Abstract
In skeletal muscle excitation–contraction (EC) coupling the sarcolemmal L-type Ca2+ channel or 1,4-dihydropyridine receptor (DHPR) transduces the membrane depolarization signal to the sarcoplasmic Ca2+ release channel RyR1 via protein–protein interaction. While it is evident that the pore-forming and voltage-sensing DHPRα1S subunit is essential for this process, the intracellular DHPRβ1a subunit was also shown to be indispensable. We previously found that the β1a subunit is essential to target the DHPR into groups of four (tetrads) opposite the RyR1 homotetramers, a prerequisite for skeletal muscle EC coupling. Earlier, a unique hydrophobic heptad repeat motif (L⋯V⋯V) in the C-terminus of β1a was postulated by others to be essential for skeletal muscle EC coupling, as substitution of these residues with alanines resulted in 80% reduction of RyR1 Ca2+ release. Therefore, we wanted to address the question if the proposed β1a heptad repeat motif could be an active element of the DHPR–RyR1 signal transduction mechanism or already contributes at the ultrastructural level i.e. DHPR tetrad arrangement. Surprisingly, our experiments revealed full tetrad formation and an almost complete restoration of EC coupling in β1-null zebrafish relaxed larvae and isolated myotubes upon expression of a β1a-specific heptad repeat mutant (LVV to AAA) and thus contradict the earlier results.
Abbreviations: EC, excitation–contraction; DHPR, 1,4-dihydropyridine receptor; RyR1, ryanodine receptor type-1; SR, sarcoplasmic reticulum; zf-, β1azebrafish β1a; rb-, β1arabbit β1a; GFP, green fluorescent protein; nt, nucleotide numbers; WT, wild-type; hpf, hours post-fertilization
Keywords: L-type voltage-gated calcium channels, DHPR–RyR1 interaction, Tetrads, Zebrafish relaxed, RNA zygote injection, Phenotype rescue, Larval motility analysis
1. Introduction
Excitation–contraction (EC) coupling is understood as the signal transduction process linking membrane depolarization to the contraction of a muscle cell. In skeletal muscle this involves a direct crosstalk between two Ca2+ channels, the plasmalemmal high voltage-activated skeletal muscle Ca2+ channel or 1,4-dihydropyridine receptor (DHPR) and the intracellular Ca2+ release channel, the ryanodine receptor type-1 (RyR1) in the sarcoplasmic reticulum (SR) membrane. Membrane depolarization induces conformational changes in the voltage-sensing DHPRα1S which are transduced to RyR1 via protein–protein interaction [1,2]. This leads to opening of RyR1 without the need of DHPR Ca2+ influx [3]. Both the channels are targeted into the muscle triad junctions where clusters of DHPR in the sarcolemma co-localize with clusters of RyR1 in closely juxtaposed SR membrane [4,5]. Ultrastructurally, DHPRs, visible as “particles” in freeze-fracture images, are arranged in groups of four (tetrads) to communicate with every other RyR1 homotetramer and thus are geometrically arranged in orthogonal arrays following the RyR1-arrays [6,7].
The skeletal muscle DHPR is a hetero-multimeric protein complex consisting of the central α1S subunit and the auxiliary subunits β1a, α2δ-1, and γ1 [8]. According to the current model, the pore-forming and voltage-sensing α1S subunit transduces the opening signal to RyR1, essentially via the intracellular loop connecting homologous repeats II and III [9,10]. The intracellular β1a subunit was shown to have multiple roles in targeting and modulating the central α1S subunit [11,12]. The lack of β1a is incompatible with skeletal muscle EC coupling, and leads to perinatal lethality in β1-null mice due to respiratory paralysis [13] or to an immotile larval phenotype in the β1-null zebrafish mutant relaxed [14,15]. As shown in zebrafish relaxed the absence of β1a specifically leads to, (i) reduction of α1S membrane targeting, (ii) severe reduction in α1S charge movement, and (iii) complete absence of the ultrastructural arrangement of DHPRs into tetrads in orthogonal arrays, a prerequisite for skeletal muscle EC coupling [14].
Despite the inability to ventilate their gills, relaxed larvae are able to survive for few days due to oxygen and metabolite diffusion via the skin [16]. This in combination with the possibility of zygote injection into the externally developing embryos makes zebrafish mutant relaxed an excellent in vivo expression system. In a reconstitution study we showed that the expression of β1a in the relaxed system restored the motile phenotype and that DHPR tetrad formation is an exclusive property of β1a [17]. Thus, specific structural elements important for the formation of tetrads must exist in β1a.
