Abstract
Purpose of review
The innate immune system is our first line of disease against infection and injury, and responsible for initiating inflammatory and immune responses to resolve infections and repair injured tissues. This review focuses on the Toll-like receptors (TLRs) of the innate immune system and their role in recognizing infection and injury, and regulating inflammatory responses in the kidney.
Recent findings
There is increasing data to support a role for TLRs in immune complex-mediated glomerulonephritis. TLR7 has emerged as a key regulator of autoantibody production in murine lupus nephritis. In addition, studies have implicated TLR recognition of endogenous molecules released during cellular necrosis as critical regulators of sterile inflammation and injury. Tonic interactions between TLRs and environmental agonists derived from commensal microbes and endogenous sources may also influence autoimmune disease and inflammatory disorders affecting the kidney.
Conclusion
Future studies to decipher the contribution of TLRs and other innate immune receptors in the regulation of inflammation, immune responses, and injury in the kidney will pave the way for novel therapeutic interventions.
Keywords: autoimmunity, endogenous agonists, inflammation, innate immunity, sterile injury, Toll-like receptors
Introduction
Toll-like receptors (TLRs) are members of a growing family of innate immune receptors and the focus of this review. Innate immune receptors detect infection and regulate inflammatory and immune responses. This traditional view of innate immunity has been transformed by in-depth study over the past several years to reveal a much broader role for the innate immune system in regulating the interactions between the host and its environment.
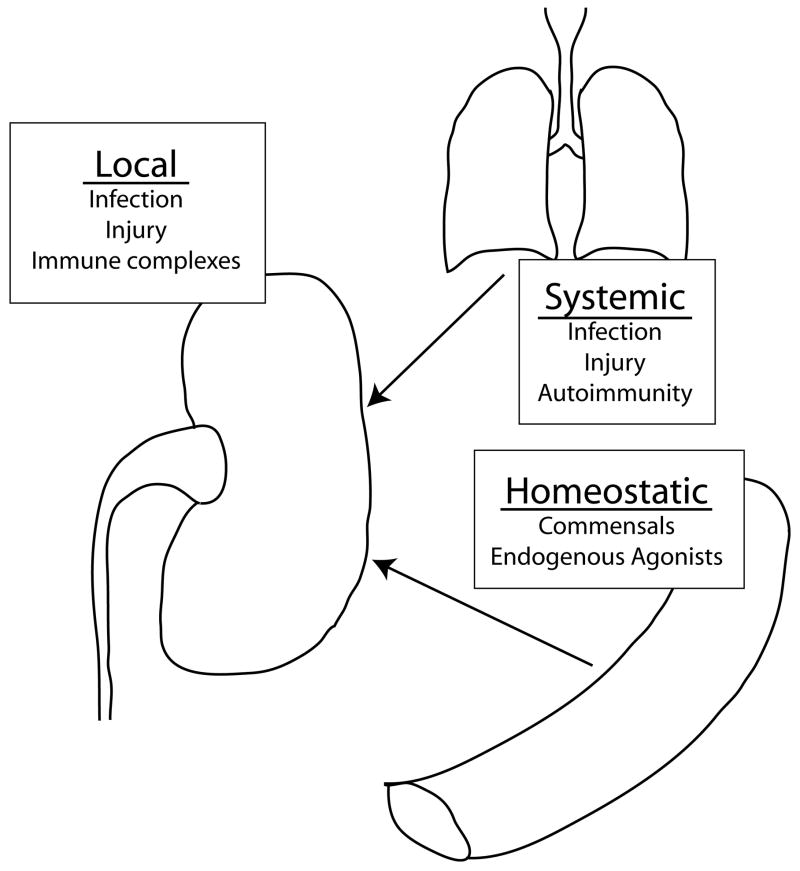
For simplicity, we can divide the innate immune system’s interaction with our environment into three classes (Fig. 1). The first is local recognition of infection and injury, in which local activation of innate immunity yields potent inflammatory responses and the initiation of immunity. The second class is systemic activation of innate immunity by infection or injury, which may indirectly affect the kidney through release of inflammatory mediators or the propagation of systemic diseases. The third class is homeostatic interactions between innate immune system and environmental stimuli. The innate immune system is constantly receiving input from our environment and these signals may influence self-nonself discrimination (allergic and autoimmune responses, and transplantation), as well as tissue healing and repair in response to injury.
