Summary
The mTORC1 signaling pathway integrates environmental conditions into distinct signals for cell growth by balancing anabolic and catabolic processes. Accordingly, energetic stress inhibits mTORC1 signaling predominantly through AMPK-dependent activation of TSC1/2. Thus, TSC1/2-/- cells are hypersensitive to glucose deprivation and this has been linked to increased p53 translation and activation of apoptosis. Herein, we show that mTORC1 inhibition during glucose deprivation prevented not only the execution of death, but also induction of energetic stress. mTORC1 inhibition during glucose deprivation decreased AMPK activation and allowed ATP to remain high, which was both necessary and sufficient for protection. This effect was not due to increased catabolic activities such as autophagy, but rather exclusively due to decreased anabolic processes, reducing energy consumption. Specifically, TSC1/2-/- cells become highly dependent on glutamate dehydrogenase-dependent glutamine metabolism via the TCA cycle for survival. Therefore, mTORC1 inhibition during energetic stress is primarily to balance metabolic demand with supply.
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder that is characterized by the development of benign tumors due to the loss of TSC1 (hamartin) or TSC2 (tuberin) and hyper-activation of the mammalian target of rapamycin (mTOR) (Kwiatkowski and Manning, 2005). mTORC1, composed of mTOR, Raptor, PRAS40 and mLST8, is an evolutionarily conserved signaling pathway that integrates a cell's extracellular environment - nutrient, energy, oxygen, and growth factor levels - into distinct and coordinated signals. The deregulation of this pathway has been associated with various conditions including cancer, inflammatory disorders, and neurological dysfunction (Shaw and Cantley, 2006). The rapamycin analogue Sirolimus, an allosteric inhibitor of mTORC1, has been tested against TSC-mutated tumors. Preliminary reports suggest that Sirolimus decreases tumor size during treatment, but cessation of therapy leads to a recovery in tumor size (Bissler et al., 2008). Thus, therapies that induce not only cytostasis, but also cytotoxicity are greatly warranted.
The mTORC1-signaling pathway is structured to allow a cell to integrate environmental conditions to balance catabolic and anabolic processes. Growth factor-activated kinases phosphorylate TSC2 leading to decreased GAP activity towards the small G protein Rheb. Rheb activates mTORC1 leading to increased cell growth in part through augmented macromolecule biosynthesis and nutrient/glucose uptake (Shaw and Cantley, 2006). Meanwhile, amino acids activate mTORC1 through Rag-mediated recruitment to endomembranes, where Rheb is active (Sancak et al., 2009). Conversely, mTORC1 is primarily inhibited via AMPK-mediated TSC activation (Inoki et al., 2003) or raptor phosphorylation (Gwinn et al., 2008) during energetic stress. Therefore, when nutrients, energy, and growth signals are abundant, TSC is inhibited, and mTORC1 is activated to stimulate cell growth; conversely, when these are lacking, mTORC1 inhibition halts cell growth and stimulates catabolic processes such as fatty acid oxidation and autophagy to provide a constant supply of nutrients in part to maintain ATP production (DeBerardinis et al., 2008).
Inoki et al. first showed that TSC1/2-/- cells are highly dependent on glucose for survival and that mTORC1 inhibition during nutrient or glucose limitation prolongs survival. Several mechanisms have been proposed for this phenomenon: AMPK-dependent activation of p53, decreased activation of survival kinases, and global increase in ER-stress pathways, all of which directly affect the execution of cell death following ATP decline (Lee et al., 2007; Ghosh et al., 2006; Ozcan et al., 2008). In view of mTORC1's role in anabolic and catabolic processes, we investigated whether mTORC1-dependent regulation of bioenergetics contributed to the hypersensitivity of TSC-/- cells to glucose deprivation; we were intrigued by the fact that TSC-/- cells express high amounts of HIF-1α, which often addicts cells to glucose, and that mTORC1 controls both autophagy and fatty acid oxidation, which provide substrates to generate energy via the TCA cycle and oxidative phosphorylation (OXPHOS) (Shaw, 2006; Buzzai et al., 2005). Further, Akt, which activates mTORC1, addicts cells to fatty acid oxidation for survival following glucose withdrawal, suggesting a metabolic role for mTORC1 in allowing TSC2-/- cells to survive glucose deprivation (Buzzai et al., 2005).
Herein, we describe the consequences of mTORC1 inhibition during energetic stress and demonstrate that mTORC1 is a critical balancer of metabolic demand with supply. Following glucose withdrawal, mTORC1 inhibition allowed TSC-/- cells to maintain ATP levels and a viable ATP/ADP ratio, and repress AMPK activation, preventing energetic stress. Contrary to expectations, the observed decrease in metabolic consumption was both necessary and sufficient to protect TSC-/- cells from glucose deprivation-induced death. Thus, mTORC1 inhibition prevents both metabolic stress and cell death in TSC-/- cells. Glucose limitation addicted TSC-/- cells to glutamine as a carbon source, and surprisingly, this reliance on glutamine is dependent on glutamate dehydrogenase (GDH), but not transaminases. These data reveal potential therapeutic strategies for the treatment of TSC and LAM pathologies.
RESULTS
mTORC1 inhibition protects TSC-/- MEFs from glucose deprivation-induced death through a p53-independent pathway
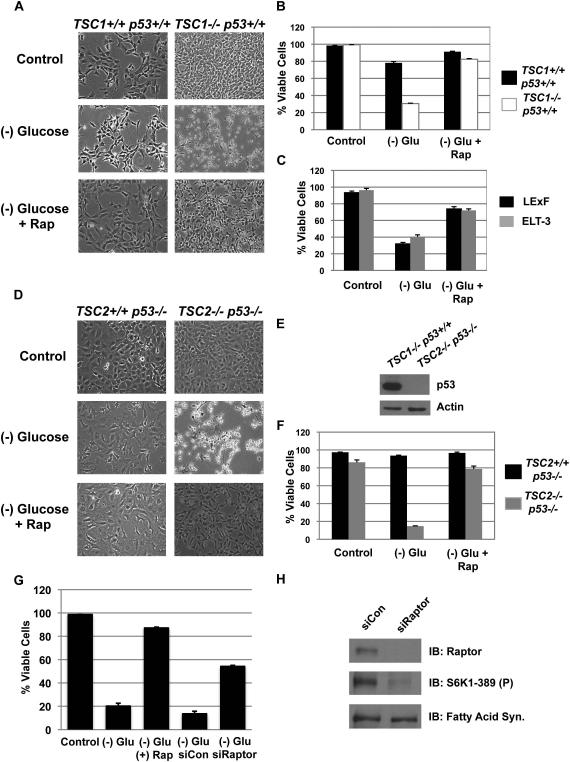
The TSC1/2 protein complex plays a critical role in integrating various environmental conditions to regulate the activity of the mTORC1 complex (Shaw and Cantley, 2006). The loss of the TSC1/2 leads to mTORC1 activation irrespective of growth factor or energy levels, and glucose deprivation of these cells leads to rapid onset of death (Inoki et al. 2003). To measure cell viability, we utilized the uptake of propidium iodide (PI) (Buzzai et al., 2005). By 48 hours of glucose deprivation, TSC1-/- MEFs died characterized by detachment from the cell substratum and membrane permeability to PI (Figure 1a & b). Conversely, TSC1 reconstituted cells remained over 80% viable following glucose deprivation and delayed the onset of death by an mTORC1 inhibition-dependent mechanism. This phenomenon was also observed in ELT-3 and LExF cells, which are both tumor cells with loss of TSC function (Figure 1c) (Inoki et al., 2003).
