Abstract
The objective of this study to design a delivery system resistant to the gastrointestinal environment for oral vaccine against porcine rotavirus. Lactococcus lactis NZ9000 was transformed with segments of vP4 of the porcine rotavirus inserted into the pNZ8112 surface-expression vector, and a recombinant L. lactis expressing VP4 protein was constructed. An approximately 27 kDa VP4 protein was confirmed by SDS-PAGE , Western blot and immunostaining analysis. BALB/c mice were immunized orally with VP4-expression recombinant L. lactis and cellular, mucosal and systemic humoral immune responses were examined. Specific anti-VP4 secretory IgA and IgG were found in feces, ophthalmic and vaginal washes and in serum. The induced antibodies demonstrated neutralizing effects on porcine rotavirus infection on MA104 cells. Our findings suggest that oral immunization with VP4-expressing L. lactis induced both specific local and systemic humoral and cellular immune responses in mice.
1. Introduction
Rotavirus is an important pathogen that causes diarrhea in human infants and in animals worldwide [1]. Rotavirus infections in pigs seriously impact the pork industry. The mortality rate in piglets is extremely variable, ranging from 0 to 50%, and is usually of the order of 0–10%; however, the loss in growth in recovered piglets is economically the most important effect of the disease.
Rotaviruses are classified in the genus rotavirus, in the family reoviridae. There are three groups of rotaviruses that affect humans and animals, which are referred to as group A, B, and C on the basis of the group-specific inner capsid protein VP6 [2]. Group A rotaviruses are the most common agents that cause diarrheal disease in the young of not only humans but also many animal species including piglets. The core of porcine rotavirus is composed of double-stranded RNA arranged in 11 genome segments. Segment 4 encodes VP4 outer capsid protein on the rotavirus surface, which not only defines viral P serotypes, but is also a potent protective immunogen [3]; VP4 protein can independently elicit neutralizing antibodies resulting in protective immunity. The antigenic functional region from the 5′ end of VP4 is encoded by a 756-bp fragment that includes the trypsin region of VP8 at the C terminus and VP5 at the N terminus [4].
Gut mucosal infection occurs primarily by the invasion route via viral replication at the tips of the villi of epithelial cells in the small intestine, leading to structural and functional changes in the epithelium. The diarrhea that results is caused by the multiple activities of the virus. Malabsorption is a generally accepted mechanism of rotavirus-induced diarrhea, which is characterized by viral replication in villus enterocytes in the small intestine, with subsequent cell lysis and attendant villus blunting, depressed level of mucosal disaccharidase, watery diarrhea, and dehydration [5, 6]. The rotavirus nonstructural protein NSP4, which has recently been suggested to have a toxin-like function, may participate in inducing intestinal inflammation [7].
Because rotaviruses are enteric pathogens, gut mucosal immune responses are likely to play an important role in protective immunity against rotavirus infection. Gut innate immunity provides the first line of defense against pathogenic microorganisms and also initiates acquired immune responses. Thus, oral vaccines present an ideal immunoprophylactic strategy for eliciting protection against this type of infection. However, an obstacle in the generation of oral vaccine formulations is maintaining immunogenicity while simultaneously avoiding being denatured in the presence of the gastric environment. Therefore, we designed a delivery system resistant to the gastrointestinal environment by engineering a L. lactis VP4 expression vector. In this study, the potential of using L. lactis to express heterologous rotavirus VP4 protein and its ability to act as an antigen delivery carrier for oral vaccination were analyzed. The immunogenicity of the recombinant VP4-expressing L. lactis was analyzed by oral administration of live bacteria in the BALB/c mice. Our data indicate that oral inoculation of VP4-expressing L. lactis can induce specifc immune responses, both in the mucosal and systemic immune systems in a mouse model study, which is useful for the subsequent evaluation of immune responses with recombinant L. lactis as a potential rotavirus oral vaccine in pigs.
2. Materials and Methods
2.1. Reagents
Rabbit anti-PRV serum, 1 : 3200 ELISA titer, was prepared as previously described in our laboratory [8]; MTT [3-(4,5-dimethylthiazol-2-y)-2,5-diphenyltetrazolium bromide], horseradish peroxidase-(HRP-) conjugated goat anti-mouse IgA, and HRP-conjugated goat anti-rabbit IgG were purchased from Sigma (St. Louis, MO). FITC-conjugated goat anti-rabbit IgG was purchased from Beijing Zhongshan Goldbridge Biotechnology Co. (Beijing, China).
2.2. Plasmids and Bacterial Strains
The lactococcal surface-expression vector pNZ8112, including the Cm resistance determinant, repA and repC replication elements, usp45 signal sequence, nisA-promoter, and the cell wall anchor motif obtained from Streptococcus pyogenes M6 protein, and the L. lactis strain NZ9000 were kindly provided by NIZO Food Research (Ede, The Netherlands). The pET-VP4 recombinant expression plasmid containing porcine rotavirus VP4 gene was constructed in our laboratory, and VP4 protein was expressed and purified as described previously [8]. JL94 isolates of rotavirus virus were propagated in MA104 cells (ATCC, Rockville, MD) as described [9].
