Abstract
The zinc(II) ion has recently been implicated in a number of novel functions and pathologies in loci as diverse as the brain, retina, small intestine, prostate, heart, pancreas and immune system. Zinc ions are a required nutrient but elevated concentrations are known to kill cells in vitro. Paradoxical observations regarding zinc’s effects have appeared frequently in the literature, and often their physiological relevance is unclear. We found that for PC-12, HeLa and HT-29 cell lines as well as primary cultures of cardiac myocytes and neurons in vitro in differing media, approximately 5 nmol/L free zinc (pZn = 8.3, where pZn is defined as − log10 [free Zn2+]) produced apparently healthy cells, but 20-fold higher or (in one case) lower concentrations were usually harmful as judged by multiple criteria. These results indicate that (1) the free zinc ion levels of media should be controlled with a metal ion buffer; (2) adding zinc or strong zinc ligands to an insufficiently buffered medium may lead to unpredictably low or high free zinc levels that are often harmful to cells; and (3) it is generally desirable to measure free zinc ion levels due to the presence of contaminating zinc in many biochemicals and unknown buffering capacity of many media.
Keywords: zinc, toxicity, HeLa, neuron, cardiomyocyte, PC-12, HeLa, HT-29
Introduction
Classically, zinc ions have been viewed biochemically as serving primarily in two roles: as a coenzyme for many enzymes of all classes and as the essential constituent of zinc fingers and related structural motifs found in numerous transcription factors.1,2 However, evidence has been accumulating of many other roles for zinc in humans and other organisms that are incompletely understood. For instance, although evidence has been presented3 that the granular zinc found in presynaptic boutons in some glutamatergic cortical neurons (notably in the mossy fibers of the hippocampus4,5) plays a role in long-term memory formation, there is as yet no consensus on its function.6 Furthermore, recent genome-wide association studies have identified mutations in the beta-cell-specific zinc transporter ZnT8 (SLC30A8) as a predisposing factor in type 2 diabetes,7 indicating that insulin-associated zinc plays an important role. Zinc has been observed to be released extracellularly and to accumulate intracellularly in models of ischemia, seizure and blunt trauma,8–11 but it remains unclear whether this is an effect or a cause of the subsequent neural damage. The origin and function of the zinc-rich drusen found in the retinas of persons with age-related macular degeneration remain obscure.12 The work of Bush and his colleagues13,14 showed that the hallmark amyloid plaques of patients with Alzheimer disease are rich in zinc as well as copper, and demonstrated that chelating drugs could reduce the plaques in animal models and ameliorate symptoms in some patients.15 It remains unclear what the role of zinc (or copper) is in this disease, but the approach is attractive. Evidence has emerged suggesting a role for zinc in prostate,16 pancreatic17 and breast cancer.18 In view of the well-known therapeutic intractability of type 2 diabetes, Alzheimer disease, age-related macular degeneration, stroke and pancreatic cancer, elucidating zinc’s biochemical role(s) in these disease processes especially may offer improved approaches for treatment.
For all these systems, a fundamental in vitro experimental approach has been to add zinc ions in some form to cultured cells or tissue and study the resulting response. Yet this approach has led to sometimes paradoxical results: for example, many workers have observed that addition of zinc to cells results in cell death,19,20 often by induction of apoptosis,21,22 whereas others have found that addition of avid, relatively selective cell-permeant zinc ion chelators, such as TPEN: N,N,N′,N′,-tetrakis(2-pyridylmethyl) ethylenediamine, also induces apoptosis23–25 and that added zinc ions seem to protect from apoptosis.26 While TPEN can also induce apoptosis with zinc bound, it remains unclear what a physiologically appropriate level or range of zinc ion concentration is. Several investigators have emphasized that while total zinc in many cells and sera is abundant for a ‘trace metal’ at tens to hundreds of micromolar, the concentration of zinc ions that are not tightly bound, especially by proteins, is much lower, and therefore the ‘free’ zinc ion concentrations in such media and tissues are also much lower.27,28 The free (other investigators have used ‘bioavailable’, ‘rapidly exchangeable’ or ‘labile’ as synonyms) zinc ion operationally represents that fraction which is relatively weakly bound and/or capable of rapidly exchanging ligands. Zn2+ in aqueous solutions is always liganded at least with water, chloride or other weak ligands; these ligands must dissociate rapidly to bind other ligands with higher affinity such as histidinyl imidazoles or thiols on cysteine. The free zinc ion concentration on the molar scale is conveniently expressed as its negative logarithm (in a manner analogous to pH) as pZn. Recently, free zinc ion concentrations in cells have been estimated29 and measured,30,31 and found to be in the picomolar regime. The goals of the present study were to determine what levels of added (total) zinc were harmful to widely used cell lines (including transformed cells as well as primary cultures of neurons and myocytes) in various media, and the relationship of free to total zinc ion concentrations under those diverse conditions. Multiple cell lines, different measures of cell toxicity, different methods of measuring free zinc and even multiple practitioners were employed to assure that the conclusions were not based on any particular cell type or method.
Relationship of free to total zinc in growth media
In typical growth media used with added serum as well as in serum itself, the free zinc ion concentration is usually orders of magnitude smaller than the total zinc concentration present, and only a small fraction of zinc that is added to the medium is actually available to interact with other parts of the system. When serum is added to the medium (typically 5–10% v/v), the relatively high affinity (KD ~ 0.1 μmol/L32) and abundant (0.3 mmol/L) albumin will bind a significant portion of the available zinc. In addition, most complete media contain ligands at millimolar concentrations with significant zinc affinity such as histidine, cysteine and phosphate. Finally, in addition to zinc already present at micromolar levels in serum, there are also usually nanomolar levels of zinc present as a contaminant in the medium ingredients.
Therefore, a zinc salt added to a growth medium, such as Neurobasal, Dulbecco’s modified Eagle’s medium (DMEM), M199 or most others, rapidly becomes bound to these other ligands, and is no longer rapidly exchangeable. The difference between added zinc ions and the free zinc ions actually present in the medium is illustrated for DMEM33 and an artificial cerebrospinal fluid (ACSF) to which zinc salts are added (Figure 1). The ACSF contains 142 mmol/L NaCl, 5 mmol/L KCl, 10 mmol/L glucose, 1 mmol/L MgCl2, 2 mmol/L CaCl2 and 10 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) at pH 7.4; it is open to the atmosphere and thus contains some carbonate/bicarbonate, but no phosphate (whose zinc salts are quite insoluble). There are no strong zinc ligands in the ACSF so adding 10 μmol/L total zinc yields about 0.3 μmol/L free zinc ion as measured by phase fluorometry using Newport Green DCF as fluorescent zinc indicator.34 In DMEM, however, 0.2 mmol/L histidine, 0.2 mmol/L cystine, 0.8 mmol/L lysine, 44 mmol/L NaHCO3, 0.9 mmol/L NaH2PO4, 9 μmol/L folate and 1 mmol/L pyruvate are all ligands for zinc ions of varying affinities. Evidently if the total zinc ion concentration in DMEM is increased to 100 μmol/L, the free zinc ion concentration still is less than 50 nanomolar. For defined media such as Neurobasal plus B-27 supplement,35 which has ingredients with known zinc ion affinities, the free zinc ion concentration can be calculated with reasonable accuracy36 using software such as MINEQL (Environmental Research Software, Hallowell, ME, USA). Since zinc levels in serum can vary at least two-fold, one can expect lot-to-lot variations in the total zinc ion present if serum is incorporated in the medium, and thus the free zinc ion concentration in these cases becomes difficult to predict.
