about a possible mismatch between metabolic requirements and blood flow locally in muscle [16] and is considered a valid [50], and reliable tool [51] for measuring local muscle oxygenation during work.
Heterogeneity of blood flow and oxygen consumption distributions within a muscle [43, 52, 53], work intensity and level of training [16] and mode of exercise [54] may influence NIRS-measurements. In this, and in previous studies [9, 10], the work and the position of the probe were standardised, to enable appropriate comparison between studies [55].
The probe was chosen to ensure that data was obtained from the trapezius, and not from underlying muscle. It may be argued that the measuring depth of the probe may not have been sufficient to accurately assess changes in %StO2. However, the muscle volume measured with NIRS is controversial, and while the signal is presumed to be obtained mostly from a tissue depth of approximately 60% of the transmitter-detector distance, a banana-shaped region of sensitivity extends both above and below this depth [17]. Since the skin overlying muscle in lean subjects contributes <5% of the signal [16], our measurement should mainly originate from muscle.
The discrepancies between the few studies that have studied the effect of low-load work on metabolism and sensitising substances, both in methodology, and subjects studied, emphasise that more independent studies are needed.
5. Conclusions
The changes in substance concentrations and oxygenation found in this study indicate normal increase in metabolism in response to work but no ongoing inflammation in subjects with TM. Our results do not support the role of glutamate as a pain mediator in the periphery.
Conflict of Interest
The authors have no conflicts of interest.
Acknowledgments
The financial support of the Swedish Agency for Innovation Systems, VINNOVA (project no. 510240) is gratefully acknowledged. Special thanks are due to laboratory technician Margaretha Marklund for qualified technical and graphical work, and to physiotherapist Åsa Svedmark for professional help with the clinical examinations. Thanks also to Dr. Lars Öhberg for help with interpreting the ultrasonographic images.
References
- 1.Bernard BP. Musculoskeletal Disorders and Workplace Factors. Cincinnati, Ohio, USA: Department of Health and Human Services National Institute for Occupational Safety and Health; 1997. [Google Scholar]
- 2.Buckle PW, Devereux JJ. The nature of work-related neck and upper limb musculoskeletal disorders. Applied Ergonomics. 2002;33(3):207–217. doi: 10.1016/s0003-6870(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 3.Bongers PM, Ijmker S, van den Heuvel S, Blatter BM. Epidemiology of work related neck and upper limb problems: psychosocial and personal risk factors (Part I) and effective interventions from a bio behavioural perspective (Part II) Journal of Occupational Rehabilitation. 2006;16(3):279–302. doi: 10.1007/s10926-006-9044-1. [DOI] [PubMed] [Google Scholar]
- 4.Hägg G. Static work loads and occupational myalgia- a new explanation model. In: Anderson PA, Hobart DJ, Danoff JV, editors. Electromyographical Kinesiology. Amsterdam, The Netherlands: Elsevier; 1991. pp. 141–144. [Google Scholar]
- 5.Johansson H, Sojka P. Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: a hypothesis. Medical Hypotheses. 1991;35(3):196–203. doi: 10.1016/0306-9877(91)90233-o. [DOI] [PubMed] [Google Scholar]
- 6.Knardahl S. Psychophysiological mechanisms of pain in computer work: the blood vessel-nociceptor interaction hypothesis. Work and Stress. 2002;16(2):179–189. [Google Scholar]
