Abstract
Atypical antipsychotic medications are increasingly used for a wide range of clinical indications in diverse populations, including privately and publicly insured youth and elderly nursing home residents. These trends heighten policy challenges for payers, patients, and clinicians related to appropriate prescribing and management, patient safety, and clinical effectiveness. For clinicians and patients, balancing risks and benefits is challenging, given the paucity of effective alternative treatments. For health care systems, regulators, and policymakers, challenges include developing the evidence base on comparative risks and benefits; defining measures of treatment quality; and implementing policies that encourage evidence-based practices while avoiding unduly burdensome restrictions.
BEFORE THE EARLY 1990s the use of antipsychotics was largely reserved for adults with severe psychotic disorders. Since then, however, the newer atypical antipsychotics, widely considered as safer than first-generation (“typical”) antipsychotics, have transformed the landscape of antipsychotic treatment. Approval of risperidone in 1993 was followed by olanzapine (1996), quetiapine (1997), ziprasidone (2001), aripiprazole (2002), and paliperidone (2006). Having all but replaced previously approved antipsychotics, the atypicals are now prescribed to a much larger and more diverse clinical population.
Recent studies have raised concerns regarding the safety and effectiveness of atypical antipsychotics in this broadened population.1 Major gaps remain in the evidence base supporting treatment within these new subpopulations. Particularly scarce are comparative safety and effectiveness studies across individual atypicals.
Medications have historically been a relatively small component of mental health spending. By 2007, however, U.S. spending for antipsychotic medications was estimated at $13.1 billion, exceeded only by lipid regulators and proton pump inhibitors.2 As the predominant payers for people with mental disorders, public programs are disproportionately affected by the costs of atypical antipsychotics. In recent years, antipsychotics have become the most costly drug class for Medicaid programs, exceeding the runner-up (antidepressants) by a wide margin.3 Since the 2006 implementation of Medicare prescription drug coverage, antipsychotics have also become a major expenditure item for Medicare Part D.
In addition to cost concerns, states and other payers have been concerned about appropriate balancing of risks and benefits in prescribing, and about whether psychopharmacological treatment is consistently preceded by appropriate assessment and followed by adequate monitoring. Of particular concern has been increased use in two populations: children with behavioral problems and elderly people with behavioral symptoms of dementia. In this paper we examine antipsychotic treatment trends within these two populations.4
Children And Adolescents
There has been an impressive increase in treatment of U.S. children and adolescents with atypical antipsychotics. During 1993–2002, medical office visits for youth that included antipsychotic prescriptions increased approximately five-fold.5 Although some indications suggest that growth in antipsychotic treatment of young people may have recently moderated, use remains high compared to historical and cross-national patterns.6
Clinical indications approved by the Food and Drug Administration (FDA) for antipsychotics in young people are limited to schizophrenia, behavioral symptoms in autism, Tourette's disorder, and mixed or manic bipolar episodes. Yet use for non-FDA-approved indications now accounts for most treatment and has been growing faster than treatment for FDA-approved indications.7
Atypical antipsychotics' adverse metabolic effects have heightened concern over growth in off-label prescribing to youth. In one recent eight-week trial, for example, mean weight gain was eight pounds for risperidone and thirteen pounds for olanzapine.8 Concerns about metabolic effects have motivated calls to implement the routine monitoring of metabolic status in young people during antipsychotic treatment.9
Youth in Medicaid
Medicaid is the nation's largest health care program for low-income Americans, jointly funded by the federal and state governments. We examined antipsychotic use among Medicaid youth in seven states (Exhibit 1). In 2004, 4.2 percent of enrollees ages 6–17 filled at least one prescription for an antipsychotic, up from 2.7 percent in 2001 (data not shown). The rate was 3.8 percent among enrollees ages 6–12, 4.7 percent among those ages 13–17, 5.8 percent among white youth, 3.4 percent among African Americans, and 2.1 percent among Hispanics. Almost all antipsychotic use was in the atypical class.
EXHIBIT 1.