2.3. Construction of the Lactococcus lactis VP4 Expression Vector
A 756-bp gene fragment encoding the main functional antigen regions of the rotavirus VP4 (1–252 amino acids, encompassing the whole VP8 and part of VP5) was obtained from the recombinant plasmid DNA pET-VP4 containing the complete VP4 gene using polymerase chain reaction (PCR) with a specific forward primer, 5′GACGCAAGCATGGCTTCGCTCATTTATAGACAA3′, containing an SphI site (underlined) and a reverse primer, 5′ATTTCTAGAAGCTCTAGAGTGCACTATCTCTCT3′, containing an XbaI site (underlined). PCR amplification conditions were as follows: 95°C for 5 minutes; 30 cycles of 95°C for 1 minute; 55°C for 1 minute; 72°C for 1 minute; and 72°C for 10 minutes for the final extension. PCR products were purified and digested by SphI and XbaI and then inserted into the corresponding sites of the pNZ8112 expression vector. Electroporation of Lactococcus lactis was carried out as previously described [10, 11]. Briefly, 10 μL of pNZ8112-VP4 plasmid DNA was added to 150 μL of L. lactis strain NZ9000, gently mixed at 4°C for 5 minutes, and subjected to a single electric pulse (25 μF of 2.5 kV/cm). The mixture was then incubated in M17 medium without Cm at 30°C anaerobically for 2 hours and was then selected on M17-agar medium containing 10 μg/mL of Cm. The respective NZ9000 transformants containing pNZ8112-VP4 plasmid DNA were extracted and subjected to restriction enzyme digestion, PCR (Figure 1), and sequencing (data not shown) was carried out for the identification. L. lactis strain NZ9000 transformed with the pNZ8112-VP4 was designated pNZ8112-VP4/NZ9000.
Figure 1.
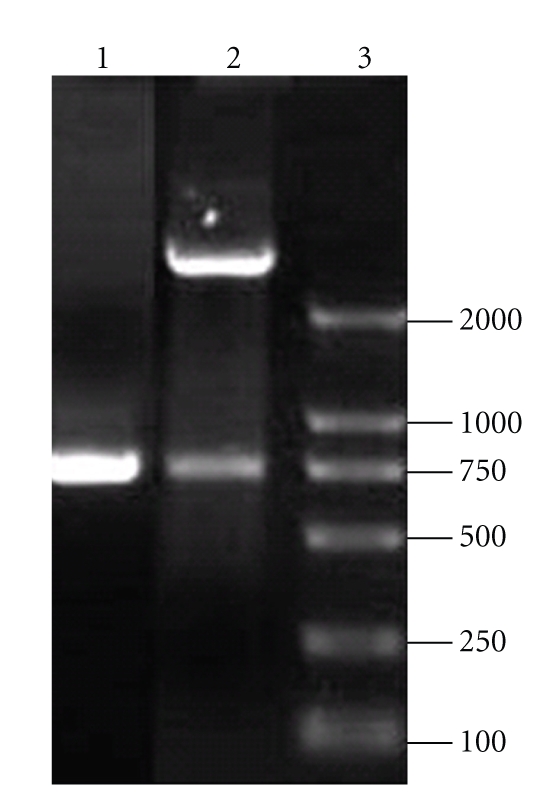
Restriction enzyme and PCR analysis of pNZ8112-VP4. Lane 1, a 756 bp VP4 fragment amplified from recombinant plasmid DNA pET-VP4; Lane 2, SphI and XbaI digestion of the recombinant expression vector pNZ8112-VP4 showing both vector and insert; Lane 3, 2000 bp DNA ladder.
2.4. VP4 Protein and Localization Analysis
To analyze the expression and localization of the VP4 fusion protein, pNZ8112-VP4/NZ9000 was inoculated in fresh M17 medium with 0.5% glucose at a ratio of 1 : 250. For the induction of nisA promoter, nisin was added to a final concentration of 10 ng/mL when grown to an optical density at 600 nm of 0.5. Growth was continued for 3 hours until an optical density at 600 nm of 1.0 was reached. Nisin-induced pNZ8112-VP4/NZ9000 was harvested by centrifugation at 12,000 g for 10 minutes at 4°C. The bacterial pellets were washed twice with sterile 50 mM Tris-Cl, pH 8.0, and incubated with 10 mg/mL lysozyme at 37°C for 60 minutes. Cell lysates were centrifuged at 15,000 g for l0 minutes, and the supernatant was maintained at −20°C for further analysis. The bacterial protein supernatant was examined by 12% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and then electrotransferred onto nitrocellulose membrane. Immunoblots were carried out using rabbit anti-VP4 serum in 10 mL at a dilution of 1 : 1000 with phosphate-buffered saline (PBS) at 37°C for 60 minutes after blocking with TBST (Tris-buffered saline, 0.05% Tween-20) buffer containing 5% skimmed milk at 4°C overnight and washed in PBS three times for 5 minutes between all steps. Immunoblots were then washed and incubated with 1 : 2000 dilution of HRP-conjugated goat anti-rabbit IgG in 10 mL for 60 minutes. Binding was visualized using Chemiluminescent Substrate reagent (Pierce) according to the manufacturer's instruction.
Immunofluorescence was used to analyze VP4 protein surface expression by pNZ8112-VP4/NZ9000 as described previously [12]. Briefly, 2 mL of 12-hour nisin-induced cultures including pNZ8112-VP4/ZN9000 and pNZ8112/NZ9000 were harvested by centrifugation at 4000 g for 10 minutes at 4°C, respectively, washed with PBS three times by centrifugation at 4000 g for 10 minutes each, resuspended in 1 mL sterile PBS 3% bovine serum albumin (BSA) containing anti-VP4 antibodies, and then incubated for 1 hour at 37°C. The cells were then pelleted and washed three times with sterile PBS 0.05% Tween 20. The cell-antibody complexes were then incubated in 2 mL of 1 : 500 dilution of FITC-conjugated goat anti-mouse IgG containing 1% Evans blue or of FITC-conjugated goat anti-mouse IgG without 1% Evans blue or 1% Evans blue only for 1 hour at 37°C in the dark. Cells were washed three times with PBS 0.05% Tween 20 and streaked onto slides and then air dried on a glass slide and fixed with cold acetone at −20°C for 30 minutes. Analysis was performed using a confocal microscope. Noninduced recombinant strains were used as negative controls.
