Abstract
DNA vaccination can induce specific CD8+ T cell immune response, but the response level is low in large mammals and human beings. Coadministration of an adjuvant can optimize protective immunity elicited by a DNA vaccine. In this study, we investigated the effect of a synthetic glucohexaose (β-glu6), an analogue of Lentinan basic unit, on specific CD8+ T cell response induced by a DNA vaccine encoding HBcAg (pB144) in mice. We found that β-glu6 promoted the recruitment and maturation of dendritic cells, enhanced the activation of CD8+ and CD4+ T cells and increased the number of specific CD8+/IFN-γ+ T cells in lymphoid and nonlymphoid tissues in mice immunized by pB144. Immunization with pB144 and β-glu6 increased the anti-HBc IgG and IgG2a antibody titer. These results demonstrate that β-glu6 can enhance the virus-specific CTL and Th1 responses induced by DNA vaccine, suggesting β-glu6 as a candidate adjuvant in DNA vaccination.
1. Introduction
Sugars are bioactive components of many plants and microorganisms. Polysaccharides and oligosaccharides of various origins (fungi, bacteria, plants, etc.) can be recognized by surface receptors of host cells, in particular macrophages and dendritic cells, and trigger host innate immune reactions [1]. Some polysaccharides and oligosaccharides extracted from plants, such as microgranular formulation of inulin, can regulate the immune response by acting as adjuvants to nonspecifically enhance cellular and humoral immune responses and activate the innate immunity through the alternative complement pathway [2].
The curative effects of Ganoderma lucidum and other mushrooms were recorded in the Compendium of Materia Medica. Their extracts have been used to prevent and treat diseases in traditional Chinese medicine. Polysaccharides and oligosaccharides are the major bioactive molecules in the extracts [3, 4]. Lentinan, a polysaccharide exacted from the fungus Lentinus edodes, possesses various biological activities, such as immune regulation, anti-infection and anti-tumoral activity [5, 6], and its usage is recorded in Chinese Pharmacopoeia [7]. Lentinan has potent ability to activate innate immune effector cells such as monocytes/macrophages and NK cells, and to stimulate cell-mediated immune response [4, 8]. Kupfahl et al. demonstrated that Lentinan enhanced the protective CD8+ T cell response against Listeria monocytogenes in the spleen of mice [9]. This suggests that Lentinan may be used as an adjuvant to enhance host's immune response. However, it is very hard to extract pure Lentinan from the fruiting body of Lentinus edodes because the extracted fungal polysaccharides and oligosaccharides have some drawbacks, such as the heterogeneous structures of the saccharides, poor solubility in water, low purity, and low yield [10]. These disadvantages limit their applicability in clinical practice.
Many bioactive polysaccharides from fungi and plants have the common structural signature of β-(1→6)-branched β-(1→3) gluco-oligosaccharides [11]. The basic unit β-(1→6)-branched β-(1→3) glucohexaose in Lentinan plays a vital role in antitumour activity in mice [12], and is endowed with the immunostimulatory effects of the whole polysaccharide. Gu et al. synthetized a β-(1→6)-branched β-(1→3) glucohexaose analogue containing a α-(1→3)-linked bond (β-glu6) (patent publication number: PCT/CN02/00478), and it shows similar effects as Lentinan on mouse spleen cell proliferation and TNF-α production [13]. The study by Dong et al. demonstrated administration of synthetic β-glu6 with hepatitis B surface antigen (HBsAg) significantly increased the antigen-specific antibody titer and the number of HBsAg-specific IL-4-producing T cell in spleen, and the ratio of anti-HBsAg IgG1/IgG2a was higher in mice immunized with HBsAg plus β-glu6 than that in mice receiving HBsAg alone, indicating a shift towards a Th2-biased response [14]. This suggests that β-glu6 can act as an adjuvant to enhance Th2 humoral immune response induced by a protein vaccine, but the adjuvant effect of β-glu6 on specific CD8+ T cell response needs to be studied.
In a DNA vaccine, it is expected that DNA encoded antigens are subjected to intracellular proteasomal degradation, resulting in peptide fragments that can be presented by MHC class I antigens to CD8+ T cells, thus mimicking viral infection [15]. Therefore, DNA vaccines have been extensively studied both for preventive and therapeutic approaches against microbe infections. Coadministration of DNA vaccine with adjuvants (such as cytokines, chemokines, CpG DNA, costimulatory molecules, ligands, etc.) is one of strategies to optimize CD8+ T cells protective immunity [16–18]. Polysaccharides from microorganisms and plants can also be used as DNA vaccines adjuvants. For example, Agaricus blazei Murill extracts containing a great quantity of polysaccharides have an ability to enhance humoral and cellular immune responses induced by plasmid DNA encoding HBcAg [19]. In mice, Lentinan was found to increase Env-specific type 1 cytokine production and cytotoxic T-lymphocyte (CTL) activities induced by DNA vaccine, encoding human immunodeficiency virus envelope glycoprotein [20]. Since the adjuvant effect of β-glu6 on the polarization of Th1/Th2 balance and the induction of antigen-specific cytotoxic T lymphocytes upon administration of a DNA vaccine is still unknown, in the present study, we have investigated effect of β-glu6 on the antigen specific CD8+ T cell response induced by a DNA vaccine encoding HBcAg.
2. Materials and Methods
2.1. Plasmid and Reagents
The eukaryotic expression plasmid pB144 was kindly provided by Prof. Yuan Wang, and was constructed by inserting a gene encoding HBcAg N'-end 144 amino acids into a vector (pcDNA3.1, Invitrogen, Carlsbad, CA, USA) under the control of the cytomegalovirus (CMV) immediate early promoter. COS-7 cells transiently transfected with pB144 significantly express HBcAg and efficiently secrete it into the cell culture supernatant [21].
