Abstract
AIM: To investigate our learning curves of orthotopic liver transplantation (OLT) in rats and the most important factor for successful surgery.
METHODS: We describe the surgical procedures for our rat OLT model, and determined the operator learning curves. The various factors that contributed to successful surgery were determined. The most important surgical factors were evaluated between successful and unsuccessful surgeries.
RESULTS: Learning curve data indicated that 50 cases were required for operator training to start a study. Operative time, blood loss, warm ischemic time, anhepatic phase, unstable systemic hemodynamic state, and body temperature after surgery significantly affected surgery success by univariate analysis, while the anhepatic phase was the most critical factor for success by multivariate analysis.
CONCLUSION: OLT in rats is the only liver transplantation model that provides clinically relevant and reliable results. Shortened anhepatic phase is key to success in this model.
Keywords: Animal model, Liver transplantation, Microsurgery, Rat, Reperfusion injury, Ultra-microsurgery
INTRODUCTION
Orthotopic liver transplantation (OLT) has become the treatment of choice for end-stage liver disease. Split OLT shows great promise for narrowing the gap between the number of patients waiting for an OLT and the number of available deceased organ donors. Living-donor OLT also addresses the severe problem of donor shortage and should ensure donor safety. However, further investigation into small-for-size grafts and small-for-size graft syndrome associated with split and living-donor OLT is needed prior to full acceptance in the clinical practice of OLT. Murine organ transplantation models, such as cardiac, lung, and kidney grafts, have been reported[1-5] and are commonly used by transplant immunity investigators. However, OLT in mice is technically very difficult, even without reconstruction of the hepatic artery (HA). Furthermore, a validated model of murine OLT is unavailable. In contrast, OLT in rats is technically accessible, producing more clinically relevant and reliable data. Hence, a comprehensive model of OLT in rats is particularly useful.
OLT in rats was first reported in 1973 using hand-suture techniques[6], while a modified model without HA reconstruction and temporal shunt of the porto-jugular veno-venous bypass was documented in 1975[7]. However, these models were not widely used due to the prolonged surgery time and technical demand. With the cuff method being introduced in 1973[8], OLT in rats without HA reconstruction became globally accepted. The pros and cons of each model were recently reported[9-23], and a combination of hand-suture and cuff methods are deemed to be key factors for successful OLT[24].
The development of clinically relevant OLT models in rats[9-23] has advanced clinical knowledge in liver transplantation[24-26]. Therefore, we will describe detailed surgical procedures of innovative OLT in rats based on two decades of experience at our centers. We will present the learning curves and important factors associated with successful OLT in rats.
MATERIALS AND METHODS
Materials
Animals: Lewis rats (RT-1l, 8-10 wk) were used as recipients. As donors, Lewis rats were used for syngeneic grafts. As a large vessel diameter is necessary for the anastomosis, male rats of 230-250 g body weight were most suitable. Rats > 300 g were avoided because of the large amount of intra-abdominal fat, making the surgical procedures more difficult.
Adequate hydration, achieved with solution injections, is important for successful surgery, particularly prior to the anhepatic phase and after allograft recirculation[27]. We used male rats because the penile vein was easily accessible for repeated intravenous injections.
The use of animals was institutionally approved in accordance with The National Institutes of Health Guide for the Care and Use of Laboratory Animals.
General instruments: Cotton swabs with optimal stiffness (cotton tipped applicators; Hardwood Products Company, Guilford, ME, USA) and soft clay (Color Mounting Clay; Hampton Research, Aliso Viejo, CA, USA) were prepared. A cylindrical warmer (Mantello, Ambulatory Surgical Warmer, medium; Kent Scientific Co., Torrington, CT, USA) was used immediately after surgery.
General anesthesia was performed with a rodent anesthesia machine (VetEquip Inc., Pleasanton, CA, USA), including an evacuation canister and induction chamber.
Agents and solutions: Chemical agents used were: heparin (heparin lithium salt, 100 unit/mg; MP Biomedicals, Cleveland, OH, USA); Cephalexin hydrate (MP Biomedicals); buprenorphine 300 μg/mL (Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA, USA); bicarbonate, 8.4% Sodium Bicarbonate Injection USP, 1 mEq/mL, 84 mg/mL (Hospira Inc., Lake Forest, IL, USA), and microfibrillar collagen, Avitene (C. R. Bard, Inc., Murray Hill, NJ, USA). The solutions used were: Lactated Ringer’s Injection USP and Ringer’s Injection USP (B. Braun Medical Inc., Irvine, CA, USA); 0.9% Sodium Chloride Injection USP (Hospira Inc.).
Surgical instruments: Basic instruments required for small animal surgery were similar to those described in previous reports[27,28]. For the anastomosis of vessels with a diameter under 0.5-1.0 mm, the instruments used were as previously described[27-29].
We have preferentially used instruments from Takasago Medical Industry Co. (Tokyo, 113-0033, Japan), Kent Scientific Co., Roboz Surgical Instrument Co., Inc. (Gaithersburg, MD, USA), and Southpointe Surgical Supply Inc. (Coral Springs, FL, USA).
Sutures used were thin silk threads (Silk Suture 7-0; Braintree Scientific Inc., Braintree, MA, USA), monofilament nylon suture (10-0 Ethilon, BV130-3, 2820G; Ethicon, Inc., Somerville, NJ, USA), monofilament polypropylene sutures (7-0 Prolene, BV-1, 8304H-X, and 8-0 Prolene, BV130-5, 8732H; Ethicon, Inc.), and absorbable thread (5-0 Coated Vicryl Plus; Ethicon, Inc.). Micro-clips (Microclip size: M, ML, and L, Horizon Ligation System; Teleflex Medical, Durham, NC, USA) and applying forceps (Microclip applicators; Teleflex Medical) were prepared.
Microscopes: A surgical loupe (2.0-3.0 × magnification) or a microscope (5-6.25 × magnification) is sufficient for microsurgery. We used a surgical microscope at 5-20 × magnification (Surgical Scope M680, Type 10445496; Leica Microsystems Inc., Bannockburn, IL, USA) for hepatic artery reconstruction at high magnifications (12.5-20 × magnification).
Micro-tubes and catheters: The micro-tubes used were polyurethane micro-tubes (Polyurethane Catheters, Straight Tip, Hydrocoat, 2 French, 20 gauge; Access Technologies, Skoki, IL, USA).
The peripheral catheters used were 14 gauge (14G Cathlon i.v. catheter; Johnson & Johnson Medical, Inc., Arlington, TX 76004-3130, USA) and 24 gauge (24 G Surflo Flush; Terumo Co., Tokyo, Japan).
Preparation of the stent tube for biliary duct and cuffs for portal vein and infra-hepatic inferior vena cava
Stent tubes for biliary duct (BD) reconstruction were made using 24 gauge peripheral catheters or 2 French polyurethane micro-tubes. A total length of 7-8 mm is sufficient.
The portal vein (PV) cuff was made using a 14 gauge peripheral catheter. First, 2-3 mm of the main body and 2 mm of the extension were made (Figure 1A).
Figure 1.
