Abstract
We report a case of hepatocellular carcinoma (HCC) that caused a severe arterioportal shunt (APS). A 49-year-old man was admitted to hospital due to esophagogastric variceal hemorrhage and HCC, and underwent endoscopic variceal ligation (EVL) and endoscopic injection sclerotherapy (EIS). He was then referred to our hospital. Abdominal computed tomography revealed a low-density lesion in the posterior segment of the liver and an intratumoral APS, which caused portal hypertension. Although the patient underwent EVL, EIS, Hassab’s operation, and transcatheter arterial embolization for APS, he vomited blood due to rupture of esophagogastric varices. Right hepatectomy was performed for the treatment of HCC and APS, although the indocyanine green retention value at 15 min after intravenous injection was poor (30%). The patient’s postoperative course was uneventful. Eventually, APS disappeared and the esophagogastric varices improved.
Keywords: Arterioportal shunt, Hepatocellular carcinoma, Esophagogastric varices
INTRODUCTION
Hepatocellular carcinoma (HCC) can easily invade the portal vein and form a direct communication between the hepatic artery and portal vein, which results in the formation of an arterioportal shunt (APS). Severe APS leads to life-threatening conditions, such as esophagogastric varices, refractory ascites, and hepatic encephalopathy, as a result of portal hypertension[1-3]. Furthermore, indocyanine green retention at 15 min after intravenous injection (ICG-R15) for APS is worse than the true value[4]. We report a patient who underwent successful hepatectomy for the treatment of HCC and an intratumoral APS that was not controlled with various treatments.
CASE REPORT
A 49-year-old man was admitted to hospital because of vomiting blood, which was diagnosed as esophagogastric varices and HCC. He underwent emergency endoscopic variceal ligation (EVL), followed by endoscopic injection sclerotherapy (EIS) three times at the hospital. The patient was then referred to our hospital for treatment of HCC.
The laboratory data obtained at the time of hospitalization in our hospital are shown in the Table 1. The Child-Pugh classification status was class B (8 points) and the degree of liver damage on the scoring system designed by the Liver Cancer Study Group of Japan[5] was class B. Abdominal dynamic computed tomography (CT) revealed a low-density lesion at any phases that existed in the posterior segment of the liver. The lesion was 11 cm in diameter and apposed the vena cava, the right hepatic vein, and the right anterior superior and inferior portal vein (Figure 1A and C). CT also demonstrated a hyper-enhanced portal vein during the arterial phase (CT attenuation of the portal vein was 284 HU and that of the proper hepatic artery was 294 HU) (Figure 1B); a filling defect caused by tumor thrombus in the posterior branch of the portal vein (Figure 1C); esophagogastric varices despite previous EIS; ascites and splenomegaly. Upper gastrointestinal endoscopy revealed severe esophagogastric varices (Figure 2). Right hepatic arteriography revealed severe intratumoral APS accompanied by reflux into the main portal vein (Figure 3A). Transarterial portography revealed non-enhancement of the portal vein due to portal hypertension (Figure 3B). The patient was diagnosed with HCC with severe intratumoral APS, which caused portal hypertension that lead to esophagogastric varices and hypersplenism.
Table 1.
Laboratory data obtained at time of hospitalization and after TAE
| At time of hospitalization | After TAE | |
| AST level (IU/L) | 86 | 70 |
| ALT level (IU/L) | 66 | 20 |
| Total bilirubin level (mg/dL) | 0.98 | 0.71 |
| Albumin level (g/dL) | 3.4 | 3.8 |
| White blood cell count (/μL) | 1800 | 3900 |
| Hemoglobin level (g/dL) | 9.9 | 9.2 |
| Hematocrit (%) | 30.7 | 29.8 |
| Platelet count (/μL) | 7.5 × 104 | 18.2 × 104 |
| Prothrombin activity (%) | 71 | 80 |
| HBsAg | Negative | - |
| HCV Ab | Positive | - |
| AFP level (ng/mL) | 5570 | - |
| PIVKA-II level (mAU/mL) | 5360 | - |
| ICG-R15 (%) | 36 | 30 |
TAE: Transcatheter arterial embolization; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; HBsAg: Hepatitis B surface antigen; HCV Ab: Hepatitis C virus antibody; AFP: α-fetoprotein; PIVKA-II: Protein induced by vitamin K absence or antagonist II; ICG-R15: Indocyanine green retention at 15 min after intravenous injection.
Figure 1.