Figure 1. mTORC1 suppression protects TSC deficient cells from glucose deprivation-induced death through a p53-independent mechanism.
A. TSC1+/+ p53+/+ and TSC1-/- p53+/+ MEFs were deprived of glucose for 48 hours with or without rapamycin, and phase microscopy was used to observe cell viability.
B. Cell viability from A was measured via propidium iodide (PI) exclusion assay.
C. ELT-3 and LExF were deprived of glucose for 72 hours with or without rapamycin.
D. Phase image of TSC2-/- p53-/- or TSC2+/+ p53-/- MEFs deprived of glucose for 60 hours.
E. Western blot of p53.
F. PI-exclusion assay from D.
G. Cell viability following Raptor knockdown.
H. Knockdown efficiency for Raptor and phospho-S6K1.
Shown is an average (+ SEM) of 3 independent experiments.
A previous report linked hyper-activation of p53 by mTORC1-dependent p53 mRNA translation and AMPK-mediated phosphorylation of p53 as the cause for glucose sensitivity of TSC-/- cells (Lee et al., 2007). To determine if p53-mediated cell death is the only mechanism for death, we deprived TSC2-/- p53-/- MEFs of glucose (Figure 1d) and observed rapid cell death at 60 hours (Figure 1e&f). We analyzed death at 60 hours instead of 48 hours because the TSC1-/- p53+/+ died at a faster rate. Rapamycin treatment or Raptor knockdown provided complete protection, suggesting that mTORC1 inhibition provides a p53-independent mechanism for cell survival (Figure 1d-h).
Next, we investigated the role of proliferation and survival kinases in addicting TSC-/- cells to glucose. Cell proliferation can regulate the sensitivity of cells to glucose deprivation, and kinases such as Akt can influence survival during energetic stress (Vander Heiden et al., 2001). In addition, Ghosh et al. showed that TSC-/- cells are hypersensitive to DNA alkylating agents through an mTORC1-dependent mechanism (Ghosh et al., 2006). To determine if decreased proliferation was sufficient to protect TSC2-/- p53-/- MEFs (TSC2-/- MEFs) from glucose deprivation-induced death, we utilized thymidine (3mM), which slowed proliferation similar to rapamycin (Figure S1b). As shown in figure S1a&c, thymidine treatment did not affect the survival of glucose-deprived cells, suggesting that mTORC1-mediated inhibition of cell proliferation is not sufficient to protect cells from death.
mTORC1 inhibition in TSC-/- also leads to the activation of PI3K-Akt, a survival kinase (Figure S1d). Accordingly, we co-treated rapamycin with LY29004, a PI3K catalytic site inhibitor, or Akt IV, an Akt inhibitor. Although LY294002 can catalytically inhibit mTOR, this was not a concern as rapamycin was used in the same experiment (Choo et al., 2008). As shown in figures S1e, f, and g, inhibition of Akt by either LY294002 or Akt IV did not affect rapamycin-mediated protection from glucose deprivation, and these cells maintained fibroblast-like morphology and remained PI-negative. Therefore, mTORC1 inhibition-induced PI3K-Akt reactivation is not required for protection from glucose deprivation.
Rapamycin maintains cellular bioenergetics in the absence of glucose
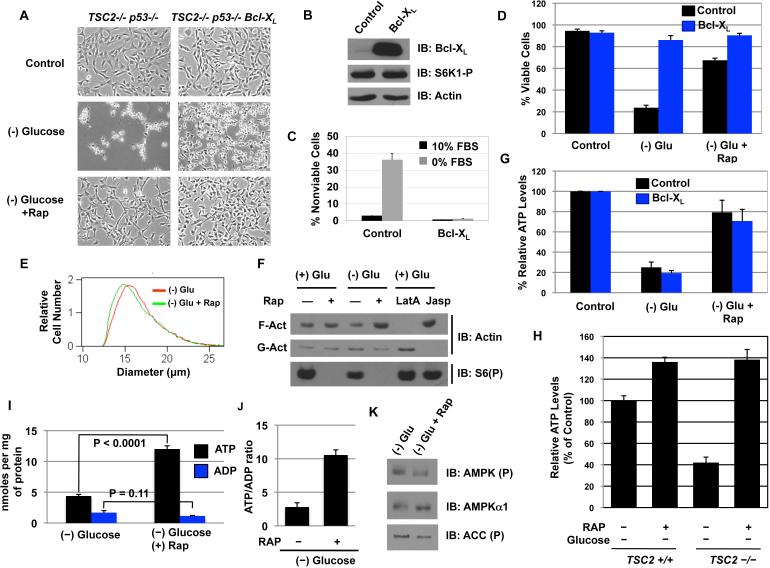
Previously, others reported that Akt-mediated protection from growth factor deprivation requires extracellular nutrients, while overexpression of Bcl-XL protects irrespective of glucose levels (Plas et al., 2001). If the protection of TSC2-/- MEFs from glucose deprivation were due to inhibition of apoptosis, then Bcl-XL overexpression should mimic the effect of rapamycin. Accordingly, Bcl-XL overexpression effectively protected cells from serum starvation (Figure 2b&c) as measured by PI-exclusion staining (Figure 2d). The cellular morphology between rapamycin- and Bcl-XL-protected TSC2-/- MEFs from glucose deprivation was distinct; while rapamycin-protected cells appeared healthy with efficient 2-D spreading and fibroblast-like morphology, the Bcl-XL protected cells appeared condensed without fibroblast-like morphology (Figure 2a), but were completely viable as glucose treatment led to rapid recovery in morphology and 2-D spreading (data not shown). Surprisingly, the decrease in 2-D spreading via glucose withdrawal was not a result of decreased cell size as the rapamycin protected Bcl-XL TSC2-/- MEFs were smaller than the Bcl-XL TSC2-/- MEFs were (Figure 2e). This defect in Bcl-XL expressing cells correlated with decreased filamentous actin (F-actin) and an increase in globular actin (G-actin) (Figure 2f), while rapamycin treatment maintained high levels of F-actin. These results were confirmed with appropriates controls; Latrunculin A (LatA), an inhibitor of actin polymerization, increased G-actin, and jasplakinolide (Jasp), a stimulator of actin polymerization, increased F-actin (Figure 2f). Therefore, the data suggest rapamycin treatment maintains the level of polymerized actin in the absence of glucose, likely leading to 2-D spreading and the fibroblast-like morphology.
Figure 2. mTORC1 inhibition maintains cellular bioenergetics in the absence of glucose.
A.The cellular morphology of TSC2-/- p53-/- control and Bcl-XL expressing cells after 48 H of glucose withdrawal.
B. The expression level of Bcl-XL and the phosphorylation of S6K1.
C. Cell viability of TSC2-/- p53-/- Bcl-XL cells at 96 hours post serum withdrawal.
D. The viability of control or Bcl-XL expressing TSC2-/- p53-/- MEFs at 60 hours of glucose deprivation. Shown is an average (+ SEM) of 3 independent experiments.
E. Cell size of TSC2-/- p53-/- Bcl-XL MEFs.
F. F- versus G- actin population after glucose deprivation in TSC2-/- p53-/- Bcl-XL MEFs. JASP = Jasplakinolide (1μM). LatA = Latrunculin A (2μM).