Characteristics Of Medicaid Youth Ages 6–17 Receiving Antipsychotic Medication, In Seven States, 2001 And 2004
| Characteristic | 2001 (N = 51,093) | 2004 (N = 88,096) |
|---|---|---|
| Age (years) | ||
| 6–12 | 54.0% | 56.2% |
| 13–17 | 46.0 | 43.8 |
|
| ||
| Sex (male) | 71.3 | 69.2 |
| Race/ethnicity | ||
| White | 46.1 | 48.9 |
| African American | 24.5 | 23.5 |
| Hispanic | 11.6 | 14.4 |
| Other | 17.8 | 13.2 |
|
| ||
| Medication class | ||
| Typical antipsychotics | 8.2 | 3.4 |
| Atypical antipsychotics | 96.5 | 99.0 |
|
| ||
| Diagnosis group | ||
| Group 1–any schizophrenia | 4.0 | 3.3 |
| Group 2–any autism or MR | 5.3 | 4.9 |
| Group 3–bipolar disorder | 14.2 | 18.7 |
| Group 4–conduct disorder and/or DBD, but not ADHD | 10.9 | 8.9 |
| Group 5–conduct disorder and/or DBD, plus ADHD | 10.4 | 9.0 |
| Group 6–ADHD | 27.5 | 29.1 |
| Group 7–anxiety or depression | 9.5 | 9.1 |
| Group 8–substance abuse | 0.4 | 0.6 |
| Group 9–adjustment-related disorders | 2.0 | 1.5 |
| Group 10–other MH disorders | 6.3 | 5.9 |
| Group 11–none of above | 9.6 | 9.1 |
SOURCE: Authors' analysis of Medicaid Analytic Extracts (MAX) data, based on any antipsychotic prescription for the target year.
NOTES: MAX states include CA, FL, GA, IL, NY, OH, and TX. Exhibit includes data on all patients meeting eligibility criteria and with a claim for an antipsychotic. Medication class proportions add to more than 100 percent because some youth received drugs in both classes. MR is mental retardation. DBD is disruptive behavior diagnoses. ADHD is attention deficit hyperactivity disorder. MH is mental health.
To understand the conditions for which antipsychotics were prescribed, we constructed a hierarchical classification of diagnoses, beginning with conditions with FDA indications for youth such as schizophrenia, autism, and bipolar disorder and progressing to other conditions such as conduct disorder, attention deficit hyperactivity disorder (ADHD), anxiety, and depression (Exhibit 1). Youth were assigned to the highest-listed diagnostic category for which they had a diagnosis within the year. In 2004, almost three-quarters of Medicaid youth treated with antipsychotics were diagnosed only with conditions for which no FDA indication existed; for privately insured youth, this proportion was more than 70 percent (Exhibit 2). These estimates are likely conservative because they do not consider dosage levels outside of the approved ranges or use outside of the age ranges associated with the approved indications.
EXHIBIT 2.
Characteristics Of Privately Insured U.S. Youth Ages 6–17 Receiving Antipsychotic Medication, Selected Years 1996–2006
| 1996 (N = 349) | 2001 (N = 4,061) | 2004 (N = 16,192) | 2006 (N = 17,523) | |
|---|---|---|---|---|
| Age (years) | ||||
| 6–12 | 37.5% | 41.7% | 42.7% | 42.3% |
| 13–17 | 62.5 | 58.3 | 57.3 | 57.7 |
| Sex (male) | 69.9 | 68.6 | 65.4 | 66.4 |
| Medication class | ||||
| Typical antipsychotics | 62.8 | 9.5 | 3.7 | 3.1 |
| Atypical antipsychotics | 47.3 | 94.5 | 97.8 | 98.4 |
| Diagnosis group | ||||
| Group 1–any schizophrenia | 8.0 | 3.6 | 2.5 | 2.2 |
| Group 2–any autism or MR | 3.4 | 4.4 | 4.4 | 5.2 |
| Group 3–bipolar disorder | 11.5 | 23.8 | 22.9 | 25.2 |
| Group 4–conduct disorder and/or DBD, but not ADHD | 6.6 | 6.3 | 4.5 | 4.5 |
| Group 5–conduct disorder and/or DBD, plus ADHD | 3.7 | 4.1 | 2.8 | 2.9 |
| Group 6–ADHD | 17.8 | 18.5 | 18.9 | 21.4 |
| Group 7–anxiety or depression | 19.5 | 18.0 | 16.7 | 16.0 |
| Group 8–substance abuse | 0.3 | 0.4 | 0.6 | 0.5 |
| Group 9–adjustment-related disorders | 2.0 | 1.4 | 1.2 | 1.5 |
| Group 10–other MH disorders | 4.6 | 6.4 | 5.7 | 6.0 |
| Group 11–none of above | 22.6 | 13.1 | 19.8 | 14.6 |
SOURCE: Authors' analysis of Thomson MarketScan data.
NOTES: Exhibit includes data on all patients meeting eligibility criteria and with a claim for an antipsychotic. MR is mental retardation. DBD is disruptive behavior diagnoses. ADHD is attention deficit hyperactivity disorder. MH is mental health.
ADHD without diagnoses for schizophrenia, autism, or bipolar disorder accounted for more than one-third of Medicaid youth receiving antipsychotics in 2004 (Exhibit 1). Among youth with externalizing disorders, antipsychotics are often used to control aggressive behavior. Yet few well-controlled clinical trials exist to guide antipsychotic treatment in this population.