Figure 1.
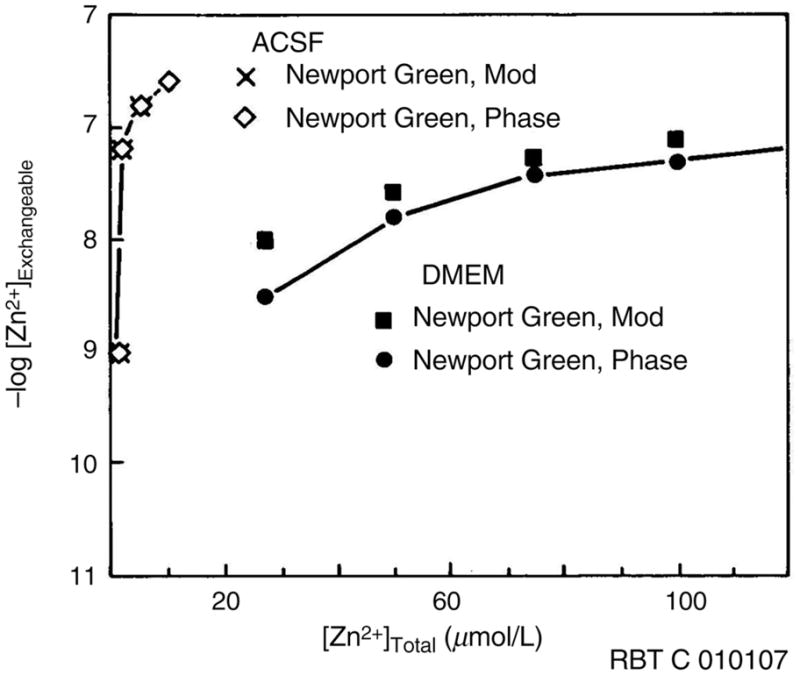
Free zinc in artificial cerebrospinal fluid (ACSF) and Dulbecco’s modified Eagle’s medium (DMEM) media measured by phase and modulation fluorescence lifetimes of Newport Green as a function of total zinc added. Reproduced with permission from Bozym et al.36
A different view has been advanced by Franklin et al.37 who obtained evidence that prostate cells expressing a characteristic zinc transporter (hZIP1) took up zinc ions from solution at similar rates whether there were free or complexed with citrate (KD ~ 10−5 at pH 7.5). In particular, they found (Figure 7) that the transporter exhibited similar high rates in the presence of 20 μmol/L ZnCl2 or 20 μmol/L ZnCl2 plus 60 μmol/L citrate, the latter of which we calculate to have a free zinc ion concentration of approximately 1.0 μmol/L. However, when complexed with ethylenediaminetetraacetic acid (KD ~ 10−13.4 mol/L at pH 7.5),38 yielding a free zinc ion concentration of less than 10−14 mol/L, the rate was almost zero. Since the published Km of the transporter is 7 μmol/L, one would ordinarily expect rapid transport at a free zinc ion concentration of 20 μmol/L but much slower transport at 1 μmol/L. These results suggest that the hZIP1 transporter recognizes and is capable to transport both ‘free’ zinc ions loosely bound to weak ligands and the (fairly stable) citrate complex. The implication is that for this transporter at least the relevant zinc concentration is much greater than the free zinc ion alone since it includes some complexed zinc, and that small molecule complexes with significant stability may insert zinc into other metalloproteins as well. While actual zinc chaperones apart from metallothioneins remain to be described, Cherezov et al.39 have proposed that some transporter proteins in bacteria can also interact with metallochaperones for zinc akin to the well-known copper chaperone proteins. This result demands confirmation and extension to other abundant metal-binding ligands.
For all the media, including those intractable to modeling, we measured the free zinc ion concentration using zinc-selective fluorescent indicator systems, including Newport Green (by changes in intensity or fluorescence lifetime);34,40 ZnPyr-1 or Zn-AF2 by intensity;41,42 or carbonic anhydrase by intensity ratio.43 Our experience with all these indicators is that they respond quickly to zinc ions in solution with rapidly exchangeable ligands such as water or chloride, but not to zinc tightly bound to albumin, other proteins, thiols or histidine; essentially, they respond to what we would term ‘free’ zinc ions.
We chose to study the apparent toxicity of zinc ions to a variety of cell types in differing media, with toxicity judged by a variety of criteria. Our goal was to understand whether zinc ions are a more potent toxin for some cell types than others, whether a range of free zinc ions exists that is non-toxic to most cells, to what degree the medium affects the apparent toxicity, and whether the apparent toxicity can be simply related to the level of free zinc ions.
Experimental section
As described below, either immortal cell lines or primary cultures in differing media were exposed to varying concentrations of total zinc, and their viability or function tested using different methods; the free zinc ions levels were also measured as zinc was added.
Rat cardiomyocytes
Briefly, the hearts from 250-g Wistar male rats were quickly removed and perfused in a nominally Ca2+-free medium for 15 min, and then with 1 mg/mL of collagenase added with 30 μmol/L CaCl2. After 20 min, the hearts were removed and cut into several pieces. The tissue was then gently dissociated through a wide-bore tip pipette. The cells were filtered on a 250-μm nylon mesh and incubated for 15 min at 37°C. Meantime, the calcium ion concentration of the incubation medium was increased step by step up to 1 mmol/L. The preparation provided at least 6 × 106 rod-shaped cells. Cells were kept at 37°C in HEPES-buffered medium adjusted to pH 7.4 and containing 117 mmol/L NaCl, 5.7 mmol/L KCl, 4.4 mmol/L NaHCO3, 1.5 mmol/L KH2PO4, 1.7 mmol/L MgCl2, 1 mmol/L CaCl2, 21 mmol/L HEPES, 11 mmol/L glucose, 10 mmol/L creatine, 20 mmol/L taurine and 0.5% bovine serum albumin. Various concentrations of ZnCl2 were added to the incubation buffer, and pZn was measured using the pZn meter as described below. Cell viability was assessed by Trypan Blue staining at different time points. All animal studies were carried out using humane methods under the supervision and with the approval of the respective Institutional Review Boards and the National Institutes of Health.