2.1.1. Plasmid Purification
The pB144 plasmid was prepared according to the protocols of QIAGEN-TIP 2500 Plasmid Mega Kit (Qiagen Corporation, Maryland, USA). A single colony from a LB/Amp plate was inoculated into LB/Amp culture medium at 1/1000, and incubated at 37°C for 12–16 hours with vigorous shaking (approx. 300 rpm). The bacterial cells were harvested by centrifugation at 6000 xg for 15 minutes at 4°C. The bacterial pellet was resuspended in Tris·Cl-EDTA buffer containing RNase A (100 μg/ml) and lyzed with NaOH (200 mM)/SDS (1%). The lysate was neutralized by the addition of acidic potassium acetate (3.0 M, pH 5.0). The supernatant of lysate was applied to the QIAGEN-tip. The binding plasmid was eluted in a high-salt buffer, then the DNA was precipitated with isopropanol. The DNA was dissolved with sterile PBS (pH 7.0). The concentration of plasmid DNA was determined spectrophotometrically.
2.1.2. Reagents
FITC-CD4 (clone GK1.5), PerCP-CD8a (clone 53–6.7), APC-IFN-γ (clone XMG1.2), PE-CD11c (clone HL3), and the Cytofix/Cytoperm Plus kit (with GolgiPlug) were purchased from BD Biosciences (Franklin Lakes, NJ, USA). The following reagents were purchased from Biolegend (San Diego, CA, USA): FITC-CD40 (clone HM40–3), FITC-CD86 (clone GL-1), FITC-MHC-II (clone M5/114.15.2), and PE-CD69 (clone H1.2F3). Brefeldin A was obtained from eBioscience (Boston, MA, USA). HISTOPAQUE-1083 and Deoxyribonuclease I were from Sigma-Aldrich (St. Louis, MO, USA). Anti-HBc ELISA kit and goat antimouse IgG-HRP were purchased from Huamei Bioengineering Co., Ltd. (Shanghai, China). Bovine anti-mouse IgG1 and anti-mouse IgG2a were purchased from the Binding Site Co., Ltd. (Birmingham, UK). Collagenase Type IV, 2-mercaptoethanol, RPMI-1640 medium, Fetal Bovine Serum (FBS), L-glutamine, penicillin, and streptomycin were obtained from GIBCO Invitrogen (Grant Island, NY, USA). Percoll was purchased from Pharmacia (Uppsala, Sweden). The peptide MGLKFRQL, representing an H-2Kb-restricted CTL epitope of the hepatitis B core antigen, was synthesized and generously provided by Dr. Rafi Ahmed (Emory University, Altanta, GA, USA).
2.2. β-(1→6)-Branched β-(1→3) Glucohexaose Analogue
β-glu6 glucohexaose was synthetized by Kong Fanzuo, Ning Jun, and Gu Jianxin, and identified with NMR, MS, and HPLC. 1HNMR (CDCl3,400 MHz): δ5.269(d, J3.2 Hz, 1H, H-1α), 4.743(d, J8.0 Hz,2H,H-1β), 4.667(c, J8.0 Hz, 2H,H-1β), 4.440(d, J8.0 Hz, 2H, H-1β), 4.152–4.081(m, 4H), 3.919–3.256(m, 32 H); 13C NMR(100 Hz, D2O):δ102.69 (2 C-1β, JC1-H1 173 Hz), 102.6 (3C-1β,JC1-H1 173 Hz), 98.92 (1C-1α, JC1-H1 164 Hz), 85.34, 84.87,92.90 (C-3), 81.96, 75.85, 75.76, 75.74, 75.57, 75.54, 75.52,75.45, 75.40, 73.73, 73.34, 73.04, 72.12, 70.98, 70.75, 69.68,69.55, 69.49, 68.86, 68.37, 68.24, 67.78 (C-2,3,4,5,6); ESMS for C36H62O31 (992.5): 991.4 [M-1]+. The details of the synthesis are illustrated in the related patent (publication number: WO03004507). The purity of the synthetic β-glu6 is more than 98%, as determined by HPLC. The endotoxin contamination in β-glu6 was under detection level by Limulus Amoebocyte Lysate (LAL) colorimetric assay (Cambrex; Walkersville, MD, USA).
2.3. Immunization of Mice with pB144 and β-glu6
Female inbred C57BL/6 mice (H-2b), aged 6–8 weeks, were purchased from B&K Universal Group Limited (Shanghai, China), and kept under pathogen-free conditions. Mice were grouped as described below, each group consisting of 15 mice. Before immunization, mice were anesthetized with 0.75% sodium pentobarbital (75 mg/kg). One group of mice was inoculated with pB144 DNA (100 μg/mouse) and synthetic β-glu6 (1 mg/kg) in phosphate buffered saline (PBS) into the tibialis anterior muscle of each hind limb, as previously described [22]. These mice then received daily intramuscular injections of synthetic β-glu6 (1 mg/kg/day in 100 μl PBS) for six days. Another group of mice were immunized with pB144 DNA alone. Control mice were administered with the same volumes of PBS. At day 30 after the first immunization with pB144, all mice were boosted with a second antigen dose, using the same immunization schedule as in the priming. At days 5, 14, 35, and 40 after priming, three mice/group were sacrificed, and peripheral blood, spleen, and liver were collected.
2.4. Lymphocyte Preparation
Mice were anesthetized and sacrificed. The peripheral blood was collected from angular vein, using 4% sodium citrate as an anticoagulant. PBMC were obtained by density gradient centrifugation over HISTOPAQUE-1083 (Sigma-Aldrich), washed three times with RPMI-1640 and counted. Mice spleens were dissociated on a 200-gauge nylon mesh. Splenocytes were collected and treated with lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.4) to eliminate red cells, washed and resuspended in RPMI-1640 with 10% heat-inactivated FBS (hereafter referred to as culture medium). Hepatic lymphocytes were isolated as previously described [23]. Briefly, the liver was perfused with 5 mL RPMI-1640 (containing 2% FBS, 2 mM L-glutamine, 100 U/mL penicillin and streptomycin, resp.) through the portal vein, then the inferior caval vein was cut and the medium was allowed to flow until the liver was free of red cells. The liver was then pressed through 200-gauge nylon mesh, fragments suspended in RPMI-1640 containing 0.05% collagenase IV and 0.001% DNase I, and digested at 37°C for 40 minutes. The liver cell suspension was collected, and mononuclear cells (MNC) were separated from parenchymal cells by centrifugation at 500 g for 10 minutes. Lymphocyte-rich cell pellets were resuspended in 44% Percoll in complete RPMI-1640 medium, gently overlaid onto 56% Percoll and centrifuged for 10 minutes at 750 g at 4°C. The pellets were resuspended in erythrocyte lysing solution (lysis buffer with the addition of 170 mM Tris, pH 7.3), washed with RPMI-1640 and re-suspended in complete RPMI-1640.