- 7.Larsson B, Björk J, Elert J, Lindman R, Gerdle B. Fibre type proportion and fibre size in trapezius muscle biopsies from cleaners with and without myalgia and its correlation with ragged red fibres, cytochrome-c-oxidase-negative fibres, biomechanical output, perception of fatigue, and surface electromyography during repetitive forward flexions. European Journal of Applied Physiology. 2001;84(6):492–502. doi: 10.1007/s004210100409. [DOI] [PubMed] [Google Scholar]
- 8.Larsson B, Björk J, Kadi F, Lindman R, Gerdle B. Blood supply and oxidative metabolism in muscle biopsies of female cleaners with and without myalgia. Clinical Journal of Pain. 2004;20(6):440–446. doi: 10.1097/00002508-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Flodgren GM, Hellström FB, Fahlström M, Crenshaw AG. Effects of 30 versus 60 min of low-load work on intramuscular lactate, pyruvate, glutamate, prostaglandin E2 and oxygenation in the trapezius muscle of healthy females. European Journal of Applied Physiology. 2006;97(5):557–565. doi: 10.1007/s00421-006-0216-7. [DOI] [PubMed] [Google Scholar]
- 10.Flodgren GM, Crenshaw AG, Gref M, Fahlström M. Changes in interstitial noradrenaline, trapezius muscle activity and oxygen saturation during low-load work and recovery. European Journal of Applied Physiology. 2009;107(1):31–42. doi: 10.1007/s00421-009-1095-5. [DOI] [PubMed] [Google Scholar]
- 11.Ungerstedt U. Microdialysis—principles and applications for studies in animals and man. Journal of Internal Medicine. 1991;230(4):365–373. doi: 10.1111/j.1365-2796.1991.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 12.Flodgren GM, Crenshaw AG, Alfredson H, et al. Glutamate and prostaglandin E2 in the trapezius muscle of female subjects with chronic muscle pain and controls determined by microdialysis. European Journal of Pain. 2005;9(5):511–515. doi: 10.1016/j.ejpain.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Rosendal L, Larsson B, Kristiansen J, et al. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain. 2004;112(3):324–334. doi: 10.1016/j.pain.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Larsson B, Rosendal L, Kristiansen J, et al. Responses of algesic and metabolic substances to 8 h of repetitive manual work in myalgic human trapezius muscle. Pain. 2008;140(3):479–490. doi: 10.1016/j.pain.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Ashina M, Stallknecht B, Bendtsen L, et al. Tender points are not sites of ongoing inflammation—in vivo evidence in patients with chronic tension-type headache. Cephalalgia. 2003;23(2):109–116. doi: 10.1046/j.1468-2982.2003.00520.x. [DOI] [PubMed] [Google Scholar]
- 16.Boushel R, Piantadosi CA. Near-infrared spectroscopy for monitoring muscle oxygenation. Acta Physiologica Scandinavica. 2000;168(4):615–622. doi: 10.1046/j.1365-201x.2000.00713.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Canadian Journal of Applied Physiology. 2004;29(4):463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 18.Sjøgaard G, Rosendal L, Kristiansen J, et al. Muscle oxygenation and glycolysis in females with trapezius myalgia during stress and repetitive work using microdialysis and NIRS. European Journal of Applied Physiology. 2010;108(4):657–669. doi: 10.1007/s00421-009-1268-2. [DOI] [PubMed] [Google Scholar]
- 19.Kuorinka I, Jonsson B, Kilbom A, et al. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Applied Ergonomics. 1987;18(3):233–237. doi: 10.1016/0003-6870(87)90010-x. [DOI] [PubMed] [Google Scholar]
- 20.Jensen BR, Schibye B, Sogaard K, Simonsen EB, Sjogaard G. Shoulder muscle load and muscle fatigue among industrial sewing-machine operators. European Journal of Applied Physiology and Occupational Physiology. 1993;67(5):467–475. doi: 10.1007/BF00376465. [DOI] [PubMed] [Google Scholar]
- 21.Rosdahl H, Hamrin K, Ungerstedt U, Henriksson J. Metabolite levels in human skeletal muscle and adipose tissue studied with microdialysis at low perfusion flow. American Journal of Physiology. 1998;274(5):E936–E945. doi: 10.1152/ajpendo.1998.274.5.E936. [DOI] [PubMed] [Google Scholar]