Metabolic risks of treating youth in the Medicaid program with antipsychotics may exceed those for youth in the general population. Because the risk of childhood obesity is inversely related to socioeconomic status, low-income children who are already at high risk for obesity and related metabolic disorders may be especially vulnerable to the adverse effects of weight gain.10 Nonpharmacological alternatives, which may involve teaching children problem-solving skills and teaching their parents to reward positive child behavior, are costly and difficult to disseminate. Given the large number of Medicaid youth treated with antipsychotics for disruptive behavior disorders, scant empirical support for efficacy, and known metabolic risks, community pharmacological treatment of Medicaid youth with externalizing disorders remains an area of specific concern.
Privately insured youth
More than half (56 percent) of U.S. children have employer-based health insurance.11 To examine antipsychotic use among privately insured youth ages 6–17, we examined Thomson MarketScan data. The overall rate of antipsychotic use is much lower in this population than in Medicaid youth, perhaps because of lower rates of mental disorders or less-aggressive treatment than among Medicaid-insured populations.12
Antipsychotic treatment rates among privately insured youth increased steadily from 1996 (0.21 percent) to 2006 (0.90 percent) (Exhibit 3). The rate in 2006 was 0.70 percent among those ages 6–12 and 1.13 percent among those ages 13–17 (data not shown). ADHD and disruptive behavior diagnoses accounted for a much smaller proportion of privately insured (26.2 percent) than Medicaid (47.0 percent) youth treated with antipsychotics, and bipolar disorder a larger share of privately insured (22.9 percent) than in Medicaid-insured youth (18.7 percent) in 2004 (Exhibits 1–2). The increase in antipsychotic treatment also appears to have been more gradual among privately insured than Medicaid children during 2001–04.13 Without structured diagnostic clinical interviews, the extent to which these populationwide differences in clinical diagnoses reflect variation in psychopathology or variation in diagnostic and treatment practices is unclear. Some treated youth in each population may have nonpsychotic prodromal (precursory) symptoms of schizophrenia or bipolar disorder.
EXHIBIT 3.
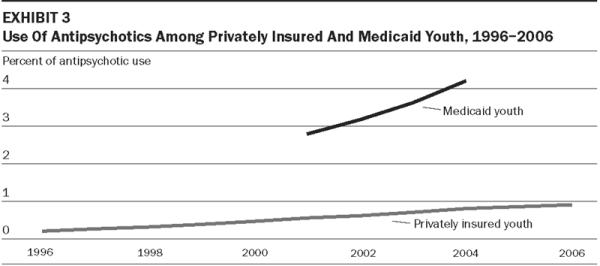
Use Of Antipsychotics Among Privately Insured And Medicaid Youth, 1996–2006
SOURCE: Authors' analyses of data from Medicaid Analytic Extracts (MAX) and Thomson MarketScan (for privately insured youth), based on any antipsychotic prescription in the target year.
Elderly People In Nursing Homes
As in youth, use of antipsychotics to manage behavioral problems increased among the elderly following introduction of the atypicals and now faces new safety and efficacy concerns. Antipsychotic drugs are widely used to treat common behavioral symptoms of dementia such as agitation, aggression, irritability, disinhibition, wandering, and anxiety.14 Controversies over use in nursing homes extend back to the first-generation era.15 Treatment rates declined in the early 1990s following regulatory reforms in the Omnibus Budget Reconciliation Act (OBRA) of 1987 but greatly increased in the mid- and late 1990s as atypicals gained market share. Despite new safety concerns, use has remained highly prevalent, perhaps because few adequate alternatives are perceived to be readily available for management of the behavioral symptoms of dementia.16 Recent efforts to reduce reliance on physical restraints appear to have been much more successful than those to reduce reliance on antipsychotics.
