Abstract
Dystroglycan is a central component of the dystrophin-glycoprotein complex (DGC) that links extracellular matrix with cytoskeleton, expressed in a variety of fetal and adult tissues. Dystroglycan plays diverse roles in development and homeostasis including basement membrane formation, epithelial morphogenesis, membrane stability, cell polarization, and cell migration. In this paper, we will focus on biological role of dystroglycan in Schwann cell function, especially myelination. First, we review the molecular architecture of DGC in Schwann cell abaxonal membrane. Then, we will review the loss-of-function studies using targeted mutagenesis, which have revealed biological functions of each component of DGC in Schwann cells. Based on these findings, roles of dystroglycan in Schwann cell function, in myelination in particular, and its implications in diseases will be discussed in detail. Finally, in view of the fact that understanding the role of dystroglycan in Schwann cells is just beginning, future perspectives will be discussed.
1. Introduction
Dystroglycan was originally isolated from skeletal muscle as one of dystrophin-associated proteins, and found to be a main component of the dystrophin-glycoprotein complex (DGC), a multimeric transmembrane protein complex [1, 2]. In skeletal muscle, α- and β-dystroglycan constitute a membrane-spanning complex and interact with various subsarcolemmal and transmembrane proteins and components of basement membrane. Thus, DGC provides the physical link between extracellular matrix and subsarcolemmal cytoskeleton, indicating its role in structural stability of sarcolemma during contraction and extension of skeletal muscles [3]. In fact, mutations in DGC components lead to progressive muscle fiber degeneration, causing various types of muscular dystrophies [4].
Dystroglycan is also expressed in many other cell types and it plays a variety of roles in nonmuscle tissues. It has been implicated in basal lamina development in mouse embryo [5], epithelial development [6], somitegenesis [7], cell polarization [8–10], carcinogenesis [11, 12], infective pathogen targeting [13–15], and development of the central nervous system (CNS) [16, 17].
In this paper, we review current understanding of dystroglycan function in Schwann cells such as ensheathment, myelination as well as maintenance of other Schwann cell structures, and the implications of these findings to peripheral nervous diseases. In broad sense, “myelination” may include entire developmental process of myelin formation from radial sorting of axons to membrane wrapping forming compact myelin as well as assembly of the nodes of Ranvier and growth of internodes, which are inextricably linked with each other [18]. However, in this paper, we will use “myelination” mainly in a rather narrow sense, as a membrane wrapping process forming compact myelin after radial sorting is completed.
2. Molecular Architecture of Dystrophin-Glycoprotein Complex (DGC) in Schwann Cells
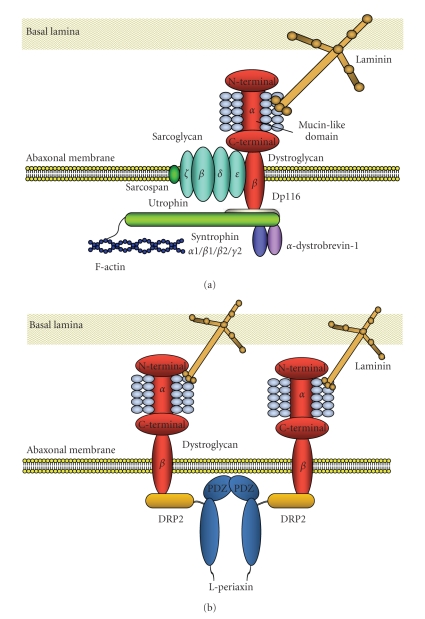
Molecular architecture of DGC in Schwann cells has been extensively studied by our group and others ([19–29], Figure 1). The biochemical analysis revealed the similarities of the fundamental molecular architecture of DGC between Schwann cells and muscle cells as well as several critical differences between them. In peripheral nerve, dystroglycan is mainly present in abaxonal membrane of both myelinating Schwann cells and nonmyelinating Schwann cells [19, 22, 24, 30, 31], while it is also expressed in perineurial cells as well as satellite cells of dorsal root ganglia [32]. As in skeletal muscle, dystroglycan in Schwann cells consists of two subunits (α and β), which are translated from a single mRNA as a propeptide that is proteolytically cleaved into two noncovalently associated proteins [2, 19, 33]. In Schwann cells, α-dystroglycan is known to bind to two of the extracellular ligands, laminin-2 (α2β1γ1), major laminin isoform in Schwann cell basement membrane, and agrin [19, 21–23]. Each of these has laminin G (LG)-like domains that mediate their high-affinity Ca2+-dependent binding to α-dystroglycan [34, 35]. While small amount of laminin-8 (α4β1γ1) is also present in mature Schwann cell basement membrane, LG domains of laminin-8 showed only a low affinity for the α-dystroglycan receptor [36]. Apparent molecular mass of α-dystroglycan in Schwann cells is 120 kDa [19], which is smaller than that of skeletal muscle (156 kDa), probably due to the difference of tissue-specific O-glycosylation within the mucin domain [4]. While the removal of N-linked glycans alters the molecular weight of α-dystroglycan by only 4 kDa [1], and does not effect on its activity as an extracellular matrix receptor [37], full deglycosylation of α-dystroglycan results in the complete loss of ligand-binding activity [37]. Thus, the sugar chains on the mucin-like domain are supposed to mediate these interactions. Actually, structural analysis of the sialylated O-linked oligosaccharides of bovine peripheral nerve α-dystroglycan revealed a high abundance of a novel O-mannosyl-type oligosaccharide, Siaα2-3Galβ1-4GlcNAcβ1-2Man-Ser/Thr (where Sia is sialic acid), and this tetrasaccharide was involved in the interaction of the α-dystroglycan with laminin [38]. In other cell types, there are evidences that α-dystroglycan binds to laminin-1 via sugar moieties other than the tetrasaccharide [39], while this is not confirmed in Schwann cells. Also, it was reported that nonglyosylated N-terminal fragment of α-dystroglycan bound to laminin-2/-4, laminin-1, fibronectin and fibrinogen [40].
Figure 1.
