Abstract
The melanocortin-3 and -4 receptors (MC3R, MC4R) have been implicated in energy homeostasis and obesity. Whereas the physiological role of the MC4R is extensively studied, little is known about the MC3R. One caveat is the limited availability of ligands that are selective for the MC3R. Previous studies identified Ac-His-DPhe(p-I)-Arg-Trp-NH2, which possessed partial agonist/antagonist pharmacology at the mMC3R while retaining full nanomolar agonist pharmacology at the mMC4R. These data allowed for the hypothesis that the DPhe position in melanocortin tetrapeptides can be used to examine ligand side-chain determinants important for differentiation of mMC3R agonist versus antagonist activity. A series of 15 DPhe7 modified Ac-His-DPhe7-Arg-Trp-NH2 tetrapeptides has been synthesized and pharmacologically characterized. Most notable results include the identification of modifications that resulted in potent antagonists/partial agonists at the mMC3R and full, potent agonists at the mMC4R. These SAR studies provide experimental evidence that the molecular mechanism of antagonism at the mMC3R differentiates this subtype from the mMC4R.
Introduction
The melanocortin receptors have been implicated in various physiological functions, including pigmentation,1–4 inflammation,5 adrenal function,2 cardiovascular function,6–8 and energy homeostasis in humans and rodents.9–11 To date, five melanocortin receptors (MC1R–MC5R) have been cloned that belong to the super family of G protein coupled receptors (GPCRs) and stimulate the cyclic AMP (cAMP) signal transduction pathway. The peptides, α-melanocyte-stimulating hormone (α-MSH),a β-MSH, γ-MSH, and ACTH are the endogenous agonists of these receptors. These ligands are derived through posttranslational processing of the precursor hormone proopiomelanocortin (POMC)12,13 and contain a common core sequence, His-Phe-Arg-Trp (residues 6–9 in α-MSH), postulated to be important for melanocortin ligand–receptor recognition and stimulation.14,15
The centrally located melanocortin-3 and -4 receptors (MC3R and MC4R)16–19 have been identified in knockout mice to be involved in energy homeostasis, obesity, and metabolism11,20,21 and are potential drug targets for the treatment of obesity and related diseases. Whereas the MC4R knockout mice are extremely obese and hyperphagic, the MC3R knockout mice possess normal body weight with increased fat mass and decreased lean body mass.11,20,21 Extensive studies have been carried out to understand the physiological role of the MC4R, but little is known about the MC3R. To date, only a limited number of ligands are available that are 100–200-fold selective for the MC3R over the MC4R subtype.22–24 However, for the use of compounds as suitable in vivo tools, a higher difference in potency (>500-fold) between the receptor subtypes is desirable. The discovery of new selective MC3R ligands will ultimately help in investigating the vast array of physiological functions of this receptor subtype and contribute to the discovery of new antiobesity agents.
Previous structure–activity relationship (SAR) studies, modifying the DPhe7 of melanocortin ligands (α-MSH numbering) such as 1 (MTII, Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2), 25 identified the MC3R/MC4R antagonists 2 (SHU9119, Ac-Nle-c[Asp-His-DNal(2′)-Arg-Trp-Lys]-NH2),26 3 (SHU8914, Ac-Nle-c[Asp-His-DPhe(p-I)-Arg-Trp-Lys]-NH2),26 and 4 [SHU9005, Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe(p-I)-Arg-Trp-Gly-Lys-Pro-Val-NH2).27 This led to the hypothesis that bulky substituents at the ligand DPhe7 position may be responsible for antagonism at the MC3 and MC4 receptors.26 Further SAR studies of the tetrapeptide template Ac-His-DPhe-Arg-Trp-NH2 led to the discovery of Ac-His-DNal(2′)-Arg-Trp-NH2 that, as anticipated, resulted in a melanocortin receptor pharmacological profile similar to that of 2, albeit with decreased potency.28 Surprisingly, modification at the DPhe position with pI, similar to 4, resulted in the tetrapeptide Ac-His-DPhe(p-I)-Arg-Trp-NH2 that possessed “mixed pharmacology” with antagonist/partial agonist activity at the mMC3R while retaining full nanomolar agonist potency and efficacy at the mMC4R.28 The difference in pharmacology of the DNal(2′)7- and DPhe(p-I)7-containing tetrapeptide ligands suggests that (i) these compounds may cause mMC3R antagonism by distinct mechanisms and (ii) these ligands may be used to investigate the determinants that discriminate between the MC3 and MC4 receptor subtypes.
The study presented herein employs ligand–receptor SAR studies in combination with computational methods to investigate (i) the molecular mechanism by which substitution of the DPhe phenyl ring at the para position with iodine causes this unique mixed mouse MC3R/MC4R pharmacological profile and (ii) the substructural determinants underlying receptor selectivity and differentiation of agonist versus antagonist activity at the mMC3R.
Results
Peptide SAR Studies
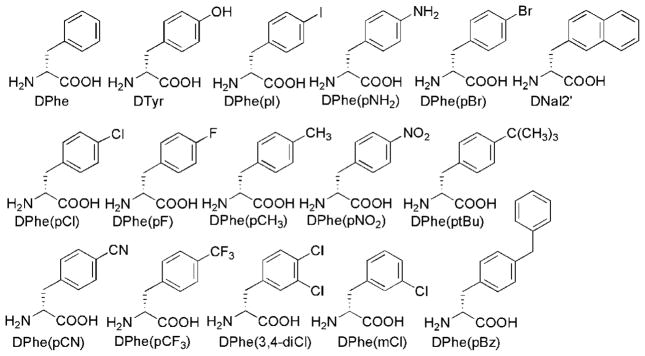
The amino acids that were used for substitution at the DPhe position in the tetrapeptide template Ac-His-DPhe-Arg-Trp-NH2 are illustrated in Figure 1. These substituents span a diverse range of electronic and polarity properties and sizes to allow a thorough assessment of the effect of the substituent physicochemical properties on melanocortin receptor functional activity.
Figure 1.
Structure of amino acid building blocks used in this study to replace DPhe in the peptide template Ac-His-Xaa-Arg-Trp-NH2.