Earlier studies, with β1a/β2a chimeras and truncation mutants expressed in murine β1-null myotubes, restricted the domain of β1a that is essential for skeletal muscle EC coupling to its C-terminus [18–20]. More precisely, a β1a-specific C-terminal hydrophobic heptad repeat motif (L478, V485, V492) was proposed to control the EC coupling activity [22]. A β1a mutant with the heptad repeat LVV exchanged to alanines could only reconstitute 20% of intracellular Ca2+ transients and also induced a significant positive shift in their voltage-dependence. Leucine heptad repeat motifs are well known to mediate protein–protein interactions [21]. On this basis it was hypothesized that the heptad repeat of β1a is involved in direct interaction with RyR1 [20,22] and thus plays an active role in the signal transduction from DHPR to RyR1 via β1a.
Moreover, since we showed that β1a is specifically required for tetrad formation [17] which is a prerequisite for EC coupling, it is also possible that already the arrangement of DHPRs into tetrads is dependent on this β1a-specific heptad repeat motif. Therefore, we wanted to address this question by comparing the ultrastructural arrangement of DHPR with functional recordings of EC coupling. If, despite the lack of the LVV motif, tetrads but no intact EC coupling could be restored, the hypothesis of β1a as a direct signal transducer – as an additional β1a function – would be supported. On the other hand, if tetrads were not formed, the conclusion would be that the LVV motif is simply involved in the initial scaffolding process which ultrastructurally enables the interaction of DHPR and RyR1 by placing them in the appropriate relative configuration.
We took advantage of the β1a-null zebrafish relaxed expression system that allows us to directly compare the effects observed in vitro to an intact in vivo muscle system. Mutant constructs from zebrafish β1a (zf-β1aAAA) and from a mammalian (rabbit) β1a (rb-β1aAAA), in which the conserved hydrophobic heptad repeat motif LVV was exchanged to alanines, were expressed in isolated relaxed myotubes and entire larvae. Our results with β1aAAA show that knock out of the LVV motif did not interfere with correct targeting of DHPR into tetrads. Furthermore, and to our surprise, heptad repeat mutants were able to restore robust intracellular Ca2+ transients in relaxed myotubes and a fully motile phenotype in relaxed larvae, thus illustrating restoration of proper DHPR–RyR1 coupling. In contrast to the earlier proposals, our results indicate that the β1a-specific C-terminal heptad repeat motif LVV is not a critical determinant of skeletal muscle EC coupling, because it is neither necessary for tetrad formation nor for DHPR–RyR1 signal transduction.
2. Materials and methods
Experimental procedures were essentially the same as described earlier in detail [17] and thus only a concise summary is given.
2.1. Zebrafish embryos
Rearing and breeding of zebrafish, heterozygous for the β1-null mutation relaxed (redts25) was performed according to the established procedures [23,24]. Freshly spawned eggs were directly used for zygote RNA microinjection and/or raised at 28 °C to be used for experiments.
2.2. Expression plasmids
The cDNAs of the β subunits and mutants were N-terminally in-frame fused to GFP cDNA and cloned into expression vector pCI-neo (Promega). Nucleotide numbers (nt) are given in parenthesis. All sequences generated and modified by PCR were checked for integrity by sequence analysis (Eurofins MWG Operon, Martinsried, Germany).
2.2.1. zf-β1aAAA
Fusion PCR was used to generate LVV/AAA substitutions (L478A, V485A, V492A) with zf-β1a cDNA (GenBank AY952462) in pCI-neo as template. The sense primer was used for T to C transition which created triplet codons GCC (nt 1452–1454) and GCG (nt 1473–1475) both coding for alanine instead of valine. With the antisense primer, triplets CTG (nt 1431–1433) and GTC (nt 1452–1454) were mutated to GCG and GCC respectively, both coding for alanine instead of leucine and valine. To gain the final construct zf-β1aAAA, the PCR-generated EarI–XbaI fusion fragment (nt 879–1803) was co-ligated with fragment EcoRV–EarI from zf-β1a (nt −748 to 879) into the EcoRV/XbaI (nt −748 to1803) cleaved zf-β1a clone.
2.2.2. rb-β1aAAA
The LVV/AAA substitutions (L478A, V485A, V492A) were created by using the fusion PCR technique with rb-β1a cDNA (GenBank NM_001082279) in pCI-neo as template. The sense primer substituted GTC (1452–1454) and GTG (1473–1475) to GCC and GCG respectively, both coding for alanine instead of valine. The antisense primer was used to replace CTG (1431–1433) and GTC (1452–1454) with GCG and GCC respectively, both coding for alanine instead of leucine and valine. The resulting BstXI–XbaI (nt 834–1801) fusion product was ligated together with fragment EcoRV–BstXI from rb-β1a (nt −763 to 834) into the EcoRV/XbaI (nt −763 to 801) cut rb-β1a to generate the final construct rb-β1aAAA.