(a) Molecular structure of DGC (traditional type), present in Cajal cytoplasmic band of myelinated Schwann cells. a- and b-dystroglycan form membrane-spanning complex in Schwann cell abaxonal membrane. Mucin-like domain of a-dystroglycan is involved in the interaction with laminin via laminin G-like domains. a-dystroglycan is noncovalently anchored to b-dystroglycan. The cytoplasmic tail of b-dystrogylcan is anchored to the cytoskeletal proteins, Dp116, Schwann cell-specific isoform of dystrophin, or utrophin, an autosomal homolog of dystrophin. Utrophin interact with f-actin at the N-teminus. Utrophin or Dp116 interacts with syntrophins (a1, b1, b2, g2) and a-dystrobrevin-1. Also at least four isoforms of sarcoglycan (b, d, e, z) form sarcoglycan complex in abaxonal membrane and interact with dystroglycan complex. Sarcospan is supposed to interact with sarcoglycan complex. Thus DGC provides the physical link between extracellular matrix and submembranous cytoskeleton in Schwann cells. (b) Molecular framework of DGC present in membrane portion directly apposed to myelin sheath (DRP2/periaxin rich plaque) of myelinated Schwann cells. In DRP2/periaxin rich plaques, dystroglycan complex intracellularly interact with DRP2, third member of dystrophin family, and periaxin, homodimeric PDZ domain-containing protein, via cytoplasmic domain of b-dystroglycan. This type of DGC lacks syntrophins and dystrobrevin.
On the other hand, α-dystroglycan is noncovalently anchored to β-dystroglycan in the Schwann cell membrane. β-dystroglycan is a type I transmembrane protein with a single transmembrane domain and a 120 amino acid long C-terminal cytoplasmic tail. The cytoplasmic tail is anchored to the cytoskeletal proteins, Dp116, Schwann cell-specific isoform of dystrophin, and utrophin, an autosomal homolog of dystrophin [19, 20, 24, 26, 41–43]. β-dystroglycan is supposed to interact with cytoskeleton via interaction with these submembranous proteins [42], while direct interaction of β-dystroglycan with f-actin was also reported [44]. α1-dystrobrevin and four syntrophin isoforms (α1, β1, β2, and γ2), which interact with dystrophin and utrophin, are also expressed in Schwann cells [25, 29]. These proteins are crucial for the formation of DGC-associated signaling scaffolds in membrane of many cell types including Schwann cell abaxonal membrane [45]. As for binding between β-dystroglycan and Dp116, 15 C-terminal amino acids of the cytoplasmic domain of β-dystroglycan are involved in the high affinity binding of Dp116, while 26 N-terminal amino acids of the cytoplasmic domain are also involved in the low affinity binding of Dp116 [24]. In Schwann cells, MMP2 and MMP9 are suggested to be involved in β-dystroglycan processing, and produce a 30 kDa fragment of β-dystroglycan [46–49].
As other membrane protein members of DGC, β-, δ-, ε-, and ζ-sarcoglycans are expressed in Schwann cells. The four isoforms of sarcoglycans in Schwann cells seem to form stable tetrameric sarcoglycan complex that associates with dystrogycan and Dp116 to constitute larger DGC complex, as in the case of skeletal muscle [25, 26, 50]. Also, sarcospan is expressed in Schwann cells as a member of DGC [25].
Recently Albrecht et al. [29] reported that molecular architecture of DGC is different between the two distinctive Schwann cell abaxonal membrane compartments, membrane covering Cajal cytoplasmic band and membrane directly apposed to myelin sheath (DRP2/periaxin rich plaque). In the former compartment, β-dystroglycan forms a complex with Dp116, utrophin, α1-dystrobrevin, α1-, β1-, β2-, and γ-syntrophins, and ABCA1 (traditional type, Figure 1(a)), and in the latter, β-dystroglycan forms a complex with L-Periaxin and DRP2 (Figure 1(b)), which was originally characterized by Sherman et al. [27, 51] and Court et al. [52]. Also, Occhi et al. found expression of DGC in node of Ranvier, and the DGC in node of Ranvier seems to be compatible with the traditional type, comprising Dp116 and utrophin, but not periaxin and DRP2 [28]. They also found that laminin-2 (α2β1γ1) and laminin-10 (α5β1γ1) are expressed in the nodal region [28].
Among these DGC components, only laminin-2 and periaxin do not depend on dystroglycan for their localization in Schwann cell abaxonal membrane [25]. Recently, a giant protein, AHNAK was reported to modify laminin binding capacity of Schwann cell abaxonal membrane probably via interaction with DGC [53], while precise molecular interaction between AHNAK and DGC remain to be elucidated.
3. Loss-of-Function Studies for DGC Components
Functions of DGC components including core components such as dystroglycans in Schwann cells were mainly revealed by loss-of-function studies in animals by targeting mutagenesis as well as gene mutation survey for human and mouse muscular dystrophies (Table 1). In particular, double knockout of two functionally related genes as well as Schwann cell-selective gene ablation system such as Cre/loxP contributed to these studies (Table 1). Therefore, the findings obtained from these studies will be described in detail.
Table 1.
Loss-of-function studies for DGC components.
