Abstract
The endothelins and their G protein-coupled receptors A and B have been implicated innumerous diseases and have recently emerged as pivotal players in a variety of malignancies. Tumors over-express the endothelin 1 (ET-1) ligand and the endothelin-A-receptor (ETAR). Their interaction induces tumor growth and metastasis by promoting tumor cell survival and proliferation, angiogenesis, and tissue remodeling. On the basis of results from xenograft models, drug development efforts have focused on antagonizing the autocrine-paracrine effects mediated by ET-1/ETAR. In this review, we discuss a novel role of the endothelin-B-receptor (ETBR) in tumorigenesis and the effect of its blockade during cancer immune therapy. We highlight key characteristics of the B receptor such as its specific overexpression in the tumor compartment; and specifically, in the tumor endothelium, where its activation by ET-1 suppresses T-cell adhesion and homing to tumors. We also review our recent findings on the effects of ETBR-specific blockade in increasing T-cell homing to tumors and enhancing the efficacy of otherwise ineffective immunotherapy.
Background
The endothelin system
The endothelin system comprises four endothelin (ET) peptide ligands, ET-1, 2, 3 (1), and the more recently discovered ET-4 (2); their two G protein-coupled receptors (GPCR), ETAR (3) and ETBR (4); and the endothelin-converting enzymes (ECEs), which catalyze the generation of the biologically active ETs. ETs derive from precursor proteins after cleavage by membrane-bound metalloproteinase ECEs (5) and are well known for their overall vasoconstricting activity. Among them, ET-1 is the most potent ligand and the most widely expressed in endothelial cells (6). The endothelin peptides exert their function through binding to their cognate receptors A and B, whereby they trigger divergent intracellular effects by activating numerous downstream signaling pathways. Members of the endothelin system have been identified in neuronal, renal, and vascular tissues, and their involvement has been well documented in an array of physiological processes such as embryonic development, reproduction, angiogenesis, and cardiovascular homeostasis (4, 7–9).
Role of the endothelin system in disease
The role of the endothelin system has been well characterized in cardiovascular and renal disorders (10–13). ET-1 is produced by endothelial cells and exerts autocrine-paracrine functions by binding to ETAR and ETBR on vascular endothelial cells and pericytes. Balanced activation of the two receptors maintains vascular tone and regulates endothelial cell proliferation (14, 15), whereas imbalance in this system contributes to the onset of hemodynamic disorders. The same applies to the renal vasculature, in which endothelins play a major role in maintaining normal vascular tone through both the A (13, 16) and B receptor (17). Endothelins and their receptors have also been implicated in pulmonary hypertension (18), asthma (19), and pulmonary fibrosis. ET-1 immunostaining was detected in normal lung epithelium and vasculature (20). ETAR is found on vascular and airway smooth muscle, whereas ETBR is mostly often found on the endothelium and smooth muscle cells. Activation of both A and B receptors on lung smooth muscle cells results in vasoconstriction, whereas ETBR activation alone leads to bronchoconstriction (21).
ETAR and ETBR are also involved in inflammatory processes. Both ETAR and ETBR expression in bronchial smooth muscle cells is increased upon experimentally induced airway inflammation (22). ETAR activation is also required for endotoxin-induced inflammation (23) or T-cell homing to the lungs after allergenic or inflammatory stimuli, whereas experimental airway inflammation is abrogated by ETAR inhibition (24, 25). The role of the endothelin axis in inflammation extends beyond the respiratory tract. ETAR activation mediates renal inflammation and transforming growth factor-β (TGF-β) production in diabetes (26). Owing to its proinflammatory properties (27, 28), ET-1 contributes to the progression of various diseases like glomerulosclerosis and atherosclerosis and the pathogenesis of autoimmune diseases such as scleroderma and lupus erythematosus (29, 30). Importantly, ET-1 is synthesized by lymphocytes and other leukocytes, and has been shown to activate the proinflammatory transcriptional factor nuclear factor-κB (NF-κB) in human monocytes via ETBR and to stimulate the production of inflammatory interleukins and tumor necrosis factor-α (TNF-β) (ref. 31). ET-1 is also a chemoattractant for monocytes in vitro, via stimulation of IL-8/CXCL8 and monocyte chemoattractant protein-1 (MCP-1)/CCL2 (32, 33).
Role of the endothelin system in cancer
Endothelin 1
The role of the endothelin system in cancer has been reviewed extensively by Bagnato and colleagues (34) and others in the field. Kusuhara and colleagues were among the first to report ET-1 overexpression by breast, colon, stomach, prostate, and glioblastoma cell lines (35, 36). Ovarian, neuroblastoma, and human papilloma virus (HPV)-positive human cervical carcinoma cell lines also overexpress ET-1 (37, 38), whereas increased immunopositivity for ET-1 was detected in vivo in human colorectal cancer (39). Compiling clinical evidence shows elevated plasma ET-1 levels in patients diagnosed with various solid tumors, including hepatocellular, gastric, and prostate cancer (40–42). Interestingly, condensed breath of patients with non small cell lung carcinoma (NSCLC) showed increased ET-1 levels (43), proposing ET-1 as an early detection marker (44). Finally, in ovarian carcinoma, high ET-1 levels were detected in ascites (45). In summary, the endothelin 1 ligand is overexpressed by many tumors.
Strong evidence suggests a role for members of the endothelin system in the growth and progression of multiple tumors. Exogenous addition of ET-1 to a range of cell lines promotes various aspects of tumorigenesis. In prostate cancer cell lines, ET-1 increased survival and proliferation (42, 46). Exposure of breast cancer cells to ET-1 led to invasive phenotype, which involved matrix metalloproteinase (MMP) activity (47). The same mechanism occurred in osteosarcoma, in which ET-1 was shown to promote MMP-2 and MMP-9 induction (48). Lastly, in colon cancer ET-1 overexpression was shown to rescue cancer cells from apoptosis and growth arrest by promoting the oncogene β-catenin (49).
ETAR
The effects of ET-1 on cancer cells are mostly mediated by ETAR. Importantly, ETAR was shown to be overexpressed in renal and cervical cancer cell lines (50, 51) as well as several cancer types in vivo including colorectal, bladder, prostate, and nasopharyngeal carcinomas (46, 52–55). ETAR is also overexpressed in approximately 85% of primary and metastatic ovarian carcinomas. In this study, all ovarian carcinoma-derived cell lines were positive for both ET-1 and ETAR mRNA (56). Concomitant up-regulation of ET-1 and ETAR on tumor cells contributes to cell autonomy and malignant progression and triggers complex pathways driving tumorigenesis, including cell proliferation, inhibition of apoptosis, matrix remodeling, invasion, and metastatic dissemination (57). ET-1/ETAR also increases invasion and migration of tumor cells through downstream effects on MMPs, cadherins, connexins, and integrins. Increased cell proliferation is mediated by increased Ca2+ uptake and activation of the PKC, PLC, MAPK, and AKT pathways. ET-1/ETAR interaction can also activate the AKT and the NF-?B pathways to promote tumor cell survival (34).