2.2.3. GFP
GFP alone was cloned into expression vector pCI-neo for standardizing experimental conditions [17].
2.3. Zygote injection of in vitro synthesized RNA
All β subunits and mutants were linearized with restriction enzyme XbaI, but GFP with NotI. Purified and linearized DNA templates were used for in vitro transcription followed by phenol/chloroform extraction and ethanol precipitation. RNA pellets were resuspended in RNAse-free water, fidelity checked on an agarose gel under denaturing conditions and the aliquots were stored at −80 °C until use. Eggs from heterozygous parental zebrafish in one-cell stage were injected within 20 min after spawning. Approximately 2.6 ng of RNA, containing 0.1% phenol red as an injection volume tracer [24] was injected per egg. The GFP fluorescence of 8-h-old healthy injected embryos was quantified using a photomultiplier system. Only proper developing injected embryos with a mean fluorescence signal exceeding 40% above uninjected control embryos were considered for further experiments.
2.4. Identification of rescued larvae
Differentiation of motility-restored homozygous relaxed larvae, used in motion analysis experiments, from the injected “normal” siblings (i.e. heterozygous and wild-type, WT) was done by keeping all injected larvae in isolation up to 5 days. During this period a gradual fallback to the paralyzed phenotype due to degradation of the injected β-RNAs and translated proteins was observed for restored relaxed but not normal larvae. Genotype confirmation of the relaxed phenotype was done by RFLP. In the case when larval tails were used for freeze-fracture electron microscopy, motility-restored relaxed larvae had to be identified immediately by RFLP test on the larval heads. For this, genomic DNA was extracted as described previously [17] and was used as PCR template to amplify a 459 bp fragment containing the relaxed mutation. Restriction enzyme digest of the PCR product with BsrI cleaved the 459 bp fragment into 279 and 180 bp fragments, only in case of WT but not in relaxed alleles.
2.5. Freeze-fracture electron microscopy
27–30 h post-fertilization (hpf) injected motile larvae were decapitated and the tails were fixed with 6% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2; both Sigma) for 30 min at room temperature. Samples were stored in 3% glutaraldehyde at 4 °C. Tails were infiltrated in 30% glycerol in water and fractured in the double replica holder, shadowed with platinum at 45 °C, and replicated with carbon in a BFA 400 Balzers freeze-fracture unit (Balzers SpA). Replicas were analyzed in an electron microscope (model 410; Philips). Only myotubes with peripheral couplings between the surface membrane and the SR were analyzed.
2.6. Digital motion analysis
27–30 hpf larvae were dechorionated using pronase and transferred into 24-well plates to keep individuals identifiable for several days. 2-min video sequences were recorded with a Sony CCD AVC-D7CE b/w camera, converted into stacks of differential images [25] and 3 × 3 median filtered to eliminate pixel noise. The mean luminance of every image reflecting larval movement was quantified by automated counting of total dynamic pixels per image and plotted against time. On this processed signal, peak detection was performed. For quantifying the larval ‘movement extent’, the cumulative dynamic pixels per peak were calculated, the mean value for all larval movements of each experimental group determined and standardized to the values obtained from normal larvae.
2.7. Primary culture of zebrafish myotubes
25–28 hpf homozygous relaxed larvae were identified by their inability to move despite tactile stimulation. Motile normal siblings were used for control experiments. 100–150 larvae were dechorionated using Pronase (Protease, Type XIV, Sigma) [23], anesthetized and decapitated and the tails digested with collagenase type I (Sigma) to dissociate muscle cells. The cells were transfected with 2 μg of plasmid cDNA using the AMAXA™ rat neonatal cardiomyocyte nucleofector kit (AMAXA Biosystems, Köln, Germany) according to the manufacturer's manual. Myocytes were cultured at 28 °C for 4–6 days for electrophysiological experiments.
