Abstract
The identification of one mechanism that causes vision loss in inherited degenerative retinal disorders revealed a new signaling molecule that represents a potential therapy for these currently untreatable diseases. This protein, called rod-derived cone viability factor (RdCVF), maintains the function and consequently the viability of cone photoreceptor cells in the retina; mice that lack this factor exhibit a progressive loss of photoreceptor cells. The gene encoding RdCVF also encodes, by differential splicing, a second product that has characteristics of a thioredoxin-like enzyme and protects both photoreceptor cells and, more specifically, its interacting protein partner, the tau protein, against oxidative damage. This signaling pathway potentially links environmental insults to an endogenous neuroprotective response.
INTRODUCTION
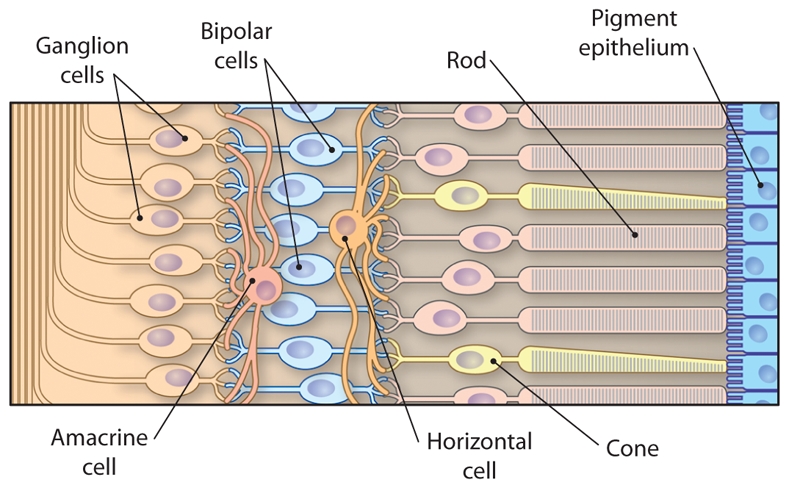
The retina, an intimate part of the brain, has proven to be an extraordinary fruitful model for neurobiologists and developmental biologists for more than a century. We now have remarkable knowledge about the physiology of this light-sensing organ (Fig. 1) and of multiple genes contributing to its development, maintenance, and functions. The complexity of the retina accounts for its vulnerability: Inherited retinal degeneration disorders, including retinitis pigmentosa (RP) and Leber congenital amaurosis (LCA), can be caused by mutations in a wide variety of genes in human and animal models. Degeneration of photoreceptors cells (neurons in the retina that convert photons into electrophysiological signals) can be caused by mutations in genes coding not only for proteins of the phototransduction cascade but also, surprisingly, in many other genes expressed more widely throughout the body. Currently, one of the major challenges for scientists and ophthalmologists working in this field is to provide both rationales for therapeutic interventions to prevent blindness and clinically relevant proofs of concept.
Fig. 1. Organization of the retina.
CREDIT: C. BICKEL/SCIENCE TRANSLATIONAL MEDICINE
TREATMENT STRATEGIES FOR DEGENERATIVE RETINAL DISEASES
Gene therapy
The successful ongoing gene therapy trial using the RPE65 gene for treating patients suffering from LCA—an autosomal recessive disorder—is at the cutting edge of translational research (1). RPE65 was identified in 1993 (2); mutations in this gene were found to cause some cases of LCA in humans in 1997 and in dog in 1998 (3–5). In 2001, the disease was successfully cured in a dog model of LCA by the delivery of a normal copy of the gene (6). The efficacy of this corrective gene therapy approach was confirmed in subsequent clinical trials conducted by three independent groups in 2008 (7, 8, 9, 10). This success story, even though it resuscitated interest in gene therapy, has to be positioned in its medical context: the RPE65 mutations cause only 2% of the autosomal inherited retinal degenerations, and consequently this approach is applicable only to a limited number of patients.
Cell replacement
Alternatively to the corrective gene therapy approach, replacement of the missing cells with functional photoreceptor cells has been attempted for decades. The major hurdle of that strategy is the need to establish synaptic connectivity between the transplanted cells and the inner neurons: the bipolar and the horizontal cells. of the grafted animal. The problem has recently been addressed by grafting photoreceptor precursor cells from a stage corresponding to the onset of expression of the transcription factor Nrl, which is involved in photoreceptor differentiation (11). Again, this discovery has to be positioned into the perspective of its implementation into clinical practice. Because that step of retinal development occurs during a fetal stage of human development, the availability of material for grafting will be a major limitation. A more effective strategy may rely on human stem cells genetically engineered to reach the proper stage of photoreceptor differentiation as represented by the onset of NRL expression, but the use of such cells is currently only a promise for the future (12).