As summarized in Table 1, compounds synthesized in this study were pharmacologically characterized at the mouse MC1 (mMC1R) and MC3–5 (mMC3R–mMC5R) receptors because it was hypothesized that different substituents at the DPhe phenyl ring might lead to distinct potency and/or functional activity at the different receptor subtypes. The mMC2R was not included because it can be stimulated only by ACTH and not by the tetrapeptides examined in this study.2 The tetrapeptides Ac-His-DPhe-Arg-Trp-NH2 (5), Ac-His-DTyr-Arg-Trp-NH2 (6), Ac-His-DPhe(p-I)-Arg-Trp-NH2 (16), and 18 [JRH887-12, Ac-His-DNal(2′)-Arg-Trp-NH2) have been previously reported28 and have been included in this study as control compounds and to complement SAR trends. Tetrapeptide 19 was designed to mimic the para and meta DPhe substitution of the DNal(2′) group of 18, whereas analogue 20 was included to investigate single substitution at the meta position. The table found in the Supporting Information summarizes the analytical characterization results for the tetrapeptides examined in this study.
Table 1.
Functional Activity of the DPhe7 (α-MSH Numbering) Modified Tetrapeptides at the Mouse Melanocortin Receptorsa
| peptide | structure | mMC1R |
mMC3R |
mMC4R |
mMC5R |
||||
|---|---|---|---|---|---|---|---|---|---|
| EC50 (nM) | fold diffb | EC50 (nM) | fold diffb | EC50 (nM) | fold diffb | EC50 (nM) | fold diffb | ||
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.088 ± 0.031 | 0.33 ± 0.08 | 0.12 ± 0.01 | 0.26 ± 0.05 | ||||
| 5 | Ac-His-DPhe7-Arg-Trp-NH2 | 112 ± 28 | 1 | 126 ± 7.8 | 1 | 2.28 ± 0.52 | 1 | 3.26 ± 0.60 | 1 |
| 6 | Ac-His-DTyr-Arg-Trp-NH2Tyr | 2600 ± 930 | 23 | 12500 ± 5700 | 99 | 390 ± 48 | 171 | 730 ± 210 | 223 |
| 7 | Ac-His-DPhe(pNH2)-Arg-Trp-NH2 | 2300 ± 300 | 21 | 16400 ± 1480 | 130 | 220 ± 15.0 | 96 | 226 ± 10.2 | 69 |
| 8 | Ac-His-DPhe(pCH3)-Arg-Trp-NH2 | 170 ± 20 | 2 | 525 ± 130 | 4 | 5.65 ± 0.88 | 2 | 5.20 ± 0.71 | 2 |
| 9 | Ac-His-DPhe(pt-Bu)-Arg-Trp-NH2 | 100 ± 13 | 1 | >100000c | 4360 ± 1300 | 1912 | 340 ± 73 | 104 | |
| 10 | Ac-His-DPhe(pBz)-Arg-Trp-NH2 | 180 ± 72 | 2 | 770 ± 108 | 6 | 63.8 ± 12.5 | 28 | 51.1 ± 3.22 | 16 |
| 11 | Ac-His-DPhe(pCN)-Arg-Trp-NH2 | 280 ± 27 | 3 | 240 ± 49 | 2 | 2.84 ± 0.26 | 1 | 2.85 ± 0.27 | 1 |
| 12 | Ac-His-DPhe(pNO2)-Arg-Trp-NH2 | 1030 ± 145 | 9 | 615 ± 44 | 5 | 13.0 ± 2.92 | 6 | 17.6 ± 2.1 | 5 |
| 13 | Ac-His-DPhe(pF)-Arg-Trp-NH2 | 53.7 ± 8.62 | −2d | 53.7 ± 5.30 | −2d | 0.45 ± 0.10 | −5d | 2.04 ± 0.27 | −2d |
| 14 | Ac–His-DPhe(pCl)-Arg-Trp-NH2 | 38.2 ± 3.3 | −3d | 84.8 ± 21.0 | −2d | 0.40 ± 0.12 | −6d | 1.47 ± 0.44 | −2d |
| 15 | Ac-His-DPhe(pBr)-Arg-Trp-NH2 | 40.8 ± 10.4 | −3d | ~50% at 100 μMe pA2 = 7.54 ± 0.12f |
antagonist Ki = 350 nM | 1.07 ± 0.46 | −2d | 2.60 ± 1.52 | 1 |
| 16 | Ac-His-DPhe(pI)-Arg-Trp-NH2 | 40.7 ± 2.73 | 3 | ~40% at 100 μMe pA2 = 7.41 ± 0.08f |
antagonist Ki = 260 nM | 3.94 ± 1.32 | 2 | 7.41 ± 0.08 | 2 |
| 17 | Ac-His-DPhe(pCF3)-Arg-Trp-NH2 | 540 ± 120 | 5 | ~25% at 100 μMe pA2 = 6.79 ± 0.15f |
antagonist Ki = 6170 nM | 12.8 ± 1.94 | 6 | 8.03 ± 1.33 | 2 |
| 18 | Ac-His-DNal(2′)-Arg-Trp-NH2 | 92.8 ± 42.7 | 1 | ~25% at 100 μMe pA2 = 6.70 ± 0.12f |
antagonist Ki = 5010 nM | pA2 = 8.28 ± 0.13 | antagonist Ki = 19 nM | 22.4 ± 5.0 | 7 |
| 19 | Ac-His-DPhe(3,4-diCl)-Arg-Trp-NH2 | 140 ± 30 | 1 | ~30% at 100 μMe pA2 = 7.42 ± 0.26f |
antagonist Ki = 260 nM | 3.40 ± 0.66 | 1 | 7.42 ± 0.26 | 2 |
| 20 | Ac-His- DPhe(m-Cl)-Arg-Trp-NH2 | 320 ± 57.3 | 3 | 1240 ± 270 | 10 | 8.64 ± 1.73 | 4 | 30.0 ± 13.6 | 9 |
The indicated errors represent the standard error of the mean from at least three independent experiments.
The fold differences are determined relative to compound 5.
Compounds possessing >100000 EC50 values were not found to possess agonist or antagonist activity at up to 100 μM concentrations.
A negative fold difference is indicative of increased potency relative to compound 5.
A percentage value indicates that some stimulatory agonist pharmacology resulted at up to 100 μM concentrations, but the maximal stimulation levels were less than the control level.
The compounds not demonstrating full agonism were assayed for antagonism using Schild pA2 analysis and the MTII peptide as agonist. Ki = −log pA2.
As seen in Table 1, all new tetrapeptides described here are 1–2 orders of magnitude more potent at the mMC4R and the mMC5R as compared to at the mMC1R and mMC3R. Thus, although none of the new analogues is the desired highly potent mMC3R-selective agonist, comparison of the functional properties of these DPhe-substituted tetrapeptides at different receptor subtypes reveals several trends that suggest structural features that underlie agonist versus antagonist activity at the mMC3R. Incorporation of hydrophilic hydrogen-bond donating groups (p-OH in peptide 6 and p-NH2 in peptide 7) results in decreases of ligand potency of about 20-fold for mMC1R and approximately 100-fold for mMC3–5R receptor subtypes. In contrast, the presence of polar electron-withdrawing and possible hydrogen-bond accepting groups (p-CN in peptide 11 or p-NO2 in peptide 12) results in similar or moderately less (10-fold) reduced potency. On the other hand, incorporation of the small hydrophobic p-CH3 group in peptide 8 is well tolerated by all receptor subtypes, whereas introduction of bulky hydrophobic groups (p-tBu in peptide 9 and p-Bz in peptide 12) decreases ligand potency for all receptor subtypes, except mMC1R. The presence of p-tBu group is much more detrimental, especially for activation of mMC3R, than p-Bz.