2.5. Flow Cytometric Analysis of Surface Markers on Lymphocytes
Coexpression of CD11c with CD40, CD86, and MHC class II on spleen leukocytes was determined by flow cytometry. Coexpression of CD69 with CD4 and CD8 on lymphocytes of spleen, liver and peripheral blood was also assessed. Briefly, cells were stained with relevant fluorochrome-labelled mAbs for 30 minutes, washed with FACScan buffer (PBS containing 2% FBS and 0.1% sodium azide), then fixed with 4% paraformaldehyde. Fluorescence profiles were generated on a FACScan flow cytometer (BD Biosciences). For each sample, 100,000 cells were collected and histogram and density plots were produced by the CellQuest software package.
2.6. Detection of IFN-γ Producing T Cells by Intracellular Cytokine Staining
At day 5, 14, and 40 after primary immunization, IFN-γ production was detected by intracellular staining. Spleen cells, PBMC, and hepatic lymphocytes were plated separately (1 × 106 cells/well) in a 96-well plate (Corning Costar; Cambridge, MA) in a final volume of 200 μl. Cells were stimulated with a specific peptide corresponding to a Kb-restricted CTL epitope of the hepatitis B core antigen (final concentration 1 μg/mL) for 5 hours at 37°C in moist atmosphere with 5% CO2, in the presence of Brefeldin A (final concentration 2 μg/mL). Cells were then stained with PerCP-conjugated anti-CD8a mAb for 30 minutes at 4°C, washed with FACS buffer (2% FBS, 0.1% sodium azide in PBS), treated with Cytofix/Cytoperm for 20 minutes, and washed with Perm/Wash Buffer (both from BD Biosciences). Cells were subsequently stained with APC-conjugated anti-mouse IFN-γ mAb for 30 minutes at 4°C, washed with Perm/Wash Buffer and FACS buffer, and fixed with 4% (w/v) paraformaldehyde. Sample data were acquired using a FACSCalibur flow cytometer (BD Biosciences).
2.7. Detection of Anti-HBc Antibodies
Blood was collected from the retroorbital plexus of mice after 4 weeks from the primary immunization and 2 weeks from boost, and serum were obtained. The titer of anti-HBc antibodies was measured by ELISA (anti-HBc ELISA kit; Diagnostic Reagent Center of Shanghai Municipal Infectious Diseases Hospital, Shanghai, China). Serum was serially diluted in PBS with 5% nonfat milk (starting from 1 : 100) and incubated in microtitre plates precoated with HBcAg for 1 hour at 37°C. Plates were then washed and further incubated (1 hour at 37°C) with 100 μL of HRP-conjugated goat anti-mouse IgG, bovine anti-mouse IgG1, or bovine anti-mouse IgG2a. After extensive washing, 50 μl of substrate were added to each well and incubated for 15 minutes at 37°C. The plates were read at 450 nm (reference wavelength 630 nm) with a BenchMark ELISA reader (Bio-Rad Laboratories, Hercules, CA, USA). The cut-off line discriminating between positive and negative antibody detection was set as 0.20 (OD 450/630). The endpoint titer of anti-HBc antibodies is reported as the reciprocal of the highest dilution at which the OD 450/630 reading is above 0.20.
2.8. Statistical Analysis
Data are presented as mean ± SD of values obtained from replicate mice within a single representative experiment. Each experiment was repeated at least three times with similar results. Results were analyzed with SPSS software and compared by ANOVA and the post-hoc analysis. A P-value of <.05 was considered as statistically significant.
3. Results
3.1. Effect of β-glu6 on PB144-Induced Maturation of CD11c+ Dendritic Cells in the Spleen
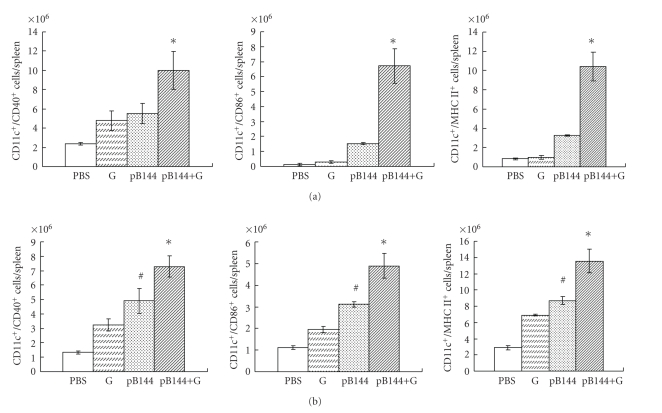
Activated dendritic cells (DC) have a strong antigen-presenting capacity. The effect of β-glu6 on pB144-induced activation of DC in mice was studied. In the spleen of mice immunized intramuscularly with pB144 plus β-glu6, the number of CD11c+ cells coexpressing CD40, CD86, or MHC-II was examined by flow cytometry. Compared with the mice immunized with pB144 alone, the populations of mature DC (CD11c+/CD40+, CD11c+/CD86+, and CD11c+/MHC-II+) in mice immunized by pB144 with β-glu6 at day 5 after priming were increased 1.8-, 4.4-, and 3.2-fold, respectively, as shown in Figure 1(a).
Figure 1.
Effect of the β -glu6 on pB144-induced CD11c+ DC maturation. Mice were injected in the hind leg muscle with β-glu6 (G, 1 mg/kg), pB144 (100 μg/mouse), pB144 together with β-glu6 (pB144+G), or PBS. At day 5 after priming (a) and day 5 after boosting (b), spleen CD11c+ cells coexpressing CD40 (left panels), CD86 (middle panels), or MHC-II (right panels) were measured by flow cytometry. Data are expressed as mean ± SD of data from 3 mice/group. *P < .05, pB144 +G versus all other groups; #P < .05, pB144 versus PBS control.