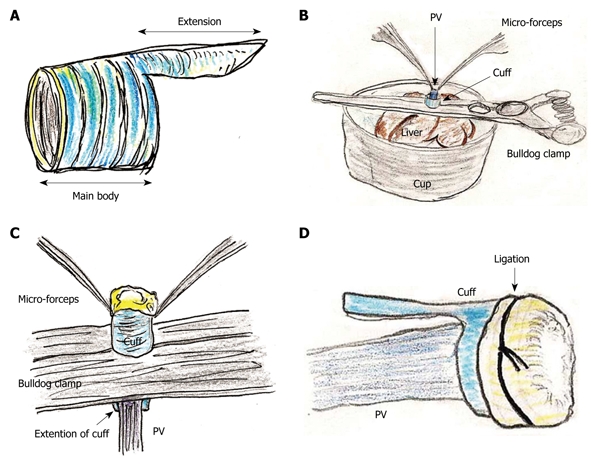
Portal vein (PV) cuff attachment. A: Cuff body with encircled chases and extension are made; B: PV trunk is induced through PV cuff. Cuff extension and PV trunk are grasped with a straight large-sized bulldog clamp. Cuff is set on the cup; C: Wall of PV trunk completely reversed using micro-forceps; D: Reversed PV wall is fixed to chase on cuff by ligation of silk thread.
The infra-hepatic inferior vena cava (IHIVC) cuff was similarly made using a conventional sterilized tube with a minimum inner diameter of 2.0-2.5 mm and a thin wall. The total cuff length should be 5-6 mm, with 3-4 mm main body and 2 mm extension.
Anesthesia
All operative procedures were performed under general anesthesia using isoflurane accompanied by oxygen, and inhalational anesthesia was induced and maintained. Isoflurane accompanied by oxygen flow at 5 L/min was used in the introduction phase and was reduced to 0.5-2.0 L/min in the maintenance phase.
After the introduction of anesthesia, the abdominal wall was shaved using electric clippers. The feet were fixed to the surgical table. Before skin incision, 2.0-2.5 mL/rat of lactated Ringer’s solution was injected intravenously (penile vein) using a 27 gauge fine needle.
Donor operation
Laparotomy: The abdominal wall was prepped with betadine. A long midline skin incision was made extending from the xiphoid process to the pubis, followed by a transverse incision. Tractions on bilateral subcostal borders and the lower abdominal walls provided maximal exposure of the abdominal cavity. Liver damage should be avoided during laparotomy. Warm saline was arbitrarily dripped onto the intraperitoneal organs to prevent drying during laparotomy.
Preparation for graft harvest: The gastrointestinal tract was moistened with warm saline and positioned to the outside of the left abdominal cavity and coated with gauze. The liver was handled delicately by blunt and soft items such as a cotton swab.
The falciform and triangular ligaments were cut, and the left inferior phrenic vein was located. This vein was skeletonized carefully and ligated with silk to prevent massive hemorrhage after liver reperfusion. The transparent membranes around the liver which fix each lobe to the surrounding organs were cut, and the PV branch communicating to the paraesophageal vessels was ligated with silk thread.
The retroperitoneum on the IHIVC was dissected, and the IHIVC was skeletonized. The right renal vein and artery were carefully isolated, ligated with silk thread, and divided close to the renal hilum. The fat tissue around the right adrenal gland was dissected. From this side, the back of the IHIVC was skeletonized from the connective tissues and the right renal artery. The IHIVC was then tunneled using blunt micro-forceps. Both lumbar and right adrenal veins were ligated. The lower lumbar vein and left adrenal vein were ligated with silk thread, and the junction of the left renal vein and the IHIVC were skeletonized.
The hepatoduodenal ligament was cut and the extra-hepatic BD was mobilized completely. Rats have no gallbladder. The BD was cut at the level of the pancreas. A biliary stent tube was inserted and fixed with silk thread. Bile was usually observed coming out of the stent tube during the donor operation.
All branches of the PV trunk were ligated by silk thread. Complete isolation and skeletonization of the PV trunk is required for the cuff method. Excess attached tissues tend to cause stenosis in the cuff.
The proper hepatic artery (PHA) and the gastroduodenal artery (GDA) were dissected. The GDA was ligated with silk thread. The PHA was further dissected toward the hepatic hilum and was isolated from the PV. The common hepatic artery (CHA) was similarly dissected from the connective tissue around the celiac and superior mesenteric arteries.
Graft harvest: Heparinization (500 units/rat) was administered intravenously. One minute after heparin injection, the CHA was ligated at the aorta. Next, the IHIVC was clamped at an upper point of the right renal vein using a micro-clamp. The mesenteric branches of the PV trunk were clamped using micro-clamps, and one of superior mesenteric branches was opened using the cut-down method. A 24 gauge peripheral catheter was subsequently inserted into the PV trunk. After confirmation of the tip position in the PV trunk, 10 mL of cold Ringer’s solution (4°C) was injected to start the hypothermic perfusion of the donor liver. A thoracotomy was performed immediately, and the thoracic supra-hepatic inferior vena cava (SHIVC) was divided. The IHIVC clamp was maintained during the cold perfusion and after the cuff attachment. The cold flush was slowly continued without high pressure, and the PV was clamped using a micro-clamp at the hepatic hilus.
After the cold wash-out, liver procurement was performed in the order of the PV trunk, diaphragm, remnant ligaments behind the SHIVC, hepatic inferior vena cava (HIVC), IHIVC, and the renal vessels. Note that the IVIHC was cut in a branch patch-fashion using the IHIVC and the left renal vein for an easy insertion of the IHIVC cuff (Figure 2A). The PV trunk was also cut in a branch patch-fashion using the portal vein trunk and the splenic vein (Figure 3A). Finally, the whole liver was harvested and immediately placed into cold Ringer’s solution (4°C).
Figure 2.
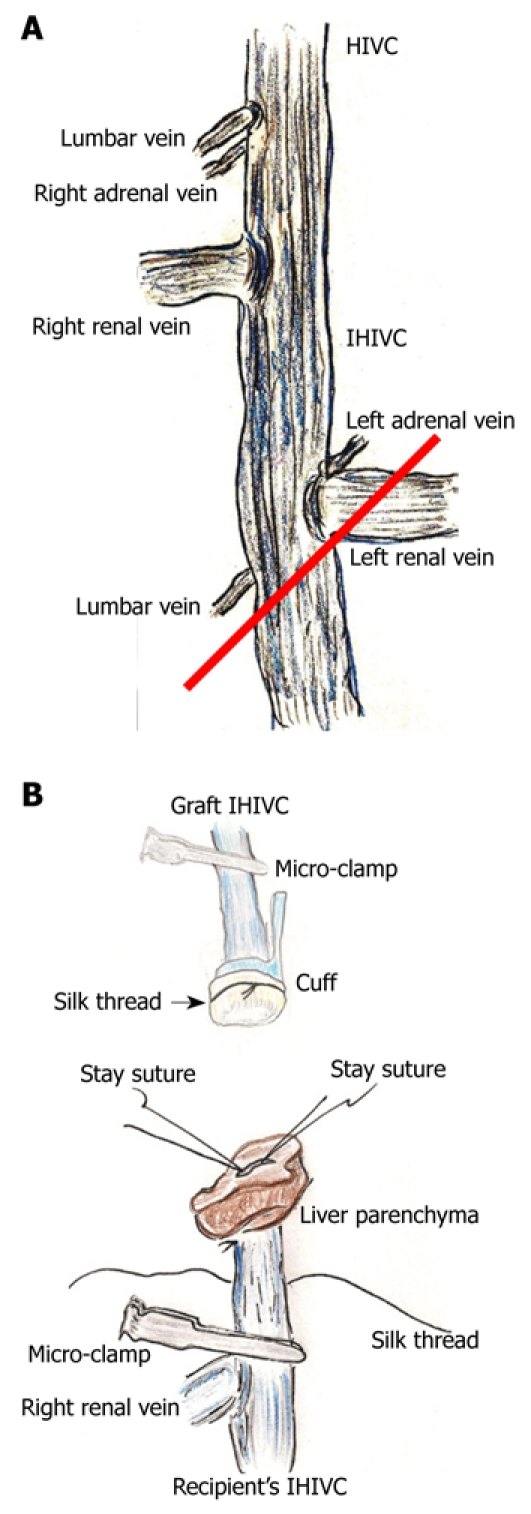
Infra-hepatic inferior vena cava (IHIVC) branches and reconstruction. A: Right adrenal and lumbar veins flow directly into IHIVC at point of lowest edge of right inferior segment. Left adrenal and the lower lumbar veins flow into the IHIVC at the junction of the left renal vein and the IHIVC. In donor operation for IHIVC reconstruction using the cuff method, IVIHC is cut in a branch patch-fashion (red line); B: Inner side of recipient’s IHIVC is easily detected by adherent liver parenchyma. Stay sutures are made bilaterally on posterior wall, and anterior wall has some allowance for cuff insertion. Stay sutures are held by a bulldog clamp and are pulled to the cranial side. Silk thread is set behind the recipient’s IHIVC beforehand. Confirmation of quality of graft IHIVC is confirmed using saline flush. Cuff is led towards the recipient’s IHIVC, and cuff is inserted into IHIVC.