| Disrupted genes (animal) | Expression level and modification of Schwann cell proteins | Nerve pathology and functional deficits | References |
|---|---|---|---|
| Schwann cell-selective Dag1 null mouse (P0-DG null mouse) | No expression of DG Reduced expressions of other DGC components except periaxin, laminin-2 |
Mild radial sorting defect in spinal roots Myelin folding/polyaxonal myelination Mild loss of myelinated axons in sciatic nerve Intact BM Lower conduction Disruption of nodes Disrupted Cajal bands Shortening of internodes |
[25, 52, 54] |
| Unknown (dy mouse) | No expression of laminin α2 Upregulation of laminin α4 |
Radial sorting defect in nerve roots and cranial nerves Mild radial sorting defect in peripheral nerves Disrupted BM Shortening of internodes Lower conduction Widened nodes of Ranvier |
[55–61] |
| Lama2/point mutation of LN domain/CxxC motif (dynmf417mouse) | Normal expression of the mutated laminin α2 chain | Radial sorting defect in nerve roots Intact BM |
[62] |
| Lama2/splicing skip of exon2 (dy2J mouse) | Normal expression of truncated laminin α2 chain (domain IV deleted) Upregulation of laminin α4 |
Radial sorting defect in nerve roots and cranial nerves Mild radial sorting defect in peripheral nerves Multiple axons myelinated by one Sc Disrupted BM Disrupted Cajal bands Shortening of internodes |
[52, 55, 57, 60, 63–65] |
| Lama2 (dy3k mouse) | No expression of laminin α2 Upregulation of laminin α4 |
A few solitary unmyelinated axons Thin myelin sheath Decreased nodal gap Lower conduction Disrupted BM |
[66] |
| Lama4 (mouse) | No expression of laminin α4 | Radial sorting defect in peripheral nerves Polyaxonal myelination Intact BM |
[67] |
| Lama2/Lama4 (dy2J/α4 null mouse) | Normal expression of truncated laminin α2 chain No expression of laminin α4 |
Severe radial sorting defect of peripheral nerves Mild radial sorting defect in spinal roots Disrupted BM |
[67] |
| Schwann cell selective Lamg1-null mouse | Severely reduced expression of all known laminin isoforms in Schwann cells | Radial sorting defect in spinal roots and peripheral nerves | [68] |
| L-Periaxin (mouse) | No expression of L-periaxin | Demyelination in sciatic nerve Myelin folding Disrupted S-L incisure Lower conduction Impaired regeneration Hyperalgesia, allodynia Disrupted Cajal bands Shortening of internodes |
[51, 69] |
| Utrophin (mouse) | No expression of utrophin | Disrupted Cajal bands Shortening of internodes |
[52] |
| Sarcoglycans (BIO14.6 hamster) | No expression of sarcoglycans Reduced expression of α-DG and Dp116 |
Myelin folding Disrupted S-L incisure |
[50] |
| Large null mouse (enr, myd) | Very low expression of Large Hypoglycosylation of α-DG |
Radial sorting defect in sciatic nerve and spinal roots Intact BM Lower conduction Disrupted Cajal bands Impaired regeneration |
[52, 70] |
| Fukutin chimera mouse | Deficiency of fukutin Hypoglycosylation of α-DG |
Radial sorting defect in sciatic nerve and spinal roots Loss of myelinated axons in nerve roots and sciatic nerve at P15–30 Multiple axons myelinated by one Sc Intact BM Disruption of NMJ |
[71] |
| Schwann cell-selective β1 integrin null mouse | No expression of β1 integrin | Radial sorting defect in sciatic nerve Disrupted BM |
[72] |
| Schwann cell-selective β4 integrin null mouse | No expression of β4 integrin | Myelin folding | [54] |
| Schwann cell-selective β4 integrin/Dag1 null mouse | No expression of DG and β4 integrin | Radial sorting defect in spinal ventral roots Severe myelin folding Demyelination Disrupted BM |
[54] |
BM: basement membrane, DG: dystroglycan, S-L: Schmidt-Lanterman, NMJ: neuromuscular junction, Sc: Schwann cell.
3.1. Dystroglycan-Null Mice
The mutant mice showed a variety of morphological and functional abnormalities including redundant myelin loops, polyaxonal myelination at postnatal day 3–14, abnormal myelin sheath folding at 12 months, and axonal loss with aging [25]. The mice also showed nodal changes such as reduced sodium channel density, disorganized microvilli as well as axonal abnormalities in this region. However, because of the redundancy caused by other genes/proteins with similar biological functions such as α6β4 integrin, this loss-of-function study did not fully reveal the roles of dystroglycan in Schwann cell function including myelination, which will be discussed in detail later.
3.2. Laminin α Chain-Null Mice
As typically observed in dy/dy mice and dy2J/dy2J mice, loss of laminin α2 chain expression in mice constantly resulted in not only congenital muscular dystrophy but also radial sorting defect in peripheral nerve characterized by the presence of bundles of naked axons (unmyelinated axons) more evident in the proximal part of the PNS [55–60, 62–64, 67]. Moreover, transgenic rescue of laminin defect in muscle of dy/dy mice revealed that the neuropathy significantly contributes to the disease phenotype [73]. Recently, Yu et al. [65] reported that laminin is necessary for Schwann cell morphogenesis, especially during radial sorting, and suggest that laminin signaling is a central regulator coordinating the processes of proliferation and morphogenesis in radial sorting.
In the spinal roots of laminin α2 chain-deficient mice, expression of laminin α4 chain was increased and expression of laminin α5 chain was preserved [61, 66, 67]. Interestingly, loss of laminin α4 chain showed bundles of naked axons more evident in distal part of the PNS [67]. Double knockout of α2 and α4 chains led to most numerous naked axons in tibial nerve, but paradoxically improved the radial sorting in spinal root [67]. Also, transgenic laminin α5 chain expression promoted myelination [67]. Laminin α1 chain is also suggested to compensate the deficiency of laminin α2 chain, and actually transgenic expression of laminin α1 chain rescued radial sorting defect of spinal roots of dy3K/dy3K mice [74, 75]. In addition, inactivation of all known laminin isoforms in Schwann cells led to radial sorting defect in both spinal roots and sciatic nerve [68]. These results indicated the complicated functional redundancy of the four kinds of laminin α chain isoforms (α1, α2, α4, and α5), or even other unrecognized proteins with similar function.
Another important finding in dy mice is the abnormal sodium channel clusters in nodes of Ranvier [28], which was identical in quality but less severe than those observed in dystroglycan-null mice [25]. This nodal abnormality was supposed to be caused by laminin-2 deficiency because laminin-2 and laminin-10 are expressed in normal nodes and paranodes [28]. Notably, laminin-α1 chain was upregulated in dy mice nodes [28].
3.3. Integrin-Null Mice
While integrin is not supposed to be a member of DGC, α6β4 and α6β1 integrins are another important laminin receptors in Schwann cells, and have been suggested to be involved in Schwann cell myelination [76–78]. Dissecting functions of those integrins is critical for delineating exact role of dystroglycan, and therefore, the studies for integrin-null mice will be reviewed in this paper.
