Abstract
AIM: To evaluate the technical failures of the Bravo pH test in a population with nonerosive gastroesophageal reflux disease.
METHODS: Over the course of a year, we prospectively studied a population of 66 nonerosive reflux disease patients who received a Bravo pH test. The number and frequency of all technical failures were documented, quantified and analyzed.
RESULTS: A total of 66 patients, with a mean age of 41.7 years, were studied. Technical failures occurred in 15.15% of the sample. The most frequent failures were due to poor data reception (4.5%), early dislodgement (4.5%) and capsule removal (6.1%).
CONCLUSION: The Bravo capsule pH test involves a low but non-negligible rate of technical problems, a fact that must always be considered by physicians.
Keywords: Bravo test, Capsule dislodgement, Nonerosive reflux disease, Poor data reception, Technical problems
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a condition characterized by heartburn and regurgitation with or without esophageal lesions[1]. It is a common condition, with a prevalence of up to 52.8%, and occurs more frequently in women than in men[2]. Erosive esophagitis and nonerosive reflux disease (NERD) are the primary presentations, with NERD being the most common phenotype[3,4]. This group of patients must undergo pH measurements to confirm the diagnosis of acid reflux, as they lack visible esophageal reflux lesions[5].
To date, the two best methods employed for objectively demonstrating the presence of acid reflux are conventional ambulatory catheter pH-metry monitoring and the Bravo capsule (Medtronic, Minneapolis, MN, USA) catheter-free pH test[6-8]. Both methods are valid and reliable for the measurement of esophageal acid exposure[9,10].
The traditional catheter pH-metry remains the more commonly used of the two methods. However, it can cause undue burden on the patient due to the discomfort and embarrassment of the transnasal pH probe placement, which is known to be uncomfortable and is poorly tolerated[11]. Although the Bravo capsule is a device that is designed to overcome the disadvantages of traditional pH monitoring, to improve acceptance of testing and to extend the period of monitoring, it is not exempt from technical problems and side effects[12,13]. The primary technical problems of this test are related to failures in transmission or early detachment of the Bravo capsule. Nevertheless, there are many case reports that describe a list of unusual and diverse problems. Both novice and expert gastroenterologists must be familiar with the various possible technical problems that can occur with this device so that they can solve them promptly and efficiently, avoiding additional complications.
In this paper, we quantify and describe our experience of the technical problems that can occur with the use and physical presence of the Bravo capsule in a sample of patients with NERD.
MATERIALS AND METHODS
Patients
We enrolled a total of 66 consecutive patients in a prospective study of 48-h ambulatory pH monitoring using the Bravo capsule, in order to record all technical failures. Before enrollment, each patient had undergone an upper endoscopy which showed an absence of lesions on the esophageal mucosa and were thus diagnosed as having NERD. Patients were asked to discontinue the use of proton pump inhibitors and histamine-2 receptor antagonists for one week prior to the study and to avoid the use of anti-acids 24 h prior to the examination.
None of the patients had significant comorbidities, such as coronary artery disease. The study was conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants.
Bravo procedure and pH recording
The Bravo capsule was placed 6 cm from the squamo-columnar junction, using standard techniques. All the capsules were placed orally. A second-look esophagoscopy documented suitable capsule attachment. Once the pH recording was initiated, patients were encouraged to engage in their usual daily activities and to consume their usual diet without restrictions. All subjects were instructed to document their food intake, sleep periods and the occurrence of GERD symptoms in a diary to complete the pH-metry evaluation. From the pH records of the first and second day, the average pH value was obtained.
To confirm any capsule dislodgment, a chest X-ray was obtained seven days after the the beginning of the study. Those patients who still showed the device in the esophagus were asked to undergo another plain radiography seven days later to record the detachment.
Assessment of technical problems and statistical analysis
Technical failures of the device were recorded in a “database” and analyzed with a statistical package. Quantitative data, including age and the fraction of time with pH < 4 on the first and second monitoring days were also added. The percentages, means and standard deviations were calculated for each variable. The analysis was performed with the statistical software package SPSS, version 9.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient population
Between July 2007 and June 2008, a total of 66 patients were enrolled in the trial. These patients included 27 men (40.9%) and 39 (59.1%) women, aged 11-73 years (mean age = 41.7 years; standard deviation = 13.3 years).
Of the total population, 31 patients reported typical gastroesophageal reflux symptoms, 15 had atypical manifestations and 20 had a mixed symptomatology.
Technical failures and recording efficacy
All of the technical failures observed in our trial are described in Table 1. In the 66 patients, 10 technical failures (15.15%) were observed. Three probes had poor data reception (4.5%), and three more presented with early dislodgement (4.5%). In four cases (6.1%), the capsule had to be removed. We considered intolerable chest pain in one case as an absolute indication for removal. On the contrary, we considered cases of detachment failure (12 d after implantation, because a Heller myotomy was to be performed), transmission failure and placement error to be relative indications for removal. In the last two cases, new capsules (3%) were reinstalled so that those patients could complete the study.