Supplementation with a trophic factor
Our approach to the question was inspired by the sequential occurrence of the clinical manifestations in the retinal degeneration disorders. In patients suffering from RP, the most common form of these genetic diseases, the vision loss develops in two successive steps. Early in adulthood, these patients lose their ability to see in dim light conditions (referred to as a loss of night vision), which corresponds to the loss of function and degeneration of rod photoreceptors. This change is felt as a minor handicap, especially in individuals affected by congenital stationary night blindness, another type of inherited retinal disease characterized exclusively by lack of rod function; in our current well-illuminated environment, these people retain an almost normal way of life (13). For patients with RP, the disease then progresses through a debilitating step resulting from loss of function and degeneration of the second class of photoreceptors, the cones, that dominate at the center of the retina and are required for color vision. Cones represent only 3 to 5% of all photoreceptors in most mammals, but their role for vision is essential. This secondary event leads to central vision loss and potentially complete blindness.
Because the cones underlie all visual functions in a lighted environment, we deemed cone rescue a clinically relevant target. We studied the mechanism of the secondary degeneration of cone photoreceptors in a commonly used model of RP, the rd1 mouse. This mouse carries a spontaneous mutation in the gene encoding the beta subunit of the rod-specific phosphodiesterase 6 (Pde6b). Recessive mutations in the corresponding gene in humans can lead to RP. Although this gene is not expressed by cone cells, they nevertheless degenerate after the loss of rods, and various hypotheses were put forth to explain this mystery. To discriminate among the possibilities, we demonstrated that grafting normal photoreceptors (97% rods) into the eye of the rod-less rd1 mouse before the cones degenerate exerts a positive effect on the host retina cones even when the cones are at some distance from the transplant (16, 17). Subsequent work in vitro showed that this paracrine protective activity was carried by molecules, most likely proteins, that are secreted in the presence of rods (18, 19). Globally, this part of our work revealed the existence of proteins that we designated as rod-derived cone viability factors (RdCVFs) and parallels the work of Punzo et al. (20). We predicted that these proteins were expressed and secreted specifically in the presence of rods in the retina and that their expression was lost following rod death in the first phase of the disease. This loss would then trigger cone degeneration through a mechanism reminiscent of a phenomenon observed during neuronal development, when the loss of trophic factors—which are required for neuronal survival—results in the death of some neurons (21). These RdCVFs would be particularly well suited for preventing the secondary degeneration of cones and for treating RP at a stage in which night blindness is associated with moderate central visual impairment, an approach that is almost independent of the causative mutations.
CHARACTERIZATION OF GENES ENCODING RdCVFs
To identify the genes encoding RdCVFs, we applied a systematic strategy based on function rather than sequence similarity, because no similar proteins were described at that time in the retina. We constructed a cDNA expression library from normal mouse retinal tissue with the assumption that the mRNAs encoding these factors would be included in it. To screen this library, in view of the very limited number of cones in mammalian retinas, we developed a cone survival assay made of dispersed cultured cells from the retina of chicken embryos. In these cultures, and in the absence of developmental cues, the retinal precursors differentiate as photoreceptors by a default pathway (22). After confirming that these cells respond to the trophic activity of the normal mouse retina (19), we screened more than 200,000 clones and identified a cDNA encoding the first 109 residues of a protein that we referred to as RdCVF (23). RdCVF protein expression is rod-dependent in the mouse; when administered by a sub-retinal injection, RdCVF prevents rd1 mouse cones from degenerating.
The cDNA we identified represents the Nucleoredoxin-like (Nxnl1) gene, which encodes two products via alternative splicing. The product identified in our screen is a truncated thioredoxin-like protein with no enzymatic activity; the other product is the full-length protein, which is predicted to have enzymatic activity (Fig. 2). Interestingly, the thioredoxin family of proteins protects against oxidative stress, a role that has been described in great detail (24). In the horseshoe crab Carcinoscorpius rotundicauda, the gene encodes a protein—RdCVFL—that comprises an entire thioredoxin fold and exhibits thiol-oxidoreductase activity (25). We subsequently identified in silico a paralogous gene in mice, Nxnl2, that also encodes both a truncated thioredoxin named RdCVF2—for which we demonstrated protective activity toward cones—and RdCVF2L, which contains an entire thioredoxin fold (26).
