Abstract
Background and Purpose
Although caffeinol (combination of low dose of caffeine and ethanol) was shown to robustly reduce stroke damage in experimental models and is now in clinical evaluation for treatment of ischemic stroke, little is known about the potential mechanism of its action.
Methods
We have used an in vivo excitotoxicity model based on intracortical infusion of NMDA and model of reversible focal ischemia to demonstrate NMDA receptor inhibition as one potential mechanism of Caffeinol anti-ischemic activity.
Results
Caffeinol reduced the size of excitotoxic lesion and substitution of ethanol in Caffeinol with CNS-1102 and MK-801, but not with MgSO4, produced treatment with strong synergistic effect that was at least as robust in reducing ischemic damage as Caffeinol. This NMDA receptor antagonist and caffeine combination showed long window of opportunity, activity in spontaneously hypertensive rats, and unlike Caffeinol was fully effective in animals chronically pre-treated with ethanol.
Conclusions
Our study suggests that anti-excitotoxic properties may underlie some of the anti-ischemic effect of Caffeinol. This study provides strong evidence that the anti-ischemic effect of NMDA receptor blockers in general can be dramatically augmented by caffeine, thus opening a possibility for new utilization of NMDA-based pharmacology in the treatment of stroke.
Keywords: Neuroprotection, excitotoxicity, NMDA-antagonist, magnesium, ethanol, caffeine
INTRODUCTION
Substantial body of evidence demonstrates that low doses of ethanol plus caffeine (Caffeinol), can effectively reduce brain damage in rodent models of focal cerebral ischemia1–3 and traumatic brain injury4. Based on these promising pre-clinical studies Caffeinol was evaluated in stroke patients during a safety and feasibility study5. While pre-clinical data indicate strong neuroprotective potential and feasibility data suggest that Caffeinol can be safely administered to stroke patients, little is known about its mechanism of action..
It is recognized that among myriad of biological effects, ethanol can effectively inhibit N-methyl-D-aspartate (NMDA) subtype of glutamate receptor6–8. It is also well-established that cerebral ischemia/reperfusion produces massive release of glutamate into the extracellular space, thereby causing activation of NMDA receptor, a process believed to lead to neurotoxicity (excitotoxicity) via calcium overload. Thus, it is possible that ethanol’s ability to inhibit NMDA receptor could represent an important component of the anti-ischemic effect of Caffeinol.
While the anti-excitotoxic potential of ethanol is intriguing, our earlier experience with ischemic stroke is that ethanol alone augments ischemic damage2. Hence, ethanol requires caffeine for its protective effect, suggesting that caffeine not only neutralizes the deleterious aspect of ethanol, but it also interacts with ethanol in such a way that leads to a superadditive synergy. The mechanism of how caffeine modifies ethanol’s effect is also unclear. The caffeine plasma levels in animals treated with Caffeinol in pre-clinical stroke studies was approximately 20µg/ml (representing approximately 3–4 cups of strong coffee), a concentration recognized to inhibit adenosine receptor (and maybe inhibition of phosphodiesterase)9.
In this study we tested the hypothesis that the anti-ischemic effect of Caffeinol is at least in part mediated through inhibition of excitotoxic damage, and that NMDA receptor inhibition by ethanol may represent an important part of Caffeinol effect. Three specific questions were posed: 1) could Caffeinol reduce excitotoxic damage mediated via NMDA receptor; 2) could substitution for ethanol with pharmacologic agent(s) that display selectivity in blocking NMDA receptor (e.g. MK-801 or CNS-1102) support superadditive synergy when combined with caffeine; and 3) could this substitution approach represent new strategy for stroke treatment.
MATERIAL AND METHODS
All procedures were in compliance with National Institute of Health guidelines for the humane care of animals and were approved by the institutional Animal Welfare Committee. No deaths or seizures were observed in any of the groups analyzed.