Table 1.
Technical failures, removals and feasibility of the Bravo capsule procedure n (%)
| Technical problems | Patients (n = 66) |
| Technical failure | 10 (15.15) |
| Transmission failure | 3 (4.5) |
| Early dislodgement | 3 (4.5) |
| Capsule removal | 4 (6.1) |
| Absolute | |
| Intolerable chest pain | 1 (1.5) |
| Relative | |
| Detachment failure | 1 (1.5) |
| Transmission failure | 1 (1.5) |
| Error in placement | 1 (1.5) |
| Capsule replacements | 2 (3.0) |
| Feasibility | |
| Fully finished tests (48 h) | 59 (89.39) |
| Half-finished tests (24 h) | 4 (6.0) |
| Tests without record (0 h) | 3 (4.5) |
Of the total sample, 89.39% completed the 48-h examination period, and 6% were able to complete only 24 h. However, 24 h was enough time to gather sufficient information to obtain a diagnosis; indeed, using 24 h of data, the Bravo test was accurate in 95% of patients. Only three (4.5%) patients lacked records.
DISCUSSION
This study describes our experience with the Bravo pH test in patients with NERD, a patient population with special features in terms of sensibility and treatment issues. Technical problems occurred in a low but non-negligible proportion (15.15%) of the sample. Our most remarkable problems were early dislodgement, poor data reception and capsule removals, which occurred at similar rates.
In early capsule dislodgement, the Bravo probe detaches from its location and falls prematurely, i.e., before the 48 h monitoring period is complete. This finding can be observed in the pH tracing as a sudden prolonged drop in the pH value line when the capsule drops into the stomach, followed by a subsequent sharp rise in the pH line as the capsule enters the small intestine (Figure 1A). This early capsule dislodgement rate of 4.5% is similar to those in other reports, which registered rates between 0% and 3.22%[6,14]. Although early dislodgement is considered a failure, it is sometimes possible to complete at least 24 h of monitoring, a time period that still allows a diagnosis of NERD (Table 1).
Figure 1.
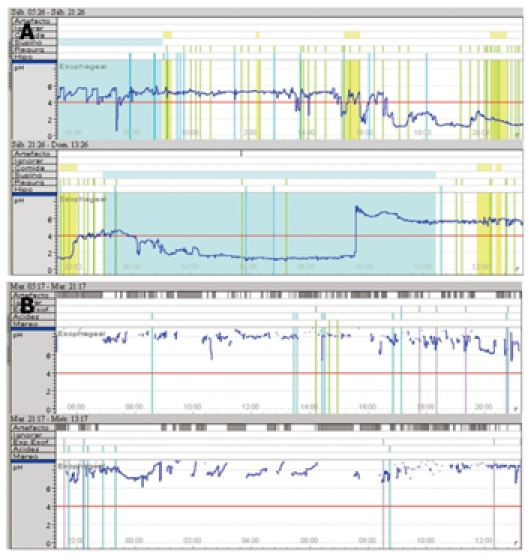
Tracings of problems. A: pH tracing with the typical prolonged drop in the pH line when the capsule drops into the stomach; B: pH tracing (in blue) showing gaps as a result of poor data reception.
In the same way, the poor data reception rate of 4.5% (3 of our 66 patients) presented a similar problem to that of early capsule dislodgment. Some authors have reported transmission failure rates of 8.2%[6]. This electromechanical flaw may be seen in the pH tracing as time periods during which data capture was interrupted and are shown as gaps on pH tracings (Figure 1B); these gaps may be interpreted as artifacts during the computerized data analysis. They are potentially attributable to malfunctions in the electronics or possibly the receiver being beyond the range of the signal emanating from the pH capsule.
The need for capsule removal is another frequently observed problem. The capsule removal frequency reached 6.1% (4 of our 66 patients), higher than previously documented frequencies, which ranged from 0% to 3.5%[6,15]. However, the higher incidence observed in our study may have been due to the fact that we considered absolute and relative indications, even though our only absolute indication was a patient with intolerable chest pain (1.5%). This symptom was the primary indication in most previous studies[12,16]. The other three patients had a relative indication. One of these patients presented with transmission failure, and another with a placement error (gastroesophageal junction) (Figure 2A). We decided to remove and replace the capsule with a new one in order to finish the test and to avoid the side effects produced by the presence of two probes in the esophagus, as the presence of the capsule produces esophageal contractions associated with pain[17,18]. Finally, a fourth capsule was retrieved twelve days after implantation, as a Heller myotomy was to be performed. Three of these capsules were retrieved by a cold snare technique, and the other by a hot snare technique (Figure 2B), as previously described[19].