- 22.Borg G. A category scale with ratio properties for intermodal and interindividual comparisons. In: Geissler H-G, et al., editors. Psychophysical Judgement and the Process of Perception. Berlin, Germany: VEB; 1982. pp. 25–34. [Google Scholar]
- 23.Melzack R, Katz J. Pain measurement in persons in pain. In: Wall PD, Melzack R, editors. Textbook of Pain. 4th edition. London, UK: Harcourt; 1999. pp. 409–411. [Google Scholar]
- 24.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979:65–70. [Google Scholar]
- 25.Carlton SM. Peripheral excitatory amino acids. Current Opinion in Pharmacology. 2001;1(1):52–56. doi: 10.1016/s1471-4892(01)00002-9. [DOI] [PubMed] [Google Scholar]
- 26.Hargreaves KM, Swift JQ, Roszkowski MT, Bowles W, Garry MG, Jackson DL. Pharmacology of peripheral neuropeptide and inflammatory mediator release. Oral Surgery, Oral Medicine, Oral Pathology. 1994;78(4):503–510. doi: 10.1016/0030-4220(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 27.Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surgery, Sports Traumatology, Arthroscopy. 1999;7(6):378–381. doi: 10.1007/s001670050184. [DOI] [PubMed] [Google Scholar]
- 28.Cairns BE, Svensson P, Wang K, et al. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. Journal of Neurophysiology. 2003;90(4):2098–2105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- 29.Rutten EPA, Engelen MPKJ, Schols AMWJ, Deutz NEP. Skeletal muscle glutamate metabolism in health and disease: state of the art. Current Opinion in Clinical Nutrition and Metabolic Care. 2005;8(1):41–51. doi: 10.1097/00075197-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Cairns BE, Dong X. The role of peripheral glutamate and glutamate receptors in muscle pain. Journal of Musculoskeletal Pain. 2008;16(1-2):85–91. [Google Scholar]
- 31.Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54(3):241–289. doi: 10.1016/0304-3959(93)90027-M. [DOI] [PubMed] [Google Scholar]
- 32.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. Journal of Physiology. 2004;557(2):599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karamouzis M, Langberg H, Skovgaard D, Bulow J, Kjaer M, Saltin B. In situ microdialysis of intramuscular prostaglandin and thromboxane in contracting skeletal muscle in humans. Acta Physiologica Scandinavica. 2001;171(1):71–76. doi: 10.1046/j.1365-201X.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- 34.Barbe MF, Barr AE. Inflammation and the pathophysiology of work-related musculoskeletal disorders. Brain, Behavior, and Immunity. 2006;20(5):423–429. doi: 10.1016/j.bbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Windhorst U. Neuroplasticity and modulation of chronic pain. In: Johansson H, Windhorst U, Djupsjöbacka M, Passatore M, editors. Chronic Work-Related Myalgia. Neuromuscular Mechanisms behind Work-Related Chronic Muscle Pain Syndromes. Gävle, Sweden: Gävle University Press; 2003. pp. 207–224. [Google Scholar]
- 36.Brooks GA. Lactate shuttles in nature. Biochemical Society Transactions. 2002;30(2):258–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 37.Kreisberg RA. Lactate homeostasis and lactic acidosis. Annals of Internal Medicine. 1980;92(2):227–237. doi: 10.7326/0003-4819-92-2-227. [DOI] [PubMed] [Google Scholar]
- 38.Stanley WC, Gertz EW, Wisneski JA. Lactate extraction during net lactate release in legs of humans during exercise. Journal of Applied Physiology. 1986;60(4):1116–1120. doi: 10.1152/jappl.1986.60.4.1116. [DOI] [PubMed] [Google Scholar]
- 39.Ashina M, Stallknecht B, Bendtsen L, et al. In vivo evidence of altered skeletal muscle blood flow in chronic tension-type headache. Brain. 2002;125(2):320–326. doi: 10.1093/brain/awf029. [DOI] [PubMed] [Google Scholar]