Compared to cells in naïve animals, at day 5 after boosting, CD11c+/CD40+, CD11c+CD86+, and CD11c+/MHC-II-+ DC increased about 3–3.5 times in the spleen of mice immunized with pB144 alone (from 1.3 × 106, 1.1 × 106, and 2.9 × 106 in naïve mice to 4.9 × 106, 3.1 × 106, and 8.7 × 106 in pB144 immunized mice), and about 4.5–5.6 times in the spleen of mice receiving pB144 plus β-glu6 (7.3 × 106, 4.9 × 106, and 13.6 × 106, resp.) (Figure 1(b)). No statistically significant difference was observed between naïve mice and mice receiving β-glu6 alone intramuscularly.
3.2. Effect of β-glu6 on PB144-Induced Recruitment and Activation of CD4+ and CD8+ T Cells
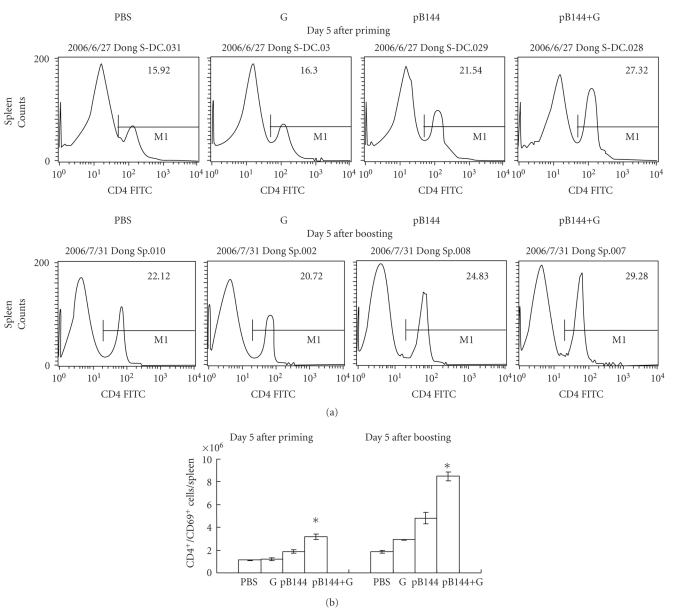
The recruitment and activation of CD4+ T cells in the spleen of mice immunized by pB144 with or without β-glu6 were evaluated. At day 5 after priming, compared to the mice immunized with pB144 alone, the percentage of CD4+ T cells in the mice immunized by pB144 with β-glu6 was higher in the spleen (from 21.5% to 27.3%) (Figure 2(a)). At day 5 after boosting, the percentage of CD4+ T cells in the mice immunized by pB144 with β-glu6 increased from 24.8% to 29.3% in the spleen (Figure 2(a)). Both at day 5 after priming and at day 5 after boosting, the number of activated CD4+/CD69+ T cells in the spleens of mice immunized by pB144 with β-glu6 was about 1.7-fold higher than that in mice immunized with pB144 alone (Figure 2(b)). The recruitment of CD4+ T cells in the liver and peripheral blood of mice immunized by pB144 with β-glu6 was of no significant difference from that in the mice immunized with pB144 alone, shown in the Supplementary Figure 1 (see in supplementary Material available online at doi:10.1155/2010/645213).
Figure 2.
Effect of β -glu6 on pB144-induced recruitment and activation of CD4+ T cells in the spleen. Mice were immunized with β-glu6 (G), pB144, pB144+G, or PBS as described in Section 2. Five days after priming and five days after boosting, the percentage of CD4+ T cells (a) and the number of CD4+/CD69+ T cells (b) in the spleen were evaluated. Data are expressed as mean of 3 mice/group. *P < .05, pB144 + G versus pB144 alone.
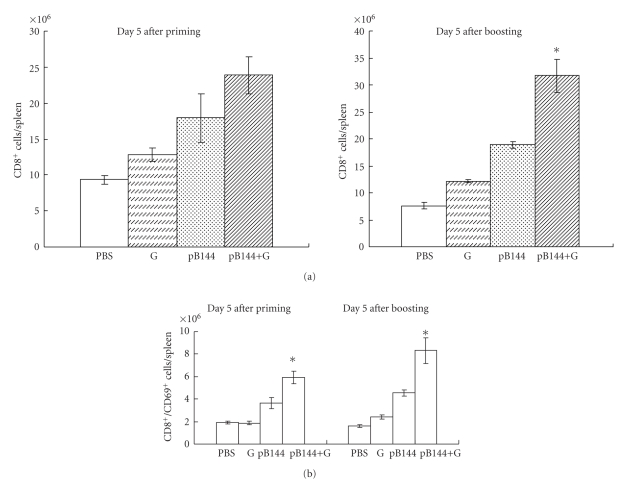
The recruitment and activation of CD8+ T cells in the spleen of mice immunized by pB144 with or without β-glu6 were evaluated. At day 5 after priming, upon treatment with β-glu6, the number of CD8+ T cells in the spleen of pB144-immunized mice was increased from 17.9 × 106 to 23.9 × 106 (Figure 3(a)). At day 5 after boosting, β-glu6 enhanced the pB144-induced recruitment of CD8+ T cells into the spleen by 1.7-fold (from 18.9 × 106 to 31.7 × 106 cells/spleen, P < .05) (Figure 3(a)). At day 5 after priming, a higher number of CD8+ T cells in splenocytes were activated by administration of pB144 with β-glu6, as compared to pB144 alone, judged by the expression of the CD69 as an activation marker (from 3.8 × 106 to 5.9 × 106 CD8+/CD69+ cells/spleen, P < .05), while activated CD8+/CD69+ T cells were increased to 8.3 × 106/spleen (P < .05 versus pB144 alone), at day 5 after boosting (Figure 3(b)). The number of CD8+ T cells in the liver and peripheral blood was not statistically different among groups (Supplementary Figure 2).
Figure 3.
Effect of β -glu6 on pB144-induced recruitment and activation of CD8+ T cells in the spleen. Mice were immunized with β-glu6 (G), pB144, pB144+G, or PBS, as described in Section 2. Five days after priming and five days after boosting, the number of CD8+ T cells (a) and CD8+/CD69+ T cells (b) in the spleen were evaluated. Data are expressed as mean ± SD of 3 mice/group. *P < .05, pB144+G versus pB144 alone.