2.5. Animal Immunizations
Eight-week old female BALB/c mice weighing 18–20 g, purchased from Harbin Veterinary Research Animal Laboratory (Harbin, China), were used as an animal model for the immune responses to pNZ8112-VP4/ZN9000 recombinant strain. The mice were randomly divided into two groups (n = 10) and were housed under standard conditions with free access to food and water. pNZ8112-VP4/NZ9000 and pNZ8112/NZ9000 were cultured and centrifuged. Cell pellets were washed once with sterile PBS and resuspended in PBS (pH 7.4) at a concentration of 1010 colony-forming units (CFU)/mL. All of the mice in the two groups orally received a dose of 109 CFU/mL. The immune protocol was performed as described previously [13]. Briefly, all the mice were administered on three consecutive days at days 0, 1, and 2. A booster immunization was administered at days 14, 15, and 16, and a second booster was given at days 28, 29, and 30.
2.6. ELISA Assays
Mouse sera were collected on days 0, 9, 25, and 41 following the first immunization and stored at −20°C until required. Feces were collected on days 0, 1, 7, 14, 17, 21, 24, 29, 31, and 35 after the first immunization, and IgA was extracted as described previously [14, 15]. Ophthalmic washes were obtained by washing eyes with 50 μL PBS on days 0, 9, 16, 25, 32, and 35 after the first immunization, and the vagina was washed with 200 mL PBS and collected on the same days as those of the ophthalmic washes. All of the samples were stored at −20°C until required. Before measurement by indirect ELISA, two identical samples from the sera, feces, as well as ophthalmic and vaginal washes were mixed together into one detection well for a total of four wells representative of eight samples, which were detected at different time points for each group. ELISA plates used to detect the sIgA levels in feces, ophthalmic and vaginal washes, and the IgG levels in sera were coated overnight at 4°C with 200 μL of 106.2 TCID50/0.2 mL porcine rotaviruses propagated on MA104 cells, and the MA104 cell cultures were used as a negative control. Wells were then washed with PBS containing 0.05% Tween 20 and blocked with 200 μL of PBS containing 5% skim milk at 37°C for 2 hours. After washing, 100 μL of fecal extracts, ophthalmic and vaginal washes, or 100-fold diluted sera samples were added and used as a primary antibody. After incubation at 37°C for 1 hour, the plates were washed three times with PBS containing 1% Tween 20, and 100 μL of 1 : 5000 diluted horseradish peroxidase-conjugated anti-mouse IgA or goat anti-mouse IgG was added to every well followed by incubation at 37°C for 1 hour. After washing three times with PBS containing 1% Tween 20, bound antibodies were visualized with 100 μL OPD-H2O2 substrate; the reaction was stopped by addition of an equal volume of 2 N H2SO4, and the optical density (OD) was measured at 490 nm using a microculture plate reader (ELX800 Bio-Tek Instruments).
2.7. Spleen Cell Proliferation Assay
The proliferation of mouse spleen cells was examined using the MTT assay. Spleen cells were isolated and purified as described [14] and were randomly divided into the control group (RPMI1640 media only), positive control group treated with 5 μg/mL of ConA, low VP4 group treated with 0.5 μg/mL of VP4 protein, and high VP4 group treated with 5 μg/mL VP4 protein. The cells were incubated in 5% CO2 at 37°C for 72 hours, and 10 μL of 5 g/L MTT was added to each well and then solubilized with 150 μL of 30% dimethyl sulfoxide (DMSO) for 10 minutes at 37°C. The optical density (OD) of each well was measured at 570 nm with an ELISA Reader (Elx800 Bio-Tek Instruments).
2.8. Determination of Neutralizing Antibodies
Neutralization was determined using the microplates [15]. Briefly, antisera of mice immunized with pNZ8112-VP4/NZ9000 and pNZ8112/NZ9000 were filtersterilized and inactivated at 56°C for 30 minutes and serially diluted at 2-fold increments from 1 : 5 to 1 : 640 by mixing 100 μL of serum with 100 μL of Eagle's medium containing 100 TCID50 of porcine rotavirus. The virus-serum mixture was incubated at 37°C in an atmosphere of 5% CO2 for 1 hour. Each diluted serum sample was tested in four replicate wells. The OD value of virus-induced cytotoxicity was measured at 96 hours after infection at 490 nm using the CytoTox 96 nonradioactive cytotoxicity assay (Promega) according to the instruction of the manufacturer, and neutralization titers were also examined for cytopathic effects (CPEs) under a microscope.
3. Results
3.1. Target Protein Identification
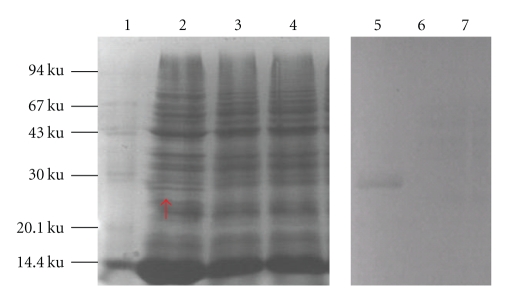
The cell lysates from nisin-induced pNZ8112-VP4/NZ9000, pNZ8112-VP4/NZ9000, and noninduced pNZ8112-VP4/NZ9000 were analyzed by SDS-PAGE and western blotting using anti-VP4 serum of porcine rotavirus. Coomassie blue gel staining revealed an approximately 27 kDa band in induced pNZ8112-VP4/NZ9000 (Figure 2, lane 2) but not in non-nisin-induced pNZ8112-VP4/NZ9000 (Figure 2, lane 3) or in pNZ8112/NZ9000 (Figure 2, lane 4). Similarly, an immunoreactive band was detected via western blot in a similar position as observed in the SDS-PAGE in induced pPNZ8112-VP4/NZ9000 (Figure 2, lane 5). As the negative control, the non-nisin-induced recombinant strain of pNZ8112-VP4/ZN9000 (Figure 2, lane 6) and induced pNZ8112/ZN9000 (Figure 2, lane 7) did not display the corresponding immunoreactive band. These results show that the nisin promoter from pNZ8112 could efficiently induce the expression of heterologous protein.