The mitogenic activity of ET-1 can also be augmented by growth factors (57). One example is the cross-signaling between ETAR and the epidermal growth factor receptor (EGFR) (ref. 34). EGFR has been identified as a downstream mediator of ETA-receptor activation by ET-1 in ovarian cancer (58). The mechanism is triggered by ET-1, which causes EGFR transactivation. This event leads to activation of the RAS/MAPK pathway and AKT activation through the formation of Shc/Grb-2 complexes (45, 58), subsequently contributing to the mitogenic signaling induced by ET-1. This cross-signaling between the EGFR and ETAR pathways provides the rationale for combining EGFR inhibitors with ETAR antagonists to treat ovarian carcinoma. It has been shown that ZD4054, a specific ETAR antagonist, reduces ET-1-induced EGFR transactivation, whereas the EGFR inhibitor gefitinib significantly inhibited EGF and ET-1 induced EGFR phosphorylation (59). This drug combination simultaneously disables multiple signaling pathways, offering improvements in ovarian carcinoma treatment (59).
ET-1 increases the expression of cyclooxygenase (COX)-1 and COX-2, prostaglandin (PG)E2, and VEGF production by ovarian cancer cells via ETAR activation (60). The effect of ET-1 on VEGF expression is mediated through HIF-1α (61). Elevated expression of ET-1 has been associated with increased VEGF expression, lymphatic vessel invasion, and unfavorable outcome in invasive ductal breast carcinoma (62). A correlation between ET-1 expression and VEGF expression has also been shown in lung cancer (63). Additionally, inhibition of human ovarian tumor growth in nude mice after treatment with the potent ETAR -selective antagonist ABT-627 was associated with reduced COX-2 and VEGF expression by the tumor (60). Thus, over-expression of the ligand ET-1 and its receptor, ETAR, account for autocrine-paracrine activation of the endothelin axis in many solid tumors, which plays important and multifaceted roles in tumor cell progression. The ETAR is therefore a very attractive target for cancer therapy.
ETBR
Investigation of the role of ET-1/ETBR in tumor cell biology has been more limited. In normal cells, ETBR counter-regulates ET-1/ETAR activity through multiple mechanisms including increasing production of nitric oxide, promoting ET-1 clearance, triggering apoptotic pathways, and blocking cell growth; but it is unclear whether such antagonism also operates in tumor cells (64). Interestingly, ETBR is overexpressed and correlates with melanoma development and progression. Expression profiling of human melanoma biopsies indicated ETBR overexpression to be associated with aggressive tumor phenotype (65), and ETBR was proposed as tumor progression marker (66). Underscoring the role of ETBR in melanoma growth, the specific antagonist BQ-788 was found to inhibit the growth of human melanoma cell lines and to reduce human melanoma tumor growth in a nude mouse model (67, 68). The B receptor is also expressed in Kaposi’s sarcoma and glioblastoma (69–71).
The role of ETBR in cancer angiogenesis has been thoroughly investigated and reviewed by Bagnato and colleagues (34). ET-1 has been shown to directly promote tumor angiogenesis by inducing endothelial cell survival, proliferation, and invasion through ETBR (61). ET-1 promotes angiogenesis also indirectly, by upregulating VEGF production in the vasculature, also through ETBR activation (72), and increases vascular permeability through VEGF in response to tissue hypoxia (73). Furthermore, ET-1 upregulates expression of the extra domain-B containing fibronectin (EDB+ FN) in human vascular endothelial cells (74, 75). EDB+ FN is a recently proposed marker of angiogenesis expressed in human cancers and in ocular neovascularization in patients with proliferative diabetic retinopathy. There is a strong correlation between ETBR and VEGF expression in a number of different tumor specimens (76).
Clinical-Translational Advances
ETAR and ETBR antagonists in cancer therapy
ETAR and ETBR represent interesting targets for cancer chemoprevention and therapy. Many receptor antagonists have been developed and undergone preclinical and clinical testing. Some compounds have preferential A or B receptor inhibitory activity, whereas others exhibit mixed A and B antagonism. Given the prominent role of ETAR in tumor cell biology, ETAR -selective antagonists have been developed more extensively than ETBR antagonists to treat malignancy (see Table 1). The first ETAR -selective peptide antagonist, BQ-123 (77), was shown to inhibit cervical cancer growth in preclinical models (78). Furthermore, nonpeptide ETAR antagonists such as Atrasentan, ZD4054, and YM598 have been shown to have a static effect on ovarian tumor growth in xenograft models (79); to delay progression of prostate cancer (80, 81); and to attenuate growth and metastasis of human gastric carcinoma (82). ETAR inhibitors are currently undergoing clinical testing for various cancer indications. A list of endothelin antagonists under preclinical or clinical development for cancer and various other indications is provided in Table 1. Notably, phase II results with Atrasentan in hormone refractory prostate cancer (HRPC) were encouraging (83). However, subsequent phase III trials in metastatic and nonmetastatic HRPC showed no significant therapeutic effects despite evidence of biologic effects on serum markers of disease burden (84, 85). Large geographic differences in the median time to progression were also noted; U.S. patients showed less gain in time to progression relative to non-U.S. patients (85). Given the role of ETBR in melanoma cells, ETBR antagonists have been tested in melanoma, in which they proved efficacious (67, 68). Interestingly, IRL1620, an ETBR agonist, was shown to improve both delivery and therapeutic efficacy of paclitaxel in breast tumor-bearing mice (86).
Table 1.