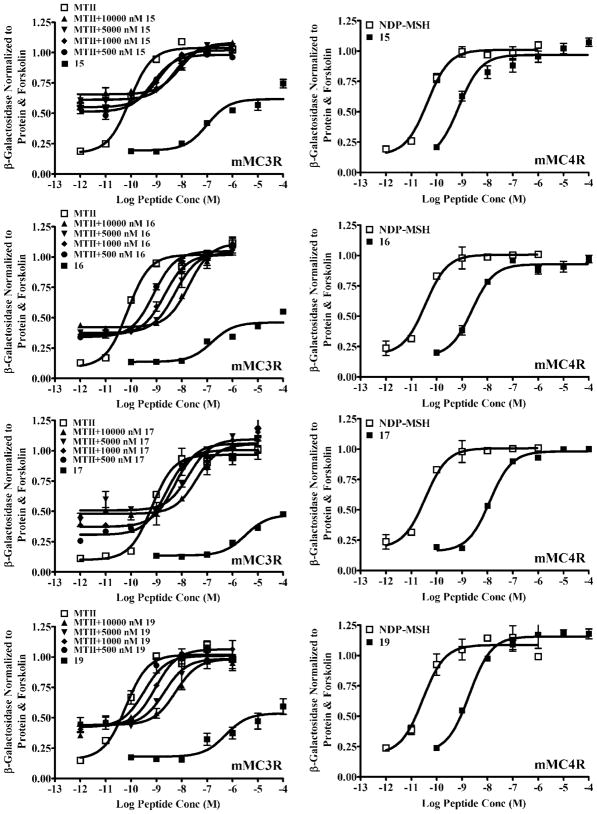
Effects of halogens included in the tetrapeptide ligands are more interesting. The incorporation of the smaller fluorine, chlorine, and bromine substituents at the para position of DPhe slightly improved ligand potency for mMC1R, mMC4R, and mMC5R (p-F in peptide 13, p-Cl in peptide 14 or p-Br in peptide 15), whereas the larger p-I substituent in peptide 16 and the p-CF3 in peptide 17 or a chlorine substituent in the meta position (3,4-diCl in peptide 19 and m-Cl in peptide 20) slightly decreases ligand potency for these receptor subtypes. The effect on functional activity of the mMC3R by halogen-substituted tetrapeptide ligands is quite different. Whereas the peptides with fluorine (peptide 13) and chlorine (peptide 14) substituents at the para position of DPhe have slightly improved agonist potency for mMC3R, the peptides with larger size halogens or –CF3 at the para position (peptide 15–17) or with chlorine in the meta position (peptide 19) function as partial agonist/antagonists or as very poor agonists [peptide 20 with DPhe(m-Cl)]. As shown in Figure 2, both the bromine-containing peptide 15 and the iodine-containing peptide 16 possess partial agonist activity in addition to competitive antagonism at the mMC3R. Comparison of halogen-containing tetrapeptides revealed that peptide 13 with DPhe(p-F) was the most potent tetrapeptide agonist for mMC3R (EC50 at 53 nM), whereas peptides 16 with DPhe(p-I) and 19 with DPhe(3,4-diCl) were the most potent tetrapeptide antagonists for the mMC3R with Ki = 260 nM (Table 1; Figure 2).
Figure 2.
Illustration of melanocortin tetrapeptides displaying partial agonist and antagonist activity at the mouse MC3R and full agonist activity at the mouse MC4R.
To better characterize the antagonistic properties of halogen-containing ligands, we compared them with the previously reported tetrapeptide 18 containing a DNal(2′) substitution for the DPhe residue. Peptide 18 displayed full agonist activity at the mMC1R and mMC5R receptors with equipotency at the mMC1R and 7-fold decreased potency at the mMC5R compared to peptide 5, whereas it had partial agonist/antagonist activity at the mMC3R (pA2 = 6.7) and antagonist activity at the mMC4R (pA2 = 8.3).28 However, 18 was a much less potent antagonist at the mMC3R than the halogen-containing tetrapeptides 15, 16, and 19 (Table 1; Figure 2).
Discussion
The study reported herein extends structure–activity relationship studies of the tetrapeptide template Ac-His-DPhe-Arg-Trp-NH2, in which DPhe was substituted by a series of natural and unnatural aromatic amino acids.28 We continued to test the hypothesis that the DPhe position can be exploited for improvement of receptor selectivity for the mMC3R versus the mMC4R, and for the differentiation of agonist versus antagonist activity at the mMC3R. For this purpose, a series of functional groups were chosen for substitution at the DPhe phenyl ring at the para and meta positions. The resulting tetrapeptides were pharmacologically characterized at the cloned mouse melanocortin MC1, MC3, MC4, and MC5 receptors (Table 1), the results of which provide insight into aspects of ligand–receptor interaction that result in either mMC3R stimulation or competitive antagonism.
Substitution with Hydrophilic Groups
Incorporation of polar substitutions with different physiochemical properties at the DPhe residue resulted in distinct pharmacological profiles at the mouse melanocortin receptors (Table 1). Tetrapeptides 6 and 7 containing hydrophilic and hydrogen-bond donating groups, p-OH and p-NH2, respectively, displayed large potency drops at all melanocortin receptors studied. However, tetrapeptides 11 and 12 with hydrophilic and H-bond accepting groups, p-CN and p-NO2, respectively, were equipotent to the parent peptide (5) at the mMC1R and had only slightly decreased agonist potencies in mMC3–5 receptors. Receptor mutagenesis of the mouse and human MC1R, MC3R, and MC4R, in combination with GPCR homology modeling of MC1R and MC4R,27,29–37 identified a putative hydrophobic receptor pocket consisting of aliphatic, aromatic, and sulfur-containing amino acid residues from transmembrane helices 3–6 of melanocortin receptors that are hypothesized to interact with the L/DPhe7 residue of melanocortin peptide ligands. The decrease in agonist potency observed for peptides 6 and 7 as well as the previously reported recovery of potency by using methoxy and ethoxy substituents30 support the hypothesis that the ligand DPhe7 residue is interacting with a hydrophobic environment in the receptor binding pocket. Furthermore, these data suggest that this receptor site is likely devoid of polar residues that may accept hydrogens from hydroxy or amino groups of the tetrapeptides and thus participate in hydrogen-bond formation between ligands and receptors. On the other hand, the better tolerance of p-CN and p-NO2 groups from peptides 11 and 12, respectively, may be explained by the presence of cysteines from transmembrane helices 3–5 in the receptor binding pockets that may serve as hydrogen donors forming hydrogen bonds with these groups. However, this mechanism remains speculative and should be experimentally verified.