Figure 2.
SDS-PAGE and western blot analysis. Total cell lysates were analyzed by SDS-PAGE and western blot. Coomassie blue gel staining shows the expression of a 27 KD protein in pNZ8112-VP4/NZ9000 induced by nisin (lane 2, as arrow indicates the VP4 protein), and no corresponding protein in non-nisin induced pNZ8112-VP4/NZ9000 (lane 3) and nisin-induced pNZ8112/NZ9000 (lane 4) can be seen. Similar results were observed by western blot. Nisin-induced pNZ8112-VP4/NZ9000 (lane 5), non-nisin-induced pNZ8112-VP4/NZ9000 (lane 6), and nisin-induced pNZ8112/NZ9000 (lane 7), lane 1, Protein Molecular Marker.
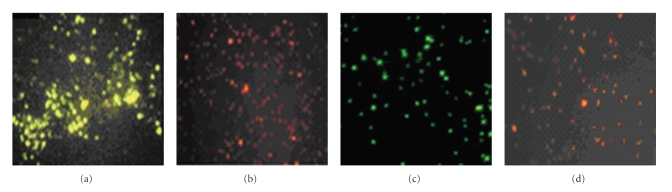
The immunofluorescence assay also confirmed the expression of VP4 protein. There was green-yellow or green fluorescence on the cell surface of nisin-induced pNZ8112-VP4/NZ9000 when incubated either with FITC-conjugated goat anti-mouse IgG containing 1% Evans blue (Figure 3(a)) or FITC-conjugated goat anti-mouse IgG (Figure 3(c)). Similarly, there was no green fluorescence in non-nisin-induced pNZ8112-VP4/NZ9000 (Figure 3(d)). There was no green fluorescence on the cell surface of nisin-induced pNZ8112/NZ9000, and the pellets was dyed red by Evans blue (Figure 3(b)). The localization analysis results suggested that VP4 protein could be exposed on the outer side of the cell wall. It could not be detected in the supernate of induced cultures of pNZ8112-VP4/NZ9000, even after concentrating 50-fold using a Millipore Ultrafree-15 column (data not shown).
Figure 3.
Cell surface expression of VP4. Nisin-induced pNZ8112-VP4/NZ9000 showed specific green fluorescence when stained either with FITC-conjugated goat anti-mouse IgG containing 1% Evans blue dye (a) or FITC-conjugated goat anti-mouse IgG without Evans blue dye (c). No green fluorescence was observed, but red pellets can be seen in induced pNZ8112-VP4/NZ9000 (d) stained with 1% Evans blue and in pNZ8112-/NZ9000 stained with FITC-conjugated goat anti-mouse IgG containing 1% Evans (b).
3.2. Anti-VP4 Responses Following Immunization
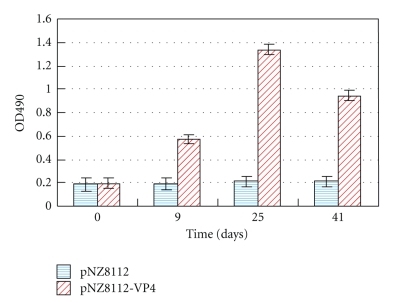
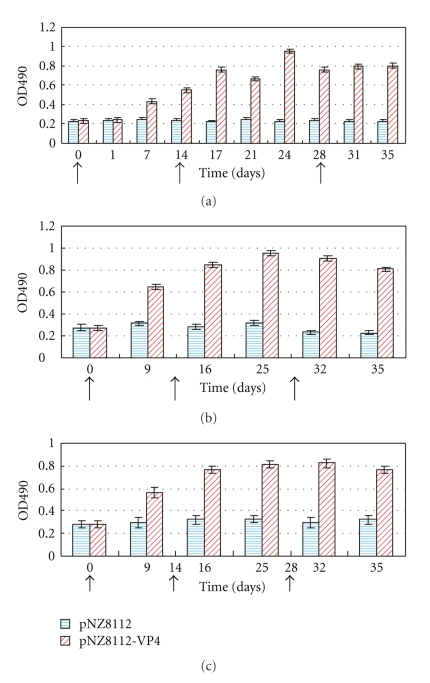
BALB/c mice were used as model animals for investigating the specific immune responses to a recombinant strain expressing VP4 antigen, and specific sIgA or IgG against porcine rotavirus VP4 were detected by indirect ELISA. To evaluate the levels in the local and systemic mucosal immune system, all mice were immunized via oral route. The results showed that there was no substantial difference in antibody titers both in sIgA and IgG between the pNZ8112-VP4/NZ9000 and pNZ8112/NZ9000 groups prior to immunization. Antigen-specific antibodies could be detected in the sera at day 9 following the first immunization. After the first booster, a high level of serum antibodies against porcine rotavirus VP4 occurred in the nisin-induced pNZ8112-VP4/NZ9000 group compared with the pNZ8112/NZ9000 group, and the specific antibody levels did not seem to increase much after a second booster when compared to the first booster; however, the levels remained high (Figure 4). Mucosal antibody sIgA was evaluated in feces (Figure 5(a)) and vaginal (Figure 5(b)) and ophthalmic washes (Figure 5(c)) in orally immunized mice. The results revealed an apparent increase in sIgA titers in these different samples at days 7–9 after the primary immunization. The higher anti-VP4 sIgA was obtained following the booster series, but a second booster did not raise the sIgA titers much higher than the first booster. These results indicate that a local and systemic mucosal sIgA and serum IgG response can be produced in mice immunized via the oral route.