Figure 3.
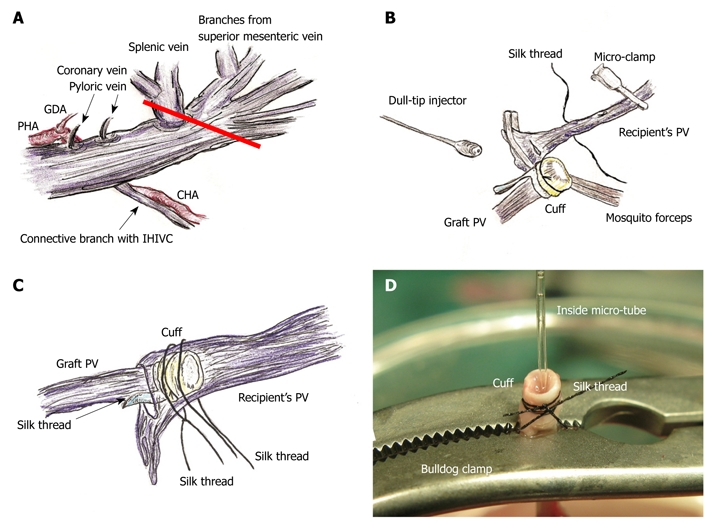
PV preparation and reconstruction. A: The splenic vein and coronary vein flow into PV trunk at the left or posterior sides of the PV. The pyloric vein flows into PV trunk at the left side of PV. The posterior side of PV trunk has a branch that connects with the IHIVC. The common hepatic artery (CHA) is located at the back of PV trunk, and the proper hepatic artery (PHA) and gastroduodenal artery branches are located at the left side of PV trunk. The PV trunk and these arteries are encased together in a thin sheath. The PV trunk is cut in a branch patch-fashion using PV trunk and splenic vein in the donor operation (red line); B: Retention of recipient PV is performed using mosquito forceps. Silk thread is set behind the recipient’s PV trunk beforehand. The natural form of the PV is confirmed using saline flush. The cuff is led onto recipient’s PV. The PV is opened using the cut-down method at the point nearest the hepatic hilus, and patency of the inner side is confirmed by saline flush; C: Cuff is inserted into recipient’s PV avoiding any torsion; D: At the back table a bulldog clamp holds PV trunk, micro-tube, and cuff extension. Because of the micro-tube inside, detection of the inner side is simple. GDA: Gastroduodenal artery.
Back table benching of donor liver graft
All procedures should be performed on crushed ice. Cold Ringer’s solution (4°C) was used as the preservation solution.
Plasty of the SHIVC: The anterior wall of the SHIVC was cut as near to the diaphragm as possible, leaving enough margin for the suture. The anterior diaphragm was completely trimmed. The posterior wall of the SHIVC was carefully detected, and the bilateral edge of the white tendon was removed. Stay sutures using polypropylene sutures were then made on the bilateral edges of the SHIVC. The bilateral stay sutures were held separately with curved bulldog clamps, and retention was achieved using the stay sutures. A key technique for SHIVC plasty involves leaving enough margin in the wall for retention with stay sutures (Figure 4A). Figure 4B illustrates the corresponding liver anatomy including the three lobes (right, left, and caudate lobes) arranged into seven segments including the right median, left median, left lateral, right superior, right inferior, superior caudate and inferior caudate segments[30,31].
Figure 4.
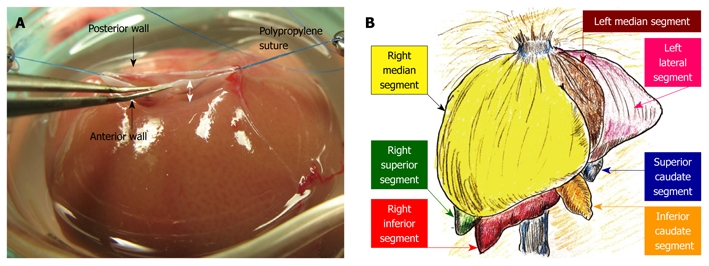
Hepatic segments and hepatic vein flows. A: The hepatic vein itself has no extra-hepatic margins for suture. The most important techniques for SHIVC plasty are (1) ensuring enough margin of the wall, and (2) retention using stay sutures. In particular, sufficient margin of the SHIVC wall (white arrow) is indispensable for confirmation of optimal out-flow; B: The liver comprises 3 lobes, which are subdivided into 7 segments. In basic anatomy, the left median segment is joined with the right median segment, and an incomplete lobulation is often detected in those segments.
Attachment of cuffs: Any fat tissue on the wall of the PV was completely removed, particularly in the portion of the cuff, to prevent considerable cuff stenosis. The PV trunk was induced through the PV cuff. The cuff extension and the PV trunk were grasped with a large straight bulldog clamp. The cuff was set on the cup/glass using the verge of the preservation cup/glass and a long bulldog clamp (Figure 1B). The wall of the PV trunk was completely reversed using micro-forceps (Figure 1C). The reversed PV wall was fixed onto the cuff with a silk ligation (Figure 1D). A clip on the hilar PV trunk was maintained during this procedure.
The IHIVC cuff was also attached using a similar procedure to that for the PV cuff. A clip on the distal side was maintained during the procedure. After attachment of the cuffs to the PV and the IHIVC, the patency of the cuffs and the closure of branches up to the clamp points were checked using a flush of Ringer’s solution through a 24 gauge catheter or dull-tip injector.
Recipient operation
Anesthesia: The induction of anesthesia and the injection of lactated Ringer’s solution were the same as for the donor operation. However, shaving was omitted or performed only over as small an area as possible to maintain body temperature after surgery.
Laparotomy: The skin incision was made only by long midline incision. Too great a volume of saline on the intraperitoneal organs causes a low body temperature after recipient surgery. As such, only warm saline was used when necessary. Temporary retention of the abdominal wall by retractors was performed sparingly to prevent limitation of thoracic movements. Direct touch was possible in the recipient operation as the native liver was not used as a graft. The gastrointestinal tract was kept moistened with warm saline.
Preparation before anhepatic phase: The procedures used to mobilize the whole liver were basically the same as for the donor operation. The cut-off point of the BD was the hepatic hilus, and the BD was isolated to the upper side of the pancreas. The GDA was ligated at the point of the root. The CHA was not dissected up to the root, but the PHA was isolated sufficiently from the PV. The PHA was ligated at the hepatic hilus. Dissection of the hepatic hilus was performed more clearly than in the donor operation to provide enough length and good mobility of the BD, PHA, and PV.