Figure 1. Toll-like receptor regulation of kidney disease.
Toll-like receptors (TLRs) can influence kidney disease through multiple mechanisms. Local activation of TLRs by infectious agents, planted agonists (i.e. in the form of immune complexes) or the generation of endogenous agonists in the setting of injury. Systemic activation of TLRs can lead to autoantibody production, such as rheumatoid factor or lupus autoantibodies, which can be deposited in the kidney. Systemic activation can also produce inflammatory mediators that can influence preexisting disease within the kidney. Homeostatic interactions between TLRs and our environment, commensal microbes and endogenous agonists, provide tonic stimulation that influences self-nonself discrimination and basal inflammatory states.
Toll-like receptor recognition of agonists
Long-awaited breakthroughs in structural studies on TLRs have demonstrated the molecular details of how some agonists bind and activate TLRs [1–3]. Structures of the TLR2/TLR1 heterodimer with triacylated lipopeptide and TLR3 with double-stranded RNA demonstrate the precise nature of ligand-induced receptor dimerization that is required for activation [1,3]. Both the TLR3 and TLR2/1 agonists bind to specific sites and form a bridge between the receptors. This bridging interaction brings together the glycan-free surfaces of the TLRs, and the dimer is further stabilized by extensive intermolecular protein–protein interactions between the TLR molecules. The general model for TLR recognition of agonists that emerges from these studies is that the individual TLRs use diverse means of ligand binding and intermolecular interactions to form dimers that have similar quaternary structure, which properly aligns the cytoplasmic domains to initiate signal transduction.
The structure of the TLR4/myeloid differentiation factor 2 (MD-2) complex with eritoran, a synthetic antagonist of lipopolysaccharide (LPS), suggests that ligand-induced conformational changes may be an additional mechanism for ligand recognition [2]. In this structure, the four acyl chains of eritoran are tightly bound in the hyprophobic pocket of MD-2 and occupy greater than 90% of the pocket’s surface. MD-2 is bound to TLR4, and there is no direct interaction between eritoran and TLR4. Because agonistic forms of LPS have upto six acyl chains, conformational changes in the MD-2 LPS complex are predicted to accommodate the additional acyl chains. It is possible that conformational differences between the MD-2/eritoran and MD-2/LPS complexes determine whether or not TLR4 dimerizes. Future structures with agonist forms of LPS should provide insight into the molecular mechanisms that induce TLR dimerization.
Local regulation of kidney disease by exogenous agonists of Toll-like receptors
Exogenous TLR agonists access the kidney through two major routes: direct infection and via circulatory system. The best studied system of direct infection is urinary tract infection and pyelonephritis. Multiple TLRs contribute to the host innate immune response to urinary tract infection by Escherichia. coli, including TLR4, TLR5, and TLR11 [4]. These clever bugs also inject Toll-interleukin-1 receptor (TIR) domain containing proteins into host cells that interfere with TLR signaling and increase virulence [5]. Despite the convincing role of TLRs in mouse models of bacterial infection, less is known about the role for these receptors in human bacterial infections of the kidney. In fact, individuals with homozygous defects in MyD88 or interleukin-1 receptor-associated kinase 4 (IRAK4), essential signaling adaptors for TLR1, 2, 4, 5, 6, 7, 8, and 9 (Fig. 2), have no reported increase in urinary tract infections [6•]. Remarkably, these individuals primarily suffered from increased susceptibility to a narrow range of pyogenic bacterial infections, most commonly Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa, and these infections were limited to childhood and early adolescence [6•, 7•]. Little is known about the role of innate immunity to polyoma virus, which has become a substantial problem in renal transplantation. One recent study on mice [8] demonstrated that MyD88 contributed to the development of long-lasting antibody-mediated immunity to polyoma. A better understanding of the role of innate immunity in controlling polyoma virus infection may lead to novel strategies to increase natural immunity to this virus and preserve renal function in kidney transplant and immunosuppressed patients.