Fig. 2. Schematic representation of the Nxnl1 gene and its two products, RdCVF and RdCVFL.
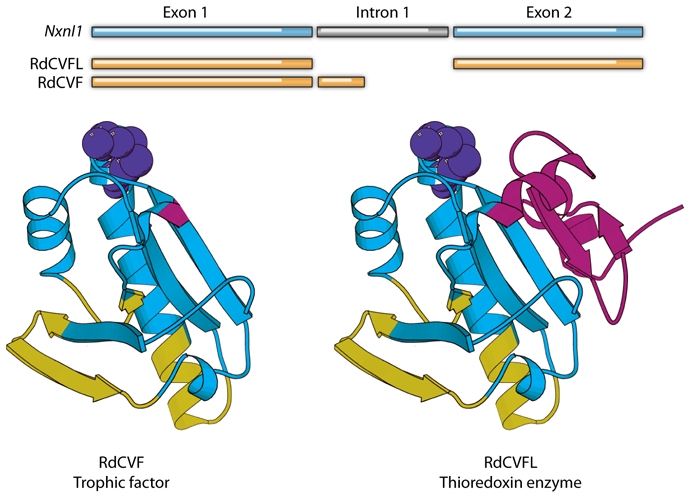
Alternative splicing generates the truncated trophic factor and the longer thioredoxin-like enzyme. In the ribbon diagrams, the thioredoxin catalytic site is shown in yellow, the shared portion of the thioredoxin fold in red, the additional thioredoxin fold (found only in RdCVFL) in green, and the RdCVF specific loops in blue.
CREDIT: C. BICKEL/SCIENCE TRANSLATIONAL MEDICINE
POTENTIAL THERAPEUTIC VALUE OF RdCVF
Because our claim is that the RdCVF could be used to treat cone degeneration in RP regardless of which rod-specific gene is mutant, we tested its therapeutic value in a different model of RP, the transgenic P23H rat. Whereas the rd1 mouse models human PDE6B mutations that account for 4% of the recessively inherited retinal degenerations, the P23H rat is a model of autosomal dominant RP in which the rats carry the human rhodopsin gene containing an RP-associated mutation that causes 25% of the dominantly inherited retinal degenerations. Sub-retinal injection of RdCVF in the P23H rat resulted in protection against the secondary degeneration of cones that normally occurs—proof of concept that this therapy would be useful for large cohorts of RP patients (27). Even more interestingly, we demonstrated that RdCVF prevents the loss of function of cone photoreceptors in this model by testing the visual function of cones using electroretinography (ERG), which measures light-induced electrical responses in the retina. The amplitude of the protective effect recorded by ERG was five orders of magnitude higher than the survival effect measured by the viability of the cones. This intriguing observation led us to analyze further the morphology of the outer segment of the cones, a specialized cytostructure containing the light-sensitive opsin receptors, which are required for vision. We demonstrated that the cone outer segment is significantly preserved by RdCVF, suggesting that its effect on survival might be an indirect consequence of the maintenance of a photoreceptor structure essential for vision (Fig. 3). This finding further enhances the therapeutic importance of RdCVF. In contrast, the only factor currently being tested in clinical trials for RP ciliary neurotrophic factor, induces an increase in the number of photoreceptor cells and a decrease in the amplitudes of light-induced electrical responses (as measured by ERG) in animal models (28). RdCVF’s mechanism most likely involves a yet unidentified cell surface receptor expressed at the surface of the cones, similar to the mechanism of the structurally-related protein TRX80 (a cytokine produced by the truncation of the thioredoxin TRX1 at a position similar to RdCVF), which binds to a receptor on peripheral blood cells (29).
Fig. 3. Maintenance of the cone outer segment by RdCVF.