Figure 4.
Specific anti-VP4 IgG in serum response after oral immunization. Mice were orally immunized with 109 CFU of pNZ8112-VP4/NZ9000 and pNZ8112-VP4/NZ9000, and the immune protocol was administered on three consecutive days: the inoculation at days 0, 1, and 2; a booster at days 14, 15, and 16; a second booster at days 28, 29, and 30. Mice sera were collected on days 0, 9, 25, and 41 following the first immunization. Sera samples were analyzed for the presence of IgG-specific VP4 antibodies by ELISA. The OD value of each well represents a mixture of two serum samples; the bars represent IgG mean OD values in four wells representative of eight samples, which were detected at different time points for each group.
Figure 5.
Anti-VP4-specific IgA responses. Specific anti-VP4 IgA was measured in fecal (a), vaginal washes (b), or lacrimal fluids (c). Mice were orally immunized with 109 CFU of pNZ8112-VP4/NZ9000 and pNZ8112-VP4/NZ9000, respectively. The immune protocol was administered on three consecutive days: immunization at days 0, 1, and 2; a booster at days 14, 15, and 16; and a second booster at days 28, 29, and 30 (the arrows on the X-axis indicate the times of vaccination and boosters). Samples from fecal extracts, vaginal washes, and lacrimal fluids were analyzed for the presence of specific Anti-VP4 IgA by ELISA. The OD value of each replicate well is the one of the mixture of two samples; the bars represent IgA mean OD values in four wells representative of eight samples, which were detected at different time points for each group.
3.3. Proliferation Assay
To explore the specific cellular immune responses of mice immunized with pNZ8112-VP4/NZ9000 against porcine rotavirus VP4, spleen cells from pNZ8112-VP4/NZ9000 and pNZ8112/NZ9000 groups following three immunizations were isolated and stimulated with purified porcine rotavirus VP4 protein at different doses in vitro. Proliferation of murine lymphocytes was detected by the MTT assay. Splenic cells from the pNZ8112-VP4/NZ9000 group significantly increased following specific stimulation with purified VP4 protein compared to the proliferation of spleen cells harvested from control mice (Table 1, P < .05).
Table 1.
Spleen cell proliferation to VP4 in immunized micea.
| Antigen dose (μg/mL) | pNZ8112b | pNZ8112-VP4c |
|---|---|---|
| 0.5 | 0.036d ± 0.002 | 0.087d ± 0.003 |
| 5.0 | 0.058d ± 0.001 | 0.971d ± 0.003 |
| Positive control (5 μg/mL Con A) | 0.871 ± 0.003 | 0.822 ± 0.002 |
| Control group (RPMI 1640) | 0.031 ± 0.002 | 0.026 ± 0.002 |
a n = 10.
bSpleen cell proliferation from mice immunized with L. lactis harboring the pNZ8112 plasmid.
cSpleen cell proliferation from mice immunized with L. lactis harboring the pNZ8112-VP4 plasmid.
d P < .05 versus control (t-test).
3.4. Neutralizing Ability of Porcine Rotavirus Infection in MA104 Cells
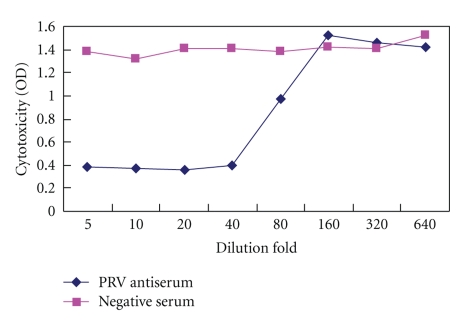
To check the neutralizing ability of porcine rotavirus infection in MA104 cells, serum samples obtained from mice immunized with pNZ8112-VP4/NZ9000 and pNZ8112/NZ9000 were incubated with porcine rotavirus at a dose of 100 TCID50 for 1 hour at 37°C in vitro and then used to infect MA104 cells. As shown in Figure 6, antiserum immunized with pNZ8112-VP4/NZ9000 inhibited cytopathicity in a dose-dependent manner. In contrast, the control serum immunized with pNZ8112/NZ9000 could not inhibit virus-induced cytopathicity, and a serum neutralization titer of 1 : 40 of pNZ8112-VP4/NZ9000-immunized mice could be determined according to the cytopathic effect (CPE) on MA104 cells infected with porcine rotaviruses.
Figure 6.
MA104 cells were infected with porcine rotaviruses (PRV) at a dose of 100 TCID50 in the presence of serial dilutions of anti-PRV antiserum or a negative serum. Anti-PRV antibodies inhibited cytopathicity in a dose-dependent manner. In contrast, the control serum could not inhibit virus induced cytopathicity.
4. Discussion
Many lactic acid bacteria (LAB) vectors used to express and deliver heterologous pathogen antigens have been constructed in recent years [16–19]. The discovery of such vectors offers a new way to explore live bacterial vehicle vaccines for the prevention of infectious diseases, especially in mucosal infectious diseases. In this paper, we constructed a surface-expression recombinant L. lactis pNZ-VP4/NZ9000, and VP4 expression was confirmed by SDS-PAGE, Western blot analysis, and immunostaining. Balb/c mice were immunized orally with recombinant L. lactis expressing VP4, and cellular, mucosal, and systemic immune responses were examined. We found that VP4-expressing recombinant L. lactis induced both local and systemic humoral and cellular immune responses against the VP4 rotavirus while using mice as model animals.