Endothelin targeting agents and their indications
| Compound | Target | Dosing | Clinical testing-indication | Phase | Cancer preclinical testing | Company |
|---|---|---|---|---|---|---|
| Atrasentan | ETAR | Oral | Prostate cancer | II–III | 51, 79, 80 | Abbott |
| Renal carcinoma | II | |||||
| Ovarian cancer | II | |||||
| FT cancer | II | |||||
| Brain tumors | I | |||||
| NSCLC | III–IV | |||||
| Malignant glioma | I | |||||
| ED | III | |||||
| HT | II | |||||
| CVD | Approved | |||||
| Ambrisentan | ETAR | Oral | PAH | Approved | — | Gilead Sciences |
| IPF | II | |||||
| USS | I | |||||
| Avosentan | ETAR | Oral | CVD | II | — | Speedel |
| DN | I–II | |||||
| BQ123 | ETAR | IV | HI | I | 36, 108, 109 | Merck Biosciences |
| PAH | II | |||||
| MI | II | |||||
| CKD | I | |||||
| HT | I | |||||
| Clazosentan | ETAR | IV | SAH | Approved | — | Actelion |
| Darusentan | ETAR | Oral | HT | III | — | Gilead Sciences |
| CAD | II | |||||
| ED | II | |||||
| Edonentan | ETAR | Oral | CHF | II | — | Bristol-Myers Squibb |
| END | II | |||||
| Sitaxsentan | ETAR | IV-Oral | PAH | III | — | Encysive Pharmaceuticals/ICOS Texas Biotech |
| CHF | II | |||||
| S-0139 | ETAR | Oral | CHF | II | — | Shionogi-GSK |
| TBC-3711 | ETAR | Oral | HT | II | — | Encysive Pharmaceuticals |
| YM598 | ETAR | Oral | Prostate | II | 82, 110 | Astellas Pharma US |
| ZD4054 | ETAR | Oral | Prostate cancer | III | 111, 112 | AstraZeneca |
| Bone metastasis | II | |||||
| NSCLC | II | |||||
| BQ788 | ETBR | IV | CVD | I | 67, 69, 113, 114 | Merck Biosciences |
| IRL-1620 | ETBR agonist | IV | — | — | 86, 99 | Sigma |
| SPI-1620 | ETBR agonist | IV | Carcinoma | I | — | Spectrum Pharmaceuticals |
| Short cervix | III | |||||
| Enrasentan | ETAR/ETBR | Oral | AHF | III | — | GlaxoSmithKline |
| Bosentan | ETAR/ETBR | Oral | Metastatic melanoma | II | 115, 116 | Actelion |
| HT | II | |||||
| PAH | Approved | |||||
| J104132 | ETAR/ETBR | Oral | CHF-HT | II | — | Banyu/Merck |
Abbreviations: AHF, acute heart failure; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; CVD, cardiovascular diseases; DN, diabetic nephropathy; ED, erectile dysfunction; END, endothelial dysfunction; FT, fallopian tube; HD, heart disease; HI, hyperinsulinemia; HT, hypertension; IPF, idiopathic pulmonary fibrosis, MD, microvascular dysfunction, MI, myocardial infarction; PAH, pulmonary arterial hypertension; PC, prostate cancer, PH, pulmonary hypertension; SAH, subarachnoid hemorrhage; USS, ulcer scleroderma, systemic.
ETBR and the tumor endothelial barrier to T-cell homing
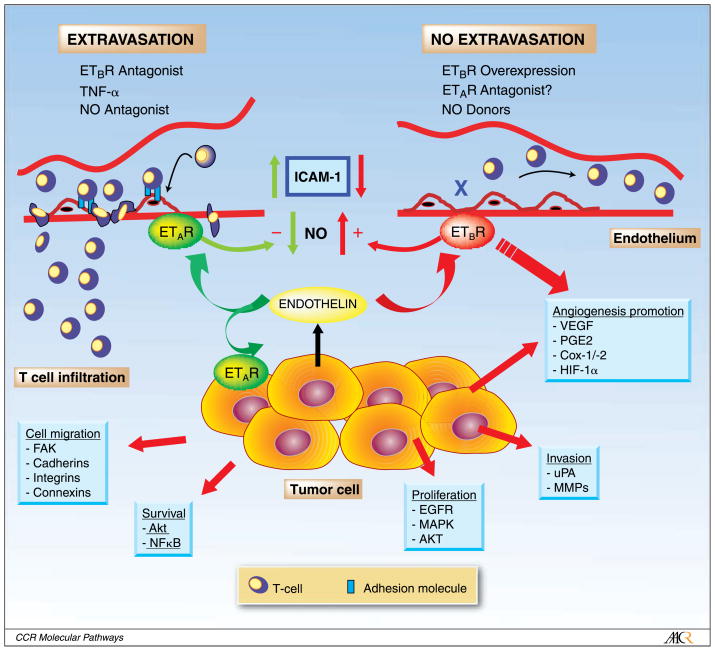
The focus of cancer therapy targeting ET-1 to date has been to antagonize the autocrine-paracrine effects of ET-1 on tumor cells, mediated mainly by ETAR. Our laboratory recently showed a novel application of ETBR blockade in tumor therapy. Specifically, ETBR blockade at the tumor endothelium proved to be therapeutically efficient for tumor immune therapy (Fig. 1) (ref. 87). The success of immune therapy depends on the ability of effector T cells to infiltrate tumors. Although current tumor vaccines have proven effective in producing an antitumor immune response as measured by blood assays, they have fallen short of clinical expectations. Endothelium is a crucial controller of T-cell trafficking in homeostasis, autoimmunity, and transplantation in humans. We showed that the endothelial barrier also exists in tumors and is partly mediated by ETBR.
Fig. 1.
Control of T-cell homing to tumors by endothelin 1 expressed by tumor cells. Opposite paracrine effects are exerted by endothelin on tumor endothelium through ETAR and ETBR. The balance between ETAR and ETBR determines the fate of antitumor T-cell response. Overexpression of ETBR in tumor endothelium results in increased angiogenesis but also suppression of ICAM-1 expression on endothelial cells, and inhibition of T-cell transendothelial migration and homing. This effect is largely mediated by NO. NO donors and possibly ETAR inhibitors exert similar effects. ETBR blockade results in increased expression of endothelial ICAM-1 and increased binding and transendothelial migration of T cells to the tumor. NO antagonists and TNF-α exert similar effects. Additional tumor-promoting autocrine effects of endothelin are established by binding to ETAR on tumor cells, resulting in enhanced tumor cell survival, proliferation, migration, and invasion. Each of these effects entail multiple signaling pathways.
This mechanism was uncovered in human ovarian cancer, in which endothelial cells were microdissected from tumors with brisk tumor-infiltrating lymphocytes (TILs) and tumors lacking TILs, to examine differences in their molecular profile. ETBR emerged as one of the few genes overexpressed in tumor endothelial cells from tumors lacking TILs by Affymetrix array analysis (87). ETBR was mostly localized to the endothelium and some stroma cells by immunostaining in human ovarian cancers. ETBR mRNA or protein overexpression was associated with absence or paucity of TILs, especially of intraepithelial (also called intratumoral) T cells (87). These are T cells infiltrating the epithelial component of the tumor (tumor islets), which predict longer survival in ovarian cancer (88). ET-1 is overexpressed in ovarian cancer cells (34), and it was found that ET-1 mRNA was significantly higher in microdissected (cytokeratin-positive) tumor cells from tumors lacking TILs relative to tumors with brisk TILs. Thus, the entire ET-1/ETBR (tumor-endothelial) paracrine axis seems upregulated in ovarian cancers lacking TILs. Importantly, we showed that recombinant human ET-1 blocks adhesion of activated T cells to human umbilical vein endothelial cells in vitro. These results establish a vascular mechanism of tumor immune evasion mediated by the endothelin system (87).