G. Cellular ATP levels in control or Bcl-XL expressing MEFs were determined after 24 hours of glucose deprivation with or without rapamycin. The control cells were plated at the same density and given fresh media at the time of glucose deprivation.
H. ATP levels were measured following 24 hours of glucose deprivation in TSC2+/+ and TSC2-/- cells.
I. ATP and ADP levels in the same conditions as H; ATP: p < 0.0001; ADP: p = 0.11
J. ATP/ADP ratio was determined from H.
K. AMPKα phosphorylation (Thr172) and ACC phosphorylation was measured 24 hours after glucose withdrawal.
The formation of thin filaments requires the binding of ATP to G-actin and eventual hydrolysis to form a stable filament bound with ADP/Pi (Korn et al., 1987). We reasoned that decreased F-actin in glucose deprived Bcl-XL TSC2-/- cells could be due to energetic deprivation from glucose withdrawal; therefore, we measured cellular ATP levels after 24 hours of glucose deprivation. As shown in figure 2g, the total ATP levels were ~20% of that of control cells. Moreover, a similar decrease was observed in Bcl-XL expressing cells, suggesting that preventing apoptosis does not prevent ATP depletion. We noticed that in both Bcl-XL expressing and vector control cells, rapamycin maintained ~60-80% of the ATP levels when compared with the control cells at 24 hours of glucose deprivation, while re-introduction of TSC2 into TSC2-/- cells also led to partial ATP maintenance (Figure 2h). The maintenance of energy was associated with increased ATP and slightly decreased ADP levels, leading to a ~4 fold increase in the ATP/ADP ratio (Figure 2i & j). Consistent with maintaining ATP levels, AMPK activation was decreased with rapamycin in glucose-deprived cells (Figure 2k).
Since cells generate ATP via glycolysis and OXPHOS, we reasoned that if this ATP maintenance was required for survival, then inhibition of OXPHOS should decrease ATP levels and survival in the absence of glucose. Accordingly, treatment with either oligomycin or antimycin A induced death in rapamycin treated and glucose deprived cells, while having no effect on cells growing in glucose (Figure S2 a & c). Importantly, incubation of rapamycin-treated and glucose-deprived cells with oligomycin for 90 minutes completely abolished ATP levels (Figure S2b). Together, these results suggest that rapamycin treatment of glucose deprived TSC2-/- cells maintains energy charge in cells through an OXPHOS-dependent mechanism, and that this may be involved in the p53-independent protection.
Glutamine, but not autophagy, is necessary for rapamycin-mediated protection and ATP maintenance in the absence of glucose
Under growth factor deprivation, cells decrease nutrient and glucose uptake and utilize autophagy, a catabolic process that provides intracellular carbons sources to maintain ATP production (Levine and Yuan, 2005; Lum et al., 2005). Since mTORC1 normally represses autophagy, failed mTORC1 inhibition following glucose withdrawal could limit ATP production. Therefore, we investigated if rapamycin-stimulated autophagy was responsible for the protection from death via glucose deprivation. As shown in figures S3a & b, rapamycin treatment induced the localization of LC3 to autophagosomes and the cleavage of LC3-I into LC3-II. To determine the role of autophagy in protection, we knocked down Beclin, the mammalian homolog of ATG6 required for rapamycin-induced autophagy. Knockdown of Beclin significantly decreased both basal and rapamycin-induced LC3-I cleavage into LC3-II (Figure S3c). Surprisingly, neither the knockdown of Beclin nor the treatment with 3-MA, which inhibits autophagy via Vps34, had any effect on rapamycin-mediated protection from glucose deprivation (Figure S3d & e). In addition, loss of ATG5, another regulator of autophagy, also did not affect rapamycin-mediated protection from glucose withdrawal, despite continued mTORC1 activation with RhebS16H, expression (Figure S3f-h). Therefore, induction of autophagy by rapamycin is not necessary for protection from glucose deprivation.
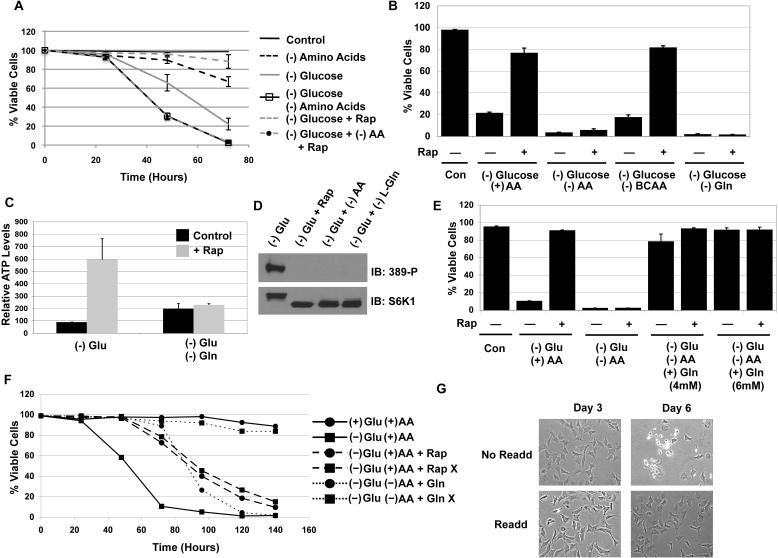
We next hypothesized that extracellular amino acids may be providing the energetic source for survival of TSC2-/- MEFs following glucose withdrawal. Deprivation of all amino acids from TSC2-/- cells led to minimal death over 3 days with 70% of the cells viable at 72 hours (Fig. 3a); conversely, less than 30% of the TSC2-/- cells were viable with glucose deprivation at 72 hours. However, when both glucose and amino acids were deprived, the cells died precipitously with essentially no viable cells by 72 hours, and this effect required the depletion of L-glutamine (Gln), but not branched chain amino acids (BCAAs), which have been reported to activate mTORC1 and provide energy in some cells (Figure 3b) (Moriwaki et al., 2004). More importantly, we observed that rapamycin failed to rescue cell death or maintain ATP levels in the absence of glutamine, suggesting that glutamine is necessary for ATP maintenance and cell survival (Figure 3b & c). We did observe that ATP level in the (-) glu cells were 50% lower than that of (-) glu/ (-) gln cells after 24 hours of deprivation (Figure 3c). When we analyzed mTORC1 activation status in these cells after 24 hours of respective deprivation, the phosphorylation of S6K1 was completely inhibited following (-) glu/(-) gln deprivation and was indistinguishable from that of rapamycin treatment (Figure 3d).
Figure 3. L-glutamine is required for rapamycin-mediated ATP maintenance in the absence of glucose.
A. TSC2-/- p53-/- MEFs were deprived of glucose, amino acids, or both with or without rapamycin.
B. Cell viability of cell with Branched chain amino acids (BCAAs), L-glutamine, or total amino depletion.
C. Total cellular ATP levels were determined after 24 hours of glucose deprivation.
D. S6K-1 (T389) phosphorylation after 24 hours of deprivation.
E. L-glutamine was added to glucose and total amino acid deprived conditions at the time of deprivation, and viability was measured at 60 H post withdrawal.