Deaths associated with antipsychotics
In the mid-2000s, evidence accumulated of increased death rates associated with antipsychotic treatment of the elderly.17 In April 2005, based on meta-analyses of randomized clinical trials, the FDA issued a public health advisory finding antipsychotic use to be associated with increased risk for death. In trials averaging eight to twelve weeks, relative risk of death increased about 60 percent, and absolute mortality increased by about 2 percent for patients treated with antipsychotics as compared with placebo-treated patients. Other meta-analyses confirmed this picture and concluded that the risk-benefit ratio is generally unfavorable.18 One large effectiveness study randomized patients with Alzheimer's disease and psychosis, aggression, or agitation to olanzapine, quetiapine, risperidone, or placebo; it found no significant group differences in time to treatment discontinuation. Time to discontinuation for lack of efficacy favored risperidone and olanzapine but was offset by greater discontinuation of these medications because of their adverse effects.19
Prevalent antipsychotic use in 2006
To examine patterns of antipsychotic use in elderly nursing home residents, we used data from the Nursing Home Minimum Data Set (MDS) for 1999 and 2006 for eight states (CA, FL, GA, IL, NJ, NY, OH, and TX), representing more than 40 percent of residents nationally. In 2006, 27.6 percent of nursing home residents had received an antipsychotic medication within the past seven days (Exhibit 4). Use rates were 28.8 percent in for-profit facilities versus 24.7 percent in not-for-profit homes. Antipsychotic treatment rates vary across hierarchical diagnostic subgroups. Among residents with dementia, those with aggressive behavioral symptoms might be considered to have stronger treatment indications, given potential risks of injury associated with this behavior; 51.2 percent of this group received antipsychotic treatment (Exhibit 4). Among those with nonaggressive behavioral symptoms, the rate was still quite high (39.5 percent). Even among dementia patients without reported behavioral symptoms, 22.6 percent received antipsychotics.
EXHIBIT 4.
Antipsychotic (AP) Use In The Past Seven Days Among Nursing Home (NH) Residents Age 65 And Older, 1999 And 2006
| 1999 |
2006 |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. NH residents in subgroup | Prop. total NH residents in subgroup | AP use rate within subgroup (row %) | Char. of NH elderly receiving AP (column %) | No. NH residents in subgroup | Prop. total NH residents in subgroup | AP use rate within subgroup (row %) | Char. of NH elderly receiving AP (column %) | |
| Total | 357,969 | 100.0 | 20.2 | 100.0a | 354,385 | 100.0 | 27.6 | 100.0b |
|
| ||||||||
| Age (years) | ||||||||
| 65–74 | 47,298 | 13.2 | 31.2 | 20.4 | 51,468 | 14.5 | 38.1 | 20.0 |
| 75–84 | 126,089 | 35.2 | 23.0 | 40.0 | 124,474 | 35.1 | 30.4 | 38.6 |
| ≥85 | 184,582 | 51.6 | 15.5 | 39.6 | 178,443 | 50.4 | 22.7 | 41.4 |
|
| ||||||||
| Sex | ||||||||
| Male | 85,738 | 24.0 | 23.3 | 27.6 | 91,343 | 25.8 | 30.9 | 28.8 |
| Female | 272,231 | 76.1 | 19.2 | 72.3 | 263,042 | 74.2 | 26.5 | 71.2 |
|
| ||||||||
| Race/ethnicity | ||||||||
| White | 297,535 | 83.1 | 19.9 | 82.0 | 275,013 | 77.6 | 27.5 | 77.2 |
| African American | 38,789 | 10.8 | 22.2 | 11.9 | 45,997 | 13.0 | 28.1 | 13.2 |
| Hispanic | 15,237 | 4.3 | 23.2 | 4.9 | 23,498 | 6.6 | 32.0 | 7.7 |
| Other | 6,408 | 1.8 | 13.8 | 1.2 | 9,877 | 2.8 | 19.7 | 2.0 |
|
| ||||||||
| Facility ownership status | ||||||||
| Governmentowned | 14,760 | 4.1 | 21.2 | 4.3 | 16,111 | 4.6 | 25.9 | 4.3 |
| Private for-profit | 261,291 | 73.0 | 21.0 | 76.0 | 250,003 | 70.6 | 28.8 | 73.5 |
| Private non-for-profit | 81,918 | 22.9 | 17.4 | 19.7 | 88,271 | 24.9 | 24.7 | 22.3 |
|
| ||||||||
| Diagnosis | ||||||||
| Group 1–schizophrenia | 14,967 | 4.2 | 74.8 | 15.5 | 18,424 | 5.2 | 81.2 | 15.3 |
| Group 2–bipolar disorder | 5,116 | 1.4 | 57.5 | 4.1 | 8,056 | 2.3 | 65.1 | 5.4 |
| Group 3–dementia and aggressive behavioral symptoms | 36,404 | 10.2 | 39.3 | 19.8 | 27,017 | 7.6 | 51.2 | 14.1 |
| Group 4–dementia and nonaggressive behavioral symptoms | 48,612 | 13.6 | 29.0 | 19.5 | 47,802 | 13.5 | 39.5 | 19.3 |
| Group 5–dementia w/o behavioral symptoms | 103,221 | 28.8 | 15.0 | 21.4 | 125,148 | 35.3 | 22.6 | 28.9 |
| Group 6–depression or anxiety disorder | 58,705 | 16.4 | 13.1 | 10.6 | 67,877 | 19.2 | 16.4 | 11.4 |
| Group 7–none | 90,944 | 25.4 | 7.3 | 9.2 | 60,061 | 17.0 | 9.3 | 5.7 |
SOURCE:Authors' analysis of Minimum Data Set (MDS) data, 1999 and 2006, for CA, FL, GA, IL, NJ, NY, OH, and TX.