Rat PC-12 cells
These cells, a rat adrenal pheochromocytoma line, were cultured on 38 mm dishes with cover slip bottoms (World Precision Instruments) on Neurobasal + B-27 without Phenol Red but with glutamine added at 37°C under a 5% CO2 humidified atmosphere as previously described.30 Free zinc ion concentrations in media were measured ratio-metrically and apoptosis assessed by Annexin V-Oregon Green staining (Invitrogen) using fluorescence microscopy, both also as previously described; free zinc ion levels were also calculated using MINEQL + (Environmental Research Software, Hallowell, ME, USA).
Mouse cortical neurons
Frontal cortex tissue was dissociated from 15- to16-day-old BALB/c/Icr murine embryos and the neurons were cultured in DMEM with 5% (v/v) horse serum on an array of 64 microelectrodes as previously described.44 This array device permits recording the electrophysiological response of scores of individual neurons as a function of time in a short period under identical conditions; transparent indium tin oxide electrodes permit simultaneous observation under the microscope with electrophysiological measurements. Spike rate histograms were acquired for several neurons following a resting period in DMEM without added serum and infusion of the zinc solution; the values of the points are the averages of scores of neurons.
HeLa cells
HeLa cells represent a human epithelial cervical adenocarcinoma line from a 31-year-old patient containing HPV-18 sequences and low p53 expression. HeLa’s are among the most widely used cells worldwide. They were cultured in Opti-MEM (Invitrogen) with 5% (v/v) heat-inactivated fetal calf serum, 2 mmol/L glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin. Following addition of zinc sulfate, free zinc in the medium was measured by pZn meter as follows: samples of media were diluted 1:1 in 50 mmol/L HEPES pH 7.5 buffer containing ZinPyr-1 to give a final concentration of the latter of 100 nmol/L. The buffer had been previously treated with Chelex-100 chelating resin (Bio-Rad, Richmond, CA, USA) to remove traces of zinc and other potentially interfering metal ions. The mixtures were allowed to equilibrate for 10 min, following which the fluorescence intensity was read and compared with zero and maximal zinc levels obtained by adding TPEN and 1 mmol/L zinc sulfate to HEPES buffer, respectively.
HT-29 Cells
Human colorectal adenocarcinoma cells (HT-29) were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM with high glucose, L-glutamine and pyridoxine hydrochloride (all Gibco/Invitrogen), with 10% (v/v) fetal bovine serum (Hyclone), 0.1 mg/mL Penicillin G and 0.1 mg/mL streptomycin sulfate, at 37°C under a 5% CO2 humidified atmosphere. Cell survival was measured by Trypan Blue staining. pZn was measured by pZn meter using Zn-AF2 (Calbiochem).
Measurements of free zinc ions
Free zinc was measured in solution using either Zn-AF2 (Calbiochem) or ZinPyr-1 by fluorescence intensity using the pZn Meter (both Neurobiotex, Galveston, TX, USA), Newport Green DCF by phase (lifetime) fluorometry (Figure 1) as previously described34 (and see below), or apoH36C-Alexa Fluor 594 human carbonic anydrase II plus Dapoxyl sulfonamide by fluorescence ratio, also as previously described30 (see below). The pZn meter was the original model (now discontinued), comprising a 470 nm LED for excitation and a spectrometer with silicon photodiode array for detection; Zn-AF2 in dimethyl sulfoxide was added to 1 mL final volume sample in 50 mmol/L HEPES pH 7.5, that was treated with Chelex-100 resin (Bio-Rad) to remove impure metal ions, to a final concentration of 200 nmol/L and the fluorescence emission integrated between 518 and 523 nm for 100 ms. Calibration was by the method of standard additions, with a typical reagent blank equivalent to approximately 2 nmol/L zinc. Note that the pZn meter is generally used in a ‘stoichiometric’ mode45 whereby the initial concentration of free zinc and indicator (Zn-AF2 or ZinPyr-1) are both above the Kd’s of the indicators (2.7 nmol/L for Zn-AF2 and ~2 nmol/L for ZinPyr-1) and there is a limited amount of zinc available, such that the indicator essentially scavenges all the available zinc and produces a signal proportional to the zinc level. If good zinc ligands are present, as in the serum used with most eukaryotic cell growth media, they essentially compete with the indicators for the free zinc ions and one observes a reduced apparent level of free zinc ions. This effect can be accounted for exactly if the concentrations of the ligands and their affinities are known;46 it is preferable to use a small amount of an indicator whose affinity is close to the free zinc ion level in an equilibrium binding mode to minimize the perturbation to the speciation of the zinc.
Newport Green DCF40 exhibits a significant increase in intensity, together with an increase in average fluorescence lifetime from 0.88 to 2.93 ns upon binding zinc (KD ~ 1.4 ±0.2 μmol/L at pH 7.4, 37°C, in 124 mmol/L NaCl, 1.75 mmol/L KCl, 10 mmol/L MOPS). Neither calcium at 4.0 mmol/L nor magnesium at 3.2 mmol/L perturb the apparent KD perceptibly.34 The change in proportion of the lifetimes corresponding to metal-free and -bound probes is a simple function of the free zinc ion concentration; the lifetime change can be easily measured by either time-domain or frequency-domain (phase) fluorometry. One can measure the fluorescence decay as a function of time or the phase angle and modulation at multiple frequencies and fit the result to two lifetimes: the proportions of free and bound equal the pre-exponential factors. Simpler is to measure the phase and modulation at a single modulation frequency (60 MHz) where the phase increases from 14 to 40 degrees as the probe saturation with zinc ion increases from 0% to 100%. The advantage of the lifetime-based measurement is its relative freedom from interference due to variations in excitation intensity or probe concentration, inner filter effects and photobleaching, all of which create errors in fluorescence intensity measurements.47 Experimentally, one simply adds a suitable (circa 1 μmol/L) concentration to the sample and measures the lifetime. Overall this is a powerful method and Newport Green DCF is a selective, responsive probe but its modest affinity limits its usefulness.