Production and analysis of excitotoxic lesions in vivo
The experiment was performed using a method we previously described10. Briefly, male Sprague Dawley rats (250–300g; Harlan, Sprague Dawley) were anesthetized with chloral hydrate (0.35g/kg). Normothermia (36.6±0.5°C) was maintained by using a thermostatically controlled heating lamp. To produce an excitotoxic lesion, NMDA (20nmols in 1µL of saline), was injected under stereotaxic guidance over 60min into the cerebral cortex. Animals were randomly divided into 5 groups: 1) NMDA alone; 2) NMDA+MK801 (a non-competitive NMDA receptor antagonist; used as positive control); 3) NMDA+caffeinol (caffeine 10mg/kg+ethanol 0.32g/kg); 4) NMDA+ethanol (0.32g/kg), and NMDA+caffeine (10mg/kg). All the above doses were shown previously to have anti-ischemic effect. Ethanol, caffeine and caffeinol were infused through the left femoral vein to reproduce conditions offering potent anti-ischemic effect. Twenty percent of the treatment was delivered as a bolus 30 minutes prior to the NMDA infusion and the remaining volume was infused over 2.5 hours. MK-801 (3 mg/kg) was injected intraperitoneally at 15 min before the onset of NMDA infusion. Forty-eight hours after the insult, rats were re-anesthetized and perfused intracardially with ice-cold saline. The dissected brains were snap-frozen in −80°C 2-methylbutan. Hematoxilin and eosin (H&E)-stained, 20µm thick serial coronal cryosections collected 200 µm apart, were used for lesion volume determination. Lesion volume was determined morphometrically. The excitotoxic lesion volume represents the integration of all surface areas from all brain sections displaying signs of damage.
Production of ischemia
Focal ischemia was induced by 180 min of reversible left MCA/CCA occlusion in Long-Evans or Spontaneously Hypertensive rats (SHR) as described previously11. Briefly, animals were anesthetized with chloral hydrate (0.45g/kg; intraperitoneally). The femoral vein was cannulated for drug administration. Core body temperature was maintained at 36.5±0.5°C during ischemia and the first hour of reperfusion. A 0.005-inch diameter stainless steel wire was placed underneath the MCA rostral to the rhinal fissure, proximal to the major bifurcation of the MCA, and distal to the lenticulostriate arteries. The CCA was occluded, and cerebral perfusion (CP) at the cortical surface, 3-mm distal to the locus of the MCA occlusion, was measured using LDF (Vesamedic, St. Paul, MN). Only animals that displayed a CP reading less than 12–15% of the initial value were included in the study. In our original studies2, we determined that under the experimental conditions employed in present study caffeinol did not affect blood pressure and other physiologic variables including temperature, pH, pO2 and pCO2. After 180 minutes of ischemia reperfusion was established by reversing the occlusion procedure. At time points indicated for each study, animals were re-anesthetized and perfused with 50ml of saline intracardially. Two mm thick coronal brain sections were cut, prior to staining with 2% 2,3,5-triphenyltetrazolium (TTC) in PBS for 30 min for infarcted tissue discrimination. Because of the rapid maturation of ischemic damage in our model of ischemia12, in most of the studies infarct volume was determined 1 or 3 days after induction of ischemia. There were no differences in core body temperature and blood gases (pH, pCO2 and pO2) (data not shown) between control animals and the groups receiving treatments as determined at 60 min after initiation of the treatment.
Infarct volume analysis
Morphometric determination of indirect infarct volume based on TTC staining was measured using a computer-based image analyzer operated by "Brain" software (Drexel University)13.
Behavioral Measurements
All behavioral tests took place in a quiet dim room by an experimenter blinded with respect to the treatment groups. The foot fault, forelimb placing and postural reflex tests were performed as described previously.14 Neurological deficit score (NDS) was measured before occlusion and 72 hours after MCA occlusion. NDS (0 to 12) was calculated by combining the score on the following 3 tests.