Substitution with Hydrophobic Aliphatic and Aromatic Groups
Peptide 8, containing the small hydrophobic p-CH3 group in DPhe, retained equal potency and full agonist activity at all melanocortin receptors, as compared to peptide 5 (Table 1). This observation is consistent with the existence of a putative hydrophobic receptor binding pocket described above, which may be large enough to tolerate small hydrophobic modifications in DPhe. Introduction of the bulky p-tBu substituent at DPhe in peptide 9 was well tolerated only at the mMC1R and resulted in significantly reduced potency at the mMC4R and mMC5R, with complete loss of stimulatory activity at the mMC3R at up to 100 μM concentrations. These results may indicate that the DPhe7 interaction site of the ligand binding pocket might be more constrained in mMC3R, therefore tolerating less bulk than in the other melanocortin receptor subtypes. The putative mMC1R binding pocket, however, seems to be more flexible for all of the tetrapeptides examined in this study. This is in agreement with previously reported results of substitutions in the tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 by various natural and unnatural aromatic amino acids,28 where all DPhe modifications resulted in full agonists at mMC1R, unlike at the other mouse melanocortin receptor isoforms. Tetrapeptide 10 with DPhe(p-Bz) resulted in high potency at the mMC1R, slightly reduced potency at the mMC3R, and 28- and 16-fold losses in potency at the mMC4 and mMC5 receptors, respectively (Table 1). The smaller changes in agonist activity of peptide 10 compared to 9 are postulated to be attributed to the methylene linker of the benzyl group, which allows increased rotational freedom of the benzyl ring and renders it less rigid as compared to tert-butyl substitution. Tetrapeptide 18, which contains the bulky and lipophilic DNal(2′) group substituted for the DPhe amino acid, has been described previously.28 This peptide behaves as a partial agonist/antagonist at the mMC3R and as an antagonist at the mMC4R but retains full agonist activity at the mMC1 and mMC5 receptors (Table 1). These data are consistent with the effect of DNal(2′) substitution for DPhe in the cyclic heptapeptide 1 that led to the hypothesis that bulky aromatic amino acid substitutions at the DPhe7 are responsible for differentiating agonist versus antagonist activities of melanocortin ligands at the MC3 and MC4 receptors.26
Incorporation of Halogen Substituents
The effect of halogen substitutents has been previously studied in linear and cyclic melanocortin peptides of different sizes. The incorporation of DPhe(p-I) into the 1 cyclic heptapeptide template led to the potent hMC3R and hMC4R partial agonist/antagonist 3, which possesses full agonist activity at the hMC1R and partial agonist activity at the hMC5R.26 Similarly, incorporation of the DPhe(p-I)7 into the linear 13-amino acid peptide NDP-MSH resulted in 4 with partial agonist and antagonist activity at the mMC3R and mMC4R.27 However, 1 with DPhe(p-F) and DPhe(p-Cl) substitutions retained agonist activity at all cloned human melanocortin receptors.26 Furthermore, substitution of the DPhe residue with DPhe(p-Cl) and DPhe(p-Br) in the linear pentapeptide template Bu-His-DPhe-Arg-Trp-Gly-NH2 resulted in full agonist activity at the hMC1 and hMC4 with similar potency as the parent pentapeptide.38 On the other hand, a pentapeptide with a dichloro-DPhe substitution, Bu-His-DPhe(3,4-diCl)-Arg-Trp-Gly-NH2, showed enhanced binding affinity, but was devoid of any stimulatory activity at up to 50 μM concentration.30
The results obtained for the series of halogenated tetrapeptides studied herein are generally consistent with the previously reported observations. Substitution with either fluorine or chlorine at the para position of the DPhe residue (peptides 13 and 14) resulted in full agonists at the mouse MC1, MC4, and MC5 receptors with slightly increased potency, as compared to the parent peptide 5 (Table 1). Interestingly, upon substitution with bromine, iodine, trifluoromethyl, or 3,4-dichloro in tetrapeptides 15, 16, 17, and 19, respectively, the ligands were converted into partial agonists/antagonists only at mMC3R while retaining agonist potencies similar to peptide 5 at the other mouse melanocortin receptor subtypes (Table 1; Figure 2). The bromine- and iodine-containing peptides displayed notable partial agonist activity (up to 50% maximal response) in addition to competitive antagonism at the mMC3R.
In tetrapeptide 19, the DPhe phenyl ring was substituted simultaneously at the meta and para positions with chlorine. This compound was designed (i) to investigate if a second chlorine substituent, which might enhance the lipophilicity of the molecule and/or decrease electron density of the phenyl ring, is needed to confer antagonist activity at the mMC3R and (ii) to mimic the DNal(2′) substitution of 18, which is an antagonist at both mMC3 and mMC4 receptors.28 Similar to peptides 15–17, tetrapeptide 19 demonstrated partial agonist/antagonist activity only at the mMC3R subtype, while retaining full and equipotent agonist activity at all other mouse melanocortin receptor subtypes (Table 1; Figure 2). To examine if this antagonist activity at the mMC3R is due to a substitution or a steric effect at the phenyl meta position, the DPhe(m-Cl)-containing tetrapeptide 20 was synthesized and characterized. This peptide, however, resulted in a full agonist at all melanocortin receptors, with slightly decreased potency at the mMC3R and mMC5R receptors and almost equipotency at the mMC1R and mMC4R receptors. It can be inferred that double substitution of the phenyl ring is necessary for antagonist activity at the mMC3R for the chlorine-containing tetrapeptides.
It is of note that the p-I-substituted tetrapeptide Ac-His-DPhe(pI)-Arg-Trp-NH2 (16) resulted in a new pharmacological profile and possessed partial agonist/antagonist activity only at the mMC3R while retaining full agonist activity at nanomolar concentrations at the centrally expressed mMC4R as well as the peripherally expressed mMC1R and mMC5R receptors.28 The difference in pharmacology of the tetrapeptide in comparison with 3 and 4 may be attributed to the additional amino acids at the N and C termini in the larger peptides that may add ligand–receptor contact points, modify the peptide secondary structure, and/or constrain the ligand core sequence “His-Phe-Arg-Trp” within the receptor binding pocket. Therefore, further systematic modifications of a pharmacophore tetrapeptide are essential to separate structural requirements imposed solely on the core sequence of melanocortin peptides. This knowledge would facilitate the development of small-molecule peptidomimetics with greater drug potential.