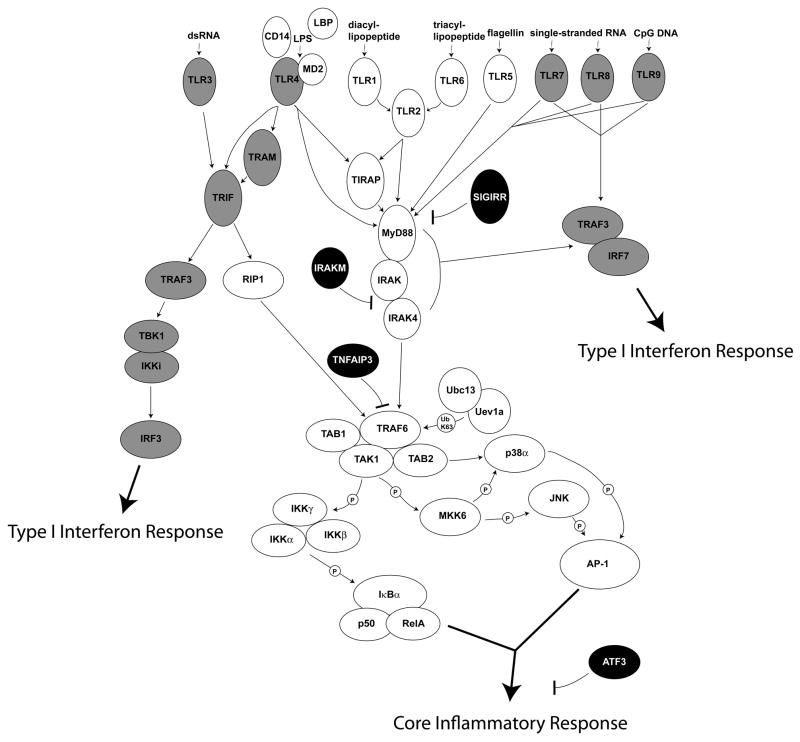
Figure 2. Toll-like receptor signaling module.
Toll-like receptors (TLRs) signal through proximal adaptors. TLR2 (+1 or 6), TLR4, TLR5, TLR7, TLR8, and TLR9 signal through MyD88 and the interleukin-1 receptor-associated kinase (IRAK) complex to activate nuclear factor-κB (NF-κB) and mitogen-activated protein (MAP) kinase pathways, leading to the expression of a broad set of genes involved in inflammatory responses. For TLR2 and TLR4, the Toll-interleukin-1 receptor (TIR) domain-containing adapter protein (TIRAP) is required for efficient and complete activation of this MyD88-dependent pathway. TLR7, TLR8, and TLR9 also activate IRF7 in a MyD88-dependent fashion leading to the expression of interferon-β(IFN-β) and type I IFN-associated genes. TLR3 signals through TIR domain-containing adapter-inducing IFN-β (TRIF), which activates the type I IFN response through IRF3 and also can activate NF-κB and MAP kinase pathways through receptor-interacting protein 1 (RIP1). TLR4 also engages the TRIF pathway and this is facilitated by TRIF-related adaptor molecule (TRAM). The TLRs that can activate type I IFN responses are shaded gray. Several negative regulator of TLR signaling are shown in black. Single Ig interleukin-1 receptor-related molecule (SIGIRR) is a TIR domain containing cell surface molecule that is believed to compete with TLRs for MyD88. IRAKM inhibits activation of the IRAK complex. TNFAIP3 inactivates TRAF6, blocking activation of NF-κB and MAP kinases. Activating transcription factor 3 (ATF3) binds to the promoters of some TLR-regulated genes and alters chromatin structure to affect gene expression.
TLR agonists may also be circulating in the blood free or as a component of immune complexes, which are then deposited in the kidneys, frequently glomeruli, causing local activation of the innate immune system. This model is mostly theoretical and although supported by circumstantial evidence, we still have very little evidence to support the deposition of TLR agonists in the kidney and whether such deposited TLR agonists contribute to kidney disease. For immune complex-mediated glomerulonephritis, there is evidence that viral agonists of TLRs may contribute to the immune complex-mediated glomerulonephritis. TLR3 mRNA is upregulated in glomerular mesangial cells in hepatitis C virus (HCV)-associated membranoproliferative glomerulonephritis [9], and TLR7 polymorphisms have been linked to chronic HCV infection [10]. In addition, HCV core and NS3 proteins have been reported to activate macrophages through TLR2 [11]. Thus, TLRs may recognize HCV in immune complexes and this could aggravate glomerulonephritis. In addition to HCV-associated glomerulonephritis, TLR agonists may also be components of other infection-associated glomerulonephritides such as postinfection glomerulonephritis and IgA nephropathy. Thus, local recognition of TLR agonists in immune deposits may affect the inflammatory activity of immune complex-mediated glomerulonephritis.