3.3. Effect of β-glu6 on PB144-Induced Antigen Specific CD8+/IFN-γ+ T Cells
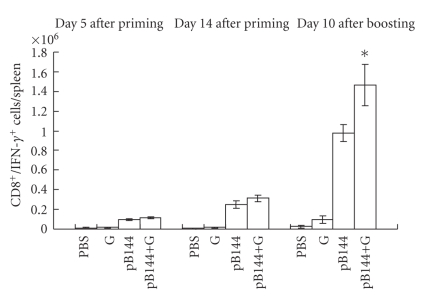
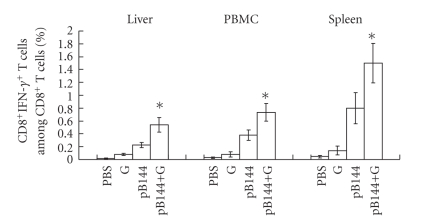
The effect of β-glu6 on antigen-specific CD8+/IFN-γ+ T cells in the mice immunized with pB144 was evaluated by intracellular cytokine staining, stimulated with the peptide MGLKFRQL, an H-2Kb-restricted CTL epitope of the hepatitis B core antigen. At day 5 and 14 after priming, β-glu6 did not increase the number of CD8+/IFN-γ+ T cells in the spleen (Figure 4). However, at day 10 after boosting, the number of CD8+/IFN-γ+ T cells was 1.5-fold higher in splenocytes from mice immunized by pB144 with β-glu6, as compared to mice immunized with pB144 alone. β-glu6 increased the percentage of CD8+/IFN-γ+ T cells induced by pB144 in the liver (0.54 ± 0.08%), PBMC (0.74 ± 0.17%), and the spleen (1.5 ± 0.3%), as compared with that in mice immunized with pB144 alone (Figure 5).
Figure 4.
Effect of β -glu6 on the CD8+/IFN-γ+T cells induced by pB144 in the spleen. Mice were immunized by pB144 with β-glu6, and boosted at day 30 after priming. At day 5 (left) and 14 (middle) after priming, and day 10 after boosting (right), splenocytes were isolated and cultured for 5 hours in the absence or in the presence of a specific peptide corresponding to Kb-restricted CTL epitope of HBcAg. Cells were then double-stained with PerCP-anti-CD8a and APC-anti-IFN-γ antibodies and analyzed cytofluorimetrically. Data expressed as mean ± SD of 3 mice/group. *P < .05, pB144+G versus pB144 alone.
Figure 5.
Effect of β -glu6 on the CD8+/IFN-γ+ T cells induced by pB144 in different organs. Mice were immunized with pB144 and treated with β-glu6, and boosted at day 30. Ten days after boosting, the liver lymphocytes, PBMC, and splenocytes were isolated and cultured for 5 hours in the absence or in the presence of a specific peptide corresponding to Kb-restricted CTL epitope of HBcAg. Cells were then double-stained with PE-anti-CD8a and APC-anti-IFN-γ antibodies and analyzed cytofluorometrically. Data are expressed as mean ± SD of 3 mice/group. *P < .05, pB144+G versus pB144 alone.
3.4. β-glu6 Enhanced the PB144-Induced Antibody Production
The effect of β-glu6 on anti-HBc antibody response induced by the DNA vaccine was evaluated by ELISA. Anti-HBc antibodies (IgG) in sera from immunized mice were detected at 4 weeks after priming and 2 weeks after boosting. At 4 weeks after priming, anti-HBcAg antibody titers in mice immunized with pB144 alone (1 : 900) were lower than that in mice receiving pB144 plus β-glu6 (1 : 2700) (Table 1). No anti-HBcAg antibodies were detected in the serum of control mice injected with PBS or β-glu6 alone. At 2 weeks after boosting, anti-HBc IgG was higher in mice immunized by pB144 with β-glu6 (1 : 24,300) than that in mice immunized with pB144 alone (1 : 8100).
Table 1.
Effect of β-glu6 on pB144-induced antibody production*.
| Immunogens | Anti-HBcAg antibody titer (Log2± SD)** | |||||
|---|---|---|---|---|---|---|
| Week 4 after priming | Week 2 after boosting | |||||
| IgG | IgG1 | IgG2a | IgG | IgG1 | IgG2a | |
| G | N.D.*** | N.D. | N.D. | N.D. | N.D. | N.D. |
| pB144 | 9.81 ± 0.21 | N.D. | N.D. | 12.98 ± 0.18 | N.D. | 11.81 ± 0.55 |
| pB144+G | 11.40 ± 0.09**** | N.D. | 6.90 ± 0.49 | 14.57 ± 0.50**** | N.D. | 12.13 ± 0.6 |
| PBS | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
*The sera from immunized mice were collected 4 weeks after priming and 2 weeks after boosting. Anti-HBcAg IgG, IgG1, and IgG2a were analyzed by ELISA. The initial dilution of each serum from immunized mouse was 1 : 100 and followed with serial of three-fold dilution for anti-HBc IgG detection, and two-fold dilution for anti-HBc IgG1, IgG2a analysis.
**The well with an absorbance OD450 ≥ 0.20 (the blank well, ~0.020) was scored as positive. The anti-HBc antibody titers are expressed as the reciprocal of the highest dilution showing a positive reaction and calculated as the mean ± standard deviation (SD) of Log2 for each group (6 mice/group).
***N.D. indicates that anti-HBc antibody in the sera of the mice is under detection level.
****P < .05, pB144 +G versus pB144 alone.
The IgG subclasses (IgG1 and IgG2a) of anti-HBc antibody were analyzed. At week 4 after priming, only low level of IgG2a was detected in mice immunized by pB144 with β-glu6. At week 2 after boosting, the mean titer of anti-HBc IgG2a was 1 : 4500 in the mice immunized by pB144 with β-glu6, and 1 : 3600 (mean) in mice receiving pB144 alone (Table 1). Anti-HBc IgG1 was under detection level in the all groups, even after boosting.