Postural Reflex Test
The degree of abnormal posture was estimated by suspending rats with their tails 10cm above a tabletop. Intact rats extended both forelimbs toward the table surface. Rats displaying this behavior were recorded as score 0. Rats that flex only the contralateral limb toward the body were recorded as 2. Rats rotating the contralateral forelimb toward the tail were graded as 4.
Forelimb Forward Placing
Animals were held by their torsos with forelimbs hanging freely. Contralateral and ipsilateral forelimb forward placing responses were induced by gently contacting the front side of the forelimb to the edge of a tabletop for 10 trials. Percent successful placing response rate was recorded as the animal successfully placed the respective forepaw on the top of the table. The NDS score was calculated as: the percent of non-placing × 4 (e.g., deficit 0 = immediate and complete placing; deficit 2 = 50% placing; deficit 4 = no placing).
Footfault
Rats were placed on a grid, with openings of 2.3cm2. As the animals traversed the grid, a footfault was scored each time the contralateral forepaw slipped through an opening in the grid. The total number of steps was also counted. The percent footfault was calculated as the number of footfaults/total steps×100. A score of 0 to 4 was given to each animal according to the severity of the deficit by calculating the percent footfaults×0.04.
Treatment groups; Ischemic stroke
Animals were randomly divided into 8 treatment groups plus control: 1) saline control; 2) Ethanol alone (0.32g/kg); 3) caffeinol (caffeine 10mg/kg + ethanol 0.32g/kg); 4) CNS1102 (1.35mg/kg), 5) CNS1102 + caffeine (1.35–0.12mg/kg + 6 mg/kg, respectively); 6) MK801 (1mg/kg); 7) MK-801 + caffeine (1mg/kg + 10mg/kg, respectively); 8) MgSO4 (67mg/kg) and 9) MgSO4 + caffeine (67mg/kg+10mg/kg, respectively). The treatments were infused through the left femoral