F. A time course measuring the viability of TSC2-/- p53-/- MEFs grown in complete, (-) glucose, (-)Glu (-)AA, and (-)glu (-)AA plus 4mM L-glutamine media. The samples labeled with X were replenished with only glutamine (2mM) starting at 48 hours and replenished every 24 hours. The Rap X sample also got 20nM of rapamycin at 0 and 72 hours.
G. Phase images were taken at 3 days and 6 days with or without L-glutamine (2mM) retreatment in (-) glucose (-) amino acids conditions.
Shown is an average (+ SEM) of 3 independent experiments.
Since glutamine is required for both survival and energy production, we asked if glutamine alone was sufficient to mediate survival without other amino acids and glucose. As shown in figures 3e and f, glutamine alone protected TSC2-/- MEFs from death despite no glucose and other amino acids, and did not require rapamycin treatment to maintain survival (Figure 3f). We observed that these cells could live up to 72 hours without any carbon source other than glutamine, but died precipitously thereafter. However, when glutamine (2mM) alone was replenished at 48 hours post deprivation and replenished every 24 hours (indicated by the X), TSC2-/- MEFs remained completely healthy and viable for 140 hours post deprivation (Figure 3f). The TSC2-/- MEFs appeared healthy and still exhibited 2-D spreading and fibroblast-like morphology even with glutamine as the only carbon source (Figure 3g). Therefore, this surprising result suggests that in the absence of glucose and all other amino acids, glutamine alone is sufficient to maintain all the processes that are necessary for cell survival.
A decrease in metabolic demand is critical for protecting TSC deficient MEFs from glucose deprivation induced death
The results presented in figure 3 suggested that L-glutamine was the energetic source during glucose deprivation, but failed to answer why mTORC1 inhibition was required for the protection considering L-glutamine is ubiquitously present in the media. We hypothesized that HIF-1α, which is overexpressed in TSC-deficient cells through enhanced translation, could be inhibiting glutamine metabolism and/or OXPHOS (Shaw, 2006). HIF-1α not only increases glucose utilization through transcribing various essential glycolytic enzymes, but also inhibits OXPHOS through various mechanisms (Denko et al., 2008). Therefore, rapamycin-induced decrease in HIF-1α could allow the TSC2-/- cells to utilize glutamine in the absence of glucose, allowing cells to maintain ATP levels. Rapamycin treatment of TSC2-/- MEFs led to significant reduction in HIF-1α protein levels by 12 hours (Figure S4a). To determine if the loss of HIF-1α was sufficient to recapitulate rapamycin's effect on viability and ATP levels, we identified 2 different HIF-1α shRNA constructs that knocked down HIF-1α to levels similar to those observed with rapamycin treatment for 24 hours (Figure S4b). As shown in figures S4c & d, knockdown of HIF-1α neither increased viability nor maintained ATP following glucose withdrawal, suggesting that reduction of HIF-1α by rapamycin is insufficient to either maintain viability or ATP during glucose deprivation.
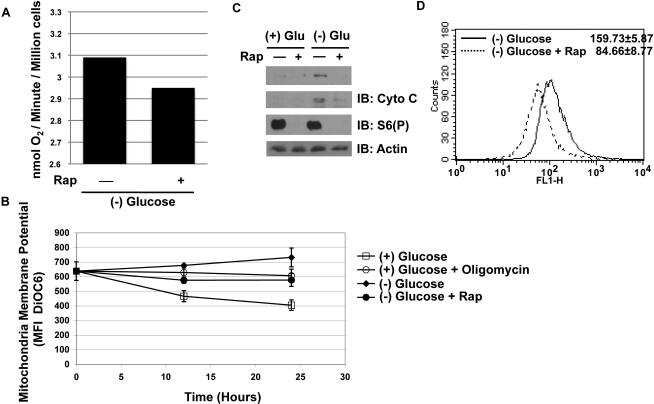
Next, we reasoned that if an increase in ATP production through OXPHOS was responsible for maintaining bioenergetics following glucose deprivation, there should be an increase in mitochondrial activity. Indeed, in cells where HIF-1α plays a negative role on OXPHOS, shRNA against HIF-1α leads to increased oxygen consumption and mitochondrial membrane potential (MMP) (Lum et al., 2007). Surprisingly, we observed a slight decrease in oxygen consumption rates, MMP, and overall mitochondria content – protein levels and mitotracker – with rapamycin treatment (Figure 4). Therefore, the maintenance of ATP per cell by rapamycin during glucose deprivation cannot be attributed to increased OXPHOS activity.
Figure 4. Mitochondrial activity is not increased in rapamycin treated TSC2-/- p53-/- MEFs.
A. TSC2-/- p53-/- MEFs were deprived of glucose for 24 hours, and the rate of oxygen consumption was measured. Oxygen consumption was measured by Clark's electrode, and the rate of consumption was determined and indicated as nmol of oxygen per minute per million cells. The experiment was conducted 3 times and yielded similar results.
B. Mitochondrial membrane potential was measure at 12 and 24 hours post treatment conditions via DiOC6 MFI via FACs. Oligomycin was used as a positive control (5μg/mL) in cells grown in glucose containing media. (n = 3)
C. PGC-1α and cytochrome c protein levels were determined at 24 hours post-deprivation.
D. Mitotracker measurement in the absence of glucose, and with or without rapamycin.
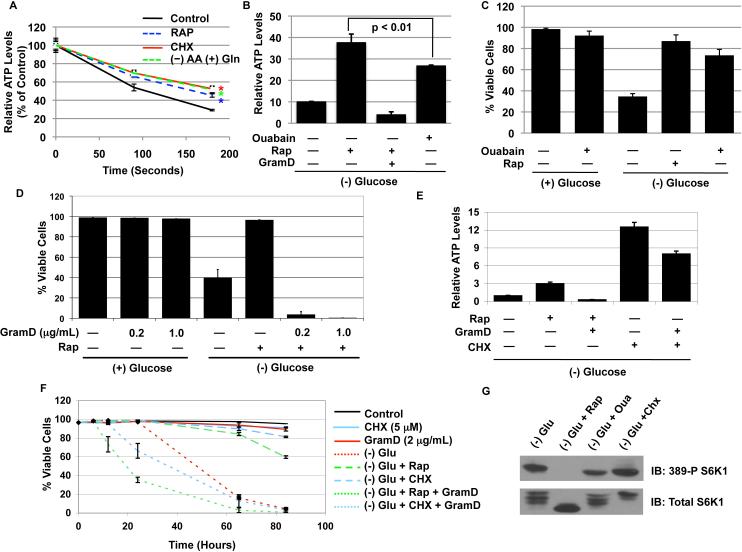
ATP and ATP/ADP levels are a function of not only metabolic production (supply), but also consumption (demand). Therefore, we next tested the hypothesis that mTORC1 activation leads to increased energy consumption, and therefore, rapamycin treatment allows significant decreases in ATP expenditure to maintain bioenergetics via OXPHOS. To measure metabolic consumption, we measured ATP levels at different time points post glucose withdrawal and oligomycin/antimycin treatment, which together block the two main modes of ATP production; within the first 3 minutes of withdrawal/inhibition, we observed statistically significant differences in ATP consumption between the tested groups. As shown in figure 5a, TSC2-/- MEFs were treated with rapamycin for 24 hours, deprived of all amino acids except glutamine for 12 hours, or treated with cycloheximide (CHX) for 12 hours, and ATP levels were measured at 90 seconds and 180 seconds post glucose withdrawal/mitochondria inhibition. At both time points, TSC2-/- cells treated with DMSO exhibited greater ATP consumption than rapamycin, (-)AA/(+)gln, and CHX groups did. The control cells consumed ATP at a rate of 0.211±0.0138 nmoles/s/mg protein, while rapamycin treated cells consumed at 0.180±0.0064 nmoles/s/mg protein with both rates having correlation coefficient values greater than 0.983. In addition, CHX treated cells and (-)AA/(+)gln cells consumed at a rate of 0.162±0.0319 nmoles ATP/s/mg protein and 0.164±0.0116 nmoles ATP/s/mg, respectively, with correlation coefficient values of greater than 0.985. Therefore, these results suggest that rapamycin, CHX treatment, and amino acid deprivation all decrease ATP consumption, with CHX and amino acid deprivation having the greatest effect.