4. Discussion
Virus-specific CD8+ T cells response plays an important role in the process of viral clearance. Herpes Simplex Virus (HSV) began to be cleared from all sites about 5 days after infection when HSV glycoprotein B-specific CD8+ T cells first appear within infected tissues [24]. The failure of inducing a virus-specific CD8+ T cell response contributes to the development of pulmonary eosinophilia and disease augmentation in formalin-inactivated respiratory syncytial virus vaccine vaccinated individuals [25]. DNA immunization has the ability to induce a strong specific CD8+ T cell response against a variety of infectious diseases [26–28].
However, clinical applications of DNA vaccines are limited by their low immunogenicity. Therefore, it is imperative to develop effective adjuvants for improving protective response in DNA vaccination. Adjuvants have been proven to optimize CD8+ T cells response induced by DNA vaccine. For examples, cytokine plasmid-delivered IL-15 enhances the longevity of CD8+ T cells induced by pB144 DNA vaccine [18]; soluble CD40L, a member of the tumor necrosis factor superfamily, augments CD8+ T cell responses induced by human immunodeficiency virus DNA vaccines [16]. Some polysaccharides extracted from plants or microorganisms possess ability to improve the protective potential of a DNA vaccine against experimental infection [19, 29].
β-glu6 is a synthetic glucohexaose containing a structure of β-(1→6)-branched β-(1→3) with an α-(1→3)-linked bond, that is an analogue of Lentinan basic unit. Lentinan was extracted from Lentinus edodes being recorded in Compendium of Materia Medica for the treatment and prevention of diseases in traditional Chinese medicine. Lentinan can strengthen the cell-mediated immune response [30, 31] and activate some innate immune effector cells such as mononuclear macrophages and NK cells [11]. Administration of Lentinan before infection can mobilize host defence and reduce mycobacterial infection [32]. The β-(1→6)-branched β-(1→3) glucohexaose is a basic unit of Lentinan, endowed with potent anticarcinoma activity. β-glu6, synthetic glucohexaose analogue, has stimulatory effects on mouse spleen cells (cell proliferation, TNF-α production), which is similar to Lentinan [12, 13]. Furthermore, β-glu6 has the advantages over Lentinan, such as possessing a defined structure, higher purity (>98% by HPLC), and availability.
Based on the study of β-glu6 as adjuvant enhancing effectivity of protein vaccine (Hepatitis B surface antigen) [14], we further investigated the adjuvant effect of β-glu6 on antigen-specific CD8+ T cell response induced by DNA vaccine encoding HBcAg (pB144) that can be used as antigen candidate of DNA vaccine against HBV. We found β-glu6 possess ability to enhance the maturation of DCs, the recruitment and activation of T cells, and to increase the number of antigen-specific CD8+/IFN-γ+ T cells induced by pB144.
DCs function as both antigen-presenting cells and antigen-producing cells in DNA vaccine immunization. Thus, triggering DC activation can improve the efficacy of genetic vaccines [33]. Immunization of HIV-1 envelope (env) DNA vaccine alone recruited few DCs to the injection site and elicited low-frequency, Env-specific immune responses in mice [34]. As an endogenous ligand, polysaccharide degradation products of the extracellular matrix produced during inflammation may activate DCs via TLR4 [35]. In the previous study, it showed administration of β-glu6 (i.p.) alone has effect on DC recruitment and maturation in the spleen [14]. Maturation of bone marrow-derived dendritic cell (MDC) induced by HBsAg was enhanced with β-glu6 treatment, and in mixed lymphocyte reaction with MDC the proliferation of T cells was increased with the treatment of β-glu6 and HBsAg (unpublished data). In the present study, β-glu6 was able to enhance the DC maturation and migration to the spleen induced by DNA vaccines. We investigated the effect of anti-TLR2 and anti-TLR4 on β-glu6 immune activity, and found that the antibody against to TLR2 or TLR4 inhibited the activities of β-glu6 inducing TNF-α secretion in a mouse macrophage cell line, RAW264.7, suggesting that β-glu6 may activate innate immune cells via TLR2 or TLR4 signal pathway, though it needs to be confirmed in vivo. DC maturation may be related with β-glu6 enhancing the HBcAg-specific CD8+ T cell response induced by DNA vaccine. Steffen Jung et al. observed that DC-depleted mice fail to mount CTL responses to infection with the intracellular bacterium Listeria monocytogenes and the rodent malaria parasite Plasmodium yoelii [36], suggesting that DC maturation play a role in specific CD8+ T cell activation.
Antigen-specific CD8+ and CD4+ T cells play a vital role in control of viral infection. They can remove infected target cells through cytotoxic or noncytotoxic function, such as producing IFN-γ and other Th1 cytokines [37]. Christine Heufler' et al. found DC produced bioactive IL-12 upon antigen-specific interaction with T cells without any other stimuli, and DC-derived IL-12 was critical for optimal proliferation and IFN-γ production by activated Thl blasts [38]. The enhancement of DC maturation, migration, and the antigen presentation increased the number of antigen-specific CD8+/IFN-γ+T cells in DNA vaccine-immunized mice [39]. Booster injections with mature DCs raised CD8+ T cell response in humans [40]. In the mice immunized by pB144 with β-glu6, the number of HBcAg-specific CD8+/IFN-γ+ T cells in lymphoid tissues (the spleen) and nonlymphoid tissue (the liver), were higher than that in the mice vaccinated by pB144 alone, suggesting that β-glu6 can amplify specific Th1 immune response induced by the DNA vaccine. With Lentinan being injected into mice intraperitoneally, the macrophage glutathione redox status and capability to produce IL-12 were improved, thus orienting toward type-1 immunity [41]. The effect of β-glu6 on the IFN-γ, IL-4 and DC-derived IL-12 production induced by pB144 needs to be further investigated.
In the present study, we found that β-glu6 increased the recruitment of CD4+and CD8+ T cells to the spleen, which was induced by pB144. With recruitment of antigen—nonspecific CD4+ and CD8+ T cells to lymphoid tissue, T cell-derived cytokines may help antigen-specific T cells augmentation.