By comparison, the FRET-based excitation ratiometric method, which uses a variant of carbonic anhydrase to recognize the zinc, is sensitive (down to picomolar free zinc concentrations), very specific (calcium at 10 mmol/L and magnesium at 50 mmol/L do not interfere) and also free of many artifacts that distort intensity measurements.48 As an excitation ratiometric measurement it is easy to perform in microscopes as well as fluorometers, and requires simpler instrumentation than lifetime measurements. Briefly, the zinc-free carbonic anhydrase is labeled with a fluorophore such as Alexa Fluor 594 and exposed to the zinc-containing sample together with a second fluorophore, Dapoxyl sulfonamide. The Dapoxyl sulfonamide is also fluorescent and is spectroscopically suited to serve as a FRET donor to the Alexa Fluor 594; it binds to the carbonic anhydrase if and only if there is zinc in the active site, and in that case instead of observing Dapoxyl sulfonamide emission with UV excitation one observes Alexa Fluor 594 emission in the red due to FRET arising from the close proximity of the Dapoxyl sulfonamide donor. In the absence of zinc, Dapoxyl sulfonamide does not bind and little UV-excited red emission is observed; the UV-excited intensity is ratioed with red emission from the Alexa Fluor 594 directly excited in the green at 540 nm. The affinity, selectivity and binding kinetics of the carbonic anhydrase can be improved by mutagenesis (reviewed in Fierke and Thompson43). Experimentally one adds the fluorescent labeled apoprotein at 50 nmol/L to 1 μmol/L together with the Dapoxyl sulfonamide (at 5 μmol/L in DMF) to the specimen and measures the ratio; usually the system equilibrates in 20 min or less.30 It is important in unbuffered solutions to use only small amounts of the protein since excess protein may scavenge up all the available free zinc ion, leading to inaccuracy. Many systems such as growth media and the interiors of cells are strongly zinc buffered,31 and in those cases the perturbation from apocarbonic anhydrase is much less.
Results
Adult rat cardiomyocytes in M199 (Korichneva)
Primary cultures of rat cardiomyocytes are a widely used model for studies of the heart and its electrophysiology; in this case they were cultured in the standard M199 medium containing 5% fetal bovine serum. The cells were exposed to the medium with varying amounts of zinc salts added to give the totals depicted in Figure 2, and the proportion of cells surviving as measured by Trypan Blue staining after 30 min is shown. The concentration of free zinc ions as measured by a pZn meter (NeuroBioTex) is also included in the figure. There is a gentle decline in cell survival as zinc is added up to the region of 50 μmol/L, corresponding to a pZn of ~6.7 (0.5 μmol/L), but with further increases corresponding to a pZn of ~6.5 there is an abrupt decline in survivability to less than 50%, and by pZn ~6.2 (total zinc ion added = 1 mmol/L) survival is almost nil. We note that this prompt measurement of cell survival does not take into account cells dying as a result of generally slower processes, such as apoptosis (see below).
Figure 2.

Survival (open circles) of adult rat cardiomyocytes and pZn (filled squares) in M199 media as a function of total zinc added
Zinc induction of apoptosis in PC-12 cells cultured in Neurobasal (Bozym and Thompson)
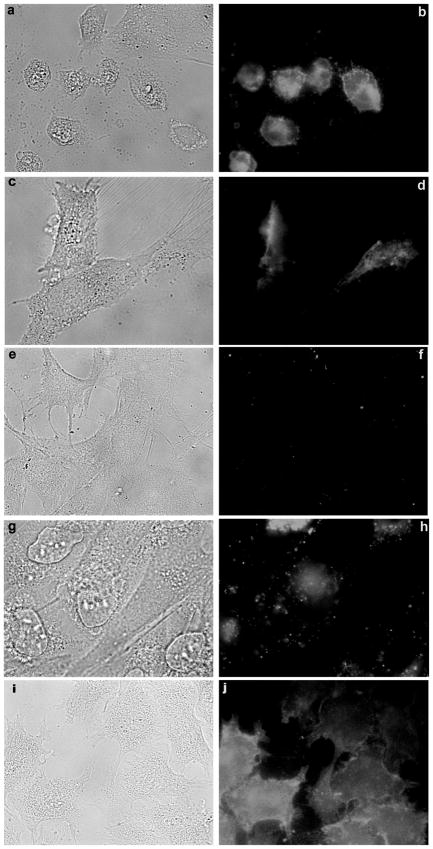
The rat pheochromocytoma cell line PC-12 is widely used as a model for neurons because nerve growth factor induces the growth of axon-like processes on the cell body. We find them of particular value because they can be grown in Neurobasal plus B-27 supplement,35 a serum-free medium with a known total zinc concentration; moreover, knowing the composition and the affinities of the ligands present, the free zinc concentration can be accurately predicted as zinc is added.36 To raise the free zinc concentration of the medium we added zinc salts to Neurobasal above the 2.6 μmol/L total zinc already present; without the addition of zinc, the free zinc ion level is about 5 nmol/L (pZn = 8.3). To lower the free zinc we added nitrilotriacetic acid (NTA) to the medium. NTA is a high-affinity zinc chelator that has very little propensity to penetrate the cell membrane because it is a polyanion. We note that while NTA is not perfectly selective for Zn(II) (pKD ~ 8.3)38 over Ca(II) or Mg(II) (6.3 and 5.6, respectively49), the small amount added perturbs the latter two metal ion concentrations only slightly. We observed the morphology of the cells for signs of toxicity or apoptosis, including rounding up, nuclear and cytoplasmic breakup, and the appearance of blebs on the cell surface. The cells were also stained with Oregon Green-Annexin V, which binds to phosphatidylserine that appears on the outside of cells as a result of apoptosis;50 thus brighter fluorescence with this stain indicates cells undergoing apoptosis. The results of these experiments are depicted in Figure 3. In Neurobasal plus B-27 without added zinc ions or chelator, the cells appear normal for many days and exhibit little or no staining with Annexin V, as expected (panels e and f). If 1 mmol/L NTA is added, the free zinc of the medium is lowered from ~5 nmol/L (pZn = 8.3) to ~280 pmol/L (pZn = 9.5), and the cells (panels c and d) begin to round up and undergo apoptosis as judged by strong Annexin V staining and the appearance of blebs. Additional NTA (to 10 mmol/L) lowers the free zinc ion to 20 pmol/L and virtually all the cells have rounded up (panels a and b); note that cells that have detached from the surface will not be observed and are thus difficult to quantitate. By comparison, treatment with NTA already stoichiometrically complexed with zinc had only a modest effect (results not shown), indicating that the frank toxicity seen with added NTA was due to chelation of zinc ions and not by some other property of the NTA. This is unlike the zinc complex of TPEN,30 which like TPEN itself is apoptogenic, albeit less potently. As zinc ions (30 μmol/L total) are added to the medium, increasing the free zinc ion concentration about 10-fold (68 nmol/L, pZn = 7.2) an increasing proportion of cells stain with Annexin V and display morphology (blebbing, apparent breakup of the nucleus, rounding up) also consistent with induction of apoptosis (panels g and h). Adding total zinc amounts (circa 1 mmol/L total) sufficient to bring free zinc ions up to 4 μmol/L (pZn = 5.4) also elicits toxicity as judged by Annexin V staining; however, these cells (panels i and j) are not rounding up. We speculate that the zinc ion concentration in this case is high enough to kill the cells by pathways more prompt than apoptosis and perhaps interfere with portions of the apoptotic process as well; for instance, zinc is a potent inhibitor of caspase-3.51,52 The much shorter exposure times used to image the Annexin V fluorescent staining in panels b, d and j underscore the intensity of Annexin V staining under those conditions. For these cells, we would conclude that free zinc ion concentrations much higher or lower than 10 nmol/L are clearly toxic. Finally, it is important to note that the stoichiometric NTA–Zn(II) complex comprises a total zinc ion concentration of approximately 1000 μmol/L but exhibits only modest toxicity, indicating that free zinc ions and not total zinc are the relevant parameter in this case.