Figure 5. mTORC1 regulates metabolic consumption, and modulating consumption is necessary and sufficient to regulate survival in the absence of glucose.
A. TSC2-/- p53-/- MEFs were incubated with rapamycin for 24 hours, cycloheximide (CHX) (5μg/mL) for 12 hours, and amino acid deprived media with glutamine, (-)AA (+)Gln, for 12 hours. Thereafter, the cells were briefly washed in (-)Glu (-)AA media and incubated with glucose free media containing oligomycin (10μg/mL) and antimycin A (2μg/mL). The (-)AA (+)Gln samples were incubated in (-)Glu, (-)AA, (+)Gln media with oligomycin and antimycin. The cells were immediately incubated in 37°C, and ATP levels were measured at indicated time points. All media used for washing and incubating was maintained at 37°C at all times. Experiments were carried out in pentuplicate, and data are presented as a percent of control, which is the ATP level prior to incubation in the glucose free/oligomycin/antimycin media. Student t-test: * p < 0.001 compared to control.
B. ATP levels were measured 24 hours after glucose deprivation in TSC2-/- p53-/- MEFs incubated with rapamycin (20nM), ouabain (1mM), and rapamycin/gramicidin D (1μg/mL). GramD = gramicidin D; Student t-test: (-) Glu compared to (-)Glu + Ouabain: p < 0.01
C. Cell viability was measured 60 Hours post glucose deprivation.
D. Cell viability for the effect of gramicidin D at the indicated concentrations was measured at 60 hours post glucose deprivation.
E. ATP levels were measured similarly to A except CHX (5μg/mL) was used. Gramicidin D (2μg/mL) was given to both samples 5 hours prior to lysis. 2μg/mL was used because we observed that a higher concentration was necessary to have a much more dramatic effect on cycloheximide treated cells.
F. Cell viability of the groups was measured at indicated time points with PI-exclusion assay.
G. The activation status of S6K1 (T389) was measured from cells deprived of glucose for 24 hours and treated with rapamycin, ouabain (1mM), and CHX (5μg/mL).
Shown is an average (+ SEM) of 3 independent experiments for all viability experiments.
If a rapamycin-mediated decrease in ATP consumption is sufficient to protect TSC-/- MEFs from glucose deprivation, then either increasing or decreasing consumption through mTOR-independent pathways should also influence cell viability following glucose withdrawal. For this purpose, we utilized regulators of the Na+/K+-ATPase, a major ATP consuming protein that pumps cellular sodium ions out and potassium ions in (Buttgereit and Brand, 1995). In the TSC2-/- MEFs, Na+/K+-ATPase activity was minimally regulated by rapamycin treatment (data not shown), allowing us to separate mTOR-dependent and -independent mechanisms. We utilized ouabain, which inhibits Na+/K+-ATPases, and gramicidin D, an ionophore that at low concentrations increases plasma membrane permeability to Na+ and K+ ions leading to increased pump activity and ATP consumption (Balaban et al., 1980; Vander Heiden et al., 1999). As shown in figure 5b, both rapamycin and ouabain treatment resulted in greater ATP levels post glucose withdrawal, although ouabain was not as effective as rapamycin. By contrast, ATP levels were significantly reduced in rapamycin/gram D treated cells, consistent with induction of energy consumption by gram D (Figure 5b). Consistent with the ATP levels, Ouabain treatment significantly delayed death following glucose withdrawal (Fig. 5c), while gramicidin D, which did not kill cells grown in the presence of glucose, rapidly induced death at the same dose in glucose-deprived cells treated with rapamycin (Figure 5d). In addition to ouabain, CHX also strongly maintained cell viability and ATP levels following glucose withdrawal (Figure 5e & f). The advantage of using CHX is that it actually increases mTORC1 activity (Figure 5g), allowing for the distinction between mTORC1-dependent and -independent regulation of survival. Consistently, CHX treatment was more effective at protecting cells from glucose withdrawal and required more time for gramicidin D treatment to induce cytotoxicity (Figure 5f); and this occurred independently of mTORC1 inhibition (Figure 5g). Therefore, rapamycin-mediated protection of glucose deprived TSC2-/- cells requires ATP maintenance, and the regulation of energy consumption is necessary and sufficient to dictate death or survival in the absence of glucose.
The role of glutamine and TCA intermediates in protecting glucose-deprived cells from death
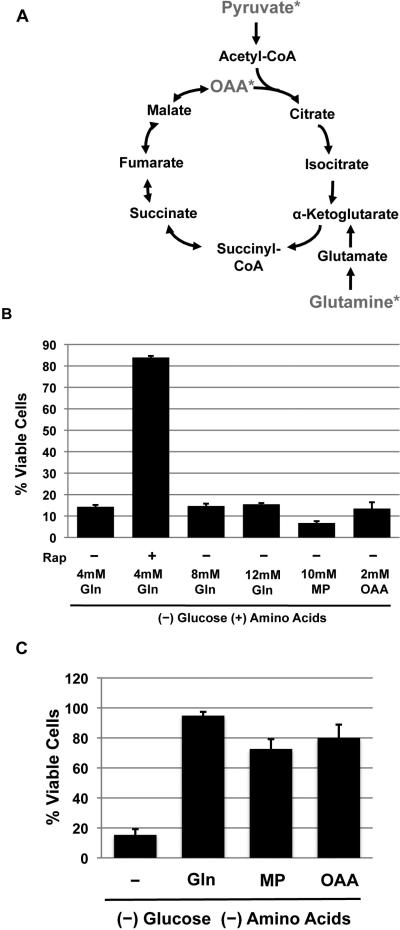
We showed previously that L-glutamine, which can be metabolized via the TCA cycle (Figure 6a), was required for rapamycin to maintain ATP levels in the absence of glucose (Figure 3). Others have shown that in the absence of growth factors, methylpyruvate (MP), a membrane permeable derivative of pyruvate that enters the TCA cycle, can compensate for the lack of glucose to meet energetic demand (Lum et al., 2005), and can protect Akt-addicted cells from glucose deprivation-induced death (Buzzai et al., 2005). However, treatment of TSC2-/- MEFs with MP or oxaloacetate (OAA) failed to provide protection in the absence of glucose (Figure 6b), and increasing concentrations of glutamine also failed to provide protection in the absence of glucose, suggesting that the lack of energetic source is not the cause for death (Figure 6b). Conversely, deprivation of glucose and amino acids, which like rapamycin treatment leads to decreased energetic consumption (Figure 5a), and subsequent treatment with OAA or MP protected the cells from death (Figure 6c). Moreover, substitution of glutamine with either OAA or MP allowed rapamycin or cycloheximide to protect cells from death (Figure S5a & b), suggesting that as long as energetic consumption is decreased, supplying carbons to the TCA cycle is sufficient to maintain ATP levels and protect cells from death. Therefore, the glutamine requirement is a matter of providing TCA cycle substrates and not providing a source of nitrogen.