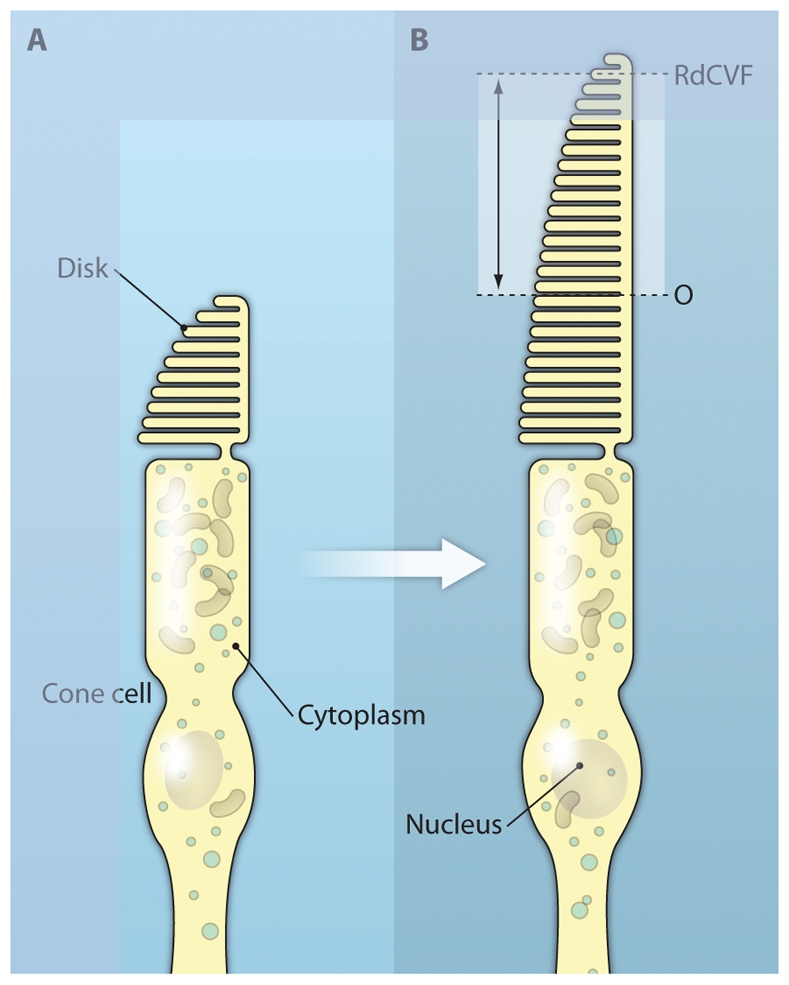
RdCVF was injected into eyes of the P23H rat, a model of autosomal dominant RP. The cone cells (green) of the RdCVF-injected animal (bottom panel) display a longer outer segment (indicated by double arrows) and smaller diameter (asterisk) than those in the vehicle-injected rat (top panel). The relative positions of the cone outer segment in each case are indicated on the diagram on the right.
PROTECTION AGAINST OXIDATIVE STRESS
Another intriguing aspect of this signaling is the fact that the trophic molecule is a member of a family of proteins that function to protect cells against oxidative stress by scavenging reactive oxygen species to maintain the appropriate intracellular redox state. The role of oxidative stress in neuronal degeneration—and more broadly in aging—is well documented. Does RdCVF participate in a redox signaling? The first insight came from the analysis of a mouse in which the Nxnl1 gene was inactivated (30). Cone function is normal in young Nxnl1−/− mice, but deteriorates month-by-month as the mice age, indicating that the gene is involved in the later (but not early) developmental stages or aging. Interestingly, the rods are also affected over time, a fact that we attribute to the enzymatic function of RdCVFL more than to the trophic activity of RdCVF. This progressive retinal degeneration is associated with increasing oxidative damage, suggesting a relation between RdCVF signaling and redox signaling. A connection is indicated more directly by the observation that cone cell function in Nxnl1−/− mice deteriorates faster than usual when these mice are kept under high oxygen pressure. We postulate that RdCVF is not only a therapeutic molecule but also a physiological signal involved in the maintenance of photoreceptors during aging and exposure to oxidative stress. This hypothesis is also supported by unpublished results demonstrating the increased susceptibility of the rod cells in Nxnl1−/− mice to light-induced damage relative to those in wild-type mice. Other groups have demonstrated that oxidative stress may play a role in cone degeneration (31). We propose that the cone degeneration in RP may result from both the loss of a protective mechanism (that is, RdCVFs) and the increased exposure to light, and oxygen from the choriocapillaries of the eye that occurs after rod loss.