The PV trunk should be isolated to provide enough length for cuff insertion. Skeletonization of the PV trunk was the same as for the donor operation, except for the splenic vein. The PV trunk was mobilized by ligating the PV branches, as for the donor operation.
For the IHIVC procedures, the portion from the HIVC to the right renal vein was completely isolated with preservation of the renal vessels. The right adrenal and lumbar veins were ligated. After cutting the dorsal membrane of the HIVC, the connective tissues at the boundary line between the SHIVC and the diaphragm were carefully dissected to provide satisfactory extensibility of the SHIVC.
Removal of native liver: A total of 2.0-2.5 mL of lactated Ringer’s solution was administered intravenously. The vascular clamps at the proximal sides were applied in the order of IHIVC and PV, starting the anhepatic phase. Anesthesia was stopped. The hilar PV was ligated. SHIVC was clamped partly including the diaphragm. The SHIVC was cut at the liver parenchyma. The PV was divided close to the hilum. The HIVC in the right inferior segment was cut at the upper point at 3-5 mm from the border line of the IHIVC and the HIVC. The native hepatectomy was completed.
Allograft implantation: The presence of bleeding points should be carefully checked using a cotton swab because secure hemostasis is very difficult after liver insertion. Placement of the graft liver is important, and should be performed based on the anatomical characteristics of the graft liver. Note that an out-flow block can make the model unusable, and the points of the stay sutures without the axis torsion should be checked again before the allograft implantation (Figure 5A).
Figure 5.
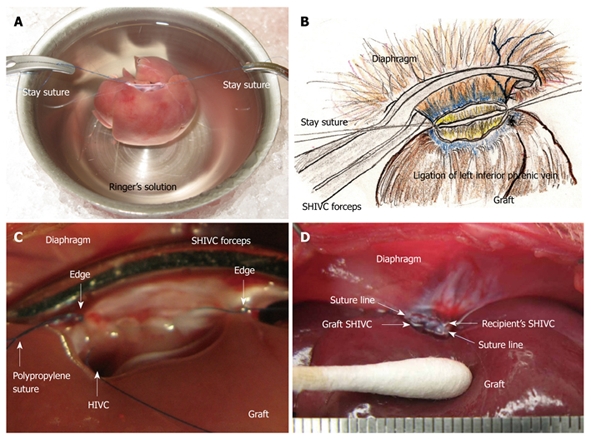
Supra-hepatic inferior vena cava (SHIVC) reconstruction. A: Note that an out-flow block can make the model unusable, and points of stay sutures without the axis torsion should be checked again before allograft implantation; B: The stay suture is placed bilaterally after careful consideration of the setup. The posterior wall straightens and the anterior wall is set as an arch; C: Finding after suture of the posterior wall is shown. HIVC is confirmed to enter into the right lobe. Too tight a ligation causes stenosis of the HIVC and disturbance of the flow. Ligations with stay sutures on both sides should be completed, although not too tightly; D: Completed SHIVC reconstruction is shown.
SHIVC reconstruction: A 5 or 10 mL syringe is deployed under the back of the recipient at the point of the SHIVC. In some cases, the movement of the thorax stops; however, the heart rate remains stable. A retractor is used for the retention of the costal bows if the respiratory movement is satisfactory. Forceps are used to grasp the diaphragm and expose the ventral surface, then to pull caudally. The forceps are fixed by soft clay at an adequate point for easy and stable sutures, and sutures are placed bilaterally. The posterior wall becomes straight and the anterior wall is set as an arch (Figure 5B). The left side is ligated, and the posterior wall is sutured from the left side using 5-6 stitches of continuous sutures. The last suture is ligated with a stay suture from the right side, avoiding over-tightening (Figure 5C). The anterior wall is then sutured from the right side using 15-20 stitches of continuous suture. The SHIVC cavity is filled with saline containing heparin by using an L-shaped injector before the complete closure. The anterior suture is then finished, and this thread is ligated not too tightly with the stay suture from the left side (Figure 5D). The back syringe and clay fixation are removed, and the retractors are released.
PV reconstruction: Retention of the recipient PV from the right side was achieved with mosquito forceps, and the forceps are fixed with soft clay. Note that too strong a retention makes it difficult to insert the cuff, despite increasing the PV length. The recipient PV was encircled beforehand with silk thread, and one knot is made for cuff fixation. The cuff was led onto the recipient PV. The PV was opened using the cut-down method at the nearest point of the hepatic hilus, and the inner side of the PV was confirmed with a saline flush (Figure 3B). The cuff was inserted into the recipient PV, avoiding any torsion of the PV (Figure 3C).
Allograft recirculation: The clamps were released in the order of the SHIVC, then the PV, and the allograft recirculation then starts. The anhepatic phase ceases. Cardiac and respiratory movements were allowed to recover, particularly in the case of a whole liver graft, and anesthesia is resumed.
IHIVC reconstruction: The inner side of the IHIVC was easily detected by the adherent liver parenchyma. Stay sutures were made bilaterally on the posterior wall, and the anterior wall had some allowances for the cuff insertion. Stay sutures were held with a bulldog clamp and were pulled to the cranial side. The recipients’ IHIVC is encircled beforehand with silk thread (Figure 2B), and one knot is made for cuff fixation. The inner side of the graft IHIVC was filled with saline. The cuff was led onto the IHIVC of the recipient liver. The cuff was inserted into the IHIVC, avoiding any IHIVC torsion. Fixation of the cuff to the IHIVC was performed. The IHIVC clamps were released in the order of the donor, then the recipient. Congestion of the right kidney and dilatation of the IHIVC were immediately resolved, and the liver color improved.
After IHIVC reflow, 2.0-2.5 mL of lactated Ringer’s solution was injected via the penile vein. If required, a total of 0.3-1.0 mEq of bicarbonate can be injected simultaneously to offset metabolic acidosis.
HA reconstruction: An atraumatic small-sized clamp was placed on the recipient’s PHA. A clamp was not needed on the graft CHA, as there was usually no back-flow. The connective tissues were completely removed. A sharp cut surface is made at the ends of the recipient’s PHA and graft CHA. An initial nylon suture was threaded through the whole layer of the CHA from the outside to the inside, and a thrusting was carried out through the whole layer of the PHA from the inside to the outside. A reverse thrusting from the PHA to the CHA is then carried out using the same thread. Next, due to the difference in vessel diameter, the recipient PHA was fed into the graft CHA, and the vessel clamp was released. One or two superficial stitches were added if bleeding occurred. This completed the “vest and pant” method (Figure 6A). Graft color was slightly improved after HA reconstruction.
Figure 6.