TNF-α is a major inflammatory cytokine implicated in carcinogenesis, tumor angiogenesis, and progression, and it is up-regulated in ovarian cancer (89). It has been previously reported that the overall TNF-α mRNA levels are similar in ovarian tumors with or without intraepithelial T cells (88). This was counterintuitive, as TNF-α is a major factor activating endothelium and promoting adhesion of T cells. It has been now found that ET-1 efficiently blocks adhesion of T cells to endothelial cells even when endothelial cells are activated with TNF-α (87). This observation explains the paradox of how tumors may exhibit inflammation yet be prohibitive to T-cell infiltration, thus establishing immune privilege even in the face of inflammation.
ET-1 was found to abrogate T-cell adhesion to endothelium via ETBR and through suppression of endothelial intercellular adhesion molecule-1 (ICAM-1) expression at base line as well as following endothelial activation with TNF-α. Furthermore, it was found that ETBR -induced suppression of ICAM-1 expression and surface clustering was mediated by nitric oxide (NO). ETBR blockade with the selective antagonist BQ-788 upregulated endothelial ICAM-1 expression, promoted ICAM-1 clustering at the cell surface, and restored adhesion of T cells to ET-1-treated endothelial cells. ICAM-1 neutralizing antibody abrogated the effect of ETBR blockade to promote T-cell adhesion to endothelium in vitro (87). These observations indicate that the endothelin system is crucial for controlling lymphocyte homing in tumors and that endothelial ETBR overexpression, which can sway the vascular ETAR/ETBR balance toward ETBR hyperactivity, results in suppression of T-cell homing. This evidence is substantiated by complementary data in lung inflammation; ETAR activation is required for endotoxin-induced inflammation (23), whereas T-cell homing to lungs in response to an inflammatory stimulus is abrogated by ETAR blockade (24, 25). Thus, vascular ETAR activation results in increased T-cell homing, whereas increased ETBR signaling facilitates immune privileged status.
ETBR blockade in cancer immune therapy
To test the activity of ETBR in controlling T-cell homing to tumors and the effects of its blockade in vivo in the context of immunotherapy, vaccine approaches that have no efficacy in delaying tumor growth were used. It was found that vaccine failure was associated with poor accumulation of T cells at the tumor site, in spite of detectable systemic antitumor immune response. ETBR blockade with specific antagonist BQ-788 greatly enhanced the efficacy of prevention and therapeutic vaccines. BQ-788 did not increase systemic immune response to the vaccine in vivo, but rather greatly enhanced T-cell infiltration in tumors following vaccine (87). This was attenuated by ICAM-1 neutralizing antibody, confirming the requirement for adhesive interactions mediated by ICAM-1 following ETBR blockade in vivo. Furthermore, BQ-788 markedly increased homing of T-cells to tumors after adoptive transfer in mice. Thus, in many tumors there is hyperactivation of a paracrine ET-1/ETBR axis established between tumor cells and endothelium, whereby tumor cells overexpress and release ET-1 whereas the tumor endothelium overexpresses ETBR. This axis tonically suppresses T-cell homing (even in the presence of tumor inflammation), and can be disrupted by ETBR blockade, which in vivo markedly enhances tumor immune therapy (87). This mechanism may not be unique to ovarian cancer. For example, ETBR is also overexpressed in breast cancer vasculature (86). Interestingly, ETBR upregulation predicts poor outcome both in breast and ovarian cancer (47, 90). The mechanisms underlying ETBR overexpression in tumor endothelium are not fully understood, but VEGF may be implicated (91, 92).
Our results argue that ETBR antagonists warrant testing in combination with passive or adoptive immunotherapy. There are unique features that render ETBR blockade an attractive strategy in cancer immunotherapy. First, as outlined above, the axis ET-1/ETBR seems to be selectively upregulated in the tumor compartment but not in normal tissues. Indeed, in mouse experiments, ETBR blockade by BQ-788 did not result in systemic inflammation or illness, and frequency of CD45+ lymphocytes or CD3+ T cells in liver, spleen, lungs, or kidneys after vaccine or adoptive T-cell transfer was not affected by BQ-788 (87). This is in contrast to current immunomodulatory approaches, which achieve systemic activation of effector cells by attenuating peripheral tolerance or other homeostatic checkpoint mechanisms and can result in significant autoimmune toxicity (93). Second, ETBR -selective antagonists, including BQ-788, have been tested in humans and are well tolerated even in patients with cardiovascular disease (94–96). Thus, ETBR can be pharmacologically perturbed with existing drugs to enhance the efficacy of immune therapy. Third, ETBR blockade is likely to have also direct antiangiogenic effects through suppression of endothelial nitric oxide. Unlike in patients with sepsis (97), NO inhibition is safe and has been well tolerated in cancer patients (98). Although the anticancer effect of ETBR (or NO) blockade as monotherapy may be modest, the concomitant administration of immunotherapy may act synergistically against angiogenesis (86, 99).
Implications for pure ETAR antagonists
Currently, on the basis of results obtained mostly with xenograft tumor models in immunodeficient mice, cancer therapy targeting ET is focused on ETAR blockade. However, previous evidence shows that ETAR signaling is required for T-cell homing (24, 25), whereas our work indicates that increased ETBR activity in tumor endothelium results in reduced T-cell homing and ETBR blockade is required to improve T-cell homing to tumors. Because of the tonic antagonism between ETAR and ETBR signaling in the vasculature, pharmacologic ETAR blockade could tilt the balance toward increased ETBR signaling in the tumor vasculature. This could possibly result in increased angiogenesis and, on the basis of our work, could suppress T-cell homing to tumors. It has been previously shown that patients with ovarian cancer whose tumors are infiltrated by intraepithelial T cells survive longer (88), a concept validated by several groups (100–103). Similar observations were made in other solid tumors. In colon cancer, tumor-infiltrating T cells predict survival better than conventional anatomical staging (104), whereas in prostate cancer TIL represent a strong independent prognosticator of longer survival (105). Although the function of tumor-infiltrating T cells is not fully understood, it is possible that they contribute to controlling tumor growth during or after conventional cancer therapy. For example, the long-term therapeutic effects of VEGF receptor 2 blockade, a major antiangiogenic pharmacologic intervention, were fully depended on CD8+ T cell infiltration in tumors (106). Furthermore, conventional chemotherapy agents have immunomodulatory effects and their long term efficacy may depend in part on immune effector mechanisms (107). If this were the case, ETAR blockade alone might increase ETBR signaling and reduce T-cell infiltration in tumors. This could negate some of the potential efficacy of cancer therapies and explain in part the failure of pure ETAR antagonists to produce significant clinical results in tumors in which TIL may affect survival. Our results argue that ETAR/ETBR mixed antagonists might offer the advantage of simultaneously targeting the tumor cell (through ETAR) and enhancing antitumor immune mechanisms (through vascular ETBR) and should be the focus of future therapy.