NOTES:Based on last full non-admission assessment in 1999 or 2006 (residents with long-term stays). “Char.” is characteristics.
N = 72,341.
N = 97,939.
In 2006, most use was for residents without an FDA diagnostic indication. Schizophrenia or bipolar disorder, the primary adult indications, accounted for only 20.7 percent. Of the remaining almost 80 percent, only 14.1 percent had dementia with aggressive behavioral symptoms (Exhibit 4).
Growth in usage rates
Antipsychotic use increased 7.4 percentage points from 1999 to 2006 (Exhibit 4). This reflects both increasing proportions of residents diagnosed with schizophrenia, bipolar disorder, dementia, depression, or anxiety disorder and an increase in antipsychotic treatment rates within each diagnostic category. Use increased despite new safety concerns, and residents diagnosed with schizophrenia, bipolar disorder, or aggressive behavioral symptoms of dementia accounted for a declining percentage of antipsychotic use, from 39.4 percent to 34.8 percent of users. Given the risk of adverse events such as strokes and increased mortality, available evidence suggests that antipsychotics should be used cautiously in the nursing home population, with treatment generally reserved for residents with schizophrenia or bipolar disorder, or severe behavioral symptoms such as aggressive behavior. Clinical recommendations further emphasize that treatment should be carefully monitored and short-term in duration and that periodic trials of antipsychotic discontinuation should be conducted.20
Discussion And Policy Implications
Antipsychotic treatment among youth and the elderly has increased and broadened in recent years to a more clinically diverse population. Although off-label use is common for many classes of drugs and does not necessarily by itself imply a quality concern, it is particularly prevalent in the antipsychotic class. The pattern of broadened use is also apparent among nonelderly adults, with use broadening beyond the traditional core treated population of people with schizophrenia among Medicaid adults (data not shown). This trend is likely to continue in the wake of new FDA indications for treatment-resistant depression for two of the atypical antipsychotics and extensive direct-to-consumer ad campaigns for these indications.
Our analyses offer little insight into clinical decision-making processes at the individual patient level. In addition, diagnostic coding might not reflect the actual clinical reasons for prescribing atypical antipsychotics, which might not map well in any event to established categories and might be more focused on problematic behavior than on underlying disorders.21 Nevertheless, the trends, patterns, and treatment rates appear to reflect major changes in the balancing of risks versus compelling clinical need in the atypical antipsychotic era.
Recent safety concerns
As clinical experience and postmarketing research with atypicals have accumulated, new safety concerns have arisen. For the elderly, the FDA's 2005 public health advisory provided a clear warning. In children and youth, although evidence of harm is less clear-cut, increased safety concerns have also emerged, particularly with respect to adverse metabolic outcomes.22 A particular concern for children has been the long-term developmental, metabolic, and other effects of treatment—a difficult issue to study with randomized clinical trials, which are typically short-term in duration. Cross-national studies suggest that treatment rates in the United States exceed those in several other industrialized countries.23
Challenges for physicians
Physicians confront difficult challenges in the appropriate use of these powerful medicines. For a broad range of patients with disturbing behavior, across the age span, physicians often must grapple with families' or facilities' expectations that “something needs to be done,” in the face of insufficient data on comparative effectiveness and long-term safety and few if any accessible, effective treatment alternatives. Despite safety concerns, prescribing patterns suggest a high level of perceived need and potential effectiveness for atypical antipsychotics in a range of populations. At the same time, broadened use has raised concerns about appropriateness, safety, and management of medication use. Areas of particular concern include concurrent prescribing of multiple antipsychotic drugs (polypharmacy); high rates of use among vulnerable populations including foster-care youth and very young children; and the adequacy of metabolic monitoring and appropriate dosing.24
Dilemmas for payers
These concerns have raised considerable dilemmas for payers, who must balance concerns about protecting clinical flexibility, respecting prescribers' autonomy, and ensuring access to needed treatments with concerns about safety, quality improvement, and cost containment. Policies such as prior authorization (PA) and preferred drug lists (PDLs), often used with other medication classes to “steer” use toward selected drugs considered preferable on grounds of safety, effectiveness, or pricing, can be difficult to apply with antipsychotics because of the heterogeneity of side-effect profiles and treatment responses among the available medications and the clinical complexities of matching individual patients and medications most suitable to them. Individual responses to medications may vary, and histories of response to particular medications may need to be considered. Thus, policies that unduly constrain providers' prescribing choices or introduce an overly burdensome “hassle factor,” such as PA policies, could have the potential to impede access to optimal treatment or to lead to dangerous clinical deterioration of patients previously stabilized on particular medication regimens.25