Group A rotaviruses, the main agents associated with significant diarrheal disease in humans and animals, are nonenveloped viruses containing 11 RNA fragments. Outer capsid proteins are of importance in infection and immunity. VP4, a nonglycosylated outer capsid protein [20], has been implicated as a virulence determinant in mice and piglets [21]. VP4 also induces neutralizing antibodies [22, 23], and antibodies directed against VP4 neutralize the virus in vitro [24, 25] and passively protect mice against heterologous rotavirus challenge in vivo [26]. Further studies have shown that VP4 effectively induces protective immunity in animals [27, 28]. Proteolytic cleavage of VP4 into VP5 and VP8 results in an enhancement of viral infectivity [29, 30]. DNA vaccines encoding the murine rotavirus proteins VP4, VP6, or VP7 can induce rotavirus-specific serum antibodies and virus-specific cytotoxic T lymphocyte responses generated by each of the three vaccines, but virus-neutralizing antibodies could be detected only in mice that were inoculated with DNA vaccines encoding for VP4 and VP7 [31]. VP6 DNA vaccine did not provide significant protection against a rotavirus challenge [32]. Therefore, the VP4 protein plays a major role in porcine rotavirus immune prophylaxis. Since the VP4 protein is associated with protective immunity, we used it as a target antigen to evaluate its immunogenicity as a potential mucosal vaccine.
Mucosal immunity is the first barrier in the prevention of infections beginning via the mouth, respiratory, and genital tracts. Stimulation of mucosal immunity is most efficiently induced following oral immunization, as it stimulates the production of sIgA and elicits systemic immunity. However, one obstacle in the development of effective oral vaccines is that target antigens are often unable to withstand denaturing in the environment of the stomach and intestinal tract before reaching the surface of the intestinal mucosa and effectively stimulating gut-associated lymphatic tissue. One strategy that can be used to overcome this is to develop a live expression vector that can both properly deliver and express the heterogenic antigen and survive in the intestinal milieu [17, 18]. We investigated the potential adhesion and persistence of cFDA-SE-labeled L. lactis NZ9000 orally fed to mice in the intestines. At day 7 following inoculation, the amounts of L. lactis NZ9000 that remained adherent to the intestinal mucosa were 69.76%, 51.59%, and 60.67% of those on the first day in the duodenum, jejunum, and ileum, respectively (data not shown). Lactococcus provides an excellent expression system, because it is safe and can persist in the intestinal mucosa. In addition, the long-term use of Lactococcus in the food industry supports the beneficial use of these kinds of organisms. L. lactis is one of the primarily utilized species in the food industry, in fermentation and in medicine. L. lactis grows rapidly and can be processed easily, making it an ideal expression vector for exogenous gene products [33–35].
Some recombinant lactobacilli expressing heterologous pathogen antigens have been constructed, such as Streptococcus pneumoniae antigens PsaA and PspA, cholera toxin B subunit, and transmissible gastroentritis coronavirus spike glycoprotein [36–38]. These organisms have been shown to be good live vehicles for the delivery of antigens for mucosal immunization. Currently, our research is under way to determine whether the recombinant Lactococcus lactis expressing porcine rotavirus VP4 protein could be used to stimulate mucosal and systemic immunization and to produce the protective anti-VP4 antibodies after mucosal vaccination in porcine model. Serial dilutions of the antisera of mice immunized with pNZ8112-VP4/NZ9000 and control sera immunized with pNZ8112/NZ9000 were mixed with an equal volume of 100 TCID50 rotavirus at 37°C for 1 hour, respectively, and MA104 cells were then infected with the virus-serum mixture. Cytopathicity was strongly inhibited by the addition of the antiserum of mice immunized with pNZ8112-VP4/NZ9000 in a dose-dependent manner. In contrast, the control sera could not inhibit virus-induced cytopathicity, indicating that the protective antigen-specific antibody responses in sera from the mice immunized orally were generated by our intervention.
IgA is the predominant antibody at the mucosal surface, as it is locally produced at a level that exceeds that of all of other immunoglobulins [39, 40]. Therefore, an oral porcine rotavirus vaccine must induce a specific mucosal IgA response. We used BALB/c mice as an animal model and evaluated the immunogenicity of VP4-expressed recombinant L. lactis following oral administration. Mucosal antibody secretory IgA (sIgA) was detected in feces vaginal and ophthalmic washes. Specific anti-VP4 sIgA can be obtained in feces via the oral route as well as in vaginal and ophthalmic washes, indicating that VP4-expressed recombinant L. lactis is able to elicit both local and systemic mucosal immune responses, which is important for specific resistance to respiratory and genital tract infectious diseases but not intestinal infectious diseases. From our experiments investigating the detection of mucosal sIgA and serum IgG after a prime vaccination, a booster at a two-week interval apparently increased the production of anti-VP4 sIgA, but a second booster did not seem to increase the sIgA titer much higher than that of the first booster. These results indicate a more rational vaccination procedure that would need to be administered to obtain a satisfactory antibody response. An oral administration regime was used in this study, which consisted of three sets of three successive daily doses of the VP4-expression recombinant L. lactis as the experimental vaccine. This protocol was adapted from the procedure of Challacombe [13], who found that this pattern of immunization was consistently effective when particulate oral vaccines were used to immunize mice. Whether this procedure is suitable for L. lactis expression in mice immunization needs to be further tested in future experiments.
Splenic cellular proliferation assay also supports the specific immune responses by immunogen VP4 induction. Following three vaccinations with VP4-expressed L. lacti in vivo, the activated lymphocytes were reexposed to the specific purified VP4 antigen and were then stimulated to initiate metabolizing cells, which can be detected with MTT. The results showed that splenic cells from the pNZ8112-VP4/NZ9000 group significantly increased following specific stimulation with purified VP4 protein compared to the proliferation of spleen cells harvested from control mice.