Schwann cell specific disruption of β1 integrin showed large bundle of naked axons in both spinal roots and sciatic nerves suggesting severe impairment of radial sorting process [72]. Notably, myelinated Schwann cells never expressed β1 integrin, suggesting that β1 integrin is not always necessary for myelination process once Schwann cells have achieved 1 : 1 relationship with large axons [72].
In contrast, Schwann cell specific disruption of β4 integrin did not affect peripheral nerve development, myelin formation, maturation, or regeneration except abnormal myelin folding with ageing [54]. However, disruption of both β4 integrin and dystroglycan showed severer hypomyelination in spinal roots than dystroglycan-null mice, with acute sign of demyelination such as myelin degeneration, macrophage infiltration and remyelination [54]. It also showed major folding abnormalities in sciatic nerve myelin, suggesting a role of dystroglycan in myelin stabilization with the cooperation of β4 integrin.
3.4. Mice with α-Dystroglycan Glycosylation Defects
Recent advances have highlighted the importance of α-dystroglycan glycosylation in dystroglycan functions. Actually, mutation in the genes encoding LARGE, POMGnT1, POMT and fukutin cause defects in α-dystroglycan glycosylation, and altered capacity for binding to laminin, agrin, and neurexin [79, 80]. As a result, the glycosylation defects lead to the human disorders, congenital muscular dystrophy 1D (MDC1D), muscle-eye-brain disease (MEB), Walker-Warburg disease (WWS), and Fukuyama congenital muscular dystrophy (FCMD), which are collectively called α-dystroglycanopathy [81–83]. Because defects in α-dystroglycan glycosylation should reduce the laminin-2 binding capacity of α-dystroglycan in not only muscle cells but also Schwann cells, Schwann cell functions associated with dystroglycan-laminin-2 interaction should be impaired in α-dystroglycanopathy. However, peripheral nerve involvement in α-dysroglycanopathy has not been extensively studied. Therefore, we embarked on the investigation whether peripheral nerve development, especially myelination, is defective or not in fukutin-deficient chimeric mice, a mouse model of FCMD [71]. As a result, we demonstrated that the sugar chain moiety and laminin-binding activity of α-dystroglycan were severely reduced, while the expression level of laminin-2 seemed to be unaffected. The fukutin chimeric mice showed cluster of naked axons and loss of myelinated fibers in both sciatic nerve and spinal roots at P30. After 20 months of age, some spinal roots showed striking loss of myelinated axons as well as degenerated axons suggesting the progressive neuropathy with aging. Also, occasionally multiple axons were myelinated by single Schwann cell, which was also found in dy2J/dy2J mice [59]. These results suggest that α-dystroglycan glycosylation plays roles in Schwann cell differentiation including ensheathment and myelination, as well as maintenance of myelin in adult mice [71].
Similar to fukutin chimeric mice, Large-null mice (enr and myd mice) showed cluster of naked axons in sciatic nerve, while the sodium channel clustering in nodes of Ranvier was unaffected [70].
Altogether, Schwann cell myelination is disturbed in α-dystroglycanopathy mice. Therefore, scrutinization of peripheral nerve involvement in human α-dystroglycanopathy will reveal more detailed characterization of those diseases.
3.5. Periaxin-Null Mice
Studies of periaxin-null mice revealed a unique aspect of DGC biological function in Schwann cells. Periaxin-null mice did not show defect in peripheral nerve development, instead they showed progressive demyelination as well as abnormal myelin sheath foldings in sciatic nerve, suggesting unstable myelin sheath [69]. Also periaxin-null mice showed abnormal Schwann cell compartmentalization, disruption of protoplasmic bands of Cajal, and impaired Schwann cell internodal growth [51, 52]. Recently, Court et al. [52] demonstrated that laminin-2, dystroglycan, utrophin axis is also required for proper Schwann cell compartmentalization, and correct internodal length.
3.6. Sarcoglycan-Null Hamster
Sarcoglycan-null hamster (BIO14.6) showed that δ-sarcoglycan induced age-dependent myelin disruption or abnormal foldings and perturbed Schmidt-Lanterman incisures [50]. Together with loss of sarcoglycan complex and reduced expression of α-dystroglycan and Dp116, these findings suggested that sarcoglycans in Schwann cells stabilize DGC-mediated transmembrane extracellular matrix-cytoskeleton linkage, and thus play a role in myelin stability.
4. Detailed Role of Dystroglycan in Schwann Cell Function
Based on the findings derived from loss-of-function studies described above and other previous studies, detailed role of dystroglycan in Schwann cell function will be discussed in detail in this section.
4.1. Role of Dystroglycan in Myelination and Myelin Maintenance
It is well known that basement membrane plays an important role in Schwann cell ensheathment and myelination [84, 85]. Therefore, the discovery in early 1990s that congenital muscular dystrophy as well as dysmyelination of nerve roots in dy/dy mice are due to lack of laminin-2 prompted us to investigate whether dystroglycan, a high affinity receptor for laminin-2, plays a role in myelination [86].
In order to address this issue, we analyzed Schwann cell expression of β-dystroglycan and laminin α2 chain during rat peripheral nerve regeneration and development. As a result, β-dystroglycan protein was present most densely in early myelinating Schwann cells, intermediately in promyelinating Schwann cells, and most faintly in the Schwann cells in the transient stage between immature Schwann cells and promyelinating Schwann cells in rats at the age of P3 [87]. Another group also reported that β-dystroglycan appear as a protein peinataly, just before myelination, exclusively in outer surface of mouse Schwann cells [74], which was consistant with our results derived from rat peripheral nerves. Also, the expression of β-dystroglycan protein dramatically increased during first week after birth of rats, and was maintained until adulthood. The expression of laminin α2 chain also increased during the first week, and continued to increase until adulthood [87]. The expression of β-dystroglycan was down-regulated by axonal degeneration in adult rats, and was induced again by axon contact during axonal regeneration [30]. These results indicate that dystroglycan is associated with myelination as well as myelin maintenance rather than radial sorting. Second, Schwann cell-specific dystroglycan-null mice showed abnormally thin myelinated fibers as well as abnormal myelin folding in which Schwann cell established 1 : 1 relationship with axon, suggesting that dystroglycan plays a role in myelination after radial sorting is completed [25, 54]. Also the dystroglycan-null mice showed mild loss of myelinated axons with aging. Third, double knockout of β4 integrin and dystroglycan revealed far severer hypomyelination than respective knockout of each of the two genes, as well as signs of acute demyelination [54]. Notably the hypomyelination caused by disruption of dystroglycan was most severe in spinal ventral roots [54]. Fourth, fukutin chimeric mice and Large-null mice showed hypomyelination as well as signs of progressive demyelinating neuropathy [70, 71]. Considering these evidences, dystroglycan no doubt plays a role in myelination as well as in myelin maintenance.