vein to reproduce conditions offering potent anti-ischemic effect and all the treatments (unless indicated otherwise) were initiated either 15 min or 30 min after the onset of stroke, as indicated. Unless indicated otherwise in the figure legend, 20% of each the treatment was delivered as a bolus and the remaining dose was infused over 2.5h.
Statistical analyses
Statistical significance was determined by t-test for two groups comparison or ANOVA followed by the Newman-Kuels test for multiple groups comparison.
RESULTS
Caffeinol reduces cortical damage produced by excitotoxic insult
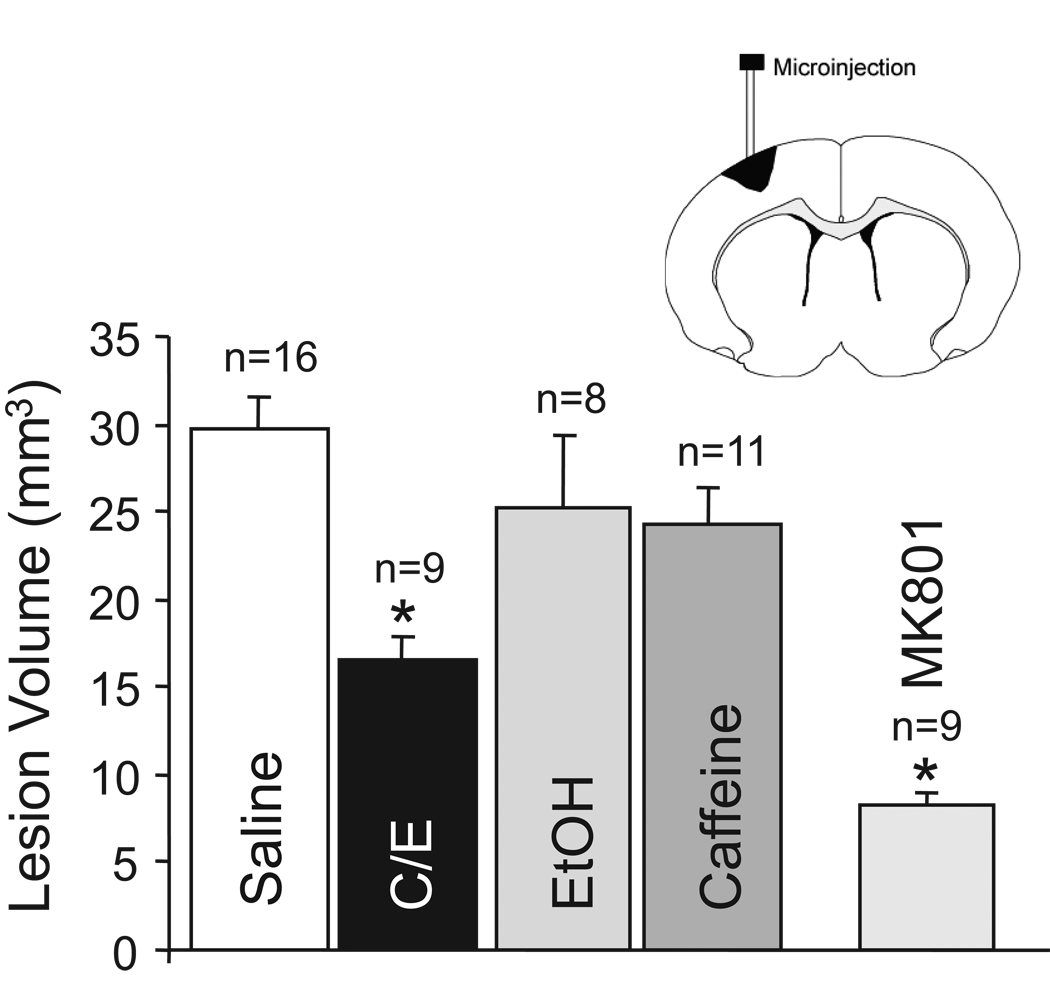
Excitotoxic cortical lesion was produced by infusing NMDA into the rat cerebral cortex as we described10. The lesion volume in animals receiving NMDA alone was 29.1 ± 7.5 mm3 (Fig 1). MK-801, a non-competitive antagonist of the NMDA receptor, administered 15 min prior to infusion of NMDA, significantly reduced the NMDA lesion volume by 72% (8.2 ± 2.5 mm3; p<0.05). The lesion volume in rats treated with caffeinol was 43% smaller (p<0.05; 16.7 ± 4.19 mm3) than the lesion volume in the NMDA-alone group (Fig 1). Neither ethanol alone nor caffeine alone affected lesion volume produced by NMDA; the lesion volumes in the ethanol-alone and caffeine-alone groups were 25.1 ± 11.7 mm3 and 24.2 ± 7.1 mm3, respectively.
Fig-1.
Caffeinol reduces excitotoxic lesion volume in rat cerebral cortex in vivo. Cortical lesion volume (mm3) produced by 100nmols of NMDA in animals pre-treated with intravenous administration of saline, Caffeinol (C/E; 0325 g/kg ethanol and 10mg/kg caffeine), ethanol (EtOH; 0.325g/kg), caffeine (10mg/kg) or MK-801 (3mg/kg). The lesion volume was measured at 48h. The data is expressed as mean±SEM. Number (N) of animals per group is indicated above the bars. *p≤0.05 from saline control. Diagram illustrates the location of the NMDA infusion/lesion.