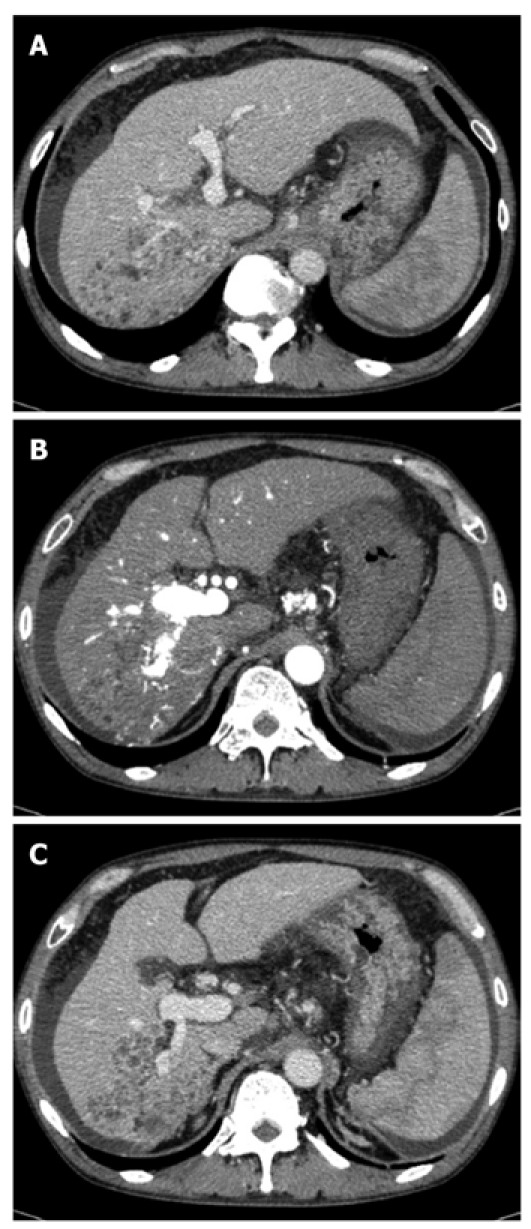
Computed tomography examination. A: Low-density lesion in the posterior segment of the liver, which pressed the vena cava and right hepatic vein and opposed the right anterior superior portal vein; B: Hyper-enhanced portal vein during the arterial phase; C: Tumor thrombus in the posterior branch of the portal vein. A tumor opposed the right anterior inferior portal vein.
Figure 2.
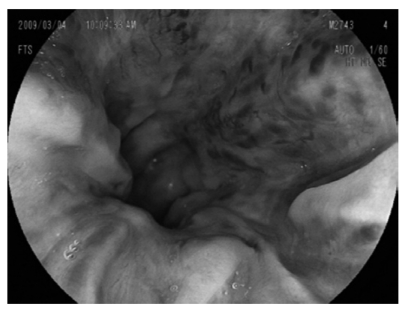
Upper gastrointestinal endoscopy revealing severe esophageal varices.
Figure 3.
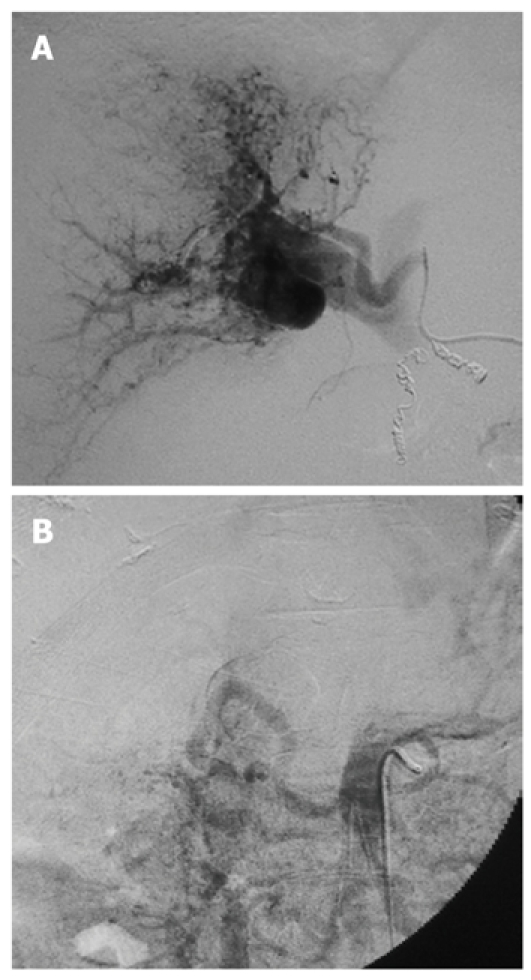
Arteriography. A: Right hepatic arteriography exhibiting the right portal vein, caused by severe intratumoral arterioportal shunt; B: Transarterial portography revealing non-enhancement of the portal vein because of portal hypertension.
We first performed Hassab’s operation (splenectomy and devascularization of the distal esophagus and proximal stomach) due to uncontrollable esophagogastric varices and hypersplenism. Despite the Hassab’s operation, the esophagogastric variceal hemorrhage prevailed. Consequently, we performed transcatheter arterial embolization (TAE) with fiber platinum coils and n-butyl 2-cyanoacrylate for the APS. Although we performed TAE twice, the esophagogastric variceal hemorrhage persisted because of recanalization of the APS.