- 40.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. Journal of Physiology. 2004;558(1):5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kallenberg LAC, Hermens HJ. Motor unit action potential rate and motor unit action potential shape properties in subjects with work-related chronic pain. European Journal of Applied Physiology. 2006;96(2):203–208. doi: 10.1007/s00421-004-1215-1. [DOI] [PubMed] [Google Scholar]
- 42.Grassi B, Quaresima V, Marconi C, Ferrari M, Cerretelli P. Blood lactate accumulation and muscle deoxygenation during incremental exercise. Journal of Applied Physiology. 1999;87(1):348–355. doi: 10.1152/jappl.1999.87.1.348. [DOI] [PubMed] [Google Scholar]
- 43.Miura H, McCully K, Nioka S, Chance B. Relationship between muscle architectural features and oxygenation status determined by near infrared device. European Journal of Applied Physiology. 2004;91(2-3):273–278. doi: 10.1007/s00421-003-0964-6. [DOI] [PubMed] [Google Scholar]
- 44.MacLean DA, Bangsbo J, Saltin B. Muscle interstitial glucose and lactate levels during dynamic exercise in humans determined by microdialysis. Journal of Applied Physiology. 1999;87(4):1483–1490. doi: 10.1152/jappl.1999.87.4.1483. [DOI] [PubMed] [Google Scholar]
- 45.Gerdle B, Hilgenfeldt U, Larsson B, Kristiansen J, Søgaard K, Rosendal L. Bradykinin and kallidin levels in the trapezius muscle in patients with work-related trapezius myalgia, in patients with whiplash associated pain, and in healthy controls—a microdialysis study of women. Pain. 2008;139(3):578–587. doi: 10.1016/j.pain.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Järvholm U, Palmerud G, Karlsson D, Herberts P, Kadefors R. Intramuscular pressure and electromyography in four shoulder muscles. Journal of Orthopaedic Research. 1991;9(4):609–619. doi: 10.1002/jor.1100090418. [DOI] [PubMed] [Google Scholar]
- 47.Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. Journal of Neuroscience Methods. 1991;40(1):31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- 48.Rosdahl H, Ungerstedt U, Jorfeldt L, Henriksson J. Interstitial glucose and lactate balance in human skeletal muscle and adipose tissue studied by microdialysis. Journal of Physiology. 1993;471:637–657. doi: 10.1113/jphysiol.1993.sp019920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visser B, Van Dieën JH. Pathophysiology of upper extremity muscle disorders. Journal of Electromyography and Kinesiology. 2006;16(1):1–16. doi: 10.1016/j.jelekin.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. Journal of Applied Physiology. 1994;77(6):2740–2747. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- 51.Van Beekvelt MCP, Colier WNJM, Wevers RA, Van Engelen BGM. Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. Journal of Applied Physiology. 2001;90(2):511–519. doi: 10.1152/jappl.2001.90.2.511. [DOI] [PubMed] [Google Scholar]
- 52.Quaresima V, Colier WNJM, van der Sluijs M, Ferrari M. Nonuniform quadriceps O2 consumption revealed by near infrared multipoint measurements. Biochemical and Biophysical Research Communications. 2001;285(4):1034–1039. doi: 10.1006/bbrc.2001.5292. [DOI] [PubMed] [Google Scholar]
- 53.Wolf U, Wolf M, Choi JH, et al. Localized irregularities in hemoglobin flow and oxygenation in calf muscle in patients with peripheral vascular disease detected with near-infrared spectrophotometry. Journal of Vascular Surgery. 2003;37(5):1017–1026. doi: 10.1067/mva.2003.214. [DOI] [PubMed] [Google Scholar]
- 54.Kennedy MD, Haykowsky MJ, Boliek CA, Esch BTA, Scott JM, Warburton DER. Regional muscle oxygenation differences in vastus lateralis during different modes of incremental exercise. Dynamic Medicine. 2006;5:8 pages. doi: 10.1186/1476-5918-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhambhani YN. Muscle oxygenation trends during dynamic exercise measured by near infrared spectroscopy. Canadian Journal of Applied Physiology. 2004;29(4):504–523. doi: 10.1139/h04-033. [DOI] [PubMed] [Google Scholar]