Acknowledgments
Grant support: National Institutes of Health (NIH) NIHR01-CA098951; NIHP50-CA083638 Ovarian Cancer SPORE; and NIHR01-CA112162. AF was supported by the National Ovarian Cancer Coalition. RJB was supported by NIH/NICHD K12-HD43459 Career Development in Women’s Health Research, and the Ovarian Cancer Research Fund.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Yanagisawa M, Masaki T. Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci. 1989;10:374–8. doi: 10.1016/0165-6147(89)90011–4. [DOI] [PubMed] [Google Scholar]
- 2.Saida K, Mitsui Y, Ishida N. A novel peptide, vasoactive intestinal contractor, of a new (endothelin) peptide family. Molecular cloning, expression, and biological activity. J Biol Chem. 1989;264:14613–6. [PubMed] [Google Scholar]
- 3.Frommer KW, Muller-Ladner U. Expression and function of ETA and ETB receptors in SSc. Rheumatology (Oxford) 2008;47(Suppl 5):27–8. doi: 10.1093/rheumatology/ken274. [DOI] [PubMed] [Google Scholar]
- 4.Meidan R, Levy N. The ovarian endothelin network: an evolving story. Trends Endocrinol Metab. 2007;18:379–85. doi: 10.1016/j tem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Valdenaire O, Rohrbacher E, Mattei MG. Organization of the gene encoding the human endothelin-converting enzyme (ECE-1) J Biol Chem. 1995;270:29794–8. doi: 10.1074/jbc.270.50.29794. [DOI] [PubMed] [Google Scholar]
- 6.Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102:2434–40. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 7.Grant K, Loizidou M, Taylor I. Endothelin-1: a multi-functional molecule in cancer. Br J Cancer. 2003;88:163–6. doi: 10.1038/sj bjc.6700750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–76. doi: 10.1146/annurev pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 9.Yanagisawa H, Hammer RE, Richardson JA, Williams SC, Clouthier DE, Yanagisawa M. Role of Endothelin-1/Endothelin-A receptor-mediated signaling pathway in the aortic arch patterning in mice. J Clin Invest. 1998;102:22–33. doi: 10.1172/JCI2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldstein C, Romero C. Role of endothelins in hypertension. Am J Ther. 2007;14:147–53. doi: 10.1097/01.pap.0000249912.02763.65. [DOI] [PubMed] [Google Scholar]
- 11.Iglarz M, Clozel M. Mechanisms of ET-1-induced endothelial dysfunction. J Cardiovasc Pharmacol. 2007;50:621–8. doi: 10.1097/FJC.0b013e31813c6cc3. [DOI] [PubMed] [Google Scholar]
- 12.Lehrke I, Waldherr R, Ritz E, Wagner J. Renal endothelin-1 and endothelin receptor type B expression in glomerular diseases with proteinuria. J Am Soc Nephrol. 2001;12:2321–9. doi: 10.1681/ASN.V12112321. [DOI] [PubMed] [Google Scholar]
- 13.Tomobe Y, Miyauchi T, Saito A, et al. Effects of endothelin on the renal artery from spontaneously hypertensive and Wistar Kyoto rats. Eur J Pharmacol. 1988;152:373–4. doi: 10.1016/0014-2999(88)90736–4. [DOI] [PubMed] [Google Scholar]
- 14.Morbidelli L, Orlando C, Maggi CA, Ledda F, Ziche M. Proliferation and migration of endothelial cells is promoted by endothelins via activation of ETB receptors. Am J Physiol. 1995;269:H686–95. doi: 10.1152/ajpheart.1995.269.2.H686. [DOI] [PubMed] [Google Scholar]
- 15.Ziche M, Morbidelli L, Donnini S, Ledda F. ETB receptors promote proliferation and migration of endothelial cells. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S284–6. [PubMed] [Google Scholar]
- 16.Katoh T, Chang H, Uchida S, Okuda T, Kurokawa K. Direct effects of endothelin in the rat kidney. Am J Physiol. 1990;258:F397–402. doi: 10.1152/ajprenal.1990.258.2.F397. [DOI] [PubMed] [Google Scholar]
- 17.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest. 2000;105:925–33. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–9. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 19.Adamicza A, Petak F, Asztalos T, Hantos Z. Effects of endothelin-1 on airway and parenchymal mechanics in guinea-pigs. Eur Respir J. 1999;13:767–74. doi: 10.1034/j.1399-3003.1999.13d12.x. [DOI] [PubMed] [Google Scholar]
- 20.Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir Res. 2001;2:90–101. doi: 10.1186/rr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagase T, Aoki T, Oka T, Fukuchi Y, Ouchi Y. ET-1-induced bronchoconstriction is mediated via ETB receptor in mice. J Appl Physiol. 1997;83:46–51. doi: 10.1152/jappl.1997.83.1.46. [DOI] [PubMed] [Google Scholar]
- 22.Granstrom BW, Xu CB, Nilsson E, Bengtsson UH, Edvinsson L. Up-regulation of endothelin receptor function and mRNA expression in airway smooth muscle cells following Sephadex-induced airway inflammation. Basic Clin Pharmacol Toxicol. 2004;95:43–8. doi: 10.1111/j.1742-7843.2004.pto950109.x. [DOI] [PubMed] [Google Scholar]
- 23.Wanecek M, Weitzberg E, Rudehill A, Oldner A. The endothelin system in septic and endotoxin shock. Eur J Pharmacol. 2000;407:1–15. doi: 10.1016/S0014-2999(00)00675-0. [DOI] [PubMed] [Google Scholar]
- 24.Sampaio AL, Rae GA, Henriques MG. Effects of endothelin ETA receptor antagonism on granulocyte and lymphocyte accumulation in LPS-induced inflammation. J Leukoc Biol. 2004;76:210–6. doi: 10.1189/jlb.1003504. [DOI] [PubMed] [Google Scholar]
- 25.Sampaio AL, Rae GA, Henriques MM. Role of endothelins on lymphocyte accumulation in allergic pleurisy. J Leukoc Biol. 2000;67:189–95. doi: 10.1002/jlb.67.2.189. [DOI] [PubMed] [Google Scholar]
- 26.Sasser JM, Sullivan JC, Hobbs JL, et al. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol. 2007;18:143–54. doi: 10.1681/ASN. 2006030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13(Suppl 5):S220–2. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- 28.Agui T, Xin X, Cai Y, Sakai T, Matsumoto K. Stimulation of interleukin-6 production by endothelin in rat bone marrow-derived stromal cells. Blood. 1994;84:2531–8. [PubMed] [Google Scholar]
- 29.Mayes MD. Endothelin and endothelin receptor antagonists in systemic rheumatic disease. Arthritis Rheum. 2003;48:1190–9. doi: 10.1002/art.10895. [DOI] [PubMed] [Google Scholar]