While α6β4 integrin was also suggested to play a role in myelination and myelin maintenance [76, 78], single disruption of β4 integrin unexpectedly did not show any abnormalities of peripheral nerve myelin except age-dependent myelin folding [54]. However double knockout of dystroglycan and β4 integrin described above showed cooperative role of β4 integrin with dystroglycan in myelination and myelin maintenance [54].
It is interesting to note that α-dystroglycan serves as a receptor for several pathogens such as Mycobacterium leprae (M.leprae), lymphocytic choriomeningitis virus and the Lassa fever virus [13–15, 88–90]. In particular, M.leprae cause acute nonimmune-mediated demyelination in mouse peripheral nerve [91], and the demyelination is at least partly mediated by ErbB2 receptor tyrosine kinase signaling [92]. However, it remains to be elucidated whether M.leprae cause demyelination via perturbation of dystroglycan function as well as whether dystroglycan has some interaction with the ErbB2 signaling, while it was reported that Lassa fever virus efficiently competes with laminin α1 and α2 chains for α-dystroglycan binding [90].
Dystroglycan is also expressed in oligodendrocyte as a laminin receptor along with β1 integrins, and may play a role in myelination by oligodendrocyte [93]. This study offered new insight into α-dystroglycanopathies that cause brain dysmyelination, as well as into the mechanism that underlies CNS myelin abnormalities caused by laminin deficiencies [93]. While structural myelin abnormalities are usually not very clear in CNS of laminin deficient human and mice [94], meticulous quantitative and morphological analysis suggested the presence of defects of CNS myelin in dy/dy mice, in particular in small-sized axons [95].
4.2. Role of Dystroglycan in Radial Sorting
It was unclear whether dystroglycan played a role in radial sorting process from the loss-of-function studies of dystroglycan [25]. However the possibility cannot be denied because radial sorting defect in spinal roots was observed in both Large-null mice and fukutin chimeric mice [70, 71]. This suggests glycosylation of α-dystroglycan may be necessary for radial sorting, although the possibility that unidentified substrate proteins of these glycosylating enzymes play a role in radial sorting cannot be denied.
On the other hand, β1 integrin null mice showed a major abnormality in radial sorting [72]. β1 integrin binds to LG domains of laminin-2 different from α-dystroglycan binding sites. Taken together, there is still a possibility that dystroglycan may cooperate with β1 integrin in radial sorting process.
4.3. Role of Dystroglycan in Development and Maintenance of Schwann Cell Structures such as Cajal Cytoplasmic Band, Internode and Node of Ranvier
The loss-of-function studies provided evidences that dystroglycan plays roles in development and maintenance of Schwann cell structures such as Cajal cytoplasmic band, internode and node of Ranvier. First, Schwann cell-specific dystroglycan null mice showed nodal changes including reduced sodium channel density and disorganized microvilli [25, 28]. Second, dystroglycan along with periaxin, utrophin and laminin-2, is necessary for compartmentalization including Cajal cytoplasmic band formation and elongation of myelin segments [52]. These studies offered new aspects of dystroglycan function in Schwann cells, and will stimulate further studies of DGC concerning internode growth as well as nodal functions.
5. Molecular Mechanisms of Dystroglycan in Schwann Cell Myelin Formation
Molecular mechanisms of how dystroglycan regulates Schwann cell myelination remain to be elucidated. One possibility is that the link between dystroglycan and laminin-2 provide anchorage between Schwann cell abaxonal membrane and basal lamina on which progression of inner lip of Schwann cells over the axonal surface is based during myelin formation [96]. Evidences that dystroglycan has binding capacities between Schwann cell abaxonal membrane and basement membrane or laminin support this idea [25, 97, 98]. Notably, basement membrane is not always necessary for myelination [60, 99]. Probably it is just because less organized extracellular matrix components, which are not visible as electron dense basement membrane, are enough for myelination. In support of this idea, Podratz et al. [99] showed abundant laminin deposition on Schwann cell abaxonal surface in spite of the lack of visible basement membrane.
Another possibility is that dystroglycan plays a role in myelination by regulating signaling from extracellular matrix, especially laminin-2, to intracellular signaling pathways or cytoskeleton. Actually, laminin signaling seems to play an essential role in Schwann cell proliferation and survival as well as cytoskeletal regulation-associated ensheathment and myelination [60, 100]. While dystroglycan is a major laminin receptor in Schwann cells along with integrins such as α6β1, α6β4, and α7β1, the receptor function of dystroglycan mediating laminin signaling has been less extensively studied compared with that of integrins. Recently, however, ample evidences are accumulating that dystroglycan is associated with cell signaling.
First, β-dystroglycan can interact with a variety of signaling proteins, at least partly via SH2 and SH3 binding sites in the C-terminus. While dephosphorylation of Y892 (pY892) in β-dystroglycan enables binding to utrophin/dystrophin [101, 102], phosphorylation of β-dystroglycan enhances recruitment of SH2/SH3 domain containing signaling-associated proteins like c-Src, Fyn, Csk, Nck, Shc, and Grb-2 [103–106]. Grb-2 is known to be involved in ERK-MAP kinase cascade and cytoskeletal organization [103]. Also β-dystroglycan can interact with FAK [104]. In addition, DAMAGE was reported to be a new member of DGC as a protein associated with α-dytrobrevin [43]. DAMAGE has a potential nuclear localization signal, 30 contiguous 12-aminoacid repeats and two MAGE homology domains, suggesting it is involved in membrane signaling [43].