Figure 6. TCA cycle intermediates can substitute for glutamine only when energetic consumption is decreased.
A. A diagram showing the TCA cycle intermediates, and the compounds used for this study are indicated with *.
B. TSC2-/- p53-/- MEFs were deprived of glucose and were given extra 4mM or 8 mM of glutamine to give a final concentration of 4mM, 8mM, and 12mM. Cell viability was measure at 60 hours post deprivation.
C. TSC2-/- p53-/- MEFs were deprived of amino acids and glucose, and given the indicated molecules. Cell viability was measured at 48 hours post deprivation.
Shown is an average (+ SEM) of 3 independent experiments.
Pharmacologic inhibition of glutamine metabolism prevents protection from glucose deprivation-induced death
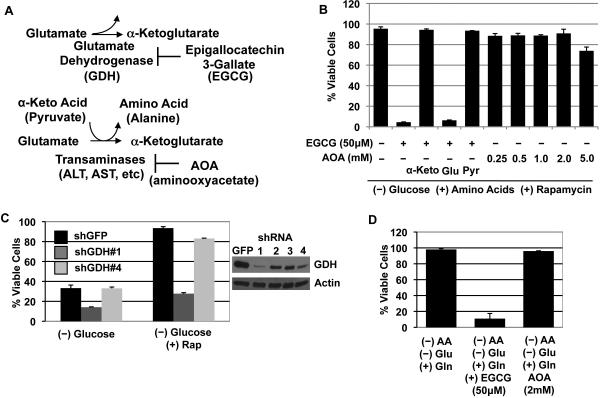
Since glutamine is important for maintaining ATP levels in the absence of glucose, we next tested if pharmacologic inhibition of glutamine metabolism can induce death. Glutamine is metabolized to α-ketoglutarate, a TCA cycle intermediate, through two deamination reactions; the first requiring glutaminase to generate glutamate and the second reaction occurring through two pathways via glutamate dehydrogenase (GDH) or transaminases, which transfer the amino group to α-ketoacids to generate amino acids such as alanine and aspartate (Figure 7a). Accordingly, both 6-Diazo-5-oxo-L-norleucine (DON), a glutamine analogue that inhibits glutaminase, and EGCG, an antioxidant in green tea extracts that inhibits glutamate dehydrogenase, effectively induced death in glucose deprived cells that were protected by rapamycin but not in cells grown in the presence of sufficient glucose (2.0mM versus 5.0 mM of glucose) (Figure S6a-c) (Li et al., 2006; Yuneva et al., 2007). EGCG's effect is specific to glutamine metabolism as supplementation of glucose- and amino acid-deprived cells with α-ketoglutarate, OAA, or pyruvate, but not glutamine or glutamate, abolished the toxic effects of EGCG (Figure S6d). This same effect was observed in cells deprived of glucose and protected with rapamycin; α-ketoglutarate and pyruvate, but not glutamate, abrogated the cytotoxic effects of EGCG (Figure 7b). The essential role of GDH in metabolizing glutamine in glucose-deprived TSC2-/- was verified with GDH targeting shRNA constructs (Figure 7c). Previous experiments showed that 0.5 mM of aminooxyacetate (AOA), a specific inhibitor of the two predominant transaminases, kills cells addicted to glutamine, and that 0.5 mM is sufficient to completely inhibit ALT and AST activities (Wise et al., 2008; Moreadith et al., 1984). However, even 5.0 mM of AOA did not kill rapamycin-protected and glucose-deprived TSC2-/- cells (Figure 7b). This effect was also evident in cells with only glutamine as the carbon source (-glu and -AA); 50μM of EGCG effectively induced glutamine-specific death after 48 hours, but 2.0 mM of AOA did not have an effect (Figure 7d). Therefore, pharmacologic inhibition of the glutamine metabolism pathway, specifically GDH, can cooperate with glucose limitation to induce cytotoxicity.
Figure 7. Pharmacologic inhibition of the glutamine metabolism pathway induces cytotoxicity under glucose limiting conditions.
A. A diagram showing the enzymes involved in glutamate metabolism (See text for more details).
B. EGCG (50μM) and AOA (0.25 to 5.0 mM) were tested for cytotoxicity of TSC2-/- cells deprived of glucose and given rapamycin (48 Hours post deprivation). Where indicated, various metabolites α-ketoglutarate (10mM), glutamate (4mM), and pyruvate (1mM) were also supplemented.
C. TSC2-/- cells were infected with shRNA constructs (#1-4) targeting GDH, and viability after 60 hours without glucose with or without rapamycin is shown.
D. TSC2-/- cells deprived of glucose and amino acids, but supplemented with glutamine (4mM) were given EGCG (50μM) or AOA (2mM).
The role of S6K1 and eIF4E/4E-BP1 in mediating rapamycin-mediated protection from glucose withdrawal
S6K1 and eIF4E/4E-BP1 are two proteins downstream of mTORC1 that controls cell growth and proliferation (Ma et al., 2009). To investigate the role of these proteins in rapamycin-mediated survival from glucose withdrawal, we overexpressed eIF4E or 4E-BP1 AA (37/46), which is a dominant negative 4E-BP1 that represses cap-dependent translation, and knocked down eIF4E and S6K1 (Figure S7a & b). Our reasoning is that if inhibition of eIF4E is important for rapamycin-mediated survival, then overexpressing eIF4E should attenuate rapamycin-mediated protection, while 4E-BP1 AA expression alone should provide some protection. As shown in figure S7a, expression of neither eIF4E nor 4E-BP1 affected rapamycin-mediated protection. In addition, we further verified this result with eIF4E knockdown, suggesting that the regulation of the eIF4E/4E-BP1 pathway is neither necessary nor sufficient for the rapamycin-mediated protection (Figure S7b). Conversely, knockdown of S6K1 provided partial protection from glucose deprivation, and this effect correlated with partial maintenance of ATP levels (Figure S7c & d). Therefore, the loss of S6K1 by rapamycin is partially sufficient to protect TSC-/- cells from glucose withdrawal-induced death.
Discussion
A p53-independent and bioenergetic-dependent mechanism for cell survival
Under energy limiting conditions, mTORC1 is primarily inhibited through a TSC1/2 dependent mechanism (Shaw and Cantley, 2006); mTORC1 inhibition prolongs the survival of energetically stressed cells by inhibiting the execution of cell death via decreased p53 translation, reduced ER-stress, and activation of survival kinases (Lee et al., 2007; Ozcan et al., 2008; Ghosh et al., 2006). While those mechanisms may delay cell death, our report provides the first evidence that mTORC1 inhibition directly reduces energetic stress by balancing metabolic demand with supply. Although mTORC1 positively controls the translation of p53 mRNA, we've determined that the loss of p53 does not prevent glucose deprivation-induced cell death in TSC2-/- deficient cells (Lee et al., 2007). By contrast, rapamycin maintains cellular bioenergetics in the absence of glucose, and this maintenance is required for rapamycin-mediated protection in both p53+/+ and p53-/- cells that have lost TSC function (Figure 2g; data not shown). This would explain how rapamycin promotes cell survival for up to 72 hours without glucose, which is the primary source for energy in TSC-deficient cells (Figure 2g; Figure S2b; data not shown). Our Bcl-XL overexpression experiments also showed that rapamycin's pro-survival effect is not exclusively through the blocking of the execution of cell death, but rather through the maintenance of cellular bioenergetics (Figure 2). Thus, inhibition of survival kinases and increase of global ER stress are likely contributors to the sensitivity of TSC-/- cells to glucose deprivation by accelerating the execution of cell death, but they ultimately are not the cause.