The laboratory data obtained after TAE are shown in the Table 1.
The Child-Pugh classification status was class B (7 points) and the degree of liver damage was class B. Because the liver function data other than ICG-R15 were good, we surmised that severe APS led to an ICG-R15 value that was worse than that of the actual liver function. Subsequent therapy for HCC and APS was required, and we performed right hepatectomy for resection of HCC and APS 4 mo after hospitalization. Although the tumor was located mainly in the right posterior section of the liver, the tumor apposed the right anterior superior and posterior portal vein and the right hepatic vein. Therefore, we selected right hepatectomy. The portal vein pressure improved after liver resection. The pre- and post-liver resection state was 55 and 37 cmH2O, respectively. Pathological examination revealed a moderately differentiated HCC in the posterior section of the liver, a tumor thrombus in the right posterior branch of the portal vein, and chronic hepatitis in the non-tumorous area. The postoperative course was uncomplicated, and APS and esophagogastric varices improved. The patient is alive at 12 mo after right hepatectomy, without esophagogastric variceal hemorrhage, and has been undergoing systemic chemotherapy for multiple recurrent HCC and lymph node metastases.
DISCUSSION
HCC is frequently associated with APS. Kido et al[6] and Ngan et al[7] have determined that APS occurs in 60% of patients with HCC, and Okuda et al[8] have reported that marked APS of the main, right or left portal vein occurs in 30% of HCC patients. Severe APS leads to or aggravates portal hypertension, which causes life-threatening conditions, such as rupture of esophagogastric varices, refractory ascites, and hepatic encephalopathy[1-3]. Luo et al[9] have reported that severe APS exhibits strong enhancement of the main portal trunk and/or the first-order branches at the hepatic arterial phase of CT. HCC with tumorous APS is frequently unenhanced at the arterial phase in dynamic CT[9,10] because APS reduces arterial flow to the tumor. In our case, the patient presented with rupture of esophagogastric varices. CT revealed a low-density lesion in the posterior segment of the liver, and tumor staining at the arterial phase was not visible because of the tumorous APS.
Endoscopic treatment is the standard treatment for esophageal varices. EVL is safer and more convenient to perform compared to EIS, but does not completely disrupt the interconnecting perforating and feeder vessels[11]. For this reason, EIS is used additionally to improve the clinical results of EVL. Hassab’s operation[12] is performed when esophagogastric varices are not controlled with endoscopic treatment and/or if hypersplenism is present. In the present case, we performed Hassab’s operation because esophagogastric varices were not controlled with endoscopic treatment and hypersplenism was present; however, the esophagogastric variceal hemorrhage prevailed. We suspect that the esophagogastric variceal hemorrhage occurred as a result of the development of submucosal collateral vessels. APS needs to be treated to improve portal hypertension caused by severe APS. Although TAE with several embolic materials such as gelatin sponge, coil, and ethanol is an effective treatment for APS, Huang et al[13] have reported that the recanalization rate of APS was 18% and 86% in the ethanol and gelatin sponge groups, respectively. In our patient, unfortunately, APS was resistant to two TAE procedures, and the esophagogastric variceal hemorrhage prevailed. Only right hepatectomy is effective for the treatment of HCC and intratumoral APS. Although the ICG-R15 value was poor (30%), we performed right hepatectomy. The ICG clearance test is one of the most commonly used liver function tests[14], and Imamura et al[15] have reported that the cutoff value of ICG-R15 that allows safe right hepatectomy is 10%, and patients with 30%-39% ICG-R15 are treated with limited hepatic resection alone. However, this is premised on the assumption that there is no portosystemic shunt and that the intrahepatic blood flow is even, and thus, the ICG-R15 value is worse than the true value in patients with APS[4]. In such cases, technetium-99m diethylenetriaminepentaacetic acid galactosyl human serum albumin (99mTc-GSA) scintigraphy demonstrates great potential and is used more widely because the results are not affected by the presence of a shunt. Many institutions have described methods for predicting hepatic functional reserve with 99mTc-GSA scintigraphy; however, a consensus on the best method has not yet been reached[16]. Thus, we did not perform 99mTc-GSA scintigraphy. We judged that the poor value of ICG-R15 resulted from APS because the value of ICG-R15 was improved by TAE for APS, despite an incompletely occluded APS (ICG-R15 was 36% before TAE and 30% after). Thus, we judged that the patient could tolerate right hepatectomy because the standard liver function test results were almost in the normal range. The patient had no major postoperative complications and his quality of life was improved due to correction of APS.