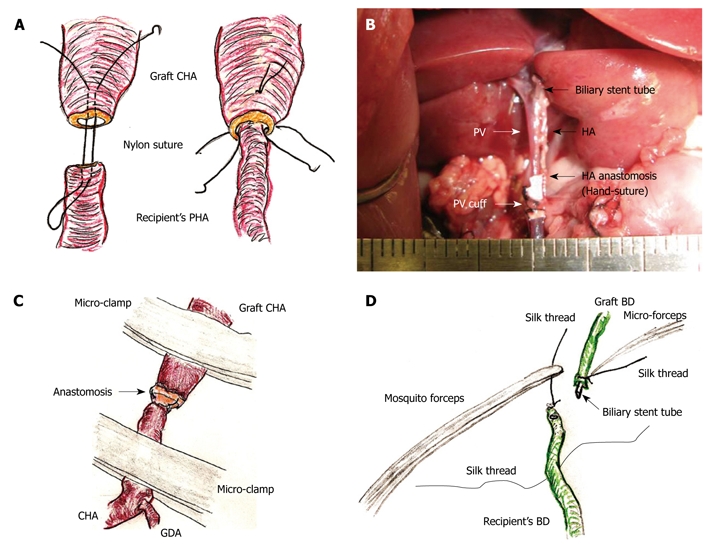
Hepatic artery (HA) and biliary duct (BD) reconstruction. A: An initial suture is made through the whole layer of the CHA from the outside to the inside, and a thrusting is then performed through the whole layer of the PHA from the inside to the outside. Subsequently, reverse thrusting from the PHA to the CHA is performed with the same thread. The recipient’s PHA is then led into the graft CHA. One or two superficial stitches can be added if bleeding occurs; B: The diameter of the recipient’s PHA is approximately 0.2 mm. Complete ultra-microsurgery allows the use of end-to-end anastomosis in HA reconstruction; C: Intermittent and alternate clamping of the HA also achieves hemostasis; D: The previously ligated silk thread of the recipient’s BD is held using mosquito forceps. The recipient’s BD is encircled beforehand with silk thread. The biliary stent tube is led into the recipient’s BD. The recipient’s BD is open using the cut-down method, and the stent tube is inserted.
In contrast, complete ultra-microsurgery allows the use of end-to-end anastomosis (Figure 6B). First, the sharp surfaces were joined as the vessel diameter in the graft CHA and the recipient CHA are similar. Next, three or four stitches were made through the whole layer using interrupted nylon sutures. Although additional superficial sutures are possible if bleeding occurs, the initial use of a cotton-like hemostatic agent with subtle compression is better. Intermittent and alternant clamping on the graft CHA and the recipient’s CHA also achieves hemostasis (Figure 6C).
Biliary reconstruction: The previously ligated silk thread of the recipient’s BD was held using mosquito forceps, and the mosquito forceps were fixed to a clay holder. The recipient’s BD was encircled with silk thread and one knot was made beforehand. The biliary stent tube was led into the recipient’s BD. The recipient’s BD was opened using the cut-down method (Figure 6D), and the stent tube was inserted. The stent tube was then fixed with ligation of silk thread, and one edge of the ligated silk thread was also reserved for the donor operation. To prevent removal of the biliary stent tube, the preserved silk threads in the donor and the recipient were ligated together.
Abdominal closure: The intraperitoneal cavity and organs were washed with warm saline, and the point of BD anastomosis was covered with the greater omentum to prevent biliary complications. The peritoneum and fascia were closed with continuous sutures using absorbable thread, and the skin layer was closed separately using the same method.
Postoperative care and observation
The recipient was warmed on a hot pad immediately after surgery. A total of 2.0-2.5 mL of lactated Ringer’s solution or maintenance solution was injected via the penile vein. An analgesic agent (0.1 mg/kg) was routinely given intramuscularly every 8 h for 3-5 d after surgery. Use of antibiotics was normally not required, although can be administered intravenously (30 mg/kg) if required.
Each transplanted recipient was allowed to recover in an individual cage to prevent injury by cage mates. Survival checks were made every 2 h until 48 h after surgery.
Statistical analysis
Data are presented as mean ± SD. Univariate and multivariate analyses were used for the between-group comparisons as follows: Mann-Whitney U test and χ2 test for unpaired variables between two groups, Kaplan-Meier method (the log-rank) for survival rates, and logistic regression analysis for factors important for surgery success. Statistical calculations were performed using SPSS Software Version 16.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
RESULTS
Learning curves
We examined the learning curves of some surgeons at our institutions. A success rate ≥ 0.80 in whole liver transplantation was considered enough to learn the basic procedures for this model.
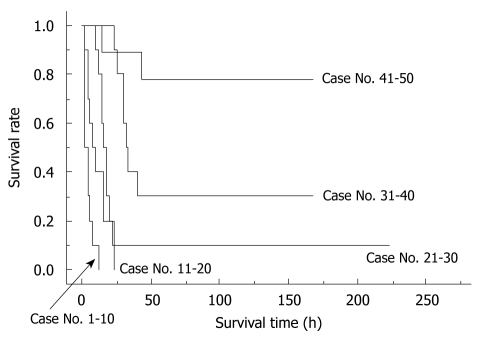
The importance of learning curves in producing reliable data using the rat OLT model has been previously reported[17]. In our experience, we observed that 30 cases are required for an initial successful OLT, and that 50 cases are required to be technically proficient for reproducible outcomes of OLT in rats. The survival curves of the first 50 cases from one surgeon can be seen in Figure 7.
Figure 7.
Learning curve in a surgeon. The initial 50 trial surgeries by one surgeon are shown. Learning curves of each surgeon showed similar patterns. Whole liver grafts were used in all cases. Preservation solution and cold ischemic time are unified as Ringer’s solution for 2 h.
Critical factors for successful surgery
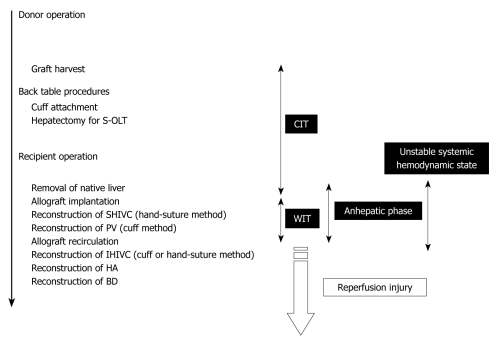
To determine the factors that were important for successful surgery, body weight (g), age difference between donor and recipient (d), abstinence before surgery, anesthesia method, operative time (min), blood loss (g), cold ischemic time (range: 0.5-4 h), organ preservation solution, warm ischemic time (the time from liver insertion to allograft recirculation; min), anhepatic phase (the time from portal clamp to allograft recirculation; min), unstable systemic hemodynamic state (the time from clamping of the IHIVC to IHIVC reflow), HA reconstruction, and body temperature immediately after surgery (°C) were collected in 100 OLT cases with whole liver grafts. The surgical timetable can be seen in Figure 8. The survival observation time was at least three days after surgery, and three day survivors were considered confirmation of successful surgery, as surgical and technical problems resulted in early deaths in the OLT model[17].
Figure 8.
Time table of OLT in rat. OLT: Orthotopic liver transplantation; CIT: Cold ischemic time; WIT: Warm ischemic time.
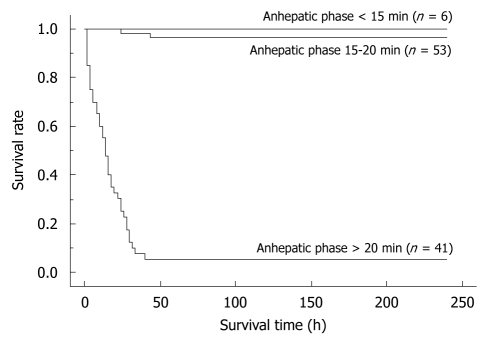
By univariate analyses, we found that operative time, blood loss, warm ischemic time, duration of anhepatic phase, unstable systemic hemodynamic state, and body temperature had a significant impact on the successful outcome of OLT in rats. Body weight, the age difference between donor and recipient, abstinence before surgery, anesthesia method, cold ischemic time, preservation solution, and hepatic artery reconstruction on the other hand, showed no difference (Table 1). When the univariate variables that showed a statistical significance were subjected to multivariate analysis, only the duration of anhepatic phase remained statistically significant in determining the successful outcome of an OLT in rats (Table 1). Furthermore, we compared the survival rates between the following groups: (1) anhepatic phase > 20 min; (2) anhepatic phase 15-20 min; and (3) anhepatic phase < 15 min. OLT recipients with an anhepatic phase > 20 min had a poor survival outcome, while those with an anhepatic phase of 15-20 min often died due to surgical issues. In contrast, recipients of an OLT with an anhepatic phase < 15 min all survived (Figure 9).