Metabolic demand, not supply, determines viability following glucose withdrawal in TSC-/- cells
When mTORC1 is inactivated, catabolic processes including autophagy and fatty acid oxidation are increased, while anabolic processes are decreased (Levine and Yuan, 2005). Therefore, the inability of TSC-/- cells to inhibit mTORC1 following glucose withdrawal could lead to accelerated death because of failed anabolic to catabolic shift. Our observation that rapamycin treatment, but not Bcl-XL overexpression, maintained cellular ATP levels following glucose withdrawal suggests that regulation of bioenergetics was involved (Figure 2g-j). One theory is that increase in rapamycin-sensitive HIF-1α expression, which is a consequence of TSC loss, may addict cells to glycolysis by increasing glucose metabolism and decreasing mitochondrial activity (Shaw, 2006). However, our data suggest that the loss of HIF-1α is not sufficient to recapitulate rapamycin-mediated protection (Figure S4). Moreover, TSC2-/- MEFs exhibited high mitochondrial activity, and at least in rapamycin treated conditions, OXPHOS was sufficient to maintain ATP levels. Thus, although HIF-1α expression is necessary for glucose addiction in some cells, its expression does not appear to be responsible for glucose addiction in TSC-/- cells.
Upon nutrient limitation, mTORC1 regulation also generates carbons through catabolic pathways to maintain basic survival processes (i.e. TCA cycle replenishment). Autophagy, which maintains survival of Bax/Bak-/- MEFs in the absence of growth factors, is activated by mTORC1 inhibition or rapamycin treatment (Lum et al., 2005). However, inhibition of autophagy did not prevent rapamycin treatment from protecting TSC-/- cells from glucose deprivation-induced death, suggesting that either carbons generated from autophagy are not effectively utilized for OXPHOS or the amount of carbons generated can not meet bioenergetic demand in the absence of decreased ATP consumption (Figure S3). The difference between Bax/Bak-/- and TSC-/- conditions may be that signaling in the former is attenuated upon growth factor withdrawal leading to low energetic demand, while the latter still exhibits potent mTORC1 activity due to TSC loss, leading to high energetic demand. This may explain why methylpyruvate can protect growth factor deprived cells, but not glucose deprived TSC2-/- cells (Lum et al., 2005; Figure 6), and why Bcl-XL overexpression in the TSC2-/- MEFs was less effective at maintaining ATP levels in the absence of glucose than that from previous reports (Vander Heiden et al., 1999). Therefore, our report suggests that simply increasing catabolism during glucose limitation is insufficient to maintain survival, and that attenuating anabolism is equally important and may be sufficient insofar as extracellular carbon sources can be attained.
We also showed that withdrawal of both glucose and glutamine forced TSC-/- cells to undergo rapid death (Figure 3). However, bioenergetic catastrophe may not be the only cause of death in this condition, as depletion of both of these carbon sources will likely lead to a significant reduction in the generation of NADPH through the inhibition of the pentose phosphate and malic enzyme pathways (DeBerardinis et al., 2008). Accordingly, we observed that GSH levels were reduced following glutamine deprivation (data not shown).
This observation may also highlight why decreased consumption, not increased production, appears to be favored by the cell. Accumulation of reactive oxygen species (ROS) resulting from increased OXPHOS may lead to necrosis when glucose levels are insufficient to support NADPH production and normal cellular redox balance; accordingly, dramatic increases in ROS are sufficient to decrease mitochondria function and contribute to eventual inhibition of OXPHOS and ATP production (Zini et al., 2007). Consistently, we observed that deprivation of glucose in TSC2-/- cells increased ROS levels, and rapamycin treatment attenuated this effect (Figure S6e). Therefore, a decrease in ATP consumption, not an increase in ATP production by OXPHOS, may be necessary for cell survival.
TSC-mTORC1 balances metabolic demand with supply
In addition to energy consumption (demand), mTORC1 may play a critical role in energy production (supply). Depending on the cell type, mTORC1 can regulate glucose transporter translocation and expression, nutrient receptor translocation, and expression of metabolic enzymes (Buller et al., 2008; DeBerardinis et al., 2008). Therefore, the TSC-mTORC1 signaling network appears to be a critical regulator of metabolic supply and demand in mammalian cells. The mTORC1-regulated processes that mediate energetic consumption remains to be determined; however, numerous processes may be involved including translation, mRNA biogenesis, and ion channels, although we observed only minor effects on ouabain-sensitive Na+/K+-ATPase activity with rapamycin (data not shown). Previous evidence has suggested that in thymocytes, DNA/RNA synthesis, protein translation, and sodium ATPases are responsible for 21, 30, and 14% of total ATP consumption (Buttgereit and Brand, 1995). Our data also suggest that S6K1, not eIF4E/4E-BP1, is at least partially involved in decreasing metabolic consumption (Figure S7c&d); future work will investigate the role of other processes, such as SKAR-dependent mRNA biogenesis, in mediating this protective effect (Ma et al., 2009).
Gwinn et al. showed recently that AMPK directly inhibits mTORC1 through raptor phosphorylation, and that this mechanism can inhibit mTORC1 in TSC deficient cells (Gwinn et al., 2008). However, our results suggest that the raptor phosphorylation mechanism is less applicable in the context of glucose deprivation, possibly because it fails to generate enough AMP to significantly activate AMPK and attenuate mTORC1 signaling in TSC deficient cells. However, double deprivation of both glucose and glutamine led to effective mTORC1 inhibition, which correlated with AMPK activation and Raptor phosphorylation (Figure 3d; data not shown).
The utilization of metabolic inhibition in targeting TSC deficient tumors
Our findings suggest that inhibiting glucose or/or glutamine metabolism may be an effective strategy to target TSC deficient tumors. Recent clinical studies involving Sirolimus have suggested that only a reversible cytostatic effect is observable during therapy; thus, modalities that can achieve tumor toxicity are greatly desired. Some unpublished data suggest that inhibition of glutamine metabolism, specifically through GDH, synergizes with glycolytic attenuation to induce robust death (data not shown). EGCG is already in several clinical trials as an anticancer agent and has been shown to be effective in limiting tumor growth in mice, although its mechanism remains largely unknown (Khan and Mukhtar, 2008). However, much caution and investigation are warranted before this combination is used because of the potential toxicity associated with GSH decrease, which accompanies EGCG treatment (data not shown). Accordingly, previous cancer clinical trials that tested glutamine analogues to prevent production of glutamate from glutamine were terminated prematurely due to excessive side effects related to nausea, hallucinations, and vomiting (Souba, 1993). Whether inhibition of GDH will be equally toxic remains unknown. However this mode of inhibition, unlike glutamine analogues, is unlikely to affect pyrimidine biosynthesis via carbamoyl phosphate synthetase (CAD); and thus far, early clinical data suggest EGCG can be tolerated by patients (Khan and Mukhtar, 2008).