- 30.Denton CP. Therapeutic targets in systemic sclerosis. Arthritis Res Ther. 2007;9(Suppl 2):S6. doi: 10.1186/ar2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nett PC, Ortmann J, Celeiro J, et al. Transcriptional regulation of vascular bone morphogenetic protein by endothelin receptors in early autoimmune diabetes mellitus. Life Sci. 2006;78:2213–8. doi: 10.1016/j lfs.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Wilson SH, Simari RD, Lerman A. The effect of endothelin-1 on nuclear factor κ B in macrophages. Biochem Biophys Res Commun. 2001;286:968–72. doi: 10.1006/bbrc.2001.5485. [DOI] [PubMed] [Google Scholar]
- 33.Abraham D, Distler O. How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res Ther. 2007;9(Suppl 2):S2. doi: 10.1186/ar2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagnato A, Rosano L. The endothelin axis in cancer. Int J Biochem Cell Biol. 2008;40:1443–51. doi: 10.1016/j biocel.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Kusuhara M, Yamaguchi K, Nagasaki K, et al. Production of endothelin in human cancer cell lines. Cancer Res. 1990;50:3257–61. [PubMed] [Google Scholar]
- 36.Ali H, Loizidou M, Dashwood M, Savage F, Sheard C, Taylor I. Stimulation of colorectal cancer cell line growth by ET-1 and its inhibition by ET(A) antagonists. Gut. 2000;47:685–8. doi: 10.1136/gut.47.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisk L, Nalivaeva NN, Turner AJ. Regulation of endothelin-converting enzyme-1 expression in human neuroblastoma cells. Exp Biol Med (Maywood) 2006;231:1048–53. [PubMed] [Google Scholar]
- 38.Berry P, Burchill S. Endothelins may modulate invasion and proliferation of Ewing’s sarcoma and neuroblastoma. Clin Sci (Lond) 2002;103(Suppl 48):322S–6S. doi: 10.1042/CS103S322S. [DOI] [PubMed] [Google Scholar]
- 39.Asham E, Shankar A, Loizidou M, et al. Increased endothelin-1 in colorectal cancer and reduction of tumour growth by ET(A) receptor antagonism. Br J Cancer. 2001;85:1759–63. doi: 10.1054/bjoc.2001.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamuta M, Ohashi M, Tabata S, et al. High plasma concentrations of endothelin-like immunoreactivities in patients with hepatocellular carcinoma. Am J Gastroenterol. 1993;88:248–52. [PubMed] [Google Scholar]
- 41.Ferrari-Bravo A, Franciosi C, Lissoni P, Fumagalli L, Uggeri F. Effects of oncological surgery on endothelin-1 secretion in patients with operable gastric cancer. Int J Biol Markers. 2000;15:56–7. doi: 10.1177/172460080001500110. [DOI] [PubMed] [Google Scholar]
- 42.Nelson JB, Hedican SP, George DJ, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995;1:944–9. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 43.Carpagnano GE, Foschino-Barbaro MP, Resta O, Gramiccioni E, Carpagnano F. Endothelin-1 is increased in the breath condensate of patients with non-small-cell lung cancer. Oncology. 2004;66:180–4. doi: 10.1159/000077992. [DOI] [PubMed] [Google Scholar]
- 44.Arun C, London NJ, Hemingway DM. Prognostic significance of elevated endothelin-1 levels in patients with colorectal cancer. Int J Biol Markers. 2004;19:32–7. doi: 10.1177/172460080401900104. [DOI] [PubMed] [Google Scholar]
- 45.Bagnato A, Spinella F, Rosano L. Emerging role of the endothelin axis in ovarian tumor progression. Endocr Relat Cancer. 2005;12:761–72. doi: 10.1677/erc.1.01077. [DOI] [PubMed] [Google Scholar]
- 46.Nelson JB, Chan-Tack K, Hedican SP, et al. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996;56:663–8. [PubMed] [Google Scholar]
- 47.Grimshaw MJ, Hagemann T, Ayhan A, Gillett CE, Binder C, Balkwill FR. A role for endothelin-2 and its receptors in breast tumor cell invasion. Cancer Res. 2004;64:2461–8. doi: 10.1158/0008-5472.CAN-03-1069. [DOI] [PubMed] [Google Scholar]
- 48.Felx M, Guyot MC, Isler M, et al. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-κB in human osteosarcoma. Clin Sci (Lond) 2006;110:645–54. doi: 10.1042/CS20050286. [DOI] [PubMed] [Google Scholar]
- 49.Kim TH, Xiong H, Zhang Z, Ren B. β-Catenin activates the growth factor endothelin-1 in colon cancer cells. Oncogene. 2005;24:597–604. doi: 10.1038/sj onc.1208237. [DOI] [PubMed] [Google Scholar]
- 50.Pflug BR, Zheng H, Udan MS, et al. Endothelin-1 promotes cell survival in renal cell carcinoma through the ET(A) receptor. Cancer Lett. 2007;246:139–48. doi: 10.1016/j.canlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Bagnato A, Cirilli A, Salani D, et al. Growth inhibition of cervix carcinoma cells in vivo by endothelin A receptor blockade. Cancer Res. 2002;62:6381–4. [PubMed] [Google Scholar]
- 52.Ali H, Dashwood M, Dawas K, Loizidou M, Savage F, Taylor I. Endothelin receptor expression in colorectal cancer. J Cardiovasc Pharmacol. 2000;36:S69–71. doi: 10.1097/00005344-200036051-00023. [DOI] [PubMed] [Google Scholar]
- 53.Herrmann E, Bogemann M, Bierer S, et al. The role of the endothelin axis and microvessel density in bladder cancer - correlation with tumor angiogenesis and clinical prognosis. Oncol Rep. 2007;18:133–8. [PubMed] [Google Scholar]
- 54.Carducci MA, Jimeno A. Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. Clin Cancer Res. 2006;12:6296s–300s. doi: 10.1158/1078-0432.CCR-06-0929. [DOI] [PubMed] [Google Scholar]
- 55.Mai HQ, Zeng ZY, Feng KT, et al. Therapeutic targeting of the endothelin a receptor in human nasopharyngeal carcinoma. Cancer Sci. 2006;97:1388–95. doi: 10.1111/j.1349-7006.2006.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bagnato A, Salani D, Di Castro V, et al. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999;59:720–7. [PubMed] [Google Scholar]
- 57.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–6. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 58.Vacca F, Bagnato A, Catt KJ, Tecce R. Transactivation of the epidermal growth factor receptor in endothelin-1-induced mitogenic signaling in human ovarian carcinoma cells. Cancer Res. 2000;60:5310–7. [PubMed] [Google Scholar]
- 59.Rosano L, Di Castro V, Spinella F, et al. Combined targeting of endothelin A receptor and epidermal growth factor receptor in ovarian cancer shows enhanced antitumor activity. Cancer Res. 2007;67:6351–9. doi: 10.1158/0008-5472.CAN-07-0883. [DOI] [PubMed] [Google Scholar]