Moreover, in nonperipheral nerve tissues, dystroglycan has been shown to be involved in several signaling pathways. Association of dystroglycan with MAPK/ERK cascade and GTPase signaling was reported [107–109]. Filopodia formation is often governed by Cdc42, a GTPase signaling-associated protein playing diverse roles in cell polarity, cytoskeletal regulation as well as cell cycle [109]. Dystroglycan plays a role in filopodia formation via forming a complex with ezrin and Dbl, and activating Cdc42 [107–109]. Fibronectin or biglycan may induce signaling via dystroglycan leading to calcium flux and alteration of cytoskeletal architecture [110]. Syntrophin contains two pleckstrin homology (PH) domains and one PDZ domain [111]. Binding of laminin to DGC induced heterotrimeric G protein binding to α-syntrophin's PDZ domain, which leads to activation of PI3K/Act signaling and alteration of intracellular Ca2+ in muscle [111–114]. Laminin-1 induced Grb binding to syntrophin, recruited Sos1/Rac1/PAK1/JNK, and eventually led to c-jun phosphorylation [115]. Recently, genetic modifier screens using Drosophila melanogaster revealed that DGC interacts with genes involved in Notch, TGF-β and EGFR signaling pathways as well as those associated with muscle function and cellular or axonal migration [116]. In Schwann cells also, laminin assembly initiated dystroglycan-dependent Src/Fyn activation and utrophin recruitment that contributed to their survival [117]. Taken together, these evidences strongly suggest that dystroglycan signaling plays diverse roles in Schwann cell functions including myelination. The hypothetical role of dystroglycan signaling in Schwann cell myelination will be discussed in detail in the section of future perspectives.
6. Human Peripheral Nervous System (PNS) Diseases Associated with DGC
Among the human diseases caused by mutation of DGC components, there are only two diseases in which peripheral nerve involvement was clearly demonstrated, MDC1A (LAMA2 mutation) and neuropathy caused by PRX mutation, which will be described in detail below. In general, peripheral nerve involvement caused by mutation of DGC components has not been studied as extensively as muscular dystrophies. Therefore, there is still a possibility that further study in the future will reveal new evidences that other DGC components play roles in the pathogenesis of human peripheral neuropathies.
6.1. MDC1A (Merosin (Laminin-2)-Deficient Congenital Muscular Dystrophy)
MDC1A is the most frequent congenital muscular dystrophy in Europe with autosomal recessive inheritance caused by LAMA2 mutation [94, 118]. Complete laminin-2 deficiency causes early-onset muscular dystrophy, peripheral neuropathy and white matter lesions in CNS. Partial laminin-2 deficiency presents variant phenotypes with later onset muscular dystrophy, or even predominant PNS or CNS abnormalities. While peripheral nerve involvement is not extensively studied in MDC1A, nerve conduction velocity is reduced in most of the patients [119–123]. As a basis for the slowed conduction velocity, abnormal sodium channel clusters were found in these patients [28]. The neuropathy is predominantly motor or sensory-motor [120–123]. Sural nerve biopsy showed mild loss of myelinated fibers, globular thickening of myelin sheath at paranodal region, myelin foldings, shortened internodes, widened nodes of Ranvier [121, 124], and compartmentalization defects [52]. Unfortunately, radial sorting defect in spinal roots in human has not been confirmed because of the absence of autopsy study.
Patients with MDC1A show striking white matter changes in T2 weighted brain magnetic resonance imaging [125], which is diffuse, bilateral, and symmetrical. It appears after the first 6 months of life, and nonprogressive [126]. However, morphological changes of cerebral white matter are not clearly demonstrated in human patients. Rather, main pathological findings in CNS are developmental anomalies such as abnormal cerebral cortical gyration, hypoplasia of vermis, hemisphere, or pons. At least, part of these abnormalities is supposed to be caused by neuronal migration defects associated with laminin-2-α-dystroglycan interaction, which are also demonstrated in α-dystroglycanopathies. The full aspects of dystroglycan roles in CNS are beyond the scope of this review, and other comprehensive reviews deal in detail with these issues [81, 127–130].
6.2. Charcot-Marie-Tooth Neuropathy Type 4F and Dejerine Sottas Neuropathy Caused by PRX (Periaxin) Mutations
Periaxin is the only one gene of members of DGC reported to be associated with human hereditary demyelinating neuropathy. Mutation of PRX causing loss of L-periaxin expression induces sensory-motor demyelinating neuropathy consistent with Charcot-Marie-Tooth neuropathy or Dejerine Sottas neuropathy [131–133]. Homozygous PRX mutation C715X causing expression of truncated form of L-periaxin showed relatively milder phenotype of Charcot-Marie-Tooth neuropathy with sensory dominant involvement [134]. Nerve biopsy showed loss of myelinated fibers, onion bulbs, focal thickening of myelin sheath, and myelin folding, which were similar to the nerve pathology of Prx-/- mice [131–134].
7. Future Perspectives
Schwann cell is one of the somatic cell types with robust regenerative capacity. The regenerative capacity of Schwann cell depends on its property of plasticity, which enables Schwann cell to change its phenotype between differentiated Schwann cell (myelinating type and nonmyelinating type) and denervated (dedifferentiated) Schwann cell [135, 136]. Recent advances have highlighted the importance of gene/protein network maintaining the identity of cell phenotype, especially from the study of embryonic stem cells [137, 138]. Then, there must be molecular network specific to each of the Schwann cell phenotypes. Definitely dystroglycan and other DGC components belong to the molecular network specific to myelinating Schwann cell phenotype, and now it is important to understand the role of dystroglycan in the context how this protein contributes to the gene/protein network. Cutting edge technologies such as DNA microarray, ultra-high-throughput sequencing and mass spectroscopy-based proteomics have made it possible to study epigenome, transcriptome, and proteome as a whole [137, 139]. Analyzing gene/protein network maintaining identity of myelinating Schwann cell phenotype, and comparing with that of dedifferentiated Schwann cell phenotype will reveal a whole framework of molecular mechanisms of myelination.