Figure 3.
Bright field (panels a, c, e, g, i) and Oregon Green-Annexin V fluorescence (panels b, d, f, h, j) micrographs of PC-12 cells exposed to Neurobasal + B-27 media with 3 μmol/L total, 20 pM free (a, b); 2.6 μmol/L total, 0.5 nmol/L free (c, d); 2.6 μmol/L total, 5 nmol/L free (e, f); 30 μmol/L total, 68 nmol/L free (g, h); and ~1 mmol/L total, 4 μmol/L free (i, j) zinc ions. Fluorescence micrograph exposure times were 30 ms (panel b), 100 ms (d), 500 ms (e), 500 ms (h) and 35 ms (j). (A color version of this figure is available in the online journal)
Cultured mouse cortical neurons (Parviz and Gross)
One of the earliest observations of cellular zinc toxicity by Yokoyama et al.19 showed that nanomolar concentrations of zinc were overtly toxic to neurons. We note that the cerebrospinal fluid for the most part lacks the abundant, fairly strong zinc ligands present in serum such as albumin53 such that a higher proportion of added zinc remains free than in serum, and may help explain the brain’s susceptibility to injury from zinc and other toxic metals. The lack of such ligands would seem to be necessary to permit zinc to function as a neuromodulator in the brain.54–56 We examined the spike rate and burst rate of cultured neurons from mouse cortex in a serum-free medium lacking albumin (DMEM) following the addition of varying amounts of a zinc salt. Previously, we found that addition of zinc ions to an array of mouse cortical neurons induced the decline and then ultimate cessation over a period of tens of minutes of both the mean spike rate and mean burst rate.44 The time for 50% of the cells to lapse into inactivity (quiescence) was plotted versus the total concentration of zinc ions added to the medium, as well as the pZn (Figure 4). While the neurons continued to function for hours at pZn below 8, even a modest increase in free zinc ion concentration to pZn = 7.5 reduced the time to quiescence two-fold or more, and further increase to pZn = 7 (100 nmol/L free Zn) reduced the time by nearly a factor of five. We note that the toxicity of zinc is indicated here by a functional (electrophysiological) criterion rather than a morphological one. Despite the different criterion, the behavior was grossly similar to that of the cardiac myocytes and PC-12 cells: as zinc ion was added the measured free zinc ion concentration was substantially lower than total zinc, with little or no effect being observed at a free zinc ion level in the range of 10 nmol/L (pZn = 8), but substantial toxicity being observed at free zinc ion concentrations above 50 nmol/L.
Figure 4.
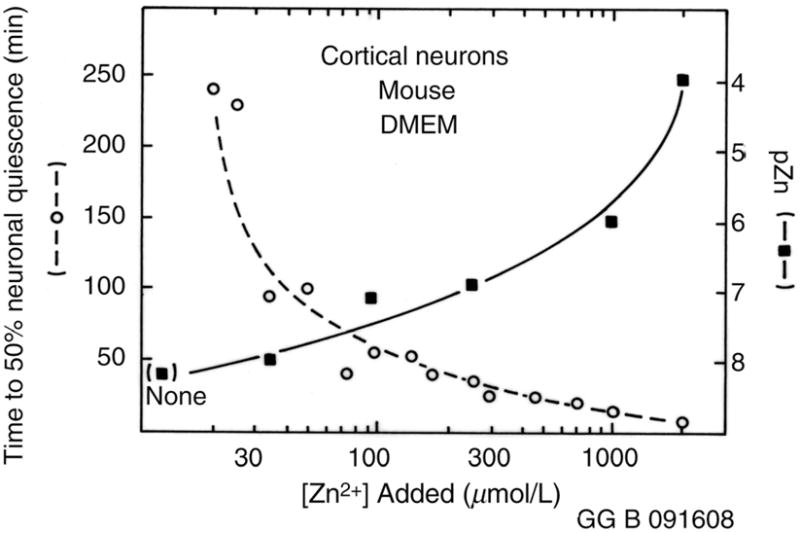
Time to neuronal quiescence for mouse cortical neurons (open circles) and pZn (filled squares) measured as a function of total added zinc in Dulbecco’s modified Eagle’s medium (DMEM) media plus serum
HeLa cells (Libert and Chimienti)
HeLa cells represent a human epithelial cell line used worldwide as a canonical immortal cell line, at least in part due to their hardiness. Derived from cervical adenocarcinoma, they have been used in over a thousand studies. We measured the proportion of cells cultured in OptiMEM with 5% serum surviving 24 h after zinc salts were added (as judged by MTT assay), as well as the free zinc ions measured by pZn meter (Figure 5). We found a more abrupt decline in cell survival with added zinc ion, with >86% survival observed as high as 200 μmol/L total zinc ion, but 300 and 400 μmol/L exhibited <2% live cells. In this case, the apparent free zinc ion concentration is higher than for the others, with the threshold for cell death appearing at pZn ~6.5.
Figure 5.
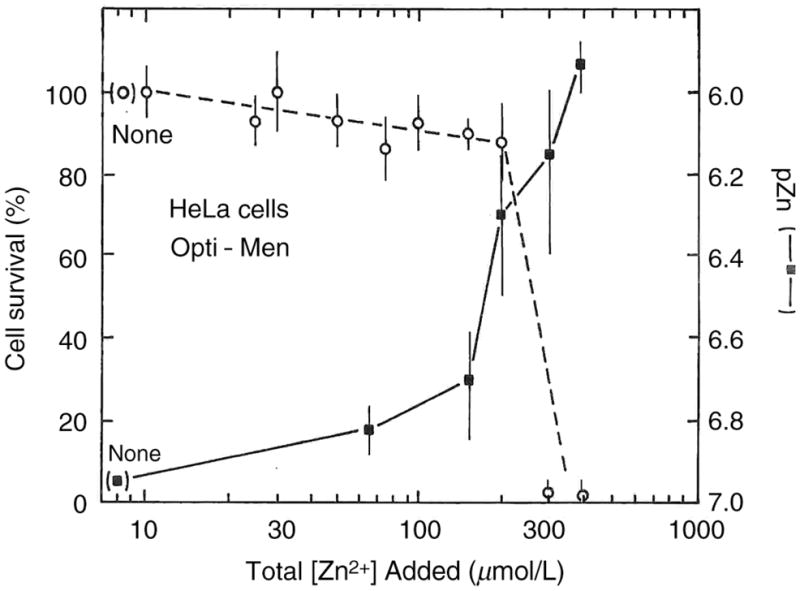
Survival of HeLa cells in Optimemmedia (open circles) and measured pZn (filled squares) as a function of total zinc added
HT-29 cells (Li and Maret)
The HT-29 human colorectal adenocarcinoma cell line was derived from a 44-year-old female patient and remains widely used. It is a p53 overexpressing line that does not express CD4. These cells cultured in DMEM plus 10% serum exhibited almost complete survival as judged by Trypan Blue staining up to ~200 μmol/L zinc ions added, but by 300 μmol/L zinc survival is down by 40%, and by 80% at 400 μmol/L (Figure 6). The measured pZn without added zinc is ~8.7, but with the addition of 300 μmol/L total zinc the pZn had declined to approximately 6.5, or about a 100-fold higher concentration than in the original medium; clearly the medium is buffering the zinc concentration strongly. These cells seem to be more resistant to zinc ion toxicity than some of the other cells, exhibiting only slight decline at pZn < 7.