Figure 2.
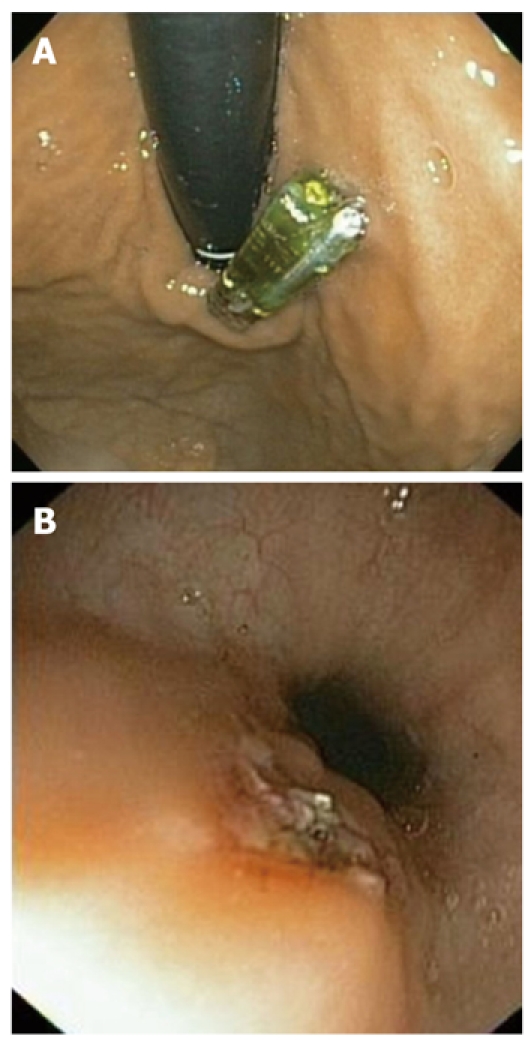
Improper delivery and removal. A: Capsule placement at an erroneous site; B: Mucosal appearance after a hot snare removal.
Other studies have reported different and unusual technical problems. Ward et al[15] observed that the capsules did not attach properly on the first attempt in at least 7 of 60 (12%) patients. Although there are some reports of major complications, we did not observe any. The main complications previously described were trauma, severe bleeding, esophageal perforations and aspiration into the bronchus[20,21]. In addition, minor incidents have been reported, such as localized inflammation, capsules not deploying from the delivery system, the plunger being broken off during delivery, mucosal tearing and probes not being correctly calibrated[22].
In conclusion, the Bravo capsule results in a low but non-negligible rate of heterogeneous technical difficulties, and although most of them are not life-threatening, gastroenterologists must be aware of these difficulties in order to interpret and address them appropriately.
COMMENTS
Background
The Bravo capsule pH test is a relatively new method for the assessment of reflux disease, and as a new method, the authors have tried to evaluate the possible technical problems that may occur during its application, course and interpretation.
Research frontiers
This article describes the most common technical failures. Gastroenterologists will find the causes of these technical failures useful in order to avoid them in the future.
Innovations and breakthrough
This trial focuses on patients with nonerosive reflux disease. Up to now, this population has not received much attention.
Applications
The present study will find application in making people aware of the possible problems and failures associated with the Bravo capsule pH test, giving people the opportunity to solve them.
Peer review
The paper is straightforward and clear.