The contribution of local vs. systemic TLR recognition to disease progression has been technically difficult to differentiate in mouse models, and the experimental approach that has been most frequently relied upon to make this distinction is the generation of bone marrow chimeras. This strategy helps to define the contribution of hematopoietic cells and nonhematopoietic cells, but falls short of defining cell-type-specific contributions. The recent generation of inducible MyD88-deficient mice provides a new tool to evaluate the cell-type-specific responses of TLRs using the Cre-LoxP system [12•] and this should provide even more details about the precise role of intrinsic TLRs in the kidney.
Local regulation of kidney disease by endogenous agonists of Toll-like receptors
Sterile injury provokes inflammation and this inflammatory response has several physiologic and pathologic outcomes. The inflammatory response to injury is critical for wound healing and repair, and defense against opportunistic pathogens. However, the resulting inflammation may also lead to excessive tissue destruction and impaired organ function. Rodent models of toxin and ischemia-induced renal injury have demonstrated that inflammation contributes to the development of renal failure [13,14]. What are the receptors and ligands that trigger inflammation in these settings of sterile kidney injury? Recent studies implicate TLR2 and TLR4 as key regulators of inflammation in ischemia/reperfusion injury (IRI), but the results are complex. TLR2-deficient mice were protected from IRI [15,16••] and using bone marrow chimeras, Leemans et al. [15] found that the TLR2 effect was mediated by nonhematopoietic cells. Shigeoka et al. [16••] tested MyD88-deficient mice, which were also protected from IRI, but surprisingly, the MyD88 effect was substantially less than that seen for TLR2-deficient mice. In contrast, Wu et al. [17••] demonstrate substantial protection from IRI in TLR4-deficient mice and MyD88-deficient mice. Yet another study by Pulskens et al. [18••] concludes that TLR4-deficient mice are protected from IRI, whereas MyD88-deficient mice and TIR domain-containing adapter-inducing IFN-β (TRIF)-deficient mice are not. This group also found that the TLR4 effect is mediated by nonhematopoietic cells [18••]. In summary, these studies have found that TLR2 and TLR4 contribute to inflammation and renal insufficiency in IRI, and when tested these effects are mediated by nonhematopoietic cells. However, the studies disagree about the contribution of MyD88, the primary signaling adaptor for TLRs (except TLR3, Fig. 2). Future studies will be needed to clarify the roles of TLRs and their signaling pathways in IRI. TLR4 expressed in nonhematopoietic cells has also been demonstrated to contribute to the inflammatory response and kidney injury secondary to cisplatin [19].
A large number of molecules have been implicated as endogenous agonists of TLRs (Table 1) [20–36], and some of these could possibly mediate IRI. Wu et al. [17••] looked for upregulation of candidate TLR4 agonists and discovered that high mobility group box 1 (HMGB1), biglycan, and hyaluronan were increased in the ischemic kidney. The ensuing model is that IRI produces endogenous TLR4 agonists that activate TLR4 expressed by resident kidney cells, leading to inflammation and renal dysfunction. Unfortunately, much of the data concerning endogenous agonists of TLRs are plagued with issues of contamination [24,37,38]. HMGB1 has been ascribed multiple mechanisms for activating TLRs, which include direct binding to TLR2 and TLR4 [30,39], binding cellular DNA to promote endocytosis by receptor for advanced glycation endproducts (RAGE) and TLR9 activation [40], and promoting CD14-dependent TLR4 recognition of LPS [41]. Recombinant biglycan activates TLR2 and TLR4 in vitro, and biglycan-deficient mice are protected from sepsis induced by LPS or yeast cell wall [42]. Biglycan has also been shown to bind tumor necrosis factor (TNF) and transforming growth factor-β (TGF-β) and this activity may be relevant in vivo. Future studies should elucidate the molecular mechanisms of TLR2 and TLR4 activation during ischemic or toxic kidney injury and the role of endogenous TLR agonist in vivo.
Table 1.