In conclusion, we reported a patient with HCC and intratumoral APS. We encountered difficulty in controlling esophagogastric varices and APS and evaluating hepatic functional reserve; however, he was treated successfully with hepatectomy. In patients with severe APS, we must estimate hepatic functional reserve, and hope that a consensus on the best method for evaluating hepatic functional reserve will be obtained.
Footnotes
Peer reviewer: Taku Aoki, MD, Division of Hepato-Biliary-Pancreatic and Transplantation Surgery, Department of Surgery, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
References
- 1.Lazaridis KN, Kamath PS. Images in hepatology. Arterio-portal fistula causing recurrent variceal bleeding. J Hepatol. 1998;29:142. doi: 10.1016/s0168-8278(98)80189-x. [DOI] [PubMed] [Google Scholar]
- 2.Morse SS, Sniderman KW, Galloway S, Rapoport S, Ross GR, Glickman MG. Hepatoma, arterioportal shunting, and hyperkinetic portal hypertension: therapeutic embolization. Radiology. 1985;155:77–82. doi: 10.1148/radiology.155.1.2983375. [DOI] [PubMed] [Google Scholar]
- 3.Velázquez RF, Rodríguez M, Navascués CA, Linares A, Pérez R, Sotorríos NG, Martínez I, Rodrigo L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520–527. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 4.Matsuyama K, Fukuda Y, Miyake H, Yogita S, Tashiro S. Experimental study of the evaluation of liver function on the opposite side during portacaval anastomosis and ligation of the left portal branch. J Med Invest. 2004;51:84–95. doi: 10.2152/jmi.51.84. [DOI] [PubMed] [Google Scholar]
- 5.Liver Cancer Study Group of Japan. General rules for the clinical and pathological study of primary liver cancer. 2nd English ed. Tokyo: Kanehara; 2003. pp. 13–14. [Google Scholar]
- 6.Kido C, Sasaki T, Kaneko M. Angiography of primary liver cancer. Am J Roentgenol Radium Ther Nucl Med. 1971;113:70–81. doi: 10.2214/ajr.113.1.70. [DOI] [PubMed] [Google Scholar]
- 7.Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. 1997;52:36–40. doi: 10.1016/s0009-9260(97)80303-0. [DOI] [PubMed] [Google Scholar]
- 8.Okuda K, Musha H, Yamasaki T, Jinnouchi S, Nagasaki Y, Kubo Y, Shimokawa Y, Nakayama T, Kojiro M, Sakamoto K, et al. Angiographic demonstration of intrahepatic arterio-portal anastomoses in hepatocellular carcinoma. Radiology. 1977;122:53–58. doi: 10.1148/122.1.53. [DOI] [PubMed] [Google Scholar]
- 9.Luo MY, Shan H, Jiang ZB, Liang WW, Zhang JS, Li LF. Capability of multidetector CT to diagnose hepatocellular carcinoma-associated arterioportal shunt. World J Gastroenterol. 2005;11:2666–2669. doi: 10.3748/wjg.v11.i17.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mamada Y, Yoshida H, Taniai N, Bandou K, Mizuguchi Y, Kakinuma D, Ishikawa Y, Akimaru K, Tajiri T, Naito Z. Major arterioportal shunt caused by hepatocellular carcinoma. J Nippon Med Sch. 2007;74:314–318. doi: 10.1272/jnms.74.314. [DOI] [PubMed] [Google Scholar]
- 11.Hou MC, Lin HC, Kuo BI, Chen CH, Lee FY, Lee SD. Comparison of endoscopic variceal injection sclerotherapy and ligation for the treatment of esophageal variceal hemorrhage: a prospective randomized trial. Hepatology. 1995;21:1517–1522. [PubMed] [Google Scholar]
- 12.Hassab MA. Gastroesophageal decongestion and splenectomy in the treatment of esophageal varices in bilharzial cirrhosis: further studies with a report on 355 operations. Surgery. 1967;61:169–176. [PubMed] [Google Scholar]
- 13.Huang MS, Lin Q, Jiang ZB, Zhu KS, Guan SH, Li ZR, Shan H. Comparison of long-term effects between intra-arterially delivered ethanol and Gelfoam for the treatment of severe arterioportal shunt in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:825–829. doi: 10.3748/wjg.v10.i6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255–1259. [PubMed] [Google Scholar]
- 15.Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16–22. doi: 10.1007/s00534-004-0965-9. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura H, Kamiyama T, Nakagawa T, Nakanishi K, Yokoo H, Tahara M, Kamachi H, Toi H, Matsushita M, Todo S. Preoperative evaluation of hepatic functional reserve by converted ICGR15 calculated from Tc-GSA scintigraphy. J Gastroenterol Hepatol. 2008;23:1235–1241. doi: 10.1111/j.1440-1746.2008.05389.x. [DOI] [PubMed] [Google Scholar]