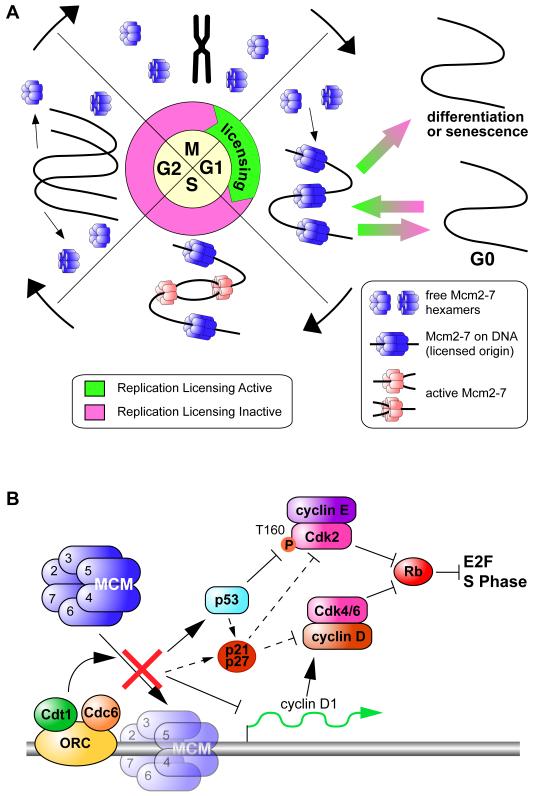
During every cell cycle, chromosomal DNA needs to be replicated accurately and completely. In order to ensure that no section of DNA is replicated more than once in a single S phase, replication origins are first ‘licensed’ by loading hexamers of MCM2-7 minichromosome maintenance proteins (Figure 1a)1,2. MCM2-7 are essential replication fork proteins and move away from each origin as it initiates, thereby leaving the origin in an unlicensed state. So long as licensing occurs only in late mitosis and early G1, no replicated origins can fire more than once in a single S phase. If too few origins are licensed, the larger distances individual replication forks have to travel increases their risk of stalling, leading to an increase in DNA strand breaks and gross chromosomal rearrangements3,4. In order to prevent this from happening, primary cells possess a “licensing checkpoint” that prevents G1 cells from entering S phase with too few licensed origins5-7. The licensing checkpoint is defective in many cancer cell lines, and a recent paper by Cook and colleagues provides some of the first evidence to show how this may occur8.
Fig 1.
The loading of MCM2-7 onto replication origins requires the activity of 3 other proteins: the origin recognition complex (ORC), Cdc6 and Cdt1 (Figure 1b)1,9. After depleting Cdc6 or Cdt1 using siRNA in immortalized human primary cells or in cancer-derived cell lines, Cook and colleagues showed that primary cells arrested in G1 while the cancer cells entered an abortive S phase. Consistent with previous reports, the arrested primary cells contained hypophosphorylated RB and low CDK2-cyclinE kinase activity, suggesting that the licensing checkpoint prevented passage through the restriction point by maintaining the RB-dependent repression of E2F. Progression into S phase is typically promoted by CDK-dependent phosphorylation of RB, relieving its repression of E2F (Figure 1b). Cook and colleagues went on to show that when the licensing checkpoint is engaged, CDK2 activity is repressed by an unusual mechanism that leads to loss of phosphorylation at Thr160 in the activation T loop of CDK2. This was accompanied by delayed nuclear accumulation of CDK2 protein in late G1. The regulation of Thr160 phosphorylation in CDK2 is not well understood, but Cook and colleagues showed that decreased Thr160 phosphorylation is dependent on p53. After p53 depletion, primary cells proceeded into S phase without the required number of licensed origins, similar to cancer cells which lack functional p53 and RB. As a consequence of entering S phase without enough licensed origins, cells synthesized DNA with reduced rate and accumulated DNA damage.
These conclusions augment previous studies showing that in primary cells, inhibition of origin licensing suppresses CDK activity required for cells to hyperphosphorylate RB and progress out of G1. This may be achieved by several partially-overlapping mechanisms (Figure 1b). Previous work has shown that activation of the licensing checkpoint is associated with induction of the CDK inhibitors p21Cip1 and p27Kip1 5,10-12. A recent study by Vaziri and colleagues showed that RNAi knockdown of components of the licensing system suppressed cyclin D1 transcription and CDK4/6 kinase activity in a manner that was independent of p27 12. They also showed that abrogation of RB activity using the HPV-E7 oncoprotein functionally abrogated the licensing checkpoint in primary cells. This suggests that several pathways contribute to the licensing checkpoint, but they all converge on suppression of G1 CDK activity required to activate E2F (Fig 1b). It would be interesting to test whether combinations of overexpressed cyclin D, constitutively phosphorylated CDK2 at Thr160 or p21/p27 knockdown could override the G1 arrest, or whether there other pathways also play a substantial role.