At the same time, it is important to study dystroglycan function focusing on specific molecular aspects such as protein interaction, signaling, transcriptional regulation including chromatin modifications, post-transcriptional modification, post-translational modification, and intracellular transport and degradation. In addition, anatomical aspects should be taken into consideration. Myelinating Schwann cell has four distint domains: internode, juxtaparanode, paranode, and node [140, 141]. Moreover, internode domain is divided into two compartments; Cajal cytoplasmic band, and compartment occupied by myelin sheath [51]. Each domain is playing a specific role in maintaining myelinating Schwann cells, and DGC is likely to play different role in each of the domain. In many of these specific aspects, dystroglycan function is just beginning to be revealed, and so many questions must be answered. A few of the mutually nonexclusive topics with high priority will be discussed below.
(1) Protein Interaction —
various proteins have been and are beginning to be revealed to interact with DGC in nonperipheral nerve tissues. Examples are extracellular matrix proteins such as fibronectin/biglycan [110, 142], perlecan [143–145], pikachurin [146], membrane proteins/receptors such as integrins [147, 148], AHNAK [53], aquaporins [149, 150], MLC1 [151], caveolins [147], Na and K channels [141, 149, 152–154], and submembranous or cytoskeletal proteins such as G proteins or other signaling-associated proteins [103, 106, 111, 113, 115], nitric-oxide synthase [147], actins [43], tubulins [155], ERMs (ezrin-radixin-moesin) [108, 109], and Par1 [10]. Dissecting their interactions with DGC in Schwann cells one by one will reveal more comprehensive molecular architecture, and specific functions associated with it.
(2) Signaling —
Myelinating Schwann cell phenotype is supposed to be maintained by signaling from both abaxonal membrane contacting extracellular matrix and adaxonal membrane apposing to axon. And the integrated signaling from both directions is supposed to maintain transcriptome or cytoskeletal structures specific to myelinating Schwann cells. Dystroglycan may play a role in signaling from abaxonal membrane via the interaction with other proteins present in extracellular matrix/abaxonal membrane/cytoskeleton, especially with laminin-2 and α6β4 integrin. Then dystroglycan-associated signaling is supposed to activate positive regulators of myelination, and inactivate negative regulators of myelination. Evidences accumulated by studies of nonperipheral nerve tissues suggest a number of hypotheses in this issue. As examples, several hypotheses will be described below.
First is about the association of dystroglycan or syntrophin with signaling-associated proteins such as Src, Fyn, Csk, Nck, Shc, and Grb2 [103–106, 108, 109, 115]. Interaction of DGC with these adaptor proteins implies that DGC may regulate Rho family GTPase signaling as well as MAPK signaling cascade [103–106, 108, 109, 115, 156]. Or yeast two hybrid screens suggested that dystroglycan can directly activate MEK or ERK, members of MAPK cascade [107]. Because it was reported that Ras signaling promotes differentiation of Schwann cells [157], dystroglycan may promote myelination through Ras signaling. However, it is controversial whether Ras/Raf/ERK signaling is promoting Schwann cell differentiation because there is a report that Ras/Raf/ERK signaling drives Schwann cell dedifferentiation [158]. On the other hand, Cdc42, one of the Rho family GTPases, was suggested to promote radial sorting and myelination [65]. So Cdc42 may be another mediator of dystroglycan signaling.
Second, genetic modifier screens suggested the association of dystroglycan with Notch signaling [116]. In Schwann cells, Notch acts as a negative regulator of myelination [135, 159]. Dystroglycan, therefore, can promote myelination via regulating Notch signaling. Notably, Notch signaling and WNT signaling seem to prevent oligodendrocyte differentiation (myelination). Notch signaling and WNT signaling respectively can induce HES5 and ID2/4, repressors of myelin genes, via transcription factor activity of NICD (Notch1 intracellular domain)/CBF1 and β-catenin/TCF7L2, and then HES5 or ID2/4 represses the transcription of myelin genes [160, 161]. Moreover, HDACs (histone deacetylases) can relieve these repressions by competing with the NICD to bind to CBF1 and competing with β-catenin to bind to TCF7L2. At the same time, the HDACs might increase the state of chromatin compaction around genes such as HES5, ID2, and ID4 that encode repressors of oligodendrocyte differentiation, thus preventing their transcription and providing permissive conditions for oligodendrocyte differentiation [160, 161].
Third, Krox20, one of the key myelin-associated transcription factors [162], might be another candidate mediator of dystroglycan signaling, because laminin signaling increases Krox-20 expression [163]. Krox 20 can inhibit c-Jun activity [164]. Also Krox 20 can suppress Notch signaling by reducing NICD post-translationally [159].
Fourth, DGC can interact with FAK [104]. FAK is an ECM-associated signaling protein and is at the crossroad of multiple signaling pathways, interacting with Rho GTPase signaling as well as MAPK signaling [165]. Effects of FAK on cytoskeletal organization were well demonstrated [165]. Moreover, FAK was recently reported to be required for radial sorting [166]. FAK can interact also with Erb2/Erb3 receptor [167], and then Erb2/Erb3 receptor can promote myelination [100]. Notably, laminin deficiency caused dramatic decrease of Erb2/Erb3 receptor phosphorylation [100]. Therefore, DGC may regulate radial sorting or myelination via interaction with FAK.
(3) Transcriptional Regulation —
little is known about transcriptional regulation of dystroglycan or other DGC components in Schwann cells. Recently, Rettino et al. [168] reported that the expression of dystroglycan was regulated by SP1 transcription factor in muscle cells, and DNA methylation as well as histone acetylation may be involved in the regulation. In addition, Miura et al. [169] reported that PPARβ/δ agonist stimulated the transcription of utrophin, which restored the expression of α1 syntrophin and β-dystroglycan at the sarcolemma of the mdx mice. However, so many issues still remain to be answered considering the extremely complicated mechanisms of transcriptional regulation exerted by various transcriptional regulators as well as chromatin modifications including DNA methylation and all kinds of histone modifications [170].