Toll-like receptors and agonists
| Receptor | Microbial agonists | Endogenous agonists | Synthetic agonists | References |
|---|---|---|---|---|
| TLR2 (+1 or 6) | Lipoproteins, peptidoglycana, lipoteichoic acidsa, glycosylphosphatidylinositol anchors, lysophosphatidylserine, lipophosphoglycan, lipoarabinomannan, lipopolysaccharide, zymosan, viruses (measles, herpes simplex, varicella zoster, cytomegalovirus, lymphocytic choriomeningitis, hepatitis C) | Hsp60b, Hsp70b, gp96b, biglycan, hyaluronan, HMGB1b, uric acidb, minimally modified LDL | diacylated and triacylated lipopeptides | [20–26] |
| TLR3 | dsRNA (>40–50 bp) | mRNA | Poly-I,C | [27,28] |
| TLR4 | Lipopolysaccharide, viruses (respiratory syncytial, mouse mammary tumor, and Coxsackie virus) | Hsp60b, Hsp70b, gp96b, Hsp22, biglycan, uric acid b, hyaluronan, fibronectin, fibrinogen, heparan sulfate, surfactant protein A, HMGB1, β-defensin, Tamm-Horsfall protein | Monophosphoryl A | [24–26, 29–31] |
| TLR5 | Flagellin | [32] | ||
| TLR7 | ssRNA | ssRNA | Imidazoquinolines, synthetic ribonucleic acids | |
| TLR8 | ssRNA | ssRNA | Imidazoquinolines, Synthetic ribonucleic acids | |
| TLR9 | CpG DNA, hemozoin | CpG DNA | CpG oligonucleotides | [33,34] |
| TLR10c | ||||
| TLR11c | Profilin | [35] | ||
| TLR12c | ||||
| TLR13c |
HMGB1, high mobility group box 1; LDL, low-density lipoprotein; TLR, Toll-like receptor.
Preparations of lipoteichoic acids and peptidoglycans may contain lipopeptides, which are the authentic TLR2 agonist.
Some studies show conflicting results, implicating contaminants as active components, or signaling through other receptors.
TLR10, which is predicted to pair with TLR2, is a pseudogene in mice. TLR11 is a pseudogene in humans, and TLR12 and TLR13 are not present in humans. Additional TLRs are present in other vertebrates, which do not have obvious orthologs in humans or mice [36].
Systemic activation of Toll-like receptors and kidney disease
Systemic activation of the innate immune can affect the kidney in multiple ways; we will focus on two pathways. The first is innate immune system activation of B lymphocytes, leading to the production of autoantibodies and immune complex-mediated glomerulonephritis. The second is the exacerbation of preexisting kidney disease by TLR agonists. Most of these data stem from mouse models, which may provide some insight into human disease.
Mouse models and human genetic studies have provided additional evidence that innate immune system regulates autoantibody production, and in particular lupus autoantibodies and rheumatoid factor. The recent advances in our understanding of the roles of TLRs in systemic lupus erythematosus (SLE) have revealed the close link between the nature of autoantigens in lupus and the ligand specificity of the TLRs, namely TLR7 and TLR9. These findings also unveiled an imperfection in the innate immune system – innate discrimination of host from pathogen is not absolute. This is true in particular for the nucleic acid TLR agonists. Thus, dsRNA (TLR3), ssRNA (TLR7 and TLR8), and CpG DNA (TLR9) are not chemical structures that are unique to microbes. The ability to discriminate host nucleic acids from microbial nucleic acids appears to rely on a combination of properties, including sequence motifs, tertiary structure, chemical modifications (i.e. cytosine methylation), and the endosomal localization of these TLRs. TLR recognition of host nucleic acids may be a promiscuity that has been tolerated throughout evolution, because the selective advantage of pathogen detection outweighs the risk of autoantibody production.
Rheumatoid factor is a common component of cryoglobulins in mixed cryoglobulinemia, which can cause membranoproliferative glomerulonephritis and is a problematic manifestation of HCV infection. Elegant studies on a mouse model demonstrated that rheumatoid factor-producing B cells were elicited in extrafollicular sites by immune complexes that contain IgG and chromatin through a mechanism requiring surface IgM, TLR7, TLR9, and MyD88 [43••]. This generation of autoreactive rheumatoid factor-producing B cells did not require T-cell-specific signals [43••]. Thus, costimulation of autoreactive B cells by autoantigens, which are TLR agonists or associated with TLR agonists, may be a common mechanism for autoantibody production.