Figure 6.
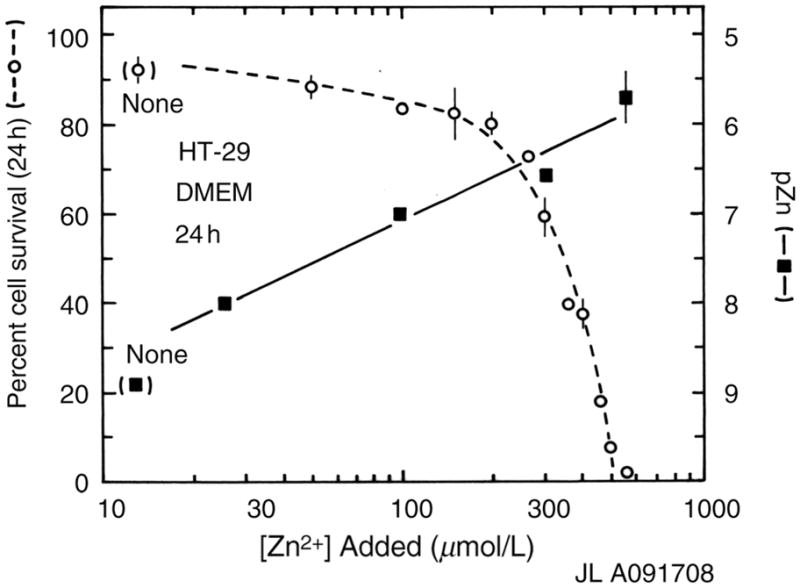
Twenty-four-hour survival of HT-29 cells in Dulbecco’s modified Eagle’s medium (DMEM) (open circles) and pZn (filled squares) as a function of added zinc ions
Discussion and conclusions
In view of the evident widespread importance of zinc ions in many different tissues, we chose to assess the injury to cells caused by a range of zinc ion concentrations in several different experimental systems to arrive at conclusions that would be broadly applicable to most cells and tissues of interest, and to assure the conclusions were not dependent on a single cell type, medium, measure of free zinc ion concentration or measure of cell viability or function. Ideally, one would test all the cell lines in all the media using all the measures of free zinc and cell function and viability, but this would comprise a rather large set of experiments. Thus we chose two primary cell cultures (cardiac myocytes and brain cortex) and three commonly used immortal cell lines (HT-29, PC-12 and HeLa); four different media (Neurobasal, DMEM, OptiMEM and M199); five different measures of zinc toxicity (Trypan Blue and Annexin V staining, MTT reduction, cell morphology and neuron function); and three different measures of free zinc level. The results are described above and summarized in Table 1. Several overt conclusions can be drawn at once. In all the media, the free zinc ion concentration as measured by any of the fluorimetric methods was typically two to three orders of magnitude below the total added zinc, such that adding ~100 μmol/L total zinc typically resulted in ~100 nmol/L free zinc (pZn = 7) being present. All the media except Optimem exhibited pZn levels of 8.6 ± 0.4 prior to addition of additional zinc, suggesting that this level is physiologically appropriate for these cell lines at least. Free levels around 100 nmol/L caused significant toxicity or loss of function, by any of the measures, for any of the cells, suggesting that adding 100 μmol/L total zinc to a cell or tissue for more than brief periods is probably generally unphysiological except in uncommon sites such as some synapses or highly zinc-buffered conditions. The sensitivity of the different cells varied significantly, which may be attributable to the very different measures of toxicity used: for instance, the neurons had nearly all ceased functioning at pZn = 7, whereas the myocytes, HT-29’s and HeLa’s all exhibited 80–95% survival at this level, but not indefinitely. These measures of toxicity represent different levels of damage to the cell, perhaps with different latencies, and zinc may affect them differentially. For instance, apoptosis is a relatively prolonged process, requiring hours in some cases, so that by another measure, the cells may be non-viable much sooner, or at lower zinc concentration. However, it may also be true that the cell types are simply varyingly sensitive to free zinc ion levels, perhaps based on their ability to take up or expel the metal ion.
Table 1.
| Cell type | Medium | pZn 50% effect* | Total Zn added for 50% effect (μmol/L)† | Viability/function measure‡ | pZn w/no Zn added§ |
|---|---|---|---|---|---|
| Cardiac myocytes | M199 | 6.4 | 280 | Survival; Trypan Blue staining | 8.4 |
| PC-12 | Neurobasal + B27 | ~7.2 | 30 | Morphology, Annexin V stain | 8.7 |
| Cortical Neurons | DMEM | 7.0 | 40 | Time to neuronal quiescence | 8.2 |
| HeLa | Optimem | 6.5 | 300 | Survival; MTT | 7.0 |
| HT-29 | DMEM | 7.5 | 350 | Survival; Trypan Blue | 9.0 |
pZn level present to observe 50% cell survival, 50% of cells undergoing apoptosis as judged by morphology (membrane blebs, rounding up and/or nuclear breakup) or Annexin V staining, or 50% of the time to reach neuronal quiescence
Total zinc concentration added to medium, in μmol/L
Measure of cell viability by Trypan Blue staining, MTT reduction, or morphology and Annexin V staining, or function by time to quiescence for 50% of the cultured neurons
pZn measured for media prior to addition of zinc ion elucidate the role of zinc in
The large difference between total and measured free zinc ion concentrations suggests that experiments seeking to elucidate the role of zinc in cellular processes should measure free zinc ion concentrations, with the caveat that some complexes may also be physiologically active. Perhaps more convenient are media with the zinc levels buffered to particular levels.36,46 Since many eukaryotic media contain serum albumin or other abundant, fairly strong zinc ligands, the zinc ion levels are functionally buffered, and it is likely that most growth media have free zinc ion concentrations in the region of a few nanomolar, though the totals will vary with the amount of serum added since serum total zinc levels themselves vary significantly. In serum-free media, care is particularly necessary since zinc buffering ligands may be inadequate, and the effects of ubiquitous contaminating zinc can be magnified. In view of the relative lack of zinc ligands in cerebrospinal fluid, caution should be exercised in studying the zinc biology of neurons in the presence of serum-containing media.