Educational and policy efforts to date
Payers and policymakers have sought to develop programs to assess the appropriateness of antipsychotic treatment. Other efforts have focused on prescriber education, such as continuing medical education programs and dissemination of guidelines. By themselves, however, such educational efforts tend to have only limited impact on prescribing behavior.26 “Academic detailing,” in which independent, academically oriented people armed with the latest empirical information meet with health care professionals in their offices, is expensive and has not been demonstrated to be effective in mental health care.27 Providing feedback to physicians on their prescribing patterns tends to have only a small to moderate impact, although this avenue may have some potential as a component of more-systematic efforts to engage prescribers in quality improvement initiatives.28
Prior authorization
PA procedures have been used by some payers, often implemented as part of “prospective drug utilization” within automated point-of-sale pharmacy payment systems. Such procedures can take a variety of forms, ranging from common edits for such parameters as appropriate dosage, therapeutic duplication, and time since last refill, to more important restrictions such as limitations related to a patient's age, diagnoses, “fail-first” requirements that require a trial of a “preferred” medication before use of others, edits for concurrent use of multiple antipsychotics, and other restrictions that are more likely to affect common prescribing practices. Such interventions, however, have been criticized for their potential “hassle factor” for clinicians.29 Thus, their potential benefits need to be carefully weighed against potential adverse effects on optimal treatment and outcomes.30 Existing PA policies vary widely in their scope. A 2005–06 study reported that about half of the states had PA policies affecting the prescribing of atypical antipsychotics, but many were of limited reach (for example, PA required for clozapine), and by mid-2006, no state had made PA changes specifically responding to the FDA's advisory on increased mortality among elderly people taking atypicals.31 The shift of dual-eligible beneficiaries (those eligible for both Medicare and Medicaid) to Part D plans may have deterred states from taking such actions.
Second-opinion programs and warning labels
Second-opinion programs also have been implemented with success in some states. This approach can help address widespread difficulties in obtaining adequate mental health specialty care and provide expert assessment for complex or treatment-unresponsive patients.32 A further route to addressing prescribing patterns perceived as problematic involves changes in FDA labeling to highlight risks, as with the FDA “black box” warnings for use of antipsychotic drugs among elderly with behavioral symptoms of dementia. However, the impact of such warnings may also be limited.33
Quality metrics
To improve the monitoring of prescribing patterns and inform efforts at quality improvement, there is a need for appropriate quality indicators. Yet despite some development of metrics, more work in this area needs to be done, and it is not clear yet that improvements have resulted.34
Despite a range of efforts by payers and policymakers to address antipsychotic prescribing, a fundamental challenge is the adequacy of the evidence base on safety and effectiveness for the clinically diverse range of patients who are treated with these agents. Particularly scarce are comparative effectiveness and safety studies that assess outcomes of individual drugs, head-to-head, in various clinical populations of concern. A few publicly funded studies of this kind have been conducted in recent years, such as the Clinical Antipsychotic Trials in Intervention Effectiveness (CATIE). These are valuable but are costly, complex, and vulnerable to methodological critique and take a long time to plan and complete. Further efforts to build the evidence base through a variety of strategies, including randomized-trial and observational methods, are greatly needed. Until more-definitive comparative safety and effectiveness data become available, policymakers and payers will be challenged to craft balanced policies in an area in which the economic and clinical stakes are considerable for patients, families, and society.
Acknowledgments
This study was supported by the Agency for Healthcare Research and Quality's (AHRQ's) Centers for Education and Research on Therapeutics (CERTs) program through award no. U18-HS016097 to Rutgers for the Center for Education and Research on Mental Health Therapeutics, with additional support from the Retirement Research Foundation (RRF) and from the National Institutes of Health (NIH), award nos. MH076206, UL1-RR024156, and UL1-RR024153. The paper represents the views of the authors and does not necessarily represent those of AHRQ, RRF, or NIH. Portions of the findings were included in presentations at the fall meeting of the National Association of State Medicaid Directors, Washington, D.C., November 2008; at the AHRQ Comparative Effectiveness Research Methods Symposium, June 2009; at a presentation at the Centers for Medicare and Medicaid Services in June 2009; and at the Annual Research Meeting of AcademyHealth, Chicago, Illinois, June 2009. The authors thank Ece Kalay for computational assistance and Judith Lucas for contributions to the research.
NOTES
- 1.See Shekelle P, et al. [accessed 1 July 2009];Comparative Effectiveness of Off-Label Use of Atypical Antipsychotics. Comparative Effectiveness Review no. 6, January 2007, http://www.effectivehealthcare.ahrq.gov/repFiles/Atypical_ Antipsychotics_Final_Report.pdf..