- 60.Spinella F, Rosano L, Di Castro V, Nicotra MR, Natali PG, Bagnato A. Inhibition of cyclooxygenase-1 and -2 expression by targeting the endothelin a receptor in human ovarian carcinoma cells. Clin Cancer Res. 2004;10:4670–9. doi: 10.1158/1078-0432.CCR-04-0315. [DOI] [PubMed] [Google Scholar]
- 61.Salani D, Taraboletti G, Rosano L, et al. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157:1703–11. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gasparini G, Weidner N, Bevilacqua P, et al. Tumor microvessel density, p53 expression, tumor size, and peritumoral lymphatic vessel invasion are relevant prognostic markers in node-negative breast carcinoma. J Clin Oncol. 1994;12:454–66. doi: 10.1200/JCO.1994.12.3.454. [DOI] [PubMed] [Google Scholar]
- 63.Ahmed SI, Thompson J, Coulson JM, Woll PJ. Studies on the expression of endothelin, its receptor subtypes, and converting enzymes in lung cancer and in human bronchial epithelium. Am J Respir Cell Mol Biol. 2000;22:422–31. doi: 10.1165/ajrcmb.22.4.3795. [DOI] [PubMed] [Google Scholar]
- 64.Lalich M, McNeel DG, Wilding G, Liu G. Endothelin receptor antagonists in cancer therapy. Cancer Invest. 2007;25:785–94. doi: 10.1080/07357900701522588. [DOI] [PubMed] [Google Scholar]
- 65.Bachmann-Brandt S, Bittner I, Neuhaus P, Frei U, Schindler R. Plasma levels of endothelin-1 in patients with the hepatorenal syndrome after successful liver transplantation. Transpl Int. 2000;13:357–62. doi: 10.1111/j.1432-2277.2000.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 66.Demunter A, DeWolf-Peeters C, Degreef H, Stas M, van den Oord JJ. Expression of the endothelin-B receptor in pigment cell lesions of the skin. Evidence for its role as tumor progression marker in malignant melanoma. Virchows Arch. 2001;438:485–91. doi: 10.1007/s004280000362. [DOI] [PubMed] [Google Scholar]
- 67.Lahav R, Heffner G, Patterson PH. An endothelin receptor B antagonist inhibits growth and induces cell death in human melanoma cells in vitro and in vivo. Proc Natl Acad Sci U S A. 1999;96:11496–500. doi: 10.1073/pnas.96.20.11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lahav R. Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma. Int J Dev Biol. 2005;49:173–80. doi: 10.1387/ijdb.041951rl. [DOI] [PubMed] [Google Scholar]
- 69.Bagnato A, Rosano L, Spinella F, Di Castro V, Tecce R, Natali PG. Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Cancer Res. 2004;64:1436–43. doi: 10.1158/0008-5472.CAN-03-2344. [DOI] [PubMed] [Google Scholar]
- 70.Kefford R, Beith JM, Van Hazel GA, et al. A phase II study of bosentan, a dual endothelin receptor antagonist, as monotherapy in patients with stage IV metastatic melanoma. Invest New Drugs. 2007;25:247–52. doi: 10.1007/s10637-006-9014-7. [DOI] [PubMed] [Google Scholar]
- 71.Egidy G, Eberl LP, Valdenaire O, et al. The endothelin system in human glioblastoma. Lab Invest. 2000;80:1681–9. doi: 10.1038/labinvest.3780178. [DOI] [PubMed] [Google Scholar]
- 72.Jesmin S, Miyauchi T, Goto K, Yamaguchi I. Down-regulated VEGF expression in the diabetic heart is normalized by an endothelin ETA receptor antagonist. Eur J Pharmacol. 2006;542:184–5. doi: 10.1016/j ejphar.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 73.Carpenter TC, Schomberg S, Stenmark KR. Endothelin-mediated increases in lung VEGF content promote vascular leak in young rats exposed to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1075–82. doi: 10.1152/ajplung.00251.2005. [DOI] [PubMed] [Google Scholar]
- 74.Bagnato A, Spinella F. Emerging role of endothelin-1 in tumor angiogenesis. Trends Endocrinol Metab. 2003;14:44–50. doi: 10.1016/S1043-2760(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 75.Khan ZA, Chan BM, Uniyal S, et al. EDB fibronectin and angiogenesis – a novel mechanistic pathway. Angiogenesis. 2005;8:183–96. doi: 10.1007/s10456-005-9017-6. [DOI] [PubMed] [Google Scholar]
- 76.Kato T, Kameoka S, Kimura T, et al. Angiogenesis as a predictor of long-term survival for 377 Japanese patients with breast cancer. Breast Cancer Res Treat. 2001;70:65–74. doi: 10.1023/A:1012534724488. [DOI] [PubMed] [Google Scholar]
- 77.Smollich M, Wulfing P. Targeting the endothelin system: novel therapeutic options in gynecological, urological and breast cancers. Expert Rev Anticancer Ther. 2008;8:1481–93. doi: 10.1586/14737140.8.9.1481. [DOI] [PubMed] [Google Scholar]
- 78.Venuti A, Salani D, Manni V, Poggiali F, Bagnato A. Expression of endothelin 1 and endothelin A receptor in HPV-associated cervical carcinoma: new potential targets for anticancer therapy. FASEB J. 2000;14:2277–83. doi: 10.1096/fj.00-0024com. [DOI] [PubMed] [Google Scholar]
- 79.Rosano L, Spinella F, Salani D, et al. Therapeutic targeting of the endothelin a receptor in human ovarian carcinoma. Cancer Res. 2003;63:2447–53. [PubMed] [Google Scholar]
- 80.Banerjee S, Hussain M, Wang Z, et al. In vitro and in vivo molecular evidence for better therapeutic efficacy of ABT-627 and taxotere combination in prostate cancer. Cancer Res. 2007;67:3818–26. doi: 10.1158/0008-5472.CAN-06-3879. [DOI] [PubMed] [Google Scholar]
- 81.Akhavan A, McHugh KH, Guruli G, et al. Endothelin receptor A blockade enhances taxane effects in prostate cancer. Neoplasia. 2006;8:725–32. doi: 10.1593/neo.06388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukui R, Nishimori H, Hata F, et al. Inhibitory effect of endothelin A receptor blockade on tumor growth and liver metastasis of a human gastric cancer cell line. Gastric Cancer. 2007;10:123–8. doi: 10.1007/s10120-007-0421-z. [DOI] [PubMed] [Google Scholar]
- 83.Carducci MA, Padley RJ, Breul J, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21:679–89. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 84.Carducci MA, Saad F, Abrahamsson PA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–66. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 85.Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with non-metastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–87. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rajeshkumar NV, Rai A, Gulati A. Endothelin B receptor agonist, IRL 1620, enhances the anti-tumor efficacy of paclitaxel in breast tumor rats. Breast Cancer Res Treat. 2005;94:237–47. doi: 10.1007/s10549-005-9000-3. [DOI] [PubMed] [Google Scholar]