Does RdCVFL, the full-length enzymatically active product of the Nxnl1 gene, function in concert with the shorter trophic form RdCVF? It is tempting to speculate that both forms work in the same regulatory system. As an attractive hypothesis, the RdCVFL enzyme could be the sensor for oxidative conditions, coupling to the trophic protein RdCVF for an environmentally adapted response. Before addressing this question directly, we searched for RdCVFL targets by screening for interacting proteins using a proteomic approach (32). We identified components of the splicing machinery and actin-binding proteins as RdCVFL binding partners. The interaction with the splicosome is perhaps related to a possible regulation of Nxnl1 splicing by its protein product RdCVFL. Among the actin-binding proteins, we are particularly interested in the interaction of RdCVFL with the microtubule-associated protein tau (a protein that also interacts with actin), which is involved in the formation of neurotoxic aggregates in the brain of patients suffering from Alzheimer’s disease. We found that tau is hyperphosphorylated in the retina of Nxnl1−/− mice and, furthermore, that rod photoreceptor degeneration in these mice is associated with tau aggregation in the retina (30, 32). In vitro, RdCVFL inhibits tau phosphorylation and limits oxidative damage of tau. In sum, we show that RdCVF signaling in the retina is a model of a defensive neuronal response against progressive oxidative damage resulting from exposure to environmental factors. This signaling might be relevant to other neurodegenerative diseases.
CONCLUSIONS
What have we learned from this translational line of research? The Nxnl1 gene and, most likely, the paralogous gene Nxnl2 are part of a newly-discovered redox signaling pathway that involves an enzymatic product and a trophic factor, both encoded by the same gene. As far as we know, this is the first time that such a phenomenon has been described in the central nervous system. Before unraveling all the fine details about this pathway, we are using this knowledge to develop a therapy aimed at preventing the loss of cone function and central vision in RP patients. Our hope is that this therapy, by restoring a physiological signal, will efficiently treat the effects of a broad range of RP mutations. The delivery of RdCVF into the patient’s eyes can be achieved through different routes, either by injection of the protein into the eye, expression from viral vectors, or delivery of RdCVF-producing cells that are encapsulated in a semi-permeable membrane to avoid attack by the immune system. An additional possibility came from our study of the Nxnl1 promoter (33). We found that under normal conditions the transcription factor VSX2 regulates the expression of Nxnl1 in bipolar cells—neurons that transmit signals from photoreceptors to ganglion cells (Fig. 1). That expression is lost in the rd1 retina after rod degeneration even if the bipolar cells themselves do not degenerate. Understanding this non-cell autonomous mechanism may allow recapitulating RdCVF expression in bipolar cells to preserve cone function.
In summary, over the last 12 years, we have reduced a clinical observation to a cellular model that allowed us to identify the molecular mechanism involved in cone protection. Taking this knowledge back to the organism, RdCVF is now in translation into a possible therapeutic agent to treat a spectrum of degenerative eye diseases. Furthermore, these studies have increased our knowledge of an intricate biological system.
Acknowledgments
Grant Support: Inserm, UPMC, ANR, EVI-GENORET (FP6), Retina France, Fédération de aveugles de France, Foundation Fighting Blindness, Novartis, Fovea Pharma,.
Footnotes
Conflicts of interest: T.L. and J-A.S. are patent-holders on the use of RdCVF and RdCVF2 for the treatment of retinal and neurological disease
References and Notes
- 1.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene Therapy for Leber’s Congenital Amaurosis is Safe and Effective Through 1.5 Years After Vector Administration. Mol Ther. 2009 Dec 1; doi: 10.1038/mt.2009.277. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J Biol Chem. 1993;268:15751–15757. [PubMed] [Google Scholar]
- 3.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 4.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre GD, Baldwin V, Pearce-Kelling S, Narfström K, Ray K, Acland GM. Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol Vis. 1998;4:23. [PubMed] [Google Scholar]