- 2.IMS Health [accessed 1 July 2009];2007 Top Therapeutic Classes by U.S. Sales. 2008 http://imshealth.com/imshealth/Global/Content/Document/Top-Line%20Industry%20Data/2006%20Top%2010%20Therapeutic%20Classes% 20by%20US%20Sales.pdf.
- 3.Esposito D, et al. Trends in Medicaid Prescription Drug Use and Costs, 1999 to 2002: Evidence from Medicaid Analytic Extract Data. AcademyHealth Annual Research Meeting; Washington, D.C.. June 2007. [Google Scholar]; Centers for Medicare and Medicaid Services [accessed 1 July 2009];Chartbook: Medicaid Pharmaceutical Benefit Use and Reimbursement in 2003. http://www.cms.hhs.gov/MedicaidDataSourcesGenInfo/downloads/Pharmacy_RX_Chartbook_2003.pdf.
- 4.For a description of our data sources, study population, and study methods, see the Methods Appendix, online at http:content.healthaffairs.org/cgi/content/full/hlthaff.28.5.w770/DC2.
- 5.Olfson M, et al. National Trends in the Outpatient Treatment of Children and Adolescents with Antipsychotic Drugs. Archives of General Psychiatry. 2006;63(no. 6):679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- 6.Constantine R, Tandon R. Changing Trends in Pediatric Antipsychotic Use in Florida's Medicaid Program. Psychiatric Services. 2008;59(no. 10):1162–1168. doi: 10.1176/ps.2008.59.10.1162. [DOI] [PubMed] [Google Scholar]
- 7.Cooper WO, et al. New Users of Antipsychotic Medications among Children Enrolled in TennCare. Archives of Pediatrics and Adolescent Medicine. 2004;158(no. 8):753–759. doi: 10.1001/archpedi.158.8.753. [DOI] [PubMed] [Google Scholar]
- 8.Sikich L, et al. Double-Blind Comparison of First- and Second-Generation Antipsychotics in Early-Onset Schizophrenia and Schizo-Affective Disorder: Findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) Study. American Journal of Psychiatry. 2008;165(no. 11):1420–1431. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correll CU. Monitoring and Management of Antipsychotic-Related Metabolic and Endocrine Adverse Events in Pediatric Patients. International Review of Psychiatry. 2008;20(no. 2):195–201. doi: 10.1080/09540260801889179. [DOI] [PubMed] [Google Scholar]
- 10.Shrewsbury V, Wardle J. Socioeconomic Status and Adiposity in Childhood: A Systematic Review of Cross-Sectional Studies, 1990–2005. Obesity. 2008;16(no. 2):275–284. doi: 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
- 11.Henry J, Kaiser Family Foundation [accessed 1 July 2009];Health Insurance Coverage of America's Children. 2007 January; http://www.allhealth.org/BriefingMaterials/HealthInsuranceCoverageofAmericasChildren-549.pdf.
- 12.Glied S, et al. Children's Access to Mental Health Care: Does Insurance Matter? Health Affairs. 1997;16(no. 1):167–174. doi: 10.1377/hlthaff.16.1.167. [DOI] [PubMed] [Google Scholar]; Martin A, et al. Datapoints: Use of Multiple Psychotropic Drugs by Medicaid-Insured and Privately Insured Children. Psychiatric Services. 2002;53(no.12):1508. doi: 10.1176/appi.ps.53.12.1508. [DOI] [PubMed] [Google Scholar]
- 13.Patel NC, et al. Trends in the Use of Typical and Atypical Antipsychotics in Children and Adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(no. 6):548–556. doi: 10.1097/01.chi.0000157543.74509.c8. [DOI] [PubMed] [Google Scholar]
- 14.Jeste DV, et al. ACNP White Paper: Update on Use of Antipsychotic Drugs in Elderly Persons with Dementia. Neuropsychopharmacology. 2008;33(no. 5):957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shorr RI, Fought RL, Ray WA. Changes in Antipsychotic Drug Use in Nursing Homes during Implementation of the OBRA-87 Regulations. Journal of the American Medical Association. 1994;271(no. 5):358–362. [PubMed] [Google Scholar]
- 16.Salzman C, et al. Elderly Patients with Dementia-Related Symptoms of Severe Agitation and Aggression: Consensus Statement on Treatment Options, Clinical Trials Methodology, and Policy. Journal of Clinical Psychiatry. 2008;69(no. 6):889–898. doi: 10.4088/jcp.v69n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider LS, Dagerman KS, Insel P. Risk of Death with Atypical Antipsychotic Drug Treatment for Dementia: Meta-Analysis of Randomized Placebo-Controlled Trials. Journal of the American Medical Association. 2005;294(no. 15):1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 18.Ibid.