Reduction of infarct volume by NMDA antagonist, CNS-1102 is augmented by caffeine
Based on our earlier work, we know that ethanol alone and caffeine alone has no benefit in our ischemic stroke model1, 2. However, when applied in combination they exert robust anti-ischemic effect1, 2. Knowing that ethanol inhibits NMDA receptor/channel, we tested whether NMDA receptor antagonist (to substitute for ethanol) in combination with caffeine could also exhibit a synergistic anti-ischemic effect similar to what we found with caffeinol. Our first experiment established that in agreement with our earlier data13, CNS-1102, a non-competitive NMDA receptor antagonist, reduced infarct volume by 41% as compared to a saline treated control group (127.2 +/− 14.7 mm3 vs. 74.22 +/− 18.6 mm3) when administered at 15 min after induction of stroke. Next, we established that caffeine alone has no effect on ischemic damage, however the combination of caffeine with CNS-1102 produced far more robust reduction of infarct volume then CNS-1102 alone (fig 2). The infarct volume was reduced by 91% (127.2 +/− 14.7 mm3 vs. 10.55 +/− 4.76 mm3) in response to caffeine plus CNS-1102 (Fig 2), indicating the synergistic effect.
Fig-2.
Infarct volume after MCA/CCA occlusion in Long Evans rats treated with saline, CNS-1102 (CNS; 0.5 mg/kg bolus+0.345mg/kg/h for 2.5h via i.v. infusion) or CNS-1102 plus caffeine (CNS+CAF; 0.5mg/kg bolus+0.345mg/kg/h CNS-1102 plus 1.66mg/kg bolus+3.33mg/kg/h caffeine, over 2.5h). Treatment was started 15min after the onset of 180min of ischemia. Infarct volume was determined at 3d. The data is expressed as mean±SEM. Number (N) of animals per group is indicated above the bars. *p≤0.05 from all the other groups.
CNS-102 plus caffeine has long window of opportunity and is effective in hypertensive rats
The objective of these experiments was to determine if caffeine and CNS-1102, similar to Caffeinol, has the extended window for effective treatment and if it can reduce infarct volume in a more severe model of ischemia in spontaneously hypertensive rats. By delaying the treatment, we established that the combination of caffeine and CNS-1102 is most effective if given early after the onset of the ischemia (Fig 3). However, caffeine plus CNS-1102 provided approximately 50% infarct volume reduction when treatment was delayed for up to 2h (Fig 3); thus being similar to the time window of opportunity demonstrated earlier for Caffeinol2. Note that the two hour window of opportunity for our MCA/CCA occlusion model is the longest of any clinically relevant approaches tested in this model (data not presented). CNS-1102 alone is ineffective in reducing infarct volume when given at 30 min after the onset of MCA/CCAo (127.2+/−14.7 mm3 for saline vs. 129.5+/−33.7mm3; n=5 for CNS-1102).
Fig-3.
Infarct volume after MCA/CCA occlusion in Long Evans rats treated with saline or CNS-1102 (0.5mg/kg bolus+0.345mg/kg/h for 2.5h) plus caffeine (1.66mg/kg bolus+3.33mg/kg/h for 2.5h) starting at 15,60,90 or 120min after the onset of MCA/CCAo. Infarct volume was determined at 3d. The data is expressed as mean±SEM. Number (N) of animals per group is indicated above the bars. *p≤0.05 from saline control.
We have also demonstrated that caffeine plus CNS-1102 was effective in reducing infarct volume in spontaneously hypertensive rats (Fig 4).
Fig-4.
Infarct volume after MCA/CCA occlusion in SHRs treated with saline or CNS-1102 (0.5 mg/kg bolus+0.345 mg/kg/h for 2.5h) plus caffeine (1.66 mg/kg bolus+3.33 mg/kg/h for 2.5h) starting at 30 min after the onset of MCA/CCAo. Infarct volume was determined at 3 days. The data is expressed as mean±SEM. Number (N) of animals per group is indicated above the bars. *p≤0.05 from saline control.
MK-801, but not MgSO4 combined with caffeine has synergistic effect in protecting brain from ischemia?