Table 1.
The important factors for successful OLT
| Metric variable (failure vs success) | P value | |
| Univariate analysis | ||
| Body weight (g) | 231.6 ± 21.5 vs 224.7 ± 13.0 | 0.0528 |
| The difference of age between donor and recipient (d) | 2.4 ± 3.4 vs 1.9 ± 3.3 | 0.3704 |
| Abstinence before surgery (yes vs no) | - | 0.5678 |
| Anesthesia (isoflurane vs diethylether) | - | 0.2861 |
| Operative time (min) | 89.8 ± 25.7 vs 59.1 ± 4.7 | < 0.0001a |
| Blood loss (g) | 8.8 ± 2.7 vs 7.6 ± 3.4 | 0.0466a |
| CIT (min) | 116.3 ± 60.8 vs 111.0 ± 42.9 | 0.6743 |
| Organ preservation solution (Ringer’s solution vs others) | - | 0.3474 |
| WIT (min) | 30.7 ± 13.7 vs 12.9 ± 1.7 | < 0.0001a |
| Anhepatic phase (min) | 41.2 ± 14.3 vs 17.2 ± 2.5 | < 0.0001a |
| Unstable systemic hemodynamic state (min) | 54.3 ± 15.5 vs 25.7 ± 4.8 | < 0.0001a |
| HA reconstruction (with vs without) | - | 0.2861 |
| Body temperature immediately after surgery (°C) | 34.1 ± 1.6 vs 36.4 ± 0.3 | < 0.0001a |
| Multivariate analysis | ||
| Operative time (min) | 0.6534 | |
| Blood loss (g) | 0.9788 | |
| WIT (min) | 0.1006 | |
| Anhepatic phase (min) | 0.0137a | |
| Unstable systemic hemodynamic state (min) | 0.9185 | |
| Body temperature immediately after surgery (°C) | 0.2984 |
P < 0.05. OLT: Orthotopic liver transplantation; CIT: Cold ischemic time; WIT: Warm ischemic time.
Figure 9.
The differences in survival rates based on the anhepatic phase length. Recipients with a long anhepatic phase had poor survival rates, while recipients with an anhepatic phase of 15-20 min often died due to surgical issues. In contrast, recipients with an anhepatic phase < 15 min all survived. Whole liver grafts were used in all cases. All cases were accompanied by HA reconstruction. Preservation solution and cold ischemic time are unified as the Ringer’s solution for 2 h. Anhepatic phase < 15 min vs anhepatic phase 15-20 min: P = 0.0830; Anhepatic phase < 15 min vs anhepatic phase > 20 min: P < 0.0001.
DISCUSSION
The cuff method cannot be used in SHIVC reconstruction. The setting for the hepatic venous flow after allograft implantation should be carefully considered, as the initial setting impacts on all subsequent procedures. An important consideration for the SHIVC setting is that it is different from humans, as the HIVC flows into the right lobes. Bilateral retention using accurate stay sutures at both edges is indispensable from the start of the procedure. With respect to operative time, a running suture of the anterior wall is faster than an interrupted suture. The thread used in the running suture should be ligated at both edges with some allowance to prevent stenosis, although if too loose a ligation causes bleeding after allograft recirculation. The clamp for the SHIVC needs an optimal bite on the thoracic side, as a shallow bite totally disturbs the SHIVC suture and slows down the surgery, although too deep a bite results in cardiac and respiratory arrest. SHIVC reconstruction should be completed using only thin SHIVC walls, and never include the liver or diaphragm in order to prevent out-flow block and thrombosis. As for hepatic vein reconstruction, in contrast to humans, rats have no extra-hepatic margins in each hepatic vein. Thus, there is no choice except for anastomosis of the SHIVC to the SHIVC. A twist of the SHIVC causes out-flow block, and out-flow complications destroy the experimental setup. Even when attempting rapid surgery due to the limitation of the anhepatic phase, all IHIVC reconstruction procedures, including adequate initial setting, smooth hand-suture, and optimal ligation, should be perfectly completed.
The cuff procedures of PV are often difficult due to its thin wall and small diameter, while the IHIVC cuff procedures are comparatively easy due to its larger diameter. In some cases of small PV, we performed the following procedure: The PV trunk is clamped using the micro-clamp near the hepatic hilus to prevent air embolism, and a soft micro-tube is inserted into the portal venous trunk from the point of the wash-out injection. Next, another micro-clamp is placed on the PV trunk and the micro-tube. At the back table, a bulldog clamp holds the PV trunk, micro-tube, and cuff extension. Due to the micro-tube inside, detection of the inner side of the vessel is very easy, even in thin and small PV cases (Figure 3D). However, care must be taken to avoid air embolism and injury of the PV trunk during this procedure. The same procedure can also be applied for the attachment of the IHIVC cuff if required.
We consider that the cuffs may cause the occlusion of blood flow in long-term survivors, although based on our experience, the cuff method produces no survival problems up to 14 d recovery. Since the PV is thin and small, kinking of the trunk, torsion of the axis, and incorrect insertion can easily occur. Our studies clearly demonstrate that shortening the anhepatic phase to less than 15 min is critical during both PV reconstruction and SHIVC reconstruction. Although we and others use a hand-suture even for PV reconstruction[15], we consider the cuff method indispensable for keeping the anhepatic phase < 15 min and for obtaining reliable data. Based on the results of the present study, we perform the late phase of the recipient operation on a hot pad to prevent hypothermia. Furthermore, we currently keep transplanted rats covered with a cylindrical warmer to reduce heat loss immediately after surgery.
The importance of training for liver transplantation was previously reported[17,18]. In the first study, 65 rat OLTs were performed by a single investigator for training. The first 39 OLT were required to master the technique, and included 23 recipients that died in the first 24 h due to technical deficits and 16 OLT to learn the technique. In our experience, 20 OLTs are required to obtain an overnight survivor, while 40 OLTs are required to obtain a one week survivor. Thus, we suggest that 40-50 OLTs per surgeon are necessary for complete learning, while more OLTs are required for an amateur microsurgeon or non-surgeon. As previously reported[17,18] surgical issues can occur, even in long-term survivors, and we consider a reliable sampling rate for assays of approximately 0.6-0.9, even when experienced microsurgeons perform the surgery. Strict elimination of unsuitable rats at the sampling time point is also important for reliable data in this model. Although a large amount of time and labor is required for even a small number of reliable samples, this model produces valuable results.
A previous study investigating the regenerative capacity of individual lobes after hepatectomy in a murine hepatectomy model demonstrated that the caudate lobes work as well as the remnant liver, especially after a 75% hepatectomy[32]. Furthermore, successful survivals were reported in recipients receiving 20% grafts without HA reconstruction[33]. The 30% graft is achieved using the caudate segments, while the 10% graft is possible in this model by using the caudate lobe. However, we did not use the caudate lobe for creating 70% or 40% grafts, as the caudate lobe showed reduced regenerative capacity in the 30% and 60% hepatectomy model compared with other lobes[32]. Based on the behavior of the caudate lobe in large hepatic remnants, we performed a 60% graft without right superior, right inferior, superior caudate, and inferior caudate segments. The set-up of 20% and 30% grafts is well established in our model for studies of small-for-size grafts.