It is currently unclear exactly how cells sense whether origins have been licensed or not. One attractive possibility for the effect on cyclin D1 transcription would be if there was a replication origin close to the cyclin D1 promoter and that the presence of MCM2-7 at that origin enhanced promoter activity. It is harder to imagine this sort of mechanism activating the pathway that leads to Thr160 dephosphorylation of CDK2. An alternative possibility is that some part of the licensing machinery, for example ORC, when unable to load MCM2-7 would instead activate a checkpoint pathway leading to CDK2 dephosphorylation.
Several studies have shown that cancer cell lines have defects in the licensing checkpoint5-7. One possible explanation for why this may be so is suggested by the observation of Cook and colleagues that Thr160 dephosphorylation of CDK2 is dependent on p53 activation8, since p53 is commonly mutated in cancers. In addition, all the different strands of the licensing checkpoint appear to converge on phosphorylation and inactivation of RB (Fig 1b). Since the RB system is often misregulated or mutated in cancers, or can be bypassed by oncogenic signalling that increases G1 CDK activity, this may provide another reason why the licensing checkpoint is defective in cancers.
Defects in the licensing checkpoint are likely to contribute to the genetic instability typically seen in cancer cells7. If cells enter S phase with too few licensed origins, each replication origin will be responsible for replicating a larger than normal amount of DNA, and will have fewer dormant origins that can be activated in response to fork stalling. These problems are likely to lead to DNA damage. Consistent with this idea, mice heterozygous for an MCM4 hypomorphic mutation and transgenic mice with lowered level of MCM2 develop mammary adenocarcinomas or lymphoma3,4.
Whatever the precise details of how it comes about, the defective licensing checkpoint seen in cancer cells could be exploited in cancer therapy7. Small-molecule inhibitors of the replication licensing system will specifically kill cancer cells, which would progress into S phase with an insufficient number of licensed origins and eventually die due to incomplete DNA replication. In contrast, normal cells would respond by activating the licensing checkpoint and arresting temporarily in G1-like state, which should be reversible when the drug is removed or metabolized.
Acknowledgements
The authors are supported by Cancer Research UK grant C303/A7399.
References
- 1.Blow JJ, Dutta A. Nat Rev Mol Cell Biol. 2005;6:476–86. doi: 10.1038/nrm1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias EE, Walter JC. Genes Dev. 2007;21:497–518. doi: 10.1101/gad.1508907. [DOI] [PubMed] [Google Scholar]
- 3.Pruitt SC, et al. Stem Cells. 2007;25:3121–32. doi: 10.1634/stemcells.2007-0483. [DOI] [PubMed] [Google Scholar]
- 4.Shima N, et al. Nat Genet. 2007;39:93–8. doi: 10.1038/ng1936. [DOI] [PubMed] [Google Scholar]
- 5.Shreeram S, et al. Oncogene. 2002;21:6624–32. doi: 10.1038/sj.onc.1205910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng D, et al. Cancer Res. 2003;63:7356–64. [PubMed] [Google Scholar]
- 7.Blow JJ, Gillespie PJ. Nat Rev Cancer. 2008;8:799–806. doi: 10.1038/nrc2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nevis KR, et al. Cell Cycle. 2009 doi: 10.4161/cc.8.12.8811. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillespie PJ, et al. BioMed Central Biochemistry. 2001;2:15. [Google Scholar]
- 10.Machida YJ, et al. J Biol Chem. 2005;280:27624–30. doi: 10.1074/jbc.M502615200. Epub 2005 Jun 7. [DOI] [PubMed] [Google Scholar]
- 11.Teer JK, et al. J Biol Chem. 2006;281:6253–60. doi: 10.1074/jbc.M507150200. [DOI] [PubMed] [Google Scholar]
- 12.Liu P, et al. Cell Cycle. 2009;8:125–36. doi: 10.4161/cc.8.1.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]