The role of innate immunity in lupus nephritis has been recently reviewed [44]. Both TLR7 and TLR9 have been implicated in autoantibody production in lupus, but recent studies on a murine lupus model (the MRL/Mplpr/lpr mouse) suggest that these receptors may have opposing roles [45]. TLR7-deficient mice did not produce anti-Smith or antiribonucleoprotein (RNP) antibodies and they were protected from lupus nephritis. In contrast, the TLR9-deficient mice did not make anti-DNA antibodies, but they had accelerated disease. Duplication of the TLR7 gene in Yaa mice was responsible for their lupus phenotype [46,47] and TLR7 was also required for the mouse model of pristine-induced lupus nephritis [48,49]. Thus, there is substantial evidence that copies of TLR7 or excess production of endogenous TLR7 agonists contribute to lupus nephritis in the mouse. Overexpression of TLR4 in mice also resulted in autoantibodies and lupus-like nephritis in C57BL/6 mice [50]. In addition, deletion of single Ig interleukin-1 receptor-related molecule (SIGIRR), a negative regulator of TLR signaling (Fig. 2), exacerbated lupus in mice [51]. These studies support a role for dysregulation of TLRs (or their agonists) in lupus and have uncovered a surprising protective role for TLR9.
The models of nephrotoxic serum-induced glomerulonephritis, membranoproliferative glomerulonephritis, in the mouse suggest that systemic activation of the innate immune system can increase inflammation and injury within the kidney. Passive transfer of antiglomerular basement membrane (GBM) antibodies induced only mild disease, which is markedly exacerbated by coadministration of TLR agonists [52]. Similarly, TLR9 agonists augmented glomerular inflammation in a mouse model of membranoproliferative glomerulonephritis [53]. These studies suggest that systemic activation of the innate immune system can aggravate preexisting kidney disease.
Homeostatic interactions between Toll-like receptors and environmental stimuli
The interaction between host and commensals is another area that has emerged in recent years as an important aspect of innate immunity. A seminal study [54] demonstrated that TLR-dependent sensing of commensal bacteria in the gut is critical for epithelial repair to toxic injury induced by dextran sodium sulfate. This study demonstrated that TLRs can sense commensal bacteria and use this as a signal to initiate wound healing and repair. More recent studies have illuminated the continuous interaction between our innate immune system and the environment. Mice that are deficient in TNFAIP3 (Fig. 2), a key negative regulator of TLR signaling, develop a spontaneous autoinflammatory disorder that involves multiple organs, including the kidney, and results in marked cachexia and early death [55]. This autoinflammatory disorder is dependent on MyD88 and commensal bacteria [56•], suggesting that tonic recognition of commensals by TLRs is kept in check by negative regulatory mechanisms. Human polymorphisms in TNFAIP3 have also been associated with SLE [57]. Interestingly, the increased anti-dsDNA antibody titer in TLR4-overexpressing mice was dependent on commensal flora [50]. In humans, MyD88 and IRAK4 are required for the efficient removal of autoreactive B cells [58••]. Finally, the development of diabetes in the nonobese diabetic (NOD) mouse was also influenced by MyD88 [59]. Thus, the development of autoantibodies and autoimmune diseases may in part be influenced by homeostatic interactions between TLRs and our environment, in particular commensal microbes and endogenous agonists. It is interesting to speculate that homeostatic interactions between TLRs and our environment are continuously regulating our inflammatory rheostat and self-nonself discrimination.
Beyond Toll-like receptors
Microbial infections are also recognized by additional families of innate immune receptors, which are increasingly recognized as important components of the innate immune response. These families include the NOD-like receptors (NLRs) and the retinoic acid-inducible gene (RIG)-like helicases (RLHs) [60,61]. These families of receptors primarily detect microbial products that gain access to the cytosol, or alterations in host cell, such as the efflux of potassium. Importantly, extracellular ATP, uric acid crystals, and calcium phosphate crystals have been shown to activate the caspase-1 inflammasome and interleukin-1B and interleukin-18 secretion through an NLR-dependent pathway [62,63]. Future studies concerning these families of innate immune receptors should provide additional insight into the role of the innate immune system in recognizing infection and injury and regulating inflammation in kidney diseases.