The 40% graft is clinically important in liver regeneration due to split and pediatric grafts from cadaveric donors for the donor shortage in the United States and the shift to left-lobe grafts in a living-donor for donor safety in Japan. It should be noted that when using our experimental model, there are distinct differences between basic anatomy and surgical anatomy. In basic anatomy, the left median segment is aggregated with the right median segment, and an incomplete lobulation is often detected in those segments (Figure 4B). However, dominating vessels of the left median segment make a common channel with those of the left lateral segment (Figure 4D). Thus, the left median segment should be handled together with the left lateral segment with respect to in-flow and out-flow in surgery. No-margin hepatectomy by the clip method causes the SHIVC/HIVC twist, especially during removal of the right median segment. The hepatic margin in the clip method prevents the SHIVC/HIVC twist and common channel injury. The parenchymal margin has no hepatic circulation (these areas are insignificant as a functional volume), and we currently use right median and left median segments as a 40% graft accompanied by the margin method. Although removal of the right median segment requires several micro-clips, the left lateral segment can usually be treated with only one micro-clip. Thus, for procedure simplicity, we recommend right median and left median segments as a 40% graft using the clip method of hepatectomy accompanied by a margin, although a 40% graft of the left median and left lateral segments without margin is ideal based on surgical anatomy. This simple and useful method with margin areas prevents unintended injury of the common channel, avoids twisting of the SHIVC/HIVC, and allows easy application to incomplete lobulation. However, the percentage of the actual graft weight to the recipient’s native liver cannot be calculated due to margin weight. Therefore, we estimated the percentages of each segment in 100 rats. A previous detailed report in a rat hepatectomy model used a ligation method with a hepatic margin[30], and we recommend the clip method with a hepatic margin in the rat S-OLT model.
With regard to another feature of the hepatic venous flow in rats, the hepatic veins of the right superior, right inferior, superior caudate, and inferior caudate segments flow directly into the HIVC (Figure 4D). Note that we never opened the IHIVC clamp during the wash-out procedure in the donor operation, as this can cause incomplete wash-out due to drainage from the PV to the HVC via these direct pathways.
Co-instantaneous reconstruction of the HA is ideal for studies focused on liver regeneration, although the omission of HA reconstruction is fine for studies focused on transplant immunity. HA reconstruction requires the most skillful ultra-microsurgery (approximately 0.1-0.3 mm vessel diameter), and extended anesthesia and operative times. For the surgeon, both finely-honed concentration and non-nervous distraction are required in microsurgery and ultra-microsurgery. If a microscope is employed, we recommend limiting its use to reconstructive procedures only to avoid fatigue. In particular, ultra-microsurgery is introduced only at limited times, while continuous microsurgery during all steps is inadvisable due to the likely loss of mental focus. If possible, preparation of the surgical equipment is better than repeating surgery in the same day to obtain enough samples[27,28].
In summary, although the rat OLT model with HA reconstruction is difficult and complicated, the model without HA reconstruction is well established[9-23]. Moreover, the model with HA reconstruction provides clinically relevant data.
COMMENTS
Background
Due to the clinical situation of liver donor shortage and donor safety, the main focus of the liver regeneration field is on patients with small-for-size liver syndrome and liver reperfusion injury. The development of clinically relevant rat orthotopic liver transplantation (OLT) models has advanced the clinical liver regeneration field. However, pseudo models of fake OLT/S-OLT, such as temporal clamp or simple hepatectomy, are still used experimentally for assessing reperfusion injury and/or small-for-size syndrome after liver transplantation due to the technical demands of the rat OLT/S-OLT model.
Research frontiers
The cold ischemic time (CIT) is a critical factor in producing reliable data using the OLT model, and also plays an important role in the mechanism of true reperfusion injury and small-for-size syndrome. Data from pseudo models that omit CIT are clinically irrelevant, and should not be translated into the actual OLT field. Clinical OLT is made possible by the phenomena of immunological tolerance even after allograft transplantation and the ability of the liver to regenerate even after initial insufficient volume. As such, these factors form the prominent focus of studies attempting to further develop the OLT field. Murine organ transplantation models, such as cardiac, lung, and kidney grafts, are well established, and are commonly used by transplant immunity investigators. New insights into the mechanisms of graft injury after OLT have also been established from experiments in small animal models. Mice are particularly suitable for laboratory assays due to the growing availability of gene-altered or knock-out animals and the development of specific agents and antibodies. However, murine OLT is the most technically difficult animal transplantation model, even when reconstruction of the hepatic artery (HA) is omitted. Furthermore, a validated model of OLT in mice is unavailable, and the authors consider that the rat OLT model produces more clinically relevant and reliable data. Hence, there is a requirement for a complete rat OLT model, including S-OLT, particularly in the field of liver regeneration.
Innovations and breakthroughs
OLT in rats is the only liver transplantation model that provides clinically relevant and reliable results. Shortened anhepatic phase is an important key to success in this model.
Applications
In summary, although the rat OLT/S-OLT with HA reconstruction model is difficult and complicated, only this model provides clinically relevant data. The authors have established this model and hope that their surgical guide will also help young researchers with an interest in the liver transplantation field.
Peer review
The article itself is well written and describes the different techniques and requirements for a successful outcome in animal liver transplantation. The illustrations are excellent and clearly portray the techniques.