2.8. Whole cell patch clamp
Immobilization-resistant intramembrane charge movement as well as intracellular Ca2+ transients were recorded from transfected (GFP-positive) myotubes. Charge movement is a measure of functional membrane expression of the L-type Ca2+ channel complex [26]. Borosilicate glass patch pipettes (Harvard Instruments) had resistances of 3.5–5 MΩ when back-filled with internal solution containing 100 mM CsAspartate, 10 mM HEPES, 0.5 mM CsEGTA, 3 mM MgATP, and 0.2 mM Fluo-4 (pH 7.4 with CsOH). The bath solution consisted of 10 mM Ca(OH)2, 100 mM l-aspartate, and 10 mM HEPES (pH 7.4 with tetraethylammonium hydroxide). Contractions of myotubes were blocked by adding 100 μM of the myosin-II blocker N-benzyl-p-toluene sulfonamide (Sigma) to the bath solution. Leak currents were subtracted by a P/4 prepulse protocol and the test pulses were preceded by a 1-s prepulse to −30 mV to inactivate endogenous T-type currents [26]. Total charge movement was calculated by integrating the ON-component of gating currents (Qon). 0.2 mM Fluo-4 was added to the patch pipette solution to measure intracellular Ca2+ release. The average fluorescence of a 10-ms time period in the plateau phase of the 200-ms test pulse was normalized to the resting fluorescence and expressed as ΔF/F0.The voltage dependence of Qon and ΔF/F0 was fitted according to the Boltzmann distribution:
where A is Qon or ΔF/F0, V1/2 is the potential at which A = Amax/2, and k is a slope factor. Data were analyzed using ClampFit 10.0 (Axon Instruments) and SigmaPlot 10.0 and 11.0 (SPSS Science, Chicago, IL) software.
2.9. Statistics
Statistical significance from experimental approaches was assessed using unpaired Student's t test or one-way analysis of variance (ANOVA), as appropriate. Data are reported as mean ± SE. p values < 0.05 were considered significant.
3. Results and discussion
3.1. Conservation of the heptad repeat motif in the C-terminus of the DHPRβ1a subunit
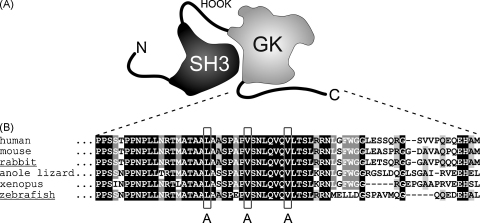
Voltage-gated Ca2+ channel β subunits are structurally organized into two conserved domains, namely the SH3 (Src homology 3) and GK (guanylate kinase) domains, which are flanked by three variable regions: the N-terminus, a HOOK region, and the C-terminus [27–30] (Fig. 1A). Amino acid sequence alignment of C-termini of different β1a subunits revealed the conservation of the previously described [22] β1a-specific heptad repeat motif (LVV) from fish to human (Fig. 1B). Murine β1-null myotubes expressing a β1a mutant with the heptad repeat motif LVV exchanged to AAA were described to have a 5-fold reduction in intracellular Ca2+ release [22]. To address the question whether this reduction is due to the lack of signal transduction from DHPR to RyR1 via β1a as proposed [20,22] or is already due to a targeting problem on the ultrastructural level, i.e. the lack of tetrad formation, we studied the heptad repeat mutation in β1-null zebrafish relaxed larvae and isolated myotubes. To validate species-independence of the results we used β1aAAA mutants derived from the native zebrafish β1a as well as from a mammalian (rabbit) β1a. Zebrafish and rabbit β1a subunits share 76% all-over amino acid identity and 68% in their C-termini.
Fig. 1.
A conserved leucine-valine heptad repeat motif in the DHPRβ1a C-terminus. (A) Cartoon of the domain organization of the β1a subunit based on crystal structure models [28–30]. (B) Sequence alignment of β1a C-termini from different vertebrate classes, from fish to human, showed the conservation of the β1a-specific leucine-valine heptad repeat motif (boxed). To test for the contribution of the heptad repeat motif in skeletal muscle EC coupling, the LVV residues (boxed) were substituted by AAA (β1aAAA) in zebrafish and rabbit β1a subunits to be expressed in the zebrafish β1-null mutant relaxed system. Sequence for human (Homo sapiens) β1a, anole lizard (Anolis carolinensis) β1a, and Xenopus (Xenopus tropicalis) β1a were extracted from genomic assemblies at http://www.ensembl.org using a BLAST search with zf-β1a or rb-β1a cDNA sequences. GeneBank accession numbers for all other sequences used are: mouse (Mus musculus) β1a, NM_031173; rabbit (Oryctolagus cuniculus) β1a, NM_001082279 and zebrafish (Danio rerio) β1a, AY952462.