To understand whether the pharmacological properties of the ligands are related to their electrostatic interactions with receptors, we analyzed the distribution of atomic charges on the ligand surface (Figure 3). Comparison of electrostatic surfaces for the tetrapeptide agonists and antagonists indicates that there is no direct correlation between the atomic charges of halogen-containing compounds and their antagonistic properties [compare agonist 14 (Figure 3A) with antagonists 16 and 19 (Figure 3C, E)]. Thus, we suggest that antagonism of bromo-, iodo-, dichloro- and trifluoromethyl-containing tetrapeptides at mMC3R may be attributed to a combination of hydrophobicity and van der Waals interactions with surrounding receptor atoms from the putative hydrophobic ligand binding pocket. Other mechanisms originating from electron-withdrawing properties of halogens and their ability to form charge transfer complexes may also contribute, but to a lesser extent. It is known that in charge transfer complexes halogens serve as acceptors and interact with a donor by transferring electronic charge.39 These interactions may occur in biological systems between halogens and the π-electron clouds of benzene rings as well as between halogens and the lone pair of oxygen, nitrogen, and sulfur and the delocalized π-electrons of peptide bonds of carboxy and amide groups.39 The magnitude of these interactions increases as Cl < Br < I, whereas fluorine is usually not involved.39 Therefore, the antagonism of the CF3-containing tetrapeptide 17 cannot be primarily attributed to charge transfer interactions, but may be largely related to its lipophilic properties.
Figure 3.
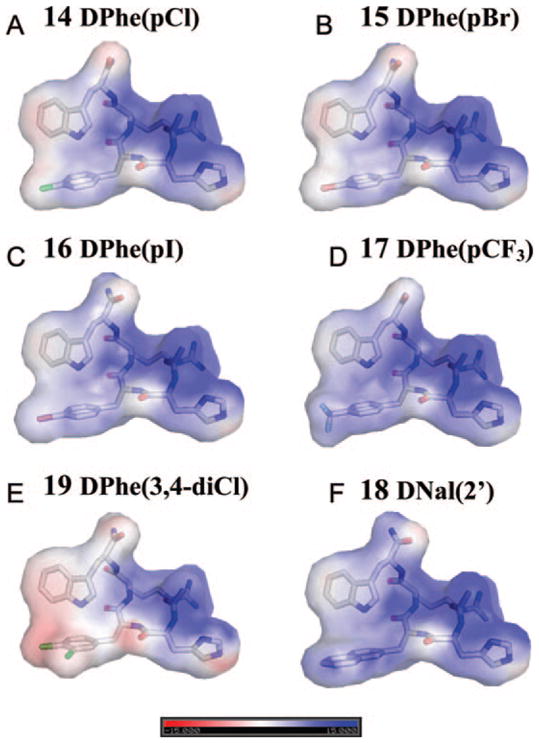
Electrostatic surface representation of tetrapeptide agonist Ac-His-(pI)DPhe-Arg-Trp-NH2 analogue 14 (A) and antagonists tetrapeptides 15–19 (B–F) of MC3 receptor colored by calculated charge from red (−15kT/e) to blue (+15kT/e) using the dielectric constant of 10. Poisson–Boltzmann electrostatic was calculated using the APBS program implemented in PyMOL.
All of the halogen-containing compounds, including tetrapeptide 17, possess high lipophilicity, which increases as F < Cl < Br < CF3 < I < 3,4-diCl. These parameters might be important considering the fact that the DPhe phenyl ring putatively interacts with aromatic and hydrophobic receptor amino acids in the melanocortin receptor binding pocket. On the other hand, it is commonly thought that van der Waals free energies of molecules are proportional to the polarizabilities.40 It has been shown that the electronic polarizability is relatively high for sulfur- and halogen-containing groups and increases as F < Cl < S < Br< I.41 Thus, the increased hydrophobicity of halogen-containing compounds together with stronger dispersion attractions between halogens (particularly –Br and –I) and sulfur-containing receptor residues from transmembrane helices 3–5 may likely play the dominant role in the stabilization of the tightly packed inactive receptor conformation. The unique pharmacological profile of the mMC3R for halogen-containing peptides may be related with tighter packing of residues within the mMC3R binding pocket in comparison with other subtypes of mouse melanocortin receptors. Indeed, peptide 9 containing the bulky hydrophobic substituent p-tBu was devoid of stimulation activity only at mMC3R, suggesting a more constrained geometry of its ligand binding pocket.
The mechanism of antagonism of halogen-containing tetrapeptides at MC3R proposed here differs from the steric hindrance mechanism previously suggested to explain the MC4R antagonism of bulky DNal(2′)-containing analogues.25 This steric mechanism was proposed on the basis of molecular modeling and mutagenesis studies of hMC4R, which indicated that DNal(2′) might hinder side-chain rotation of conserved Trp258 from the transmembrane helix 6 and Leu133 from the transmembrane helix 3 (hMC4R numbering), locking the receptor in the inactive state.37,47
Melanocortin Receptor Selectivity
The study presented here provides a better understanding that substitution at the ligand DPhe phenyl ring is a major contributor in switching receptor function of the mMC3R. Furthermore, modifications of DPhe also result in slight changes in receptor subtype specificities. The unsubstituted parent tetrapeptide 5 used in this study was 50–60-fold selective for the mMC4 and mMC5 receptors over the mMC1 and mMC3 receptors (Table 1). The same pattern of receptor selectivity was maintained for most of the ligands characterized in this study (Table 1). Substitution with the hydrophilic electron-donating groups (–OH and –NH2) did not result in substantial selectivity changes between the receptor subtypes. This observation may be consistent with the hypothesis that all melanocortin receptors possess a common hydrophobic ligand binding site that interacts with the DPhe residue of peptide ligands.27,29–37
Interestingly, substitution with the hydrophilic electron-withdrawing groups (–NO2 and –CN) resulted in increased selectivity for mMC4R and mMC5R over the skin mMC1R. In addition, incorporation of hydrophobic electron-withdrawing halogens (–F and –Cl) at the para position of the DPhe phenyl ring increased the selectivity of the peptides for the mMC4R relative to the mMC1R and mMC3R by 200-fold. Substitution with –Cl at the DPhe meta position increased selectivity by 140-fold only for the MC4R over the mMC3R. In summary, substitutions at the DPhe phenyl ring led to slight changes in receptor subtype specificities, and this knowledge is valuable for further SAR studies of melanocortin ligands.