CNS-1102 plus caffeine has strong neuroprotective effect. In order to determine if this neuroprotection is limited to CNS-1102 only; we tested other NMDA antagonists for their interaction with caffeine. For this study we decided to use prototypic NMDA antagonist MK-801 and clinically relevant MgSO4, the agent previously proposed to demonstrate anti-ischemic effect through blocking NMDA receptor. Treatments were executed at 30 min after the induction of ischemia. Under these experimental conditions the combination of caffeine and MK-801, in a similar fashion to CNS-1102, showed strong synergistic effect by reducing infarct volume by 84% (128.3+/−9.4mm3 vs. 20.2+/−9.2mm3) (Fig 5). Note that with 30 min post-treatment, MK-801 alone showed no effect on infarct volume, and that the effect of MK-801 plus caffeine, though not statistically different, appeared to be more potent than Caffeinol (Fig 5). Surprisingly, MgSO4, either alone or in combination with MK-801 had no effect on infarct volume (Fig 5).
Fig-5.
Infarct volume after MCA/CCA occlusion in Long Evans rats treated intravenously with: (1) saline; (2) ethanol (EtOH; 0.65g/kg); (3) ethanol (0.65g/kg) plus caffeine (10 mg/kg); (4) MK-801 (1mg/kg); (5) MK-801 (1mg/kg) plus caffeine (10mg/kg); (6) MgSO4 (67mg/kg) and (7) MgSO4 (67mg/kg) plus caffeine (10mg/kg). All treatments were started at 30 min after the onset of ischemia. Infarct volume was determined at 3d. The data is expressed as mean±SEM. Number (N) of animals per group is indicated above the bars. *p≤0.05 from saline control.
MK-801 plus caffeine is not sensitive to ethanol-induced tolerance
Our previous studies demonstrated that chronic consumption of ethanol (but not caffeine) prior to ischemia significantly reduced Caffeinol’s neuroprotective potency1. Here, we tested whether chronic ethanol could also compromise the neuroprotective effect of MK-801 plus caffeine. Our study showed that ethanol pretreatment did not weaken the protective effect of MK-801 plus caffeine with respect to infarct volume reduction and neurological deficit (NDS) (Fig 6). In agreement with our earlier study, pretreatment with ethanol significantly reduced the effect of Caffeinol (Fig 6).
Fig-6.
Infarct volume after MCA/CCA occlusion in Long Evans rats pretreated (Pretr) with water (groups 1 and 2) or ethanol (0.325g/kg - groups 3 and 4) for 14 consecutive days prior to ischemia and then intravenously treated with: (1) saline; (2 and 3) caffeinol - 0325g/kg ethanol and 10mg/kg caffeine; and (4) 10mg/kg caffeine plus 1mg/kg MK-801) at 30 min after the onset of MCA/CCAo. Infarct volume was determined at 3d. The data is expressed as mean±SEM. Number (N) of animals per group is indicated above the bars. * p≤0.05 from all remaining groups.
DISCUSSION
Except for thrombolysis with tissue plasminogen activator (t-PA) there is no effective, approved, treatment for ischemic stroke 15. We have recently demonstrated that a low dose of ethanol plus caffeine (Caffeinol) produces remarkably potent neuroprotective effect in rodent models of ischemic stroke1, 2 and that this treatment can be administered safely to humans5 including in combination with hypothermia16. A randomized, placebo-controlled trial is needed to determine the efficacy effect of this combination in stroke patients.
Our present study was in part designed to investigate the mechanism of action of Caffeinol in relevance to NMDA receptor inhibition. While ethanol is known to have a myriad of biological effects, its role in NMDA-receptor inhibition may have potential relevance to the pathophysiology of stroke. It is well established that ischemia causes a massive release of excitatory neurotransmitters and that various inhibitors of NMDA receptors are capable of reducing ischemia-induced damage in various models of ischemic stroke17. The effect of ethanol on NMDA receptor inhibition appears to take place at low micromolar concentrations of ethanol and is primarily mediated via interaction with NR2B and NR2A subunits, the receptors that are abundant in stroke-affected cerebral cortex18. Thus it is intuitive to suggest that inhibition of NMDA receptor by ethanol could in part play an important role in the anti-ischemic effect of Caffeinol. Unexpectedly, ethanol alone instead of being neuroprotective augmented ischemic damage2 (and Fig 5), suggesting that besides its potential beneficial components associated with NMDA receptor inhibition, ethanol may produce some adverse effects that ultimately extinguishes its beneficial effect. It could be in part that the role of caffeine in Caffeinol is to ameliorate deleterious aspects of ethanol, as caffeine alone has no beneficial role is our ischemia model2. Ongoing studies are aimed at determining this effect of caffeine.