- 6.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 7.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe, McDonnell NJ, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 9.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, Flotte TR, Fishman GA, Heon E, Stone EM, Byrne BJ, Jacobson SG, Hauswirth WW. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JI, Hauck B, Zelenaia O, Zhu X, Raffini L, Coppieters F, De Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun J, McDonnell JW, Leroy BP, Simonelli F, Bennett J. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLarenv RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 12.Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright AF. A searchlight through the fog. Nat Genet. 1997;17:132–134. doi: 10.1038/ng1097-132. [DOI] [PubMed] [Google Scholar]
- 14.Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17:175–205. doi: 10.1016/s1350-9462(97)00012-8. [DOI] [PubMed] [Google Scholar]
- 15.Sahel JA, Mohand-Said S, Léveillard T, Hicks D, Picaud S, Dreyfus H. Rod-cone interdependence: implications for therapy of photoreceptor cell diseases. Prog Brain Res. 2001;131:649–661. doi: 10.1016/s0079-6123(01)31051-8. [DOI] [PubMed] [Google Scholar]
- 16.Mohand-Said S, Hicks D, Simonutti M, Tran-Minh D, Deudon-Combe A, Dreyfus H, Silverman MS, Ogilvie JM, Tenkova T, Sahel J. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res. 1997;29:290–297. doi: 10.1159/000268027. [DOI] [PubMed] [Google Scholar]
- 17.Mohand-Said S, Hicks D, Dreyfus H, Sahel JA. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch Ophthalmol. 2000;118:807–811. doi: 10.1001/archopht.118.6.807. [DOI] [PubMed] [Google Scholar]
- 18.Mohand-Said S, Deudon-Combe A, Hicks D, Simonutti M, Forster V, Fintz AC, Léveillard T, Dreyfus H, Sahel JA. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc Natl Acad Sci U S A. 1998;95:8357–8362. doi: 10.1073/pnas.95.14.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fintz AC, Audo I, Hicks D, Mohand-Said S, Léveillard T, Sahel J. Partial characterization of retina-derived cone neuroprotection in two culture models of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2003;44:818–825. doi: 10.1167/iovs.01-1144. [DOI] [PubMed] [Google Scholar]
- 20.Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oppenheim RW. The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci. 1989;12:252–255. doi: 10.1016/0166-2236(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 22.Adler R, Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989;243:391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- 23.Léveillard T, Mohand-Saïd S, Lorentz O, Hicks D, Fintz AC, Clérin E, Simonutti M, Forster V, Cavusoglu N, Chalmel F, Dollé P, Poch O, Lambrou G, Sahel JA. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36:755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- 24.Lillig CH, Holmgren A. Thioredoxin and related molecules--from biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 25.Wang XW, Liou YC, Ho B, Ding JL. An evolutionarily conserved 16-kDa thioredoxin-related protein is an antioxidant which regulates the NF-kappaB signaling pathway. Free Radic Biol Med. 2007;42:247–259. doi: 10.1016/j.freeradbiomed.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 26.Chalmel F, Léveillard T, Jaillard C, Lardenois A, Berdugo N, Morel E, Koehl P, Lambrou G, Holmgren A, Sahel JA, Poch O. Rod-derived Cone Viability Factor-2 is a novel bifunctional-thioredoxin-like protein with therapeutic potential. BMC Mol Biol. 2007;8:74. doi: 10.1186/1471-2199-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Mohand-Said S, Danan A, Simonutti M, Fontaine V, Clerin E, Picaud S, Léveillard T, Sahel JA. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther. 2009;17:787–795. doi: 10.1038/mt.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlichtenbrede FC, MacNeil A, Bainbridge JW, Tschernutter M, Thrasher AJ, Smith AJ, Ali RR. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10:523–527. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- 29.Pekkari K, Holmgren A. Truncated thioredoxin: Physiological functions and mechanism. Antioxid Redox Signal. 2004;6:53–61. doi: 10.1089/152308604771978345. [DOI] [PubMed] [Google Scholar]
- 30.Cronin T, Raffelsberger W, Lee-Rivera I, Jaillard C, Niepon ML, Kinzel B, Clérin E, Petrosian A, Picaud S, Poch O, Sahel JA, Leveillard T. The disruption of the Rod derived Cone Viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress. Cell Death Diff. doi: 10.1038/cdd.2010.2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fridlich R, Delalande F, Jaillard C, Lu J, Poidevin L, Cronin T, Perrocheau L, Millet-Puel G, Niepon ML, Poch O, Holmgren A, Van Dorsselaer A, Sahel JA, Léveillard T. The thioredoxin-like protein rod-derived cone viability factor (RdCVFL) interacts with TAU and inhibits its phosphorylation in the retina. Mol Cell Proteomics. 2009;8:1206–1218. doi: 10.1074/mcp.M800406-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reichman S, Kalathur RK, Lambard S, Ait-Ali N, Yang Y, Lardenois A, Ripp R, Poch O, Zack DJ, Sahel JA, Léveillard T. The homeobox gene CHX10/VSX2 regulates RdCVF promoter activity in the inner retina. Hum Mol Genet. 2009;19:250–261. doi: 10.1093/hmg/ddp484. [DOI] [PMC free article] [PubMed] [Google Scholar]