- 19.Schneider LS, et al. Effectiveness of Atypical Antipsychotic Drugs in Patients with Alzheimer's Disease. New England Journal of Medicine. 2006;355(no. 15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 20.Salzman, et al. Elderly Patients with Dementia-Related Symptoms [Google Scholar]; Jeste, et al. ACNP White Paper [Google Scholar]
- 21.First MB, Pincus HA, Schoenbaum M. Issues for DSM-V: Adding Problem Codes to Facilitate Assessment of Quality of Care. American Journal of Psychiatry. 2009;166(no. 1):11–13. doi: 10.1176/appi.ajp.2008.08010016. [DOI] [PubMed] [Google Scholar]
- 22.Sikich, et al. Double-Blind Comparison [Google Scholar]; Correll Monitoring and Management [Google Scholar]
- 23.Zito JM, et al. A Three-Country Comparison of Psychotropic Medication Prevalence in Youth. Child and Adolescent Psychiatry and Mental Health. 2008;2(no. 1):26. doi: 10.1186/1753-2000-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilmer TP, et al. Antipsychotic Polypharmacy Trends among Medicaid Beneficiaries with Schizophrenia in San Diego County, 1999–2004. Psychiatric Services. 2007;58(no. 7):1007–1010. doi: 10.1176/ps.2007.58.7.1007. [DOI] [PubMed] [Google Scholar]; Zito JM, et al. Psychotropic Medication Patterns among Youth in Foster Care. Pediatrics. 2008;121(no. 1):e157–e163. doi: 10.1542/peds.2007-0212. [DOI] [PubMed] [Google Scholar]; Kogut SJ, Yam F, Dufresne R. Prescribing of Antipsychotic Medication in a Medicaid Population: Use of Polytherapy and Off-Label Dosages. Journal of Managed Care Pharmacy. 2005;11(no. 1):17–24. doi: 10.18553/jmcp.2005.11.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law MR, Ross-Degnan D, Soumerai SB. Effect of Prior Authorization of Second-Generation Antipsychotic Agents on Pharmacy Utilization and Reimbursements. Psychiatric Services. 2008;59(no. 5):540–546. doi: 10.1176/ps.2008.59.5.540. [DOI] [PubMed] [Google Scholar]
- 26.Farmer AP, et al. Printed Education Materials: Effects on Professional Practice and Health Care Outcomes. Cochrane Database of Systematic Reviews. 2008;16(no. 3):CD004398. doi: 10.1002/14651858.CD004398.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Soumerai SB, Avorn J. Principles of Educational Outreach (`Academic Detailing') to Improve Clinical Decision Making. Journal of the American Medical Association. 1990;263(no. 4):549–556. [PubMed] [Google Scholar]
- 28.Jamtvedt G, et al. Audit and Feedback: Effects on Professional Practice and Health Care Outcomes. Cochrane Database of Systematic Reviews. 2006;19(no. 2):CD000259. doi: 10.1002/14651858.CD000259.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Wilk JE, et al. Medicare Part D Prescription Drug Benefits and Administrative Burden in the Care of Dually Eligible Psychiatric Patients. Psychiatric Services. 2008;59(no. 1):34–39. doi: 10.1176/ps.2008.59.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Law, et al. Effect of Prior Authorization [Google Scholar]; West JC, et al. Medication Access and Continuity: The Experiences of Dual-Eligible Psychiatric Patients during the First Four Months of the Medicare Prescription Drug Benefit. American Journal of Psychiatry. 2007;164(no. 5):789–796. doi: 10.1176/ajp.2007.164.5.789. [DOI] [PubMed] [Google Scholar]
- 31.Polinski JM, Wang PS, Fischer MA. Medicaid's Prior Authorization Program and Access to Atypical Antipsychotic Medications. Health Affairs. 2007;26(no. 3):750–760. doi: 10.1377/hlthaff.26.3.750. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JN, et al. Second Opinions Improve ADHD Prescribing in a Medicaid-Insured Community Population. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(no. 7):740–748. doi: 10.1097/CHI.0b013e3181a2b2ed. [DOI] [PubMed] [Google Scholar]
- 33.Harris G. Use of Antipsychotics in Children Is Criticized. New York Times. 2008 November 18; [Google Scholar]
- 34.National Committee for Quality Assurance . The State of Health Care Quality 2006. NCQA; Washington: 2006. [Google Scholar]; Bremer RW, et al. Pay for Performance in Behavioral Health. Psychiatric Services. 2008;59(no. 12):1419–1429. doi: 10.1176/ps.2008.59.12.1419. [DOI] [PubMed] [Google Scholar]