When the antigen is endogenously produced upon intramuscular DNA vaccination, β-glu6 improved anti-HBc antibody production, in mice which the major IgG subclass of anti-HBc antibody was IgG2a and IgG1 were detected scarcely, and amplified the HBcAg-specific CD8+ T cell response induced by DNA vaccine, that indicates a bias towards a Th1 immune response. In the previous study, β-glu6 improved anti-HBsAg IgG1 antibody production and the number of HBsAg-specific IL-4-producing T cells in spleen in mice immunized with HBsAg protein vaccine [14], indicating that β-glu6 can act as an adjuvant for a protein vaccine shifting towards a Th2-biased response. The roles of route of antigen entry, the physical form of antigen, the type of adjuvant and the dose of antigen in controlling the type of Th-cell differentiation have been reported [42]. Apparently, β-glu6 can amplify different types of responses, depending on the type of the vaccine utilized. Recent work suggests that dendritic cell subsets contribute significant polarizing influences on T helper differentiation, but how this comes about is less clear. Mosmann and Coffman indicated a single APC type may influence the Th1/Th2 ratio by providing different accessory signals to Th cells, depending on the physical nature of the antigen encountered [33, 43]. The physical type of vaccine and DC distinct roles in immunization may contribute to β-glu6 skewing Th1/Th2 immune response induced by vaccine that warrant to study.
Because mice is a species already known to respond very well to DNA immunization, the effect of β-glu6 has yet to be proved in another animal species refractory to DNA immunization, like nonhuman primates. Since Lentinan is recorded in Chinese Pharmacopoeia as an immunopotentiator, the plasticity of the adjuvant effects displayed by β-glu6, which contains the basic bioactive unit of Lentinan, makes it a suitable candidate adjuvant for different types of vaccines, providing its potential clinical application.
Supplementary Material
Online Supplemental Material includes effect of β-glu6 on pB144-induced recruitment of T cells. The recruitment of CD4+ T cells in the liver and peripheral blood of mice immunized by pB144 with β-glu6 is shown in the Supplementary Figure 1. The number of CD8+ T cells in the liver and peripheral blood is illustrated in Supplementary Figure 2.
Acknowledgments
J. Wang, S.-F. Dong, and C.-H. Liu contributed equally to this work. This work was supported by the Program of Ministry of Science and Technology of China (2010DFA32100, 2008ZX10004-014, and 2008ZX10002-002), and the International Science and Technology Cooperation Program of Shanghai Science and Technology Commission (074307038).
References
- 1.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. Journal of Experimental Medicine. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar JC, Rodríguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–3762. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki T, Takasuka N. Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes. Carbohydrate Research. 1976;47(1):99–104. doi: 10.1016/s0008-6215(00)83552-1. [DOI] [PubMed] [Google Scholar]
- 4.Tsukagoshi S, Hashimoto Y, Fujii G, Kobayashi H, Nomoto K, Orita K. Krestin (PSK) Cancer Treatment Reviews. 1984;11(2):131–155. doi: 10.1016/0305-7372(84)90005-7. [DOI] [PubMed] [Google Scholar]
- 5.Borchers AT, Keen CL, Gershwini ME. Mushrooms, tumors, and immunity: an update. Experimental Biology and Medicine. 2004;229(5):393–406. doi: 10.1177/153537020422900507. [DOI] [PubMed] [Google Scholar]
- 6.Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity. Proceedings of the Society for Experimental Biology and Medicine. 1999;221(4):281–293. doi: 10.1046/j.1525-1373.1999.d01-86.x. [DOI] [PubMed] [Google Scholar]
- 7.The Chinese Pharmacopoeia 2005. Beijing, China: Chinese Pharmacopoeia Commission, Chemical Industry Press; 2005. [Google Scholar]
- 8.Zhang L, Li X, Xu X, Zeng F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydrate Research. 2005;340(8):1515–1521. doi: 10.1016/j.carres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Kupfahl C, Geginat G, Hof H. Lentinan has a stimulatory effect on innate and adaptive immunity against murine Listeria monocytogenes infection. International Immunopharmacology. 2006;6(4):686–696. doi: 10.1016/j.intimp.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Fang JN, Wang SC. Research advances of Lentinan. Journal of Clinical Pharmacology. 1997;32:332–334. [Google Scholar]
- 11.Smith JE, Rowan NJ, Sulivan R. Medicinal mushrooms: their therapeutic properties and current medical usage with special emphasis on cancer treatments. Cancer Research UK, University of Strathclyde, 2002, http://www.icnet.uk/labs/med_mush/med_mush.html.
- 12.Ning J, Zhang W, Yi Y, et al. Synthesis of β-(1 → 6)-branched β-(1 → 3) glucohexaose and its analogues containing an α-(1 → 3) linked bond with antitumor activity. Bioorganic and Medicinal Chemistry. 2003;11(10):2193–2203. doi: 10.1016/s0968-0896(03)00118-4. [DOI] [PubMed] [Google Scholar]
- 13.Yan J, Zong H, Shen A, et al. The β-(1 → 6)-branched β-(1 → 3) glucohexaose and its analogues containing an α-(1 → 3)-linked bond have similar stimulatory effects on the mouse spleen as Lentinan. International Immunopharmacology. 2003;3(13-14):1861–1871. doi: 10.1016/j.intimp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Dong SF, Chen JM, Zhang W, et al. Specific immune response to HBsAg is enhanced by β-glucan oligosaccharide containing an α-(1 → 3)-linked bond and biased towards M2/Th2. International Immunopharmacology. 2007;7(6):725–733. doi: 10.1016/j.intimp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Mölder T, Adojaan M, Kaldma K, Ustav M, Sikut R. Elicitation of broad CTL response against HIV-1 by the DNA vaccine encoding artificial multi-component fusion protein MultiHIV-Study in domestic pigs. Vaccine. 2009;28(2):293–298. doi: 10.1016/j.vaccine.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 16.Stone GW, Barzee S, Snarsky V, et al. Multimeric soluble CB40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. Journal of Virology. 2006;80(4):1762–1772. doi: 10.1128/JVI.80.4.1762-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Han Q, Zhang N, et al. HBcAg18-27 epitope fused to HIV-Tat49-57 adjuvanted with CpG ODN induces immunotherapeutic effects in transgenic mice. Immunology Letters. 