Many questions relevant for a good mucosal vaccine using the L. lactis recombinant strain of pNZ8112-VP4/NZ9000 remain to be investigated in the near future, such as dosage of inoculation, level of expression for the target antigen, optimal time of administration, and the reactivity of different animals. This is only a preliminary study using mice as model animals, and thus it is necessary to further investigate the immunogenicity and immunoprotection of porcine rotavirus VP4-expressed recombinant L. lactis in pigs.
The data presented in this report demonstrated that the use of L. lactis as an expression vector for the rotavirus VP4 surface antigen was safe and effective following oral vaccination in mice. The mice after administration are all in good condition. None of the mice exhibited any ill effects or died. Oral immunization elicited specific cellular and humoral immunity, including anti-VP4 sIgA, which is important for the development of a potential oral vaccine against porcine rotavirus infection initiated at mucosal surfaces. Future experiments will focus on testing VP4-expressed recombinant L. lactis vaccine in a porcine model of the disease as a means of establishing the formulation's effectiveness against infections in its natural host. Nevertheless, given its probiotic effects and harmless nature, lactic acid bacteria would make a very appropriate oral vaccine carrier to delivery heterologous antigens.
5. Conclusions
In this report, we found that using L. lactis as an expression vector for rotavirus VP4 surface antigen was safe and effective following oral vaccination in mice. Oral immunization elicited specific cellular and humoral immunity, including anti-VP4 sIgA. The protective antigen-specific antibody responses in sera from the mice immunized orally were generated by the neutralization assay in MA104 cells infected with porcine rotavirus, which is important in the control of infections initiated at mucosal surfaces. Future studies designed to evaluate the efficacy of VP4-expressed recombinant L. lactis in pigs are needed.
Acknowledgments
This work was supported by Grant no.2006BAD06A07 from the National Natural Sciences Funds of China and Program CXZ008 for Innovative Research Team of Northeast Agricultural University.
References
- 1.Li GP, Yi CR, Li FC, et al. Investigation of rotavirus infection in swine. Fujian Journal of Animal Husbandry and Veterinary Medicine. 1999;21(3):44–45. [Google Scholar]
- 2.Paul PS, Lyoo YS. Immunogens of rotaviruses. Veterinary Microbiology. 1993;37(3-4):299–317. doi: 10.1016/0378-1135(93)90031-2. [DOI] [PubMed] [Google Scholar]
- 3.Gorziglia M, Larralde G, Kapikian AZ, Chanock RM. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(18):7155–7159. doi: 10.1073/pnas.87.18.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansen K, Svensson L. Neutralization of rotavirus and recognition of immunologically important epitopes on VP4 and VP7 by human IgA. Archives of Virology. 1997;142(7):1491–1498. doi: 10.1007/s007050050175. [DOI] [PubMed] [Google Scholar]
- 5.Shaw DP, Morehouse LG, Solorzano RF. Experimental rotavirus infection in three-week-old pigs. American Journal of Veterinary Research. 1989;50(11):1961–1965. [PubMed] [Google Scholar]
- 6.Dewey CE, Carman S, Pasma T, Josephson G, McEwen B. Relationship between group A porcine rotavirus and management practices in swine herds in Ontario. The Canadian Veterinary Journal. 2003;44(8):649–653. [PMC free article] [PubMed] [Google Scholar]
- 7.Ball JM, Mitchell DM, Gibbons TF, Parr RD. Rotavirus NSP4: a multifunctional viral enterotoxin. Viral Immunology. 2005;18(1):27–40. doi: 10.1089/vim.2005.18.27. [DOI] [PubMed] [Google Scholar]
- 8.Song Y, Li YJ. Expression of vp4 gene from porcine rotavirus in E. coli. Virologica Sinica. 2004;(12):41–45. [Google Scholar]
- 9.Chen SH, Wang XS, Shi DF, et al. Isolation and characterization of porcine rotavirus. Chinese Journal of Preventive Veterinary Medicine. 2004;26(1):42–44. [Google Scholar]
- 10.Josson K, Scheirlinck T, Michiels F, et al. Characterization of a gram-positive broad-host-range plasmid isolated from Lactobacillus hilgardii. Plasmid. 1989;21(1):9–20. doi: 10.1016/0147-619x(89)90082-6. [DOI] [PubMed] [Google Scholar]
- 11.Holo H, Nes IF. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Applied and Environmental Microbiology. 1989;55(12):3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes-Perez NG, Bermúdez-Humarán LG, Le Loír Y, et al. Mice immunization with live lactococci displaying a surface anchored HPV-16 E7 oncoprotein. FEMS Microbiology Letters. 2003;229(1):37–42. doi: 10.1016/S0378-1097(03)00778-X. [DOI] [PubMed] [Google Scholar]
- 13.Challacombe SJ. Salivary antibodies and systemic tolerance in mice after oral immunization with bacterial antigens. Annals of the New York Academy of Sciences. 1983;409:177–193. doi: 10.1111/j.1749-6632.1983.tb26868.x. [DOI] [PubMed] [Google Scholar]
- 14.Aattouri N, Bouras M, Tome D, Marcos A, Lemonnier D. Oral ingestion of lactic-acid bacteria by rats increases lymphocyte proliferation and interferon-γ production. British Journal of Nutrition. 2002;87(4):367–373. doi: 10.1079/bjnbjn2001527. [DOI] [PubMed] [Google Scholar]
- 15.Veiga E, de Lorenzo V, Fernández LA. Neutralization of enteric coronaviruses with Escherichia coli cells expressing single-chain Fv-autotransporter fusions. Journal of Virology. 2003;77(24):13396–13398. doi: 10.1128/JVI.77.24.13396-13398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maassen CBM, Laman JD, Heijne den Bak-Glashouwer MJ, et al. Instruments for oral disease-intervention strategies: recombinant Lactobacillus casei expressing tetanus toxin fragment C for vaccination or myelin proteins for oral tolerance induction in multiple sclerosis. Vaccine. 1999;17(17):2117–2128. doi: 10.1016/s0264-410x(99)00010-9. [DOI] [PubMed] [Google Scholar]