Nevertheless, our present experiments implicate NMDA receptor as a component of caffeinol’s mode of action. First, we demonstrated that in analogy to what we have seen after ischemic stroke, Caffeinol (but not ethanol or caffeine alone) was capable of reducing damage produced by intracortical infusion of NMDA, a well-validated model of excitotoxic damage. Though the anti-excitotoxic effect of Caffeinol was not as robust as its anti-ischemic effect, the resulting protection in this model was certainly substantial. Second, we demonstrated that ethanol in Caffeinol could be successfully substituted by NMDA antagonist to deliver robust neuroprotection equivalent to that of Caffeinol. Specifically we showed that caffeine in combination with non-competitive NMDA antagonist CNS-1102 or MK-801 was capable of reducing infarct volume far more potently than NMDA antagonist itself. We have determined that the time-window for effective treatment for the combination of caffeine plus CNS-1102 is at least 2 hours. In our past experience with at least 50 different clinically relevant compounds only Caffeinol demonstrated 2h window of opportunity for effective treatment in this model of ischemia2. It was unexpected, however, that MgSO4, a compound with NMDA receptor blocking capacity, used at a concentration equivalent to the FAST-MAG pilot trial in humans and the range of concentration effective in experimental studies19, alone or in combination with caffeine did not show neuroprotective effect in our studies. Though we don’t have an explanation for the lack of effect of MgSO4, one potential explanation is that the treatment was initiated too late to offer any beneficial effect. We have previously demonstrated efficacy of MgSO4 in the same ischemia model with the treatment initiated at 15 min after the MCA/CCAo (unpublished results). Note that MK-801 under the same experimental conditions as MgSO4 also did not show efficacy, suggesting a short time window of opportunity for NMDA-blocking therapy. However, in contrast to MgSO4, which did not show effect in combination with caffeine, MK-801 in combination with caffeine reduced infarct volume by over 80% when applied under the same experimental conditions. It is possible that the lack of synergy with caffeine may suggest that the protective mechanism of action of MgSO4 may not involve modulation of NMDA.
In summary, our data add evidence supporting the neuroprotective activity of Caffeinol by demonstrating the effect of this combination on modulating NMDA receptor mediated damage. Furthermore, our data emphasize that the low dose of caffeine in the Caffeinol combination plays a pivotal role in amplifying the anti-excitotoxic activity of ethanol, and suggests that similar doses of caffeine might be linked to other anti-excitotoxic therapies to enhance their effect.