3.2. Intact ultrastructural organization of DHPRs in tetrads in relaxed larvae expressing β1aAAA
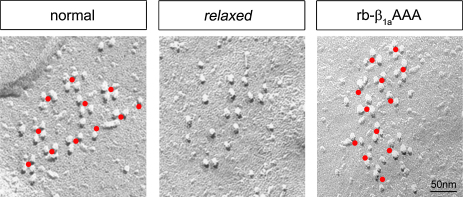
To test for a possible ultrastructural mistargeting of the channel complex as a consequence of the AAA mutation, we injected the RNA coding for rb-β1aAAA into relaxed zygotes and analyzed the orientation of DHPR particles opposite the RyR1 in 27–30 hpf larval tail muscles by freeze-fracture electron microscopy (Fig. 2). While freeze-fracture replicas of untransfected relaxed larvae showed arbitrarily arranged DHPR particles within all clusters (Fig. 2, middle panel, from an archive of 109 images from 14 fractured fish tails), replicas of relaxed larvae expressing rb-β1aAAA revealed a correct arrangement of DHPR particles in tetrads organized in orthogonal arrays (Fig. 2, right panel, from 43 images from 3 fish tails) comparable to myotubes from normal larvae (Fig. 2, left panel, from an archive of 151 images from 8 fish tails). Thus, rb-β1aAAA is able to restore the correct ultrastructural arrangement of DHPRs in tetrads, essential for skeletal muscle EC coupling. At this point our results, showing full restoration of tetrad formation in combination with the severely reduced EC coupling postulated earlier [22], would apparently suggest an additional function of β1a, i.e. as an active signal-transmitting element in the EC coupling process.
Fig. 2.
Intact DHPR tetrad formation with β1aAAA mutant expressed in relaxed myotubes. Freeze-fracture replicas of tail muscle tissue of normal zebrafish larvae (left panel) revealed the arrangement of DHPR particles in tetrads (indicated by red dots) organized in orthogonal arrays. In contrast, the β1-null mutant relaxed (middle panel) lacks DHPR tetrad formation. Comparable to normal larvae, relaxed larvae zygote-injected with in vitro synthesized rb-β1aAAA RNA, displayed correct assembly of DHPR particles in tetradic arrays (right panel). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.3. Full motility restoration in relaxed larvae expressing β1aAAA
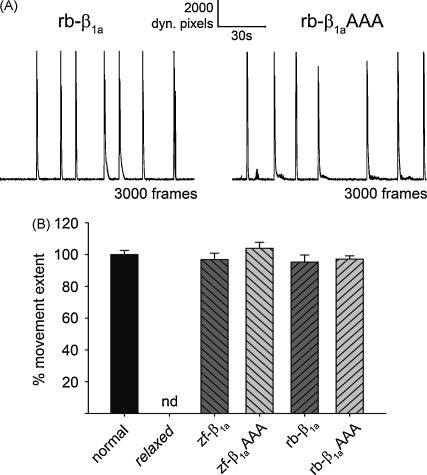
Surprisingly, relaxed larvae that were zygote-injected with either zf- or rb-β1aAAA mutant RNA displayed vigorous movements (Movie S1 and S2 in Supplementary Material). To further test whether the larval motility gives an indication of the reduced Ca2+ release as reported [20,22], a detailed movement analysis was performed (Fig. 3). 2-min videos of individual larva were recorded, converted into differential images and total number of dynamic pixels per frame were plotted against time (Fig. 3A). Interestingly, relaxed larvae expressing β1aAAA mutant revealed movement profiles similar to relaxed larvae expressing WT β1a, with double-peaks representing larval muscle contraction and relaxation [17]. Movement extent was quantified and the values obtained for rescued relaxed larvae were standardized to uninjected normal larvae (100 ± 3%, n = 114). The movement extents of relaxed larvae expressing either zf-β1aAAA (104 ± 4%, n = 30) or rb-β1aAAA (97 ± 2%, n = 65) were indistinguishable (p > 0.05) from uninjected normal larvae and from WT β1a expressing larvae, with 97 ± 4%, n = 49, for zf-β1a and 95 ± 5%, n = 21, for rb-β1a (Fig. 3B).
Fig. 3.
Full restoration of motility in relaxed larvae zygote-injected with β1aAAA mutant RNA. (A) Representative plots of total dynamic pixels per frame of 2-min video recordings of spontaneous larval movements. Relaxed larvae zygote-injected with either rb-β1a (left) or rb-β1aAAA RNA (right) revealed a similar movement profile. (B) To quantify larval movement extent, mean values of cumulative dynamic pixels per movement for each experimental group were calculated and standardized to those of normal larvae. Relaxed larvae zygote-injected with either zf-β1aAAA or rb-β1aAAA mutant RNA showed full recovery of larval movement extent, indistinguishable (p > 0.05) from normal larvae or from relaxed larvae injected with either zf-β1a or rb-β1a. One-way ANOVA revealed overall non-significance (p = 0.62, F(4,274) = 0.66). Uninjected relaxed larvae never showed any motility (nd, not detectable).