Conclusion
These recent studies underscore the importance of the innate immune system in kidney disease. A more detailed understanding of the role of the innate immune system in sterile injury will help to clarify the receptors, signaling pathways, and agonists that regulate this response. There are also potential roles for modulating the innate immune system to promote the clearance of unwanted infections, such as polyoma virus infection, and to modulate tissue repair due to injury. Finally, we are now beginning to appreciate the existence of tonic signaling through TLRs via ligands that emanate from commensal bacteria or endogenous molecules, and that dysregulation of this homeostasis can influence inflammatory and autoimmune diseases. Future studies to decipher the contribution of TLRs and other innate immune receptors in the regulation of inflammation, immune responses, and injury in the kidney will pave the way for novel therapeutic interventions.
Acknowledgments
K.D.S. is supported by grants from the US National Institutes of Health.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Jin MS, Kim SE, Heo JY. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Kim HM, Park BS, Kim JI. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Botos I, Wang Y. Structural basis of Toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song J, Abraham SN. TLR-mediated immune responses in the urinary tract. Curr Opin Microbiol. 2008;11:66–73. doi: 10.1016/j.mib.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirl C, Wieser A, Yadav M. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008;14:399–406. doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- 6•.von Bernuth H, Picard C, Jin Z. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. See Ref. [7•] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Ku CL, von Bernuth H, Picard C. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. These two studies (Ref.[6] and Ref.[7]) describe patients with homozygous deficiencies in MyD88 or IRAK4, and the susceptibility of these individuals to infections by primarily Streptococcus pneumoniae and some additional pyogenic bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guay HM, Andreyeva TA, Garcea RL. MyD88 is required for the formation of long-term humoral immunity to virus infection. J Immunol. 2007;178:5124–5131. doi: 10.4049/jimmunol.178.8.5124. [DOI] [PubMed] [Google Scholar]
- 9.Wornle M, Schmid H, Banas B. Novel role of Toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am J Pathol. 2006;168:370–385. doi: 10.2353/ajpath.2006.050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schott E, Witt H, Neumann K. Association of TLR7 single nucleotide polymorphisms with chronic HCV-infection and response to interferon-a-based therapy. J Viral Hepat. 2008;15:71–78. doi: 10.1111/j.1365-2893.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479–487. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 12•.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. These authors have generated a conditional knockout of MyD88 and use it in this study to demonstrate that this mouse will be very useful for dissecting cell-type-specific contributions of the TLR pathway to kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int Suppl. 2004;91:S56–S61. doi: 10.1111/j.1523-1755.2004.09109.x. [DOI] [PubMed] [Google Scholar]
- 14.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 15.Leemans JC, Stokman G, Claessen N. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Shigeoka AA, Holscher TD, King AJ. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. These four studies (Ref. [15,16••–18••]) provide compelling evidence that TLR2 and TLR4 contribute to inflammatory injury that is triggered during IRI in mice. Circumstantial evidence is introduced to support the role of endogenous agonists, which warrant further investigation. Conflicting data about the role of TLR signaling adaptor MyD88 is also presented. [DOI] [PubMed] [Google Scholar]
- 17••.Wu H, Chen G, Wyburn KR. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. These four studies (Ref. [15,16••–18••]) provide compelling evidence that TLR2 and TLR4 contribute to inflammatory injury that is triggered during IRI in mice. Circumstantial evidence is introduced to support the role of endogenous agonists, which warrant further investigation. Conflicting data about the role of TLR signaling adaptor MyD88 is also presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Pulskens WP, Teske GJ, Butter LM. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS ONE. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. These four studies (Ref. [15,16••–18••]) provide compelling evidence that TLR2 and TLR4 contribute to inflammatory injury that is triggered during IRI in mice. Circumstantial evidence is introduced to support the role of endogenous agonists, which warrant further investigation. Conflicting data about the role of TLR signaling adaptor MyD88 is also presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Ramesh G, Uematsu S. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol. 2008;19:923–932. doi: 10.1681/ASN.2007090982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi O, Kaufmann A, Grote K. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi O, Kawai T, Muhlradt PF. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi O, Sato S, Horiuchi T. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 23.Zahringer U, Lindner B, Inamura S. TLR2 - promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology. 2008;213:205–224. doi: 10.1016/j.imbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 25.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Finberg RW, Wang JP, Kurt-Jones EA. Toll like receptors and viruses. Rev Med Virol. 2007;17:35–43. doi: 10.1002/rmv.525. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 28.Kariko K, Ni H, Capodici J. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 29.Poltorak A, He X, Smirnova I. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 30.Apetoh L, Ghiringhelli F, Tesniere A. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 31.Saemann MD, Weichhart T, Zeyda M. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest. 2005;115:468–475. doi: 10.1172/JCI22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi F, Smith KD, Ozinsky A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 33.Coban C, Ishii KJ, Kawai T. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemmi H, Takeuchi O, Kawai T. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 35.Yarovinsky F, Zhang D, Andersen JF. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 36.Roach JC, Glusman G, Rowen L. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsan MF, Baochong G. Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J Endotoxin Res. 2007;13:6–14. doi: 10.1177/0968051907078604. [DOI] [PubMed] [Google Scholar]
- 39.Park JS, Gamboni-Robertson F, He Q. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 40.Tian J, Avalos AM, Mao SY. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 41.Youn JH, Oh YJ, Kim ES. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J Immunol. 2008;180:5067–5074. doi: 10.4049/jimmunol.180.7.5067. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer L, Babelova A, Kiss E. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Herlands RA, Christensen SR, Sweet RA. T cell-independent and Toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. This study elucidates the mechanism, whereby TLR agonists as components of immune complexes initiate the development of rheumatoid factor-producing B cells, independent of T cells and outside of B-cell follicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allam R, Anders HJ. The role of innate immunity in autoimmune tissue injury. Curr Opin Rheumatol. 2008;20:538–544. doi: 10.1097/BOR.0b013e3283025ed4. [DOI] [PubMed] [Google Scholar]
- 45.Christensen SR, Shupe J, Nickerson K. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Deane JA, Pisitkun P, Barrett RS. Control of Toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fairhurst AM, Hwang SH, Wang A. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur J Immunol. 2008;38:1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee PY, Kumagai Y, Li Y. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savarese E, Steinberg C, Pawar RD. Requirement of Toll-like receptor 7 for pristane-induced production of autoantibodies and development of murine lupus nephritis. Arthritis Rheum. 2008;58:1107–1115. doi: 10.1002/art.23407. [DOI] [PubMed] [Google Scholar]
- 50.Liu B, Yang Y, Dai J. TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J Immunol. 2006;177:6880–6888. doi: 10.4049/jimmunol.177.10.6880. [DOI] [PubMed] [Google Scholar]
- 51.Lech M, Kulkarni OP, Pfeiffer S. TIR8/SIGIRR prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J Exp Med. 2008;205:1879–1888. doi: 10.1084/jem.20072646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu Y, Xie C, Chen J. Innate stimuli accentuate end-organ damage by nephrotoxic antibodies via Fc receptor and TLR stimulation and IL-1/TNF-alpha production. J Immunol. 2006;176:632–639. doi: 10.4049/jimmunol.176.1.632. [DOI] [PubMed] [Google Scholar]
- 53.Anders HJ, Banas B, Linde Y. Bacterial CpG-DNA aggravates immune complex glomerulonephritis: role of TLR9-mediated expression of chemokines and chemokine receptors. J Am Soc Nephrol. 2003;14:317–326. doi: 10.1097/01.asn.0000042169.23931.73. [DOI] [PubMed] [Google Scholar]
- 54.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Lee EG, Boone DL, Chai S. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Turer EE, Tavares RM, Mortier E. Homeostatic MyD88-dependent signals cause lethal inflammation in the absence of A20. J Exp Med. 2008;205:451–464. doi: 10.1084/jem.20071108. This study demonstrates that TLRs are constantly exposed to signals from commensal bacteria and that TNFAIP3 (A20) is a crucial negative regulator of this tonic activation of TLR signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Musone SL, Taylor KE, Lu TT. Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nat Genet. 2008;40:1062–1064. doi: 10.1038/ng.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Isnardi I, Ng YS, Srdanovic I. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. These authors find that patients with deficiencies in MyD88 or IRAK4 fail to delete autoreactive B lymphocytes, suggesting that interactions between TLRs and the environment regulate B-cell development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wen L, Ley RE, Volchkov PY. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Martinon F, Petrilli V, Mayor A. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 63.Mariathasan S, Weiss DS, Newton K. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]