2010;127(2):143–149. doi: 10.1016/j.imlet.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Dong SF, Sun SH, Wang Y, Li GD, Qu D. Coimmunization with IL-15 plasmid enhances the longevity of CD8 T cells induced by DNA encoding hepatitis B virus core antigen. World Journal of Gastroenterology. 2006;12(29):4727–4735. doi: 10.3748/wjg.v12.i29.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Shao HJ, Su YB. Coimmunization of Agaricus blazei Murill extract with hepatitis B virus core protein through DNA vaccine enhances cellular and humoral immune responses. International Immunopharmacology. 2004;4(3):403–409. doi: 10.1016/j.intimp.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Wierzbicki A, Kiszka I, Kaneko H, et al. Immunization strategies to augment oral vaccination with DNA and viral vectors expressing HIV envelope glycoprotein. Vaccine. 2002;20(9-10):1295–1307. doi: 10.1016/s0264-410x(01)00480-7. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Liu J, Kong Y, Wang Y, Li G. DNA immunization with fusion genes containing HCV core region and HBV core region. Science in China, Series C. 1999;42(2):171–177. doi: 10.1007/BF02880053. [DOI] [PubMed] [Google Scholar]
- 22.Lu M, Isogawa M, Xu Y, Hilken G. Immunization with the gene expressing woodchuck hepatitis virus nucleocapsid protein fused to cytotoxic-T-lymphocyte-associated antigen 4 leads to enhanced specific immune responses in mice and woodchucks. Journal of Virology. 2005;79(10):6368–6376. doi: 10.1128/JVI.79.10.6368-6376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Dong Z, Zhou R, Luo D, Wei H, Tian Z. Isolation of lymphocytes and their innate immune characterizations from liver, intestine, lung and uterus. Cellular & Molecular Immunology. 2005;2(4):271–280. [PubMed] [Google Scholar]
- 24.Van Lint A, Ayers M, Brooks AG, Coles RM, Heath WR, Carbone FR. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. Journal of Immunology. 2004;172(1):392–397. doi: 10.4049/jimmunol.172.1.392. [DOI] [PubMed] [Google Scholar]
- 25.Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. Journal of Immunology. 2007;179(8):5415–5424. doi: 10.4049/jimmunol.179.8.5415. [DOI] [PubMed] [Google Scholar]
- 26.Okuda K, Bukawa H, Hamajima K, et al. Induction of potent humoral and cell-mediated immune responses following direct injection of DNA encoding the HIV type 1 env and rev gene products. AIDS Research and Human Retroviruses. 1995;11(8):933–943. doi: 10.1089/aid.1995.11.933. [DOI] [PubMed] [Google Scholar]
- 27.Qu D, Lanier G, Zheng HY, Yu MW, Howard CR, Ahmed R. Localization of CD8+ cells specific for hepatitis B virus surface protein in the liver of immunized mice. Journal of Medical Virology. 2008;80(2):225–232. doi: 10.1002/jmv.21039. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Usherwood EJ, Surman SL, Hogg TL, Woodland DL. Long-term CD8+ T cell memory to Sendai virus elicited by DNA vaccination. Journal of General Virology. 1999;80(6):1393–1399. doi: 10.1099/0022-1317-80-6-1393. [DOI] [PubMed] [Google Scholar]
- 29.Mussalem JS, Vasconcelos JRC, Squaiella CC, et al. Adjuvant effect of the Propionibacterium acnes and its purified soluble polysaccharide on the immunization with plasmidial DNA containing a Trypanosoma cruzi gene. Microbiology and Immunology. 2006;50(4):253–263. doi: 10.1111/j.1348-0421.2006.tb03791.x. [DOI] [PubMed] [Google Scholar]
- 30.Maeda YY, Chihara G. Lentinan, a new immuno-accelerator of cell-mediated responses. Nature. 1971;229(5287):p. 634. doi: 10.1038/229634a0. [DOI] [PubMed] [Google Scholar]
- 31.Hamuro J, Chihara G. Lentinan, a cell-oriented immunopotentiator: its experimental and clinical applications and possible mechanism of immune modulation. In: Fenichel RL, Chirigos MA, editors. Immune Modulation Agents and Their Mechanisms. New York, NY, USA: Marcel Deeker; 1984. [Google Scholar]
- 32.Markova N, Kussovski V, Drandarska I, Nikolaeva S, Georgieva N, Radoucheva T. Protective activity of Lentinan in experimental tuberculosis. International Immunopharmacology. 2003;3(10-11):1557–1562. doi: 10.1016/S1567-5769(03)00178-4. [DOI] [PubMed] [Google Scholar]
- 33.Takashima A, Morita A. Dendritic cells in genetic immunization. Journal of Leukocyte Biology. 1999;66(2):350–356. doi: 10.1002/jlb.66.2.350. [DOI] [PubMed] [Google Scholar]
- 34.Sumida SM, McKay PF, Truitt DM, et al. Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines. Journal of Clinical Investigation. 2004;114(9):1334–1342. doi: 10.1172/JCI22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. Journal of Experimental Medicine. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature Reviews Immunology. 2005;5(3):215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 38.Heufler C, Koch F, Stanzl U, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. European Journal of Immunology. 1996;26(3):659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 39.Kim TW, Hung CF, Boyd D, et al. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life with intracellular targeting strategies. Journal of Immunology. 2003;171(6):2970–2976. doi: 10.4049/jimmunol.171.6.2970. [DOI] [PubMed] [Google Scholar]
- 40.Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N. Mature dendritic cells boost functionally superior CD8+ T-cell in humans without foreign helper epitopes. Journal of Clinical Investigation. 2000;105(6):R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murata Y, Shimamura T, Tagami T, Takatsuki F, Hamuro J. The skewing to Th1 induced by lentinan is directed through the distinctive cytokine production by macrophages with elevated intracellular glutathione content. International Immunopharmacology. 2002;2(5):673–689. doi: 10.1016/s1567-5769(01)00212-0. [DOI] [PubMed] [Google Scholar]
- 42.Romagnani S. The Th1/Th2 paradigm. Immunology Today. 1997;18(6):263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 43.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual Review of Immunology. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplemental Material includes effect of β-glu6 on pB144-induced recruitment of T cells. The recruitment of CD4+ T cells in the liver and peripheral blood of mice immunized by pB144 with β-glu6 is shown in the Supplementary Figure 1. The number of CD8+ T cells in the liver and peripheral blood is illustrated in Supplementary Figure 2.