3.4. Proper intracellular Ca2+ release in relaxed myotubes expressing β1aAAA
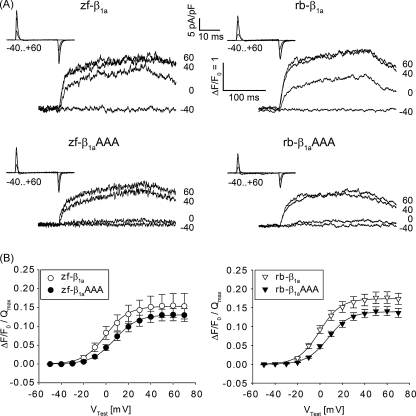
The above result was quite unexpected, because a perfect restoration of motility in larvae zygote-injected with β1aAAA mutant is not consistent with the 80% reduction in Ca2+ release as described from β1aAAA expressing murine β1a-null myotubes [20,22]. Therefore, we analyzed relaxed myotubes transfected with WT β1a and β1aAAA mutants from zebrafish and rabbit by patch clamp (Fig. 4). Unlike other vertebrates, skeletal muscle of higher teleost fish – and thus zebrafish – have no DHPR inward Ca2+ currents [31] and thus, a possible contamination of intracellular Ca2+ release recordings by extracellular Ca2+ influx is impossible so that our data shows pure skeletal-muscle-type EC coupling. Contrary to the earlier results of the Coronado laboratory but in agreement with our motility restoration data, both β1aAAA mutants were able to restore pronounced intracellular Ca2+ release in response to 200-ms test pulses to potentials of −50 to +70 mV (Fig. 4A). In order to correct for different expression levels, indicated by small differences in Qmax (Table 1), ΔF/F0 values were normalized to Qmax (Fig. 4B). Values of ΔF/F0/Qmax were identical (p > 0.05) between zf-β1aAAA (0.14 ± 0.02 mV; n = 14) and zf-β1a (0.16 ± 0.03; n = 9) but slightly smaller in rb-β1aAAA (0.14 ± 0.01; n = 41) versus rb-β1a (0.18 ± 0.02 mV; n = 23; p = 0.044). Both β1aAAA mutants induced a small shift in the voltage-dependence of intracellular Ca2+ transients towards more positive potentials, that was slightly significant (p = 0.036) for zf-β1aAAA (8.94 ± 2.32 mV; n = 14) compared to zf-β1a (−0.06 ± 3.49 mV; n = 9), but highly significant (p = 0.003) for rb-β1aAAA (7.56 ± 0.97 mV; n = 41) in comparison to rb-β1a (1.69 ± 1.81 mV; n = 23).
Fig. 4.
The β1aAAA mutation has only a minor effect on intracellular Ca2+ transients. (A) Representative intracellular Fluo-4 Ca2+ recordings from relaxed myotubes expressing zebrafish and rabbit WT β1a (upper panel) or β1aAAA mutant subunits (lower panel). Pronounced intracellular Ca2+ transients in response to 200-ms test pulses with similar kinetics were recorded with all constructs. Differences in transient size with β1aAAA can be explained by slight differences in expression levels as indicated by differences in intramembrane charge movement recordings (insets). (B) Voltage dependence curves of ΔF/F0 were corrected for differences in expression levels by normalizing ΔF/F0 to Qmax. Transients were slightly shifted towards more positive potentials for the β1aAAA constructs.
Table 1.
| Qmax (nC/μF) | V1/2 − Q (mV) | (ΔF/F0)max | V1/2 − (ΔF/F0)max (mV) | |
|---|---|---|---|---|
| rb-β1a | 11.07 ± 0.77 (n = 22) | −4.49 ± 0.86 | 1.96 ± 0.17 (n = 23) | 1.68 ± 1.81 |
| rb-β1aAAA | 8.80 ± 0.59 (n = 35) | −2.29 ± 0.64 | 1.25 ± 0.11 (n = 41) | 7.80 ± 0.93 |
| zf-β1a | 11.13 ± 1.72 (n = 12) | −3.97 ± 2.19 | 1.75 ± 0.36 (n = 9) | −0.06 ± 3.49 |
| zf-β1aAAA | 10.93 ± 1.00 (n = 18) | −3.39 ± 1.06 | 1.45 ± 0.18 (n = 14) | 8.91 ± 2.32 |
Values of maximal intramembrane charge movement (Qmax), maximal intracellular Ca2+ release ((ΔF/F0)max) and the corresponding half-maximal activation potentials, recorded from relaxed myotubes transfected with either β1a or β1aAAA mutant subunits from zebrafish and rabbit.