- 17.Scheppler L, Vogel M, Zuercher AW, et al. Recombinant Lactobacillus johnsonii as a mucosal vaccine delivery vehicle. Vaccine. 2002;20(23-24):2913–2920. doi: 10.1016/s0264-410x(02)00229-3. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira MLS, Monedero V, Miyaji EN, Leite LCC, Ho PL, Pérez-Martínez G. Expression of Streptococcus pneumoniae antigens, PsaA (pneumococcal surface antigen A) and PspA (pneumococcal surface protein A) by Lactobacillus casei. FEMS Microbiology Letters. 2003;227(1):25–31. doi: 10.1016/S0378-1097(03)00645-1. [DOI] [PubMed] [Google Scholar]
- 19.Ho PS, Wang JK, Lee YK. Intragastric administration of Lactobacillus casei expressing transmissible gastroentritis coronavirus spike glycoprotein induced specific antibody production. Vaccine. 2005;23(11):1335–1342. doi: 10.1016/j.vaccine.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias CF, Lopez S, Espejo RT. Gene protein products of SA11 simian rotavirus genome. Journal of Virology. 1982;41(1):42–50. doi: 10.1128/jvi.41.1.42-50.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Offit PA, Blavat G, Greenberg HB, et al. Molecular basis of rotavirus virulence: role of gene segment 4. Journal of Virology. 1986;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshino Y, Sereno MM, Midthun K, Flores J, Kapikian AZ, Chanock RM. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Offit PA, Blavat G. Identification of the two rotavirus genes determining neutralization specificities. Journal of Virology. 1986;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns JW, Greenberg HB, Shaw RD, Estes MK. Functional and topographical analyses of epitopes on the hemagglutinin (VP4) of the simian rotavirus SA11. Journal of Virology. 1988;62(6):2164–2172. doi: 10.1128/jvi.62.6.2164-2172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniguchi K, Urasawa S, Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. Journal of General Virology. 1985;66(5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- 26.Offit PA, Shaw RD, Greenberg HB. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. Journal of Virology. 1986;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackow ER, Vo PT, Broome R, Bass D, Greenberg HB. Immunization with baculovirus-expressed VP4 protein passively protects against simian and murine rotavirus challenge. Journal of Virology. 1990;64(4):1698–1703. doi: 10.1128/jvi.64.4.1698-1703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Offit PA, Clark HF, Blavat G, Greenberg HB. Reassortant rotaviruses containing structural proteins VP3 and VP7 from different parents induce antibodies protective against each parental serotype. Journal of Virology. 1986;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estes MK, Graham DY, Mason BB. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. Journal of Virology. 1981;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espejo RT, Lopez S, Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. Journal of Virology. 1981;37(1):156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrmann JE, Chen SC, Fynan EF, et al. Protection against rotavirus infections by DNA vaccination. Journal of Infectious Diseases. 1996;174(supplement 1):S93–S97. doi: 10.1093/infdis/174.supplement_1.s93. [DOI] [PubMed] [Google Scholar]
- 32.García-Díaz A, López-Andújar P, Rodríguez Díaz J, et al. Nasal immunization of mice with a rotavirus DNA vaccine that induces protective intestinal IgA antibodies. Vaccine. 2004;23(4):489–498. doi: 10.1016/j.vaccine.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Raha AR, Varma NRS, Yusoff K, Ross E, Foo HL. Cell surface display system for Lactococcus lactis: a novel development for oral vaccine. Applied Microbiology and Biotechnology. 2005;68(1):75–81. doi: 10.1007/s00253-004-1851-8. [DOI] [PubMed] [Google Scholar]
- 34.Wang K, Huang L, Kong J, Zhang X. Expression of the capsid protein of porcine circovirus type 2 in Lactococcus lactis for oral vaccination. Journal of Virological Methods. 2008;150(1-2):1–6. doi: 10.1016/j.jviromet.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Que YAI, Haefliger J-A, Francioli P, Moreillon P. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infection and Immunity. 2000;68(6):3516–3522. doi: 10.1128/iai.68.6.3516-3522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira MLS, Monedero V, Miyaji EN, Leite LCC, Ho PL, Pérez-Martínez G. Expression of Streptococcus pneumoniae antigens, PsaA (pneumococcal surface antigen A) and PspA (pneumococcal surface protein A) by Lactobacillus casei. FEMS Microbiology Letters. 2003;227(1):25–31. doi: 10.1016/S0378-1097(03)00645-1. [DOI] [PubMed] [Google Scholar]
- 37.Slos P, Dutot P, Reymund J, et al. Production of cholera toxin B subunit in Lactobacillus. FEMS Microbiology Letters. 1998;169(1):29–36. doi: 10.1111/j.1574-6968.1998.tb13295.x. [DOI] [PubMed] [Google Scholar]
- 38.Ho PS, Kwang J, Lee YK. Intragastric administration of Lactobacillus casei expressing transmissible gastroentritis coronavirus spike glycoprotein induced specific antibody production. Vaccine. 2005;23(11):1335–1342. doi: 10.1016/j.vaccine.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandtzaeg P. Distribution and characteristics of mucosal immuno globulin-producing cells. In: Pearay OL, Metecky H, editors. Handbook of Mucosal Immunology. Boston, Mass, USA: Academic Press; 1994. pp. 251–279. [Google Scholar]
- 40.Kilian M, Russel MW. Functions of mucosal immunoglobulins. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee JR, Bienenstock J, editors. Handbook of Mucosal Immunology. San Diego, Calif, USA: Academic Press; 1994. pp. 127–143. [Google Scholar]