Footnotes
Supported by The work at the Mayo Clinic, Florida was supported by grants to Nguyen JH from the Deason Foundation, Sandra and Eugene Davenport, Mayo Clinic CD CRT-II, and NIH R01NS051646-01A2; the work in Kyoto University Graduate School of Medicine was supported by grants of the Japan Society for the Promotion of Science, No. C20591523 and the Uehara Memorial Foundation, No. 200940051, Tokyo, 171-0033, Japan
Peer reviewers: Yasuhiko Sugawara, MD, Artificial Organ and Transplantation Division, Department of Surgery, Graduate School of Medicine University of Tokyo, Tokyo 113-8655, Japan; Christopher Christophi, Professor and Head of The University of Melbourne Department of Surgery, Austin Hospital, Melbourne, 145 Studley Road, Victoria 3084, Australia
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP
References
- 1.Jungraithmayr WM, Korom S, Hillinger S, Weder W. A mouse model of orthotopic, single-lung transplantation. J Thorac Cardiovasc Surg. 2009;137:486–491. doi: 10.1016/j.jtcvs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Niimi M. The technique for heterotopic cardiac transplantation in mice: experience of 3000 operations by one surgeon. J Heart Lung Transplant. 2001;20:1123–1128. doi: 10.1016/s1053-2498(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 3.Squiers EC, Kelley SE, West JC. Small bowel transplantation in the mouse: development of a model. Microsurgery. 1992;13:345–347. doi: 10.1002/micr.1920130612. [DOI] [PubMed] [Google Scholar]
- 4.Tori M, Ito T, Matsuda H, Shirakura R, Nozawa M. Model of mouse pancreaticoduodenal transplantation. Microsurgery. 1999;19:61–65. doi: 10.1002/(sici)1098-2752(1999)19:2<61::aid-micr4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R. Improved techniques for kidney transplantation in mice. Microsurgery. 1995;16:103–109. doi: 10.1002/micr.1920160212. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Charters AC, Chandler JG, Orloff MJ. A technique for orthotopic liver transplantation in the rat. Transplantation. 1973;16:664–669. doi: 10.1097/00007890-197312000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Charters AC 3rd, Orloff MJ. Simplified technic for orthotopic liver transplantation in the rat. Am J Surg. 1975;130:38–40. doi: 10.1016/0002-9610(75)90453-5. [DOI] [PubMed] [Google Scholar]
- 8.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 9.Aguirrezabalaga J, Arnal F, Marini M, Centeno A, Fernandez-Selles C, Rey I, Gomez M. Auxiliary liver transplantation with portal arterialization in the rat: description of a new model. Microsurgery. 2002;22:21–26. doi: 10.1002/micr.22003. [DOI] [PubMed] [Google Scholar]
- 10.Ariyakhagorn V, Schmitz V, Olschewski P, Polenz D, Boas-Knoop S, Neumann U, Puhl G. Improvement of microsurgical techniques in orthotopic rat liver transplantation. J Surg Res. 2009;153:332–339. doi: 10.1016/j.jss.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Chansmorn C, Lineaweaver WC, Tonken H, Zhang F, Campagna-Pinto D, Newlin L, Yim K, Buncke HJ. Primary common bile duct anastomosis in the rat using microsurgical techniques. Microsurgery. 1994;15:857–864. doi: 10.1002/micr.1920151207. [DOI] [PubMed] [Google Scholar]
- 12.Delrivière L, Gibbs P, Kobayashi E, Goto S, Kamada N, Gianello P. Technical details for safer venous and biliary anastomoses for liver transplantation in the rat. Microsurgery. 1998;18:12–18. doi: 10.1002/(sici)1098-2752(1998)18:1<12::aid-micr4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Dippe B, Kreisel D, Petrowsky H, Richter O, Krueger S, von Heimburg D, Schneider M, Hanisch E, Wenisch HJ, Encke A. Simplified microvascular suture techniques for rat liver transplantation as a microsurgical model with arterial blood supply. Transpl Int. 1992;5 Suppl 1:S357–S361. doi: 10.1007/978-3-642-77423-2_108. [DOI] [PubMed] [Google Scholar]
- 14.Goto S, Kamada N, Delriviere L, Kobayashi E, Lord R, Ware F, Hara Y, Edwards-Smith C, Shimizu Y, Vari F. Orthotopic liver retransplantation in rats. Microsurgery. 1995;16:167–170. doi: 10.1002/micr.1920160310. [DOI] [PubMed] [Google Scholar]
- 15.Inoue S, Tahara K, Shimizu H, Yoshino H, Suzuki C, Kaneko T, Hakamata Y, Takahashi M, Murakami T, Kaneko M, et al. Rat liver transplantation for total vascular reconstruction, using a suture method. Microsurgery. 2003;23:470–475. doi: 10.1002/micr.10168. [DOI] [PubMed] [Google Scholar]
- 16.Knoop M, Bachmann S, Keck H, Steffen R, Neuhaus P. Experience with cuff rearterialization in 600 orthotopic liver grafts in the rat. Am J Surg. 1994;167:360–363. doi: 10.1016/0002-9610(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi E, Kamada N, Goto S, Miyata M. Protocol for the technique of orthotopic liver transplantation in the rat. Microsurgery. 1993;14:541–546. doi: 10.1002/micr.1920140812. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi E, Yoshida Y, Nozawa M, Hishikawa S, Yamanaka T, Miyata M, Fujimura A. Auxiliary heterotopic liver transplantation in the rat: a simplified model using cuff technique and application for congenitally hyperbilirubimemic Gunn rat. Microsurgery. 1998;18:97–102. doi: 10.1002/(sici)1098-2752(1998)18:2<97::aid-micr7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Müller V, Ott R, Tannapfel A, Hohenberger W, Reck T. Arterialization of the portal vein in liver transplantation: a new microsurgical model in the rat. Transplantation. 2001;71:977–981. doi: 10.1097/00007890-200104150-00024. [DOI] [PubMed] [Google Scholar]
- 20.Tan F, Chen Z, Zhao Y, Liang T, Li J, Wei J. Novel technique for suprahepatic vena cava reconstruction in rat orthotopic liver transplantation. Microsurgery. 2005;25:556–560. doi: 10.1002/micr.20161. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Tahara K, Hakamata Y, Mutoh H, Murakami T, Takahashi M, Kusama M, Kobayashi E. Auxiliary partial liver grafting in rats: effect of host hepatectomy on graft regeneration, and review of literature on surgical technique. Microsurgery. 2002;22:371–377. doi: 10.1002/micr.10061. [DOI] [PubMed] [Google Scholar]
- 22.Dippe BE, Broelsch CE, Krueger SB, Richter ON, Petrowsky H, Kreisel D, Von Heimburg DO, Schneider M, Hanisch EW, Wenisch HJ. An improved model for rat liver transplantation including arterial reconstruction and simplified microvascular suture techniques. J Invest Surg. 1992;5:361–373. doi: 10.3109/08941939209012452. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Scott MH. Six models of heterotopic rat liver transplantation: introducing a reverse circulation model. Microsurgery. 1986;7:91–94. doi: 10.1002/micr.1920070210. [DOI] [PubMed] [Google Scholar]
- 24.Defamie V, Laurens M, Patrono D, Devel L, Brault A, Saint-Paul MC, Yiotakis A, Barbry P, Gugenheim J, Crenesse D, et al. Matrix metalloproteinase inhibition protects rat livers from prolonged cold ischemia-warm reperfusion injury. Hepatology. 2008;47:177–185. doi: 10.1002/hep.21929. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi M, Tokunaga Y, Fujita T, Tanaka K, Yamaoka Y, Ozawa K. The effects of cold preservation on steatotic graft viability in rat liver transplantation. Transplantation. 1993;56:282–287. doi: 10.1097/00007890-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Oshima K, Yabata Y, Yoshinari D, Takeyoshi I. The effects of cyclooxygenase (COX)-2 inhibition on ischemia-reperfusion injury in liver transplantation. J Invest Surg. 2009;22:239–245. doi: 10.1080/08941930903040080. [DOI] [PubMed] [Google Scholar]
- 27.Mehdorn MH, Muller GH. Microsurgical exercises. New York: Thieme Medical Publishers Inc; 1989. [Google Scholar]
- 28.Timm S, Timmermann W, Hamelmann WH. Microsurgical technique for vascular anastomoses. New York: Springer; 1998. [Google Scholar]
- 29.Sukop A, Tvrdek M, Dusková M, Kufa R, Válka J, Veselý J, Stupka I. History of upper extremity replantation in the Czech Republic and worldwide. Acta Chir Plast. 2004;46:99–104. [PubMed] [Google Scholar]
- 30.Madrahimov N, Dirsch O, Broelsch C, Dahmen U. Marginal hepatectomy in the rat: from anatomy to surgery. Ann Surg. 2006;244:89–98. doi: 10.1097/01.sla.0000218093.12408.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikfarjam M, Malcontenti-Wilson C, Fanartzis M, Daruwalla J, Christophi C. A model of partial hepatectomy in mice. J Invest Surg. 2004;17:291–294. doi: 10.1080/08941930490502871. [DOI] [PubMed] [Google Scholar]
- 32.Inderbitzin D, Studer P, Sidler D, Beldi G, Djonov V, Keogh A, Candinas D. Regenerative capacity of individual liver lobes in the microsurgical mouse model. Microsurgery. 2006;26:465–469. doi: 10.1002/micr.20271. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka H, Hashizume K, Enosawa S, Suzuki S. Successful transplantation of a 20% partial liver graft in rats: a technical innovation. J Surg Res. 2003;110:409–412. doi: 10.1016/s0022-4804(02)00090-2. [DOI] [PubMed] [Google Scholar]