We also found that GDH, but not transaminases, was the critical enzyme responsible for glutamate metabolism under glucose-limited conditions (Figure 7b & c). This is consistent with work from Yang et al., who recently showed that glutamine-addicted cells required GDH for protection from glycolysis inhibition (Yang et al., 2009). These authors showed that in c-myc-overexpressing and glutamine-addicted cells, GDH inhibition during glucose limitation induced cell death, suggesting that GDH may actually be the critical enzyme in balancing glycolysis and glutaminolysis in many different cell types. Our work further adds to this theory and suggests that the limitation with mTORC1 hyperactivation may be the dependence of tumors on GDH to meet bioenergetic demand during glucose limitation. Although future work will be necessary to determine the exact mechanism, it is tempting to hypothesize that the reduction of pyruvate and other α-keto acid production, which occurs upon glucose withdrawal, limits transaminase activity, requiring cells to use GDH.
Conclusion
In conclusion, we have shown that the TSC-mTORC1 signaling pathway is a critical balancer of metabolic demand with supply, and that this addicts TSC-/- cells to glucose. These results suggest that mTORC1 inhibition in TSC-/- cells not only alters the execution of death from energetic stress, but also directly inhibits the cause. While much work has identified catabolic processes such as autophagy and fatty acid oxidation as survival mechanisms during nutrient deprivation, our work has shown the importance of decreasing metabolic consumption for survival. Thus, decreased ATP consumption is sufficient to maintain survival if substrates for ATP production via the TCA cycle are maintained. These findings suggest a new consideration in determining the use of mTORC1 inhibitors to treat tumors in the clinic. Although much work involving toxicity remains, methods that limit tumor metabolism, such as GDH (EGCG) inhibition, may be an effective mode to kill TSC-/- tumors; thus further in vivo investigation is warranted.
Experimental Procedures
Cell lines and culture
TSC2-/- p53-/- and TSC1-/- p53+/+ MEFs were kindly provided by Drs. Brendan Manning and David Kwitakowski (Harvard Medical School). ELT-3 cells were provided by Dr. Cheryl Walker (University of Texas). LExF cells were provided by Dr. K.L. Guan (UCSD). The MEFs and ELT-3 cells were cultured in DMEM with 10% FBS (Dialyzed for deprivation experiments - Gibco) and P/S. The LExF cells were cultured in DMEM/F12 with 10% FBS. All DMEM lacking glucose, amino acids, L-glutamine or combinations were made from formulations provided by sigma. All extra energetic additives that are often added to some DMEM formulations such as sodium pyruvate and succinate were excluded.
Cell viability and metabolic assays
Cell viability was determined via propidium iodide (PI) exclusion assay (1μg/mL). ATP and ADP levels were determined as previously described (Vander Heiden et al., 1999). Mitochondria membrane potential and total mitochondria were determined with DiOC6 and Mitotracker stains, respectively. More details provided in supplemental text.
Cell size measurements
TSC2-/- MEFs were grown on 10cm plates and treated as indicated, trypsinized, and resuspended in PBS, pH 7.2 in a total of 10mL. Cell diameter was immediately analyzed on a Z2 Coulter Counter, and results were graphed in Coulter®AccuComp® Software, gating between 12um and 28um.
Statistics
An unpaired, 2-tail student t-test was used.
Supplementary Material
Acknowledgments
We thank members of the Blenis’ laboratory for critical discussions and technical assistance. We are also grateful to D. Kwiatkowski, B. Manning, J. Brugge, W. Kaelin Jr., C. Walker, N. Mizushima, and KL Guan for providing reagents, and S. Soltoff for advice and discussions regarding Na+K+ ATPase assays. AYC would also like to thank J. Brugge and L. Gehrke for discussions. This work was funded by the NIH Grant GM51405 to JB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balaban RS, Mandel LJ, Soltoff SP, Storey JM. Coupling of active ion transport and aerobic respiratory in isolated renal tubules. Proc. Natl. Acad. Sci. USA. 1980;77:447–51. doi: 10.1073/pnas.77.1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller CL, Loberg RD, Fan MH, Zhu Q, Park JL, Vesely E, Inoki K, Guan KL, Brosius FC., 3rd A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am. J. Physiol. Cell. Physiol. 2008;295:836–43. doi: 10.1152/ajpcell.00554.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 1995;312:163–7. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzai M, Bauer DE, Jones RG, Deberardins RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24:4165–73. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl. Acad. Sci. USA. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metabolism. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer. 2008;8:705–13. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Tergaonkar V, Rothlin CV, Correa RG, Bottero V, Bist P, Verma IM, Hunter T. Essential role of tuberous sclerosis genes TSC1 and TSC2 in NF-kappaB activation and cell survival. Cancer Cell. 2006;10:215–26. doi: 10.1016/j.ccr.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Khan N, Mukhtar H. Multitargeted therapy by green tea polyphenols. Cancer Lett. 2008;269:269–80. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn ED, Carlier MF, Pantaloni D. Actin polymerization and ATP hydrolysis. Science. 1987;238:638–44. doi: 10.1126/science.3672117. [DOI] [PubMed] [Google Scholar]
- Lee CH, Inoki K, Karbowniczek M, Petroulakis E, Sonenberg N, Henske EP, Guan KL. Constitutive mTOR activation in TSC mutants sensitizes cells to energy starvation and genomic damage via p53. EMBO J. 2007;26:4812–23. doi: 10.1038/sj.emboj.7601900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Yuan J. Autophagy in cell death: an innocent convict? J. Clin. Invest. 2005;115:2679–88. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Allen A, Kwagh J, Doliba NM, Qin W, Najafi H, Collins HW, Matschinsky FM, Stanley CA, Smith TJ. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J. Biol. Chem. 2006;281:10214–21. doi: 10.1074/jbc.M512792200. [DOI] [PubMed] [Google Scholar]
- Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, Simon MC, Thompson CB. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–49. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, Bauer DE, Kong M, Harris MH, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–48. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell. Biol. 2009;10:307–18. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Moreadith RW, Lehninger AL. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J. Biol. Chem. 1984;259:6215–21. [PubMed] [Google Scholar]
- Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem. Biophys. Res. Commun. 2004;313:405–9. doi: 10.1016/j.bbrc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell. 2008;29:541–51. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 2001;276:12041–8. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Sabatini DM. Rag proteins regulate amino-acid-induced mTORC1 signalling. Biochem. Soc. Trans. 2009;37:289–90. doi: 10.1042/BST0370289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Shaw RJ. Glucose metabolism and cancer. Curr. Opin. Cell. Biol. 2006;18:598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Souba WW. Glutamine and cancer. Ann. Surg. 1993;218:715–28. doi: 10.1097/00000658-199312000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol. Cell. Biol. 2001;21:5899–912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-XL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell. 1999;3:159–67. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18782–7. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Sudderth J, Dang T, Bachoo RG, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell. Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini R, Berdeaux A, Morin D. The differential effects of superoxide anion, hydrogen peroxide and hydroxyl radical on cardiac mitochondrial oxidative phosphorylation. Free Rad. Res. 2007;41:1159–1166. doi: 10.1080/10715760701635074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.