- 87.Buckanovich RJ, Facciabene A, Kim S, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intra-tumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 89.Merogi AJ, Marrogi AJ, Ramesh R, Robinson WR, Fermin CD, Freeman SM. Tumor-host interaction: analysis of cytokines, growth factors, and tumor-infiltrating lymphocytes in ovarian carcinomas. Hum Pathol. 1997;28:321–31. doi: 10.1016/S0046-8177(97)90131-3. [DOI] [PubMed] [Google Scholar]
- 90.Wulfing P, Diallo R, Kersting C, et al. Expression of endothelin-1, endothelin-A, endothelin-B receptor in human breast cancer and correlation with long-term follow-up. Clin Cancer Res. 2003;9:4125–31. [PubMed] [Google Scholar]
- 91.Salani D, Di Castro V, Nicotra MR, et al. Role of endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol. 2000;157:1537–47. doi: 10.1016/S0002-9440(10)64791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1α in ovarian carcinoma cells. J Biol Chem. 2002;277:27850–5. doi: 10.1074/jbc M202421200. [DOI] [PubMed] [Google Scholar]
- 93.Hodi FS, Dranoff G. Combinatorial cancer immunotherapy. Adv Immunol. 2006;90:341–68. doi: 10.1016/S0065-2776(06)90009-1. [DOI] [PubMed] [Google Scholar]
- 94.Halcox JP, Nour KR, Zalos G, Quyyumi AA. Endogenous endothelin in human coronary vascular function: differential contribution of endothelin receptor types A and B. Hypertension. 2007;49:1134–41. doi: 10.1161/HYPERTENSIONAHA.106.083303. [DOI] [PubMed] [Google Scholar]
- 95.Cardillo C, Kilcoyne CM, Waclawiw M, Cannon RO, III, Panza JA. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension. 1999;33:753–8. doi: 10.1161/01.hyp.33.2.753. [DOI] [PubMed] [Google Scholar]
- 96.Cowburn PJ, Cleland JG, McDonagh TA, McArthur JD, Dargie HJ, Morton JJ. Comparison of selective ET(A) and ET(B) receptor antagonists in patients with chronic heart failure. Eur J Heart Fail. 2005;7:37–42. doi: 10.1016/j.ejheart.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 97.López A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 98.Ng QS, Goh V, Milner J, et al. Effect of nitric-oxide synthesis on tumour blood volume and vascular activity: a phase I study. Lancet Oncol. 2007;8:111–8. doi: 10.1016/S1470-2045(07)70001-3. [DOI] [PubMed] [Google Scholar]
- 99.Cemazar M, Wilson I, Prise VE, Bell KM, Hill SA, Tozer GM. The endothelin B (ETB) receptor agonist IRL 1620 is highly vasoconstrictive in two syngeneic rat tumour lines: potential for selective tumour blood flow modification. Br J Cancer. 2005;93:98–106. doi: 10.1038/sj.bjc.6602672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leffers N, Lambeck AJ, de Graeff P, et al. Survival of ovarian cancer patients overexpressing the tumour antigen p53 is diminished in case of MHC class I down-regulation. Gynecol Oncol. 2008;110:365–73. doi: 10.1016/j ygyno.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 103.Shah CA, Allison KH, Garcia RL, Gray HJ, Goff BA, Swisher EM. Intratumoral T cells, tumor-associated macrophages, and regulatory T cells: Association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecol Oncol. 2008;109:215–9. doi: 10.1016/j.ygyno.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 104.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 105.Vesalainen S, Lipponen P, Talja M, Syrjanen K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A:1797–803. doi: 10.1016/0959-8049(94)E0159-2. [DOI] [PubMed] [Google Scholar]
- 106.Manning EA, Ullman JG, Leatherman JM, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–9. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 107.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang WM, Zhou J, Ye QJ. Endothelin-1 enhances proliferation of lung cancer cells by increasing intracellular free Ca2+ Life Sci. 2008;82:764–71. doi: 10.1016/j.lfs.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 109.Schmidt BL, Pickering V, Liu S, et al. Peripheral endothelin A receptor antagonism attenuates carcinoma-induced pain. Eur J Pain. 2007;11:406–14. doi: 10.1016/jejpain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 110.Yuyama H, Koakutsu A, Fujiyasu N, et al. Inhibitory effects of a selective endothelin-A receptor antagonist YM598 on endothelin-1-induced potentiation of noci-ception in formalin-induced and prostate cancer-induced pain models in mice. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S479–82. doi: 10.1097/01.fjc.0000166309.63808.5f. [DOI] [PubMed] [Google Scholar]
- 111.Rosano L, Di Castro V, Spinella F, Nicotra MR, Natali PG, Bagnato A. ZD4054, a specific antagonist of the endothelin A receptor, inhibits tumor growth and enhances paclitaxel activity in human ovarian carcinoma in vitro and in vivo. Mol Cancer Ther. 2007;6:2003–11. doi: 10.1158/1535-7163.MCT-07-0151. [DOI] [PubMed] [Google Scholar]
- 112.Rosano L, Di Castro V, Spinella F, Decandia S, Natali PG, Bagnato A. ZD4054, a potent endothelin receptor A antagonist, inhibits ovarian carcinoma cell proliferation. Exp Biol Med (Maywood) 2006;231:1132–5. [PubMed] [Google Scholar]
- 113.Grimshaw MJ, Naylor S, Balkwill FR. Endothelin-2 is a hypoxia-induced autocrine survival factor for breast tumor cells. Mol Cancer Ther. 2002;1:1273–81. [PubMed] [Google Scholar]
- 114.Kikuchi K, Nakagawa H, Kadono T, et al. Decreased ET(B) receptor expression in human metastatic melanoma cells. Biochem Biophys Res Commun. 1996;219:734–9. doi: 10.1006/bbrc.1996.0303. [DOI] [PubMed] [Google Scholar]
- 115.Eberl LP, Valdenaire O, Saintgiorgio V, Jeannin JF, Juillerat-Jeanneret L. Endothelin receptor blockade potentiates FasL-induced apoptosis in rat colon carcinoma cells. Int J Cancer. 2000;86:182–7. doi: 10.1002/(SICI)1097-0215(20000415)86:2<182::AID-IJC6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 116.Bien S, Riad A, Ritter CA, et al. The endothelin receptor blocker bosentan inhibits doxorubicin-induced cardiomyopathy. Cancer Res. 2007;67:10428–35. doi: 10.1158/0008-5472.CAN-07-1344. [DOI] [PubMed] [Google Scholar]