Conclusions
By using a combination of tetrapeptide ligand SAR studies and melanocortin receptor pharmacology, we have investigated the requirements at the tetrapeptide DPhe7 position for the differentiation of antagonist activity versus agonist activity at the mMC3R. We propose the following hypothesis by which a combination of van der Waals forces, charge transfer interactions, and hydrophobicity might cause antagonism at the mMC3R by halogen-containing tetrapeptides that appears to be distinct from the DNal(2′)7-containing peptides. The results from these studies highlight the differential receptor activation and inactivation mechanism of the mMC3R versus the mMC4R and may be valuable for future drug design aspects and ligand substructural considerations for melanocortin receptor ligands. Additionally, we report new compounds with mixed pharmacology at the mouse MC3 and MC4 receptors (tetrapeptides 15, 17, and 19), which may be more metabolically stable and therefore useful for in vivo studies to differentiate the physiological roles of the MC3 and MC4 receptors.
Experimental Section
Peptide Synthesis
Peptide synthesis was performed using standard Fmoc methodology42 on a semiautomated synthesizer (LabTech, Louisville, KY). The amino acids Fmoc-Tyr(tBu), Fmoc-His(Trt), Fmoc-DPhe, Fmoc-D-p-Cl-Phe-OH, Fmoc-D-p-F-Phe-OH, Fmoc-D-p-Phe(NO2), and Fmoc-Trp(Boc) were purchased from Peptides International (Louisville, KY). Fmoc-p-amino-D-Phe(Boc)-OH, Fmoc-4-bromo-D-Phe-OH, Fmoc-p-Me-D-Phe-OH, Fmoc-p-tBu-D-Phe-OH, Fmoc-4-cyano-D-Phe-OH, Fmoc-p-Bz-D-Phe-OH, and Fmoc-(3,4diCl)DPhe-OH were purchased from Bachem (Torrance, CA). Fmoc-4-iodo-D-phenylalanine, Fmoc-D-3-Cl-Phe-OH, and Fmoc-D-4-trifluoromethylphenylalanine were purchased from Synthetech (Albany, OR). The coupling reagents 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and 1-hydroxybenzotriazole (HOBt) were purchased from Peptides International. Glacial acetic acid (HOAc), dichloromethane (DCM), methanol (MeOH), acetonitrile (ACN), and anhydrous ethyl ether were purchased from Fisher (Fair Lawn, NJ). N,N-Dimethylformamide (DMF) was purchased from Burdick and Jackson (McGaw Park, IL). Trifluoroacetic acid (TFA), pyridine, piperidine, and acetic anhydride were purchased from Sigma (St. Louis, MO). N,N-Diisopropylethylamine (DIEA) and triisopropylsilane (Tis) were purchased from Aldrich (Milwaukee, WI). All reagents and chemicals were of ACS grade or better and were used without further purification.
The peptides were assembled on rink-amide-MBHA resin, purchased from Peptides International. The synthesis was performed using a 16-well Teflon reaction block. Approximately 200 mg of resin (0.1 mmol) was added to each reaction block well. The resin was allowed to swell for 2 h in DCM and deprotected using 20% piperidine in DMF for 2 min followed by 18 min 20% piperidine incubation at 450 rpm. A positive Kaiser test was performed indicating free amine groups on the resin.43 The growing peptide chain was added to the amide-resin using the general amino acid cycle as follows: a 3-fold excess of amino acid (0.1 mmol scale) starting from the C terminus was added, containing the coupling reagents HOBt (0.1 mmol) and HBTU (0.1 mmol) in DMF. Subsequently, 90 μL of DIEA was added and the reaction well volume was brought up to 5 mL using DMF. The coupling reaction was mixed for 2 h at 450 rpm, followed by emptying of the reaction block by vacuum. After the coupling cycle, the reaction block was emptied and the Nα-Fmoc-protected peptide resin was washed with DMF (8 mL, three times). Nα-Fmoc deprotection was performed by the addition of 8 mL of 20% piperidine in DMF and mixed for 2 min at 450 rpm followed by 18 min deprotection at 450 rpm. The reaction well was washed with DMF (8 mL, three times), and the next coupling cycle was performed as described above. Following Nα-Fmoc deprotection of the final amino acid, acetylation of the Nα amine was performed by the addition of 4 mL of acetic anhydride and 2 mL of pyridine to the reaction block wells and mixed for 30 min at 450 rpm. The acetylated peptide resin was washed with DCM (8 mL, five times) and dried thoroughly prior to cleavage from the resin. Deprotection of the amino acid side chains and cleavage of the acetylated-peptide-resin was performed with 8 mL of cleavage cocktail (95% TFA, 2.5% water, and 2.5% Tis) for 2 h at 450 rpm. The cleavage product was emptied from the reaction block into a cleavage block containing the collection vials. The resin was washed with 1.5 mL of cleavage cocktail for 5 min at 450 rpm and added to the previous cleavage solution. The peptides was transferred to 50 mL conical tubes and precipitated with cold (4 °C) anhydrous ethyl ether (up to 50 mL). The flocculent peptide was pelleted by centrifugation (Sorval Super T21 high-speed centrifuge using the swinging bucket rotor) at 4000 rpm for 5 min, the ether was decanted off, and the peptide was washed one time with cold anhydrous ethyl ether and again pelleted. The crude peptide was dried in vacuo for 24 h. A 15–30 mg sample of crude peptide was purified by RP-HPLC using a Shimadzu chromatography system with a photodiode array detector and a semipreparative RP-HPLC C18 bonded silica column (Vydac 218TP1010, 1.0 cm × 25 cm) and lyophilized. The purified peptides were analytically characterized by RP-HPLC using a two-solvent system and mass spectrometry (University of Florida Protein Core Facility). The Supporting Information shows the analytical data of the tetrapeptides synthesized in this study.
Functional Characterization at the Mouse Melanocortin Receptors
For the cAMP response element (CRE)/β-galactosidase reporter gene assays44 the mMC1 and MC3–5 receptor sequences were cloned into the pCDNA3 mammalian expression vector and stably expressed in HEK293 cells. Herby HEK293 cells were transfected with pCDNA3 expression vector containing the corresponding melanocortin receptor DNA (20 μg) using the calcium phosphate method.45 Stable receptor populations were generated using G418 selection method (1 mg/mL) for the subsequent bioassay analysis. HEK293 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% newborn calf serum and plated 1 day prior to transfection at (1–2) × 10−6 cells/100 mm dish.