The evident toxicity to PC-12 cells when the free zinc ion concentration is lowered to subnanomolar levels by NTA (Figure 3, panels a, b, c and d) was not unexpected since other groups had observed induction of apoptosis by the reduction of the intracellular free zinc ion concentration with cell-permeant chelators such as TPEN (reviewed in Truong-Tran et al.57), and we had previously observed that reduction of intracellular free zinc ions occurred upon treatment with an extracellular chelator.58 Nevertheless, we view the data in Figure 3 as an important result, since they suggest that a lack or shortage of free zinc ion, perhaps induced by the presence of a strong chelator, could also be toxic to cells. We note that the measured pZn of all the media except Optimem was in the range of 8.2–9.0, even though in DMEM and M199 the only source of zinc ions is from the added serum or perhaps contamination; none is included in formulation. These results indicate caution is necessary when studying the effects of reduced total zinc on cells, perhaps as a model of zinc deficiency. While the morphological changes and appearance of Annexin V staining are clear evidence of apoptosis, an extensive data set was not collected and the observation must be repeated in this system and others. Certainly, these should include primary cultures of skin cells as well as other canonical cell lines. Those experiments are underway.
Acknowledgments
Some of these data were presented in preliminary form at the SPIE Conference on Ultrasensitive Clinical Diagnostics and Zinc Signals 2006. This work was supported by NIH (RO1 EB 003924 to R B T and C J F; 1RO1 HL 77305-05 to I K); and the Texas Advanced Technology Program (G G and M P).
Footnotes
Competing interests statement: The pZn meter used in some of these studies is a product of NeuroBioTex; investigators from academic institutions received no compensation from NeuroBioTex for their contributions to this paper. NeuroBioTex has also licensed the fluorescence technology used in zinc determination using variants of carbonic anhydrase.
References
- 1.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–5. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Hough CJ, Frederickson CJ, Sarvey JM. Induction of mossy fiber –CA3 long term potentiation requires translocation of synaptically released zinc. J Neurosci. 2001;21:8015–25. doi: 10.1523/JNEUROSCI.21-20-08015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maske H. Naturwissenschaften. 1955;42:424. [Google Scholar]
- 5.Frederickson CJ, Klitenick MA, Manton WI, Kirkpatrick JB. Cytoarchitectonic distribution of zinc in the hippocampus of man and the rat. Brain Res. 1983;273:335–9. doi: 10.1016/0006-8993(83)90858-2. [DOI] [PubMed] [Google Scholar]
- 6.Weiss JH, Sensi SL, Koh J-Y. Zn(II): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 7.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Monpetit A, Psheszhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–5. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 8.Tonder N, Johansen FF, Frederickson CJ, Zimmer J, Diemer NH. Possible role of zinc in the selective degeneration of dentate hilar neurons after cerebral ischemia in the adult rat. Neurosci Lett. 1990;109:247–52. doi: 10.1016/0304-3940(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 9.Suh SW, Chen JW, Motamedi M, Bell B, Listiak K, Pons NF, Danscher G, Frederickson CJ. Evidence that synaptically-released zinc contributes to neuronal injury after traumatic brain injury. Brain Res. 2000;852:268–73. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- 10.Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Muelle RN, Zeng Y, Balaji RV, Thompson RB, Frederickson CJ, Sarvey JM, de Valdenebro M, Prough DS, Zornow MH. Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia, and reperfusion. Exp Neurol. 2006;198:285–93. doi: 10.1016/j.expneurol.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Frederickson CJ, Koh J-Y, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neuroscience. 2005;6:449–62. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 12.Lengyel I, Flinn JM, Peto T, Linkous DH, Cano K, Bird AC, Lanzirotti A, Frederickson CJ, van Kuijk FJGM. High concentration of zinc in sub-retinal pigment epithelial deposits. Exp Eye Res. 2007;84:772–80. doi: 10.1016/j.exer.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Bush AI, Pettingell WH, Multhaup G, Paradis Md, Vonsattel J-P, Gusella JF, Beyreuther K, Masters CL, Tanzi RE. Rapid induction of Alzheimer AB amyloid formation by zinc. Science. 1994;265:1464–7. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 14.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim YS, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zhang H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper–zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–76. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie CW, Bush AI, MacKinnon A, MacFarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis A, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with Iodochlorhydroxyquin (Clioquinol) targeting A-beta amyloid deposition and toxicity in Alzheimer disease. Arch Neuorol. 2003;60:1685–91. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 16.Costello LC, Franklin RB. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate. 1998;35:285–96. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci. 2007;104:18636–41. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogstrand C, Kille P, Nicholson RI, Taylor KM. Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol Med. 2009;15:101–11. doi: 10.1016/j.molmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama M, Koh J, Choi DW. Brief exposure to zinc is toxic to cortical neurons. Neurosci Lett. 1986;71:351–5. doi: 10.1016/0304-3940(86)90646-4. [DOI] [PubMed] [Google Scholar]
- 20.Weiss JH, Hartley DM, Koh J-Y, Choi DW. AMPA receptor activation potentiates Zn neurotoxicity. Neuron. 1993;10:43–9. doi: 10.1016/0896-6273(93)90240-r. [DOI] [PubMed] [Google Scholar]
- 21.Telford W, Fraker PJ. Preferential induction of apoptosis in mouse CD4+CD8+ alpha betaTCR10CD3e thymocytes by zinc. J Cell Physiol. 1995;164:259–70. doi: 10.1002/jcp.1041640206. [DOI] [PubMed] [Google Scholar]
- 22.Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH. Zn2+ induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J Biol Chem. 2001;276:47524–9. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- 23.Zalewski P, Forbes IJ, Giannakis C. Physiological role for zinc in prevention of apoptosis (gene-directed death) Biochem Int. 1991;24:1093–101. [PubMed] [Google Scholar]