Acknowledgments
Supported by National Institute of Health, National Institute of Neurological Disorders and Stroke grant 1R01NS040974. Drs. Grotta, Aronowski and Mr. Strong holds patent for Caffeinol use in stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Aronowski J, Strong R, Shirzadi A, Grotta JC. Ethanol plus caffeine (caffeinol) for treatment of ischemic stroke. Preclinical experience. Stroke. 2003;10:10. doi: 10.1161/01.STR.0000068170.80517.B3. [DOI] [PubMed] [Google Scholar]
- 2.Strong R, Grotta JC, Aronowski J. Combination of low dose ethanol and caffeine protects brain from damage produced by focal ischemia in rats. Neuropharmacology. 2000;39:515–522. doi: 10.1016/s0028-3908(99)00156-2. [DOI] [PubMed] [Google Scholar]
- 3.Belayev L, Khoutorova L, Zhang Y, Belayev A, Zhao W, Busto R, Ginsberg MD. Caffeinol confers cortical but not subcortical neuroprotection after transient focal cerebral ischemia in rats. Brain Res. 2004;1008:278–283. doi: 10.1016/j.brainres.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Dash PK, Moore AN, Moody MR, Treadwell R, Felix JL, Clifton GL. Post-trauma administration of caffeine plus ethanol reduces contusion volume and improves working memory in rats. J Neurotrauma. 2004;21:1573–1583. doi: 10.1089/neu.2004.21.1573. [DOI] [PubMed] [Google Scholar]
- 5.Piriyawat P, Labiche LA, Burgin WS, Aronowski JA, Grotta JC. Pilot dose-escalation study of caffeine plus ethanol (caffeinol) in acute ischemic stroke. Stroke. 2003;10:10. doi: 10.1161/01.STR.0000067706.23777.04. [DOI] [PubMed] [Google Scholar]
- 6.Dildy JE, Leslie SW. Ethanol inhibits nmda-induced increases in free intracellular ca2+ in dissociated brain cells. Brain Res. 1989;499:383–387. doi: 10.1016/0006-8993(89)90789-0. [DOI] [PubMed] [Google Scholar]
- 7.Lovinger DM, White G, Weight FF. Ethanol inhibits nmda-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 8.Chandler LJ, Sumners C, Crews FT. Ethanol inhibits nmda receptor-mediated excitotoxicity in rat primary neuronal cultures. Alcohol Clin Exp Res. 1993;17:54–60. doi: 10.1111/j.1530-0277.1993.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 9.Fredholm BB. Astra award lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76:93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Ou Z, Grotta JC, Waxham N, Aronowski J. Peroxisome-proliferator-activated receptor-gamma (ppargamma) activation protects neurons from nmda excitotoxicity. Brain Res. 2006;1073–1074:460–469. doi: 10.1016/j.brainres.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 11.Aronowski J, Strong R, Grotta JC. Reperfusion injury: Demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Aronowski J, Cho KH, Strong R, Grotta JC. Neurofilament proteolysis after focal ischemia; when do cells die after experimental stroke? J Cereb Blood Flow Metab. 1999;19:652–660. doi: 10.1097/00004647-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Aronowski J, Ostrow P, Samways E, Strong R, Zivin JA, Grotta JC. Graded bioassay for demonstration of brain rescue from experimental acute ischemia in rats. Stroke. 1994;25:2235–2240. doi: 10.1161/01.str.25.11.2235. [DOI] [PubMed] [Google Scholar]
- 14.Zhao X, Liu SJ, Zhang J, Strong R, Aronowski J, Grotta JC. Combining insulin-like growth factor derivatives plus caffeinol produces robust neuroprotection after stroke in rats. Stroke. 2005;36:129–134. doi: 10.1161/01.STR.0000149624.87661.18. [DOI] [PubMed] [Google Scholar]
- 15.Group Nr-PSS. Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group [see comments] N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 16.Martin-Schild S, Hallevi H, Shaltoni H, Barreto AD, Gonzales NR, Aronowski J, Savitz SI, Grotta JC. Combined neuroprotective modalities coupled with thrombolysis in acute ischemic stroke: A pilot study of caffeinol and mild hypothermia. J Stroke Cerebrovasc Dis. 2009;18:86–96. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 18.Lovinger DM. Developmental decrease in ethanol inhibition of n-methyl-d-aspartate receptors in rat neocortical neurons: Relation to the actions of ifenprodil. J Pharmacol Exp Ther. 1995;274:164–172. [PubMed] [Google Scholar]
- 19.Muir KW. Magnesium in stroke treatment. Postgrad Med J. 2002;78:641–645. doi: 10.1136/pmj.78.925.641. [DOI] [PMC free article] [PubMed] [Google Scholar]