For the (CRE)/β-galactosidase reporter gene assay, HEK293 cells stably expressing the mouse MC1, or MC3–5 receptors were transfected with 4 μg of CRE/β-galactosidase reporter gene as previously described.28,44,46–48 Twenty-four hours after transfection, 5000–15000 cells were plated into collagen-treated 96-well plates and incubated overnight. Forty-eight hours after transfection, the cells were stimulated with 100 μL of peptide synthesized (10−4–10−12 M) or forskolin (10−4 M) control in assay medium (DMEM containing 0.1 mg/mL BSA and 0.1 mM isobutylmethylxanthine) for 6 h. The antagonistic properties of these compounds were evaluated by the ability of these ligands to competitively displace the 1 agonist in a dose-dependent manner, at up to 10 μM concentrations. After stimulation, the assay medium was aspirated and 50 μL of lysis buffer (250 mM Tris-HCl, pH 8.0, and 0.1% Triton X-100) was added. The plates were stored at −80 °C overnight. The plates containing the cell lysates were thawed the following day. For relative protein determination, aliquots of 10 μL were taken from each well and transferred to another 96-well plate. The relative protein was determined by adding 200 μL of 1:5 dilution Bio-Rad G250 protein dye/water to the 10 μL cell lysate sample, and the OD595 was measured on a 96-well plate reader (Molecular Devices). To the cell lysate plates was added 40 μL of phosphate-buffered saline with 0.5% BSA to each well. Next, 150 μL of substrate buffer [60 mM sodium phosphate, 1 mM MgCl2, 10 mM KCl, 5 mM β-mercaptoethanol, 2 mg/mL of o-nitrophenyl-β-D-galactopyranoside (ONPG)] was added to each well, and the plates were incubated at 37 °C. The sample absorbance, OD405, was measured using a 96-well plate reader (Molecular Devices).
Data Analysis
Data points were normalized both to the relative protein content and receptor independent forskolin control values that indicate the maximal observable stimulation levels in the different cell lines. The assays were performed using duplicate data points and repeated in at least three independent experiments. Data analysis, EC50 and pA2 estimates, and their associated standard errors of the mean49 were determined using the PRISM program (v4.0, GraphPad Inc.). Antagonistic properties were determined by the ability of each of those peptides to competitively displace the 1 agonist in a dose-dependent manner. The pA2 values were generated using the Schild analysis method.50
Visualization of Electrostatic Surfaces of Tetrapeptide Ligands
Electrostatic properties of proteins and peptide ligands can be visualized using the coloring of the solvent-accessible molecularsurfacebyelectrostaticpotential. ThePoisson–Boltzmann equation of electrostatics was calculated using the Adaptive Poisson–Boltzmann Solver (APBS) algorithm implemented into PyMOL.51 Partial atomic charges of unusual amino acids, such as DPhe7 substitutents, which were not amenable to automatic assignment by APBS algorithm, were assigned by QUANTA. The dielectric constant ε = 80 was used for the calculation of the electrostatic potential of proteins, and the dielectric constant ε = 10 was used for the ligands.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants DK57080 (C.H.-L.) and DA03910 (H.I.M.) and an American Diabetes Association Research Award (C.H.-L.).
Footnotes
Abbreviations: ACTH, adrenocorticotropic hormone; AGRP, Agouti-related protein; BAL, backbone amide linker resin; cAMP, cyclic 5′-adenosine monophosphate; DCM, dichloromethane; DMF, N,N-dimethylformamide; DMS, dimethyl sulfide; GPCR, G protein coupled receptor; MC1R, melanocortin-1 receptor; MC2R, melanocortin-2 receptor; MC3R, melanocortin-3 receptor; MC4R, melanocortin-4 receptor; MC5R, melanocortin-5 receptor; MCR, melanocortin receptor; MeOH, methanol; MSH, melanocyte-stimulating hormone; nM, nanomolar; POMC, proopiomelanocortin; SAR, structure–activity relationship; SEM, standard error of the mean; TFA, trifluoroacetic acid; α-MSH, α-melanocyte-stimulating hormone; β-MSH, β-melanocyte-stimulating hormone; γ-MSH, γ-melanocyte-stimulating hormone; μM, micromolar.
Supporting Information Available: Table of analytical data for the peptides synthesized in this study. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- 2.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 3.Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- 4.Lu D, Willard D, Patel IR, Kadwell S, Overton L, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 5.Catania A, Airaghi L, Colombo G, Lipton JM. α-Melanocyte-stimulating hormone in normal human physiology and disease states. Trends Endocrinol Metab. 2000;11:304–308. doi: 10.1016/s1043-2760(00)00296-4. [DOI] [PubMed] [Google Scholar]
- 6.Ni XP, Bhargava A, Pearce D, Humphreys MH. Modulation by dietary sodium intake of melanocortin 3 receptor mRNA and protein abundance in the rat kidney. Am J Physiol Regul Integr Comp Physiol. 2006;290:R560–567. doi: 10.1152/ajpregu.00279.2005. [DOI] [PubMed] [Google Scholar]
- 7.Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of γ-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111:1251–1258. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayan H, Ni XP, Almog S, Humphreys MH. Suppression of γ-melanocyte-stimulating hormone secretion is accompanied by salt-sensitive hypertension in the rat. Hypertension. 2003;42:962–967. doi: 10.1161/01.HYP.0000097601.83235.F8. [DOI] [PubMed] [Google Scholar]
- 9.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 10.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 11.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 12.Mains RE, Eipper BA. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976;251:4115–4120. [PubMed] [Google Scholar]