- 24.Zalewski PD, Forbes IJ, Betts WH. Correlation of apoptosis with change in intracellular labile Zn(II) using Zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)acetic acid], a new specific fluorescent probe for Zn(II) Biochem J. 1993;296:403–8. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chimienti F, Seve M, Richard S, Mathieu J, Favier A. Role of cellular zinc in programmed cell death: temporal relationship between zinc depletion, activation of caspases, and cleavage of Sp family transcription factors. Biochem Pharmacol. 2001;62:51–62. doi: 10.1016/s0006-2952(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 26.Waring P, Egan M, Braithwaite A, Mullbacher N, Siaarda A. Apoptosis induced in macrophages and T-blasts by the mycotoxin sporodesmin and protection by zinc salts. Int J Pharmacol. 1990;12:445–57. doi: 10.1016/0192-0561(90)90028-l. [DOI] [PubMed] [Google Scholar]
- 27.Magneson GR, Puvathingal JM, Ray WJ. The concentrations of free Mg2+ and free Zn2+ in equine blood plasma. J Biol Chem. 1987;262:11140–8. [PubMed] [Google Scholar]
- 28.Prasad AS, Oberleas D. Zinc in human serum: evidence for an amino acid-bound fraction. J Lab Clin Med. 1968:1006. [Google Scholar]
- 29.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–92. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 30.Bozym RA, Thompson RB, Stoddard AK, Fierke CA. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem Biol. 2006;1:103–11. doi: 10.1021/cb500043a. [DOI] [PubMed] [Google Scholar]
- 31.Krezel A, Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem. 2006;11:1049–62. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 32.Masuoka J, Saltman P. Zn(II) and Cu(II) binding to serum albumin: a comparative study of dog, bovine, and human albumin. J Biol Chem. 1994;269:25557–61. [PubMed] [Google Scholar]
- 33.Dulbecco R, Freeman G. Plaque formation by the polyoma virus. Virology. 1959;8:396–7. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- 34.Thompson RB, Peterson D, Mahoney W, Cramer M, Maliwal BP, Suh SW, Frederickson CJ. Fluorescent zinc indicators for neurobiology. J Neurosci Methods. 2002;118:63–75. doi: 10.1016/s0165-0270(02)00144-9. [DOI] [PubMed] [Google Scholar]
- 35.Price PJ, Brewer GJ. Serum-free media for neural cell cultures. In: Federoff S, Richardson A, editors. Protocols for Neural Cell Culture. 3. Totowa, NJ: Humana Press; 2001. pp. 255–64. [Google Scholar]
- 36.Bozym R, Hurst TK, Westerberg N, Stoddard A, Fierke CA, Frederickson CJ, Thompson RB. Determination of zinc using carbonic anhydrase-based fluorescence biosensors. In: Brand L, Johnson M, editors. Fluorescence Spectroscopy. San Diego: Academic Press; 2008. pp. 287–309. [DOI] [PubMed] [Google Scholar]
- 37.Franklin RB, Ma J, Zou J, Guan Z, Kukoyi BI, Feng P, Costello LC. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J Inorg Biochem. 2003;96:435–42. doi: 10.1016/s0162-0134(03)00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krezel A, Maret W. Dual nanomolar and picomolar Zn(II) binding properties of metallothionein. J Am Chem Soc. 2007;129:10911–21. doi: 10.1021/ja071979s. [DOI] [PubMed] [Google Scholar]
- 39.Cherezov V, Hofer N, Szebenyi DME, Kolaj O, Wall JG, Gillilan R, Srinivasan V, Jaroniec CP, Caffrey M. Insights into the mode of action of a putative zinc transporter CzrB in Thermus thermophilus. Structure. 2008;16:1378–88. doi: 10.1016/j.str.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium. 2002;31:245–51. doi: 10.1016/S0143-4160(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 41.Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard SJ. fluorescent sensors for Zn2+ based on a fluorescein platform: synthesis, properties, and intracellular distribution. J Am Chem Soc. 2001;123:7831–41. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]
- 42.Hirano T, Kikuchi K, Urano Y, Higuchi T, Nagano T. Highly zinc-selective fluorescent sensor molecules suitable for biological applications. J Am Chem Soc. 2000;122:12399–400. [Google Scholar]
- 43.Fierke CA, Thompson RB. Fluorescence-based biosensing of zinc using carbonic anhydrase. BioMetals. 2001;14:205–22. doi: 10.1023/a:1012980628412. [DOI] [PubMed] [Google Scholar]
- 44.Parviz M, Gross GW. Quantification of zinc toxicity using neuronal networks on microelectrode arrays. NeuroToxicology. 2007;28:520–31. doi: 10.1016/j.neuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Weber G. Protein Interactions. New York: Chapman & Hall; 1992. [Google Scholar]
- 46.Thompson RB, Frederickson CJ, Fierke CA, Westerberg NM, Bozym RA, Cramer ML, Hershfinkel M. Practical aspects of fluorescence analysis of free zinc ion in biological systems: pZn for the biologist. In: Thompson RB, editor. Fluorescence Sensors and Biosensors. Boca Raton, FL: CRC Press; 2006. pp. 351–76. [Google Scholar]
- 47.Szmacinski H, Lakowicz JR. Lifetime-based sensing. In: Lakowicz JR, editor. Topics in Fluorescence Spectroscopy Vol. 4: Probe Design and Chemical Sensing. New York: Plenum; 1994. pp. 295–334. [Google Scholar]
- 48.Thompson RB, Cramer ML, Bozym R, Fierke CA. Excitation ratiometric fluorescent biosensor for zinc ion at picomolar levels. J Biomed Opt. 2002;7:555–60. doi: 10.1117/1.1501886. [DOI] [PubMed] [Google Scholar]
- 49.Smith R, Martell AE. NIST Critical Stability Constants of Metal Complexes, Standard Database 46. Gaithersburg, MD: National Institute of Standards and Technology, U.S. Department of Commerce; 1973. [Google Scholar]
- 50.Yao PM, Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the Fas pathway. J Biol Chem. 2000;275:23807–13. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- 51.Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, Hannun YA. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. J Biol Chem. 1997;272:18530–3. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 52.Maret W, Jacob C, Vallee BL, Fischer EH. Inhibitory sites in enzymes: zinc removal and reactivation by thionein. Proc Natl Acad Sci. 1999;96:1936–40. doi: 10.1073/pnas.96.5.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palm R, Strand T, Hallmans G. Zinc, total protein, and albumin in CSF of patients with cerebrovascular diseases. Acta Neurol Scand. 1986;74:308–13. doi: 10.1111/j.1600-0404.1986.tb03520.x. [DOI] [PubMed] [Google Scholar]
- 54.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–3. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 55.Celentano JJ, Gyenes M, Gibbs TT, Farb DH. Negative modulation of the gamma-aminobutyric acid response by extracellular zinc. Mol Pharmacol. 1991;40:766–73. [PubMed] [Google Scholar]
- 56.Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1–NR2A receptors. J Neurosci. 1997;17:5711–25. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. BioMetals. 2001;14:315–30. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- 58.Frederickson CJ, Suh SW, Koh J-Y, Cha YK, Thompson RB, LaBuda CJ, Balaji RV, Cuajungco MP. Depletion of intracellular zinc from neurons by use of an extracellular chelator in vivo and in vitro. J Histochem Cytochem. 2002;50:1659–62. doi: 10.1177/002215540205001210. [DOI] [PubMed] [Google Scholar]