Footnotes
Peer reviewer: Fabio Pace, Professor, Division of Gastroenterology, “L. Sacco” University Hospital, University of Milan, Via G. B. Grassi, 74, Milano 20157, Italy
S- Editor Wang YR L- Editor Webster JR E- Editor Lin YP
References
- 1.Pace F, Bazzoli F, Fiocca R, Di Mario F, Savarino V, Vigneri S, Vakil N. The Italian validation of the Montreal Global definition and classification of gastroesophageal reflux disease. Eur J Gastroenterol Hepatol. 2009;21:394–408. doi: 10.1097/MEG.0b013e32830a70e2. [DOI] [PubMed] [Google Scholar]
- 2.Fass R. Gastroesophageal reflux disease revisited. Gastroenterol Clin North Am. 2002;31:S1–10. doi: 10.1016/s0889-8553(02)00046-8. [DOI] [PubMed] [Google Scholar]
- 3.Fass R. Erosive esophagitis and nonerosive reflux disease (NERD): comparison of epidemiologic, physiologic, and therapeutic characteristics. J Clin Gastroenterol. 2007;41:131–137. doi: 10.1097/01.mcg.0000225631.07039.6d. [DOI] [PubMed] [Google Scholar]
- 4.Long JD, Orlando RC. Nonerosive reflux disease. Minerva Gastroenterol Dietol. 2007;53:127–141. [PubMed] [Google Scholar]
- 5.DeVault KR. Catheter-based pH monitoring: use in evaluation of gastroesophageal reflux disease symptoms (on and off therapy) Gastrointest Endosc Clin N Am. 2005;15:289–306. doi: 10.1016/j.giec.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98:740–749. doi: 10.1111/j.1572-0241.2003.07398.x. [DOI] [PubMed] [Google Scholar]
- 7.Pandolfino JE. Bravo capsule pH monitoring. Am J Gastroenterol. 2005;100:8–10. doi: 10.1111/j.1572-0241.2005.41183.x. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical Excellence (NICE) Catheterless oesophageal pH monitoring. July 2006. Accessed January 12. 2009. Available from: http://www.nice.org.uk/nicemedia/pdf/ip/IPG187guidance.pdf. [Google Scholar]
- 9.Gillies RS, Stratford JM, Booth MI, Dehn TC. Oesophageal pH monitoring using the Bravo catheter-free radio capsule. Eur J Gastroenterol Hepatol. 2007;19:57–63. doi: 10.1097/MEG.0b013e3280116eec. [DOI] [PubMed] [Google Scholar]
- 10.Schneider JH, Kramer KM, Königsrainer A, Granderath FA. Ambulatory pH: monitoring with a wireless system. Surg Endosc. 2007;21:2076–2080. doi: 10.1007/s00464-007-9301-1. [DOI] [PubMed] [Google Scholar]
- 11.Fass R, Hell R, Sampliner RE, Pulliam G, Graver E, Hartz V, Johnson C, Jaffe P. Effect of ambulatory 24-hour esophageal pH monitoring on reflux-provoking activities. Dig Dis Sci. 1999;44:2263–2269. doi: 10.1023/a:1026608804938. [DOI] [PubMed] [Google Scholar]
- 12.Ahlawat SK, Novak DJ, Williams DC, Maher KA, Barton F, Benjamin SB. Day-to-day variability in acid reflux patterns using the BRAVO pH monitoring system. J Clin Gastroenterol. 2006;40:20–24. doi: 10.1097/01.mcg.0000190753.25750.0e. [DOI] [PubMed] [Google Scholar]
- 13.Lee YC, Wang HP, Chiu HM, Huang SP, Tu CH, Wu MS, Lin JT. Patients with functional heartburn are more likely to report retrosternal discomfort during wireless pH monitoring. Gastrointest Endosc. 2005;62:834–841. doi: 10.1016/S0016-5107(05)00317-2. [DOI] [PubMed] [Google Scholar]
- 14.Bhat YM, McGrath KM, Bielefeldt K. Wireless esophageal pH monitoring: new technique means new questions. J Clin Gastroenterol. 2006;40:116–121. doi: 10.1097/01.mcg.0000196188.57543.75. [DOI] [PubMed] [Google Scholar]
- 15.Ward EM, Devault KR, Bouras EP, Stark ME, Wolfsen HC, Davis DM, Nedrow SI, Achem SR. Successful oesophageal pH monitoring with a catheter-free system. Aliment Pharmacol Ther. 2004;19:449–454. doi: 10.1111/j.1365-2036.2004.01868.x. [DOI] [PubMed] [Google Scholar]
- 16.Prakash C, Jonnalagadda S, Azar R, Clouse RE. Endoscopic removal of the wireless pH monitoring capsule in patients with severe discomfort. Gastrointest Endosc. 2006;64:828–832. doi: 10.1016/j.gie.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin SB, Gerhardt DC, Castell DO. High amplitude, peristaltic esophageal contractions associated with chest pain and/or dysphagia. Gastroenterology. 1979;77:478–483. [PubMed] [Google Scholar]
- 18.Traube M, Albibi R, McCallum RW. High-amplitude peristaltic esophageal contractions associated with chest pain. JAMA. 1983;250:2655–2659. [PubMed] [Google Scholar]
- 19.de Hoyos A, Esparza EA, Loredo ML. Cold and hot snare endoscopic techniques for removal of the Bravo pH monitoring capsule. Digestion. 2009;79:14–16. doi: 10.1159/000194945. [DOI] [PubMed] [Google Scholar]
- 20.von Renteln D, Kayser T, Riecken B, Caca K. An unusual case of Bravo capsule aspiration. Endoscopy. 2008;40 Suppl 2:E174. doi: 10.1055/s-2007-995804. [DOI] [PubMed] [Google Scholar]
- 21.Fajardo NR, Wise JL, Locke GR 3rd, Murray JA, Talley NJ. Esophageal perforation after placement of wireless Bravo pH probe. Gastrointest Endosc. 2006;63:184–185. doi: 10.1016/j.gie.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Food and Drug Administration. MAUDE database. Accessed January 20. 2009. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.cfm. [Google Scholar]