- 13.Eipper BA, Mains RE. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980;1:1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- 14.Castrucci AM, Hadley ME, Sawyer TK, Wilkes BC, al-Obeidi F, et al. α-melanotropin: the minimal active sequence in the lizard skin bioassay. Gen Comp Endrocrinol. 1989;73:157–163. doi: 10.1016/0016-6480(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 15.Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, et al. α-Melanotropin: the minimal active sequence in the frog skin bioassay. J Med Chem. 1987;30:2126–2130. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 16.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, et al. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 17.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, et al. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- 18.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 19.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, et al. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci US A. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 21.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 22.Ballet S, Mayorov AV, Cai M, Tymecka D, Chandler KB, et al. Novel selective human melanocortin-3 receptor ligands: use of the 4-amino-1,2,4,5-tetrahydro-2-benzazepin-3-one (Aba) scaffold. Bioorg Med Chem Lett. 2007;17:2492–2498. doi: 10.1016/j.bmcl.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. D-Amino acid scan of γ-melanocyte-stimulating hormone: importance of Trp(8) on human MC3 receptor selectivity. J Med Chem. 2000;43:4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 24.Mayorov AV, Cai M, Chandler KB, Petrov RR, Van Scoy AR, et al. Development of cyclic γ-MSH analogues with selective hMC3R agonist and hMC3R/hMC5R antagonist activities. J Med Chem. 2006;49:1946–1952. doi: 10.1021/jm0510326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Obeidi F, Castrucci AM, Hadley ME, Hruby VJ. Potent and prolonged acting cyclic lactam analogues of α-melanotropin: design based on molecular dynamics. J Med Chem. 1989;32:2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 26.Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, et al. Cyclic lactam α-melanotropin analogues of Ac-Nle4-cyclo[Asp5,D-Phe7,Lys10] α-melanocyte-stimulating hormone-(4–10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 27.Haskell-Luevano C, Cone RD, Monck EK, Wan YP. Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: identification of agouti-related protein (AGRP), melanocortin agonist and synthetic peptide antagonist interaction determinants. Biochemistry. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- 28.Holder JR, Bauzo RM, Xiang Z, Haskell-Luevano C. Structure–activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH(2) at the mouse melanocortin receptors. Part 2. Modifications at the Phe position. J Med Chem. 2002;45:3073–3081. doi: 10.1021/jm010524p. [DOI] [PubMed] [Google Scholar]
- 29.Chen M, Aprahamian CJ, Celik A, Georgeson KE, Garvey WT, et al. Molecular characterization of human melanocortin-3 receptor ligand-receptor interaction. Biochemistry. 2006;45:1128–1137. doi: 10.1021/bi0521792. [DOI] [PubMed] [Google Scholar]
- 30.Fleck BA, Chen C, Yang W, Huntley R, Markison S, et al. Molecular interactions of nonpeptide agonists and antagonists with the melanocortin-4 receptor. Biochemistry. 2005;44:14494–14508. doi: 10.1021/bi051316s. [DOI] [PubMed] [Google Scholar]
- 31.Haskell-Luevano C. The Melanocortin Receptors. Humana Press; Totowa, NJ: 2000. In vitro mutagenesis studies of melanocortin receptor coupling and ligand binding; pp. 263–306. [Google Scholar]
- 32.Haskell-Luevano C, Sawyer TK, Trumpp-Kallmeyer S, Bikker JA, Humblet C, et al. Three-dimensional molecular models of the hMC1R melanocortin receptor: complexes with melanotropin peptide agonists. Drug Des Discov. 1996;14:197–211. [PubMed] [Google Scholar]
- 33.Hogan K, Peluso S, Gould S, Parsons I, Ryan D, et al. Mapping the binding site of melanocortin 4 receptor agonists: a hydrophobic pocket formed by i3.28(125), i3.32(129), and i7.42(291) is critical for receptor activation. J Med Chem. 2006;49:911–922. doi: 10.1021/jm050780s. [DOI] [PubMed] [Google Scholar]
- 34.Nickolls SA, Cismowski MI, Wang X, Wolff M, Conlon PJ, et al. Molecular determinants of melanocortin 4 receptor ligand binding and MC4/MC3 receptor selectivity. J Pharmacol Exp Ther. 2003;304:1217–1227. doi: 10.1124/jpet.102.044974. [DOI] [PubMed] [Google Scholar]
- 35.Pogozheva ID, Chai BX, Lomize AL, Fong TM, Weinberg DH, et al. Interactions of human melanocortin 4 receptor with nonpeptide and peptide agonists. Biochemistry. 2005;44:11329–11341. doi: 10.1021/bi0501840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Dickinson C, Haskell-Luevano C, Gantz I. Molecular basis for the interaction of [Nle4,D-Phe7]melanocyte stimulating hormone with the human melanocortin-1 receptor. J Biol Chem. 1997;272:23000–23010. doi: 10.1074/jbc.272.37.23000. [DOI] [PubMed] [Google Scholar]
- 37.Yang YK, Fong TM, Dickinson CJ, Mao C, Li JY, et al. Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochemistry. 2000;39:14900–14911. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- 38.Danho W, Swistok J, Cheung AW, Kurylko G, Franco L, et al. Structure-activity relationship of linear peptide Bu-His6-DPhe7-Arg8-Trp9-Gly10-NH2 at the human melanocortin-1 and -4 receptors: DPhe7 and Trp9 substitution. Bioorg Med Chem Lett. 2003;13:649–652. doi: 10.1016/s0960-894x(02)01052-1. [DOI] [PubMed] [Google Scholar]
- 39.Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. 5. Wiley Interscience; New York: 1988. [Google Scholar]
- 40.McLachlan AD. Three-body dispersion forces. Mol Phys. 1963;6:423–427. [Google Scholar]
- 41.Miller KJ. Additivity methods in molecular polarizability. J Am Chem Soc. 1990;112:8533–8542. [Google Scholar]
- 42.Carpino LA, Han GY. 9-Fluorenylmethoxycarbonyl amino-protecting group. J Org Chem. 1972;37:3404–3409. [Google Scholar]
- 43.Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 44.Chen W, Shields TS, Stork PJ, Cone RD. A colorimetric assay for measuring activation of Gs- and Gq-coupled signaling pathways. Anal Biochem. 1995;226:349–354. doi: 10.1006/abio.1995.1235. [DOI] [PubMed] [Google Scholar]
- 45.Chen CA, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- 46.Holder JR, Bauzo RM, Xiang Z, Haskell-Luevano C. Structure-activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH(2) at the mouse melanocortin receptors. 1. Modifications at the His position. J Med Chem. 2002;45:2801–2810. doi: 10.1021/jm0104872. [DOI] [PubMed] [Google Scholar]
- 47.Holder JR, Xiang Z, Bauzo RM, Haskell-Luevano C. Structure-activity relationships of the melanocortin tetrapeptide Ac-His-D-Phe-Arg-Trp-NH2 at the mouse melanocortin receptors. 4. Modifications at the Trp position. J Med Chem. 2002;45:5736–5744. doi: 10.1021/jm020296e. [DOI] [PubMed] [Google Scholar]
- 48.Holder JR, Xiang Z, Bauzo RM, Haskell-Luevano C. Structure–activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the mouse melanocortin receptors. Part 3: modifications at the Arg position. Peptides. 2003;24:73–82. doi: 10.1016/s0196-9781(02)00278-4. [DOI] [PubMed] [Google Scholar]
- 49.Ott RL, Longnecker M. An Introduction to Statistical Methods and Data Analysis. 5. Duxbury; Pacific Grove, CA: 2001. [Google Scholar]
- 50.Schild HO. Pa, a new scale for the measurement of drug antagonism. Br J Pharmacol Chemother. 1947;2:189–206. doi: 10.1111/j.1476-5381.1947.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci US A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.