In comparison to the earlier study by the Coronado laboratory, the effects of the LVV/AAA mutation on size and voltage dependence of intracellular Ca2+ transients observed in our experiments (Table 1) are even minor than the effects that were observed with their control mutant D5ALAc, where 3 amino acids out of step to the LVV motif were exchanged to alanines. D5ALAc yielded 23% reduced (ΔF/F0)max values with a 24 mV right-shift in voltage dependence upon 200-ms test pulses [22]. Thus the LVV residues of the heptad repeat motif, like the residues exchanged in the D5ALAc mutant, are rather functionally irrelevant. Rather, the β1a C-terminus is an optimized structure in itself and consequently any change in the C-terminus affects the general folding and hence protein functioning. However, as shown in the in vivo motility data, these minor changes are not sufficient to show quantifiable effects on the extent of spontaneous larval movements which mirrors the skeletal muscle EC coupling.
4. Conclusion
Our results do not support an essential role of the β1a-specific hydrophobic C-terminal heptad repeat LVV in skeletal muscle EC coupling as both structural (tetrad formation) and functional interactions are restored with β1aAAA in reconstitution studies. Intracellular Ca2+ transients resulting from activation of RyR1 by the DHPR are very little affected by the β1a heptad repeat mutation, and these minor changes do not influence spontaneous muscle activity. Relaxed larvae expressing β1aAAA mutants displayed motility indistinguishable from those injected with WT β1a. The reasons underlying the discrepancy between our results and those from the earlier study [20,22] are not clear. Besides putative differences in the expression systems, a second and perhaps more likely possibility for this incongruency might be crucial differences between the clones employed. To elucidate this dissonance we intended to include analyzes from the original murine β1a heptad repeat mutant clone D5ALA [22]. Unfortunately we were unsuccessful due to the disappearance of the original clone (Dr. Timothy J. Kamp and Dr. David Sheridan, personal communications, 2008). According to our novel finding that the β1a heptad repeat motif is not the key determinant of DHPR–RyR1 coupling, doors are reopened for further in-depth structural–functional studies on the role of the DHPRβ1a subunit in skeletal muscle EC coupling.
Conflict of interest
None.
Acknowledgements
The authors thank Sandra Schleret and Nosta Glaser for excellent technical help, and Dr. Valentina Di Biase for expert help with freeze-fractures. We also thank Dr. Timothy J. Kamp (Dept. of Medicine, University of Wisconsin Medical School, Madison) for a series of efforts in searching for the originally published mouse β1a heptad repeat mutant clone D5ALA. We are grateful to Thorsten Schwerte (Inst. of Zoology, University of Innsbruck) for expert help with the digital motion analysis. This work was supported by the Austrian Science Fund (FWF-16098-B04, FWF-DK-W1101-B12 to M.G.), the Medizinische Forschungsförderung Innsbruck (MFI-6180 to J.S.), and NIH RO1 HL48093 (to C.F.A.).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ceca.2010.04.003.
Appendix A. Supplementary data
References
- 1.Schneider M.F., Chandler W.K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation–contraction coupling. Nature. 1973;242:244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- 2.Rios E., Brum G. Involvement of dihydropyridine receptors in excitation–contraction coupling in skeletal muscle. Nature. 1987;325:717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong C.M., Bezanilla F.M., Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N′-tetracetic acid. Biochim. Biophys. Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- 4.Flucher B.E., Andrews S.B., Fleischer S., Marks A.R., Caswell A., Powell J.A. Triad formation: organization and function of the sarcoplasmic reticulum calcium release channel and triadin in normal and dysgenic muscle in vitro. J. Cell Biol. 1993;123:1161–1174. doi: 10.1083/jcb.123.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X.H., Protasi F., Takahashi M., Takeshima H., Ferguson D.G., Franzini-Armstrong C. Molecular architecture of membranes involved in excitation–contraction coupling of cardiac muscle. J. Cell Biol. 1995;129:659–671. doi: 10.1083/jcb.129.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block B.A., Imagawa T., Campbell K.P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 1988;107:2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franzini-Armstrong C., Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- 8.Arikkath J., Campbell K.P. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe T., Beam K.G., Adams B.A., Niidome T., Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation–contraction coupling. Nature. 1990;346:567–569. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- 10.Takekura H., Paolini C., Franzini-Armstrong C., Kugler G., Grabner M., Flucher B.E. Differential contribution of skeletal and cardiac II-III loop sequences to the assembly of dihydropyridine-receptor arrays in skeletal muscle. Mol. Biol. Cell. 2004;15:5408–5419. doi: 10.1091/mbc.E04-05-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bichet D., Cornet V., Geib S. The I-II loop of the Ca2+ channel α1 subunit contains an endoplasmic reticulum retention signal antagonized by the β subunit. Neuron. 2000;25:177–190. doi: 10.1016/s0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- 12.Dolphin A.C. β subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 13.Gregg R.G., Messing A., Strube C. Absence of the β subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the α1 subunit and eliminates excitation–contraction coupling. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schredelseker J., Di Biase V., Obermair G.J. The β1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17219–17224. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W., Saint-Amant L., Hirata H., Cui W.W., Sprague S.M., Kuwada J.Y. Non-sense mutations in the dihydropyridine receptor β1 gene, CACNB1, paralyze zebrafish relaxed mutants. Cell Calcium. 2006;39:227–236. doi: 10.1016/j.ceca.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Pelster B, Burggren W.W. Disruption of hemoglobin oxygen transport does not impact oxygen-dependent physiological processes in developing embryos of zebrafish (Danio rerio) Circ. Res. 1996;79:358–362. doi: 10.1161/01.res.79.2.358. [DOI] [PubMed] [Google Scholar]
- 17.Schredelseker J., Dayal A., Schwerte T., Franzini-Armstrong C., Grabner M. Proper restoration of excitation–contraction coupling in the dihydropyridine receptor β1-null zebrafish relaxed is an exclusive function of the β1a subunit. J. Biol. Chem. 2009;284:1242–1251. doi: 10.1074/jbc.M807767200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beurg M., Ahern C.A., Vallejo P. Involvement of the carboxy-terminus region of the dihydropyridine receptor β1a subunit in excitation–contraction coupling of skeletal muscle. Biophys. J. 1999;77:2953–2967. doi: 10.1016/S0006-3495(99)77128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheridan D.C., Cheng W., Ahern C.A. Truncation of the carboxyl terminus of the dihydropyridine receptor β1a subunit promotes Ca2+ dependent excitation–contraction coupling in skeletal myotubes. Biophys. J. 2003;84:220–237. doi: 10.1016/S0006-3495(03)74844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coronado R., Ahern C.A., Sheridan D.C., Cheng W., Carbonneau L., Bhattacharya D. Functional equivalence of dihydropyridine receptor α1S and β1a subunits in triggering excitation–contraction coupling in skeletal muscle. Biol. Res. 2004;37:565–575. doi: 10.4067/s0716-97602004000400010. [DOI] [PubMed] [Google Scholar]
- 21.Surks H.K., Mochizuki N., Kasai Y. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Ialpha. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan D.C., Cheng W., Carbonneau L., Ahern C.A., Coronado R. Involvement of a heptad repeat in the carboxyl terminus of the dihydropyridine receptor β1a subunit in the mechanism of excitation–contraction coupling in skeletal muscle. Biophys. J. 2004;87:929–942. doi: 10.1529/biophysj.104.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westerfield M. 4th ed. University of Oregon Press; Eugene: 2000. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) [Google Scholar]
- 24.Nüsslein-Volhard C., Dahm R. Oxford University Press; New York: 2002. Zebrafish. [Google Scholar]
- 25.Schwerte T., Pelster B. Digital motion analysis as a tool for analyzing the shape and performance of the circulatory system in transparent animals. J. Exp. Biol. 2000;203:1659–1669. doi: 10.1242/jeb.203.11.1659. [DOI] [PubMed] [Google Scholar]
- 26.Adams B.A., Tanabe T., Mikami A., Numa S., Beam K.G. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346:569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- 27.Hanlon M.R., Berrow N.S., Dolphin A.C., Wallace B.A. Modeling of a voltage-dependent Ca2+ channel β subunit as a basis for understanding its functional properties. FEBS Lett. 1999;445:366–370. doi: 10.1016/s0014-5793(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y.H., Li M.H., Zhang Y. Structural basis of the α1–β subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- 29.Opatowsky Y., Chen C.C., Campbell K.P., Hirsch J.A. Structural analysis of the voltage-dependent calcium channel β subunit functional core and its complex with the α1 interaction domain. Neuron. 2004;42:387–399. doi: 10.1016/s0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- 30.Van Petegem F., Clark K.A., Chatelain F.C., Minor D.L., Jr. Structure of a complex between a voltage-gated calcium channel β-subunit and an α-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schredelseker J., Shrivastav M., Dayal A., Grabner M. Non-Ca2+-conducting Ca2+ channels in fish skeletal muscle excitation–contraction coupling. Proc. Natl. Acad. Sci. U.S.A. 2010;107:5658–5663. doi: 10.1073/pnas.0912153107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.