(4) Node of Ranvier —
While dystroglycan-null mice showed variety of nodal abnormalities including disorganized microvilli and reduced sodium channel density, the molecular mechanism remains to be unknown. In order to address this issue, it will be important to study the interaction of dystroglycan with node-associated proteins. For example, ERMs are expressed in the microvilli of many cell types including those of Schwann cells [171]. In nonperipheral nervous tissues, it is known that dystroglycan plays a role in filopodia formation via interaction with ezrin, and subsequent activation of Cdc42 [108, 109]. Then Cdc42 is supposed to induce cytoskeletal changes necessary for filopodia formation [108, 109]. Therefore, dystroglycan may be associated with Schwann cell microvilli formation through interaction with ERMs.
(5) Cell Polarity —
While Schwann cells do not belong to epithelial cells, Schwann cells have many molecular and structural properties similar to epithelial cells [140, 172]. In particular, cell polarity is a central feature shared by Schwann cells with epithelial cells [140, 172]. Several lines of evidences suggest that dystroglycan plays a role in maintaining cell polarity of not only epithelial cells but also Schwann cells [8–10, 30, 172]. First, in Wallerian degeneration, Schwann cell phenotype changes from myelinating type to dedifferentiated type [135]. During this process, the cell polarity specific to myelinating Schwann cells is lost along with the dissociation of basement membrane from abaxonal membrane as well as downregulation of dystroglycan and laminin-2 [30]. This finding suggests that dystroglycan-laminin-2 interaction is involved in cell polarity maintenance of myelinating Schwann cells. Second, Cdc42, which seems to have several links with dystroglycan signaling, is one of the cental regulators of cell polarity in epithelial cells as well as glial cells [172]. Third, cell polarity protein Par-1 not only regulates the basolateral localization of DGC, but also is required for the formation of a functional DGC in epithelial cells [10]. Analyzing molecular interactions of DGC with cell polarity-associated proteins will reveal further detailed function of dystroglycan.
8. Conclusion
Dystroglycan plays diverse roles in Schwann cells such as myelination and maintenance of myelin and nodal structures. However, the molecular mechanisms on which dystroglycan functions are based are just beginning to be revealed. Analyzing gene/protein network as a whole system using cutting edge technologies as well as individual studies focusing on a variety of biological aspects specific to Schwann cells hold promise for elucidating molecular mechanism of dystroglycan functions, and eventual development of effective treatments for human peripheral neuropathies.
References
- 1.Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 2.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355(6362):696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 3.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. Journal of Cell Science. 2006;119(2):199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 5.Williamson RA, Henry MD, Daniels KJ, et al. Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Human Molecular Genetics. 1997;6(6):831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- 6.Durbeej M, Larsson E, Ibraghimov-Beskrovnaya O, Roberds SL, Campbell KP, Ekblom P. Non-muscle α-dystroglycan is involved in epithelial development. Journal of Cell Biology. 1995;130(1):79–91. doi: 10.1083/jcb.130.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidalgo M, Sirour C, Bello V, Moreau N, Beaudry M, Darribère T. In vivo analyzes of dystroglycan function during somitogenesis in Xenopus laevis. Developmental Dynamics. 2009;238(6):1332–1345. doi: 10.1002/dvdy.21814. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Edgar D, Fässler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Developmental Cell. 2003;4(5):613–624. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- 9.Deng W-M, Schneider M, Frock R, et al. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130(1):173–184. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- 10.Masuda-Hirata M, Suzuki A, Amano Y, et al. Intracellular polarity protein PAR-1 regulates extracellular laminin assembly by regulating the dystroglycan complex. Genes to Cells. 2009;14(7):835–850. doi: 10.1111/j.1365-2443.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- 11.Henry MD, Cohen MB, Campbell KP. Reduced expression of dystroglycan in breast and prostate cancer. Human Pathology. 2001;32(8):791–795. doi: 10.1053/hupa.2001.26468. [DOI] [PubMed] [Google Scholar]
- 12.Sgambato A, Brancaccio A. The dystroglycan complex: from biology to cancer. Journal of Cellular Physiology. 2005;205(2):163–169. doi: 10.1002/jcp.20411. [DOI] [PubMed] [Google Scholar]
- 13.Cao W, Henry MD, Borrow P, et al. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282(5396):2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 14.Rambukkana A, Yamada H, Zanazzi G, et al. Role of α-dystroglycan as a Schwann cell receptor for Mycobacterium leprae. Science. 1998;282(5396):2076–2079. doi: 10.1126/science.282.5396.2076. [DOI] [PubMed] [Google Scholar]
- 15.Rambukkana A, Kunz S, Min J, Campbell KP, Oldstone MBA. Targeting Schwann cells by nonlytic arenaviral infection selectively inhibits myelination. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):16071–16076. doi: 10.1073/pnas.2232366100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore SA, Saito F, Chen J, et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418(6896):422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- 17.Schröder JE, Tegeler MR, Großhans U, et al. Dystroglycan regulates structure, proliferation and differentiation of neuroepithelial cells in the developing vertebrate CNS. Developmental Biology. 2007;307(1):62–78. doi: 10.1016/j.ydbio.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nature Reviews Neuroscience. 2005;6(9):683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 19.Matsumura K, Yamada H, Shimizu T, Campbell KP. Differential expression of dystrophin, utrophin and dystrophin-associated proteins in peripheral nerve. FEBS Letters. 1993;334(3):281–285. doi: 10.1016/0014-5793(93)80695-q. [DOI] [PubMed] [Google Scholar]
- 20.Byers TJ, Lidov HGW, Kunkel LM. An alternative dystrophin transcript specific to peripheral nerve. Nature Genetics. 1993;4(1):77–81. doi: 10.1038/ng0593-77. [DOI] [PubMed] [Google Scholar]
- 21.Yamada H, Shimizu T, Tanaka T, Campbell KP, Matsumura K. Dystroglycan is a binding protein of laminin and merosin in peripheral nerve. FEBS Letters. 1994;352(1):49–53. doi: 10.1016/0014-5793(94)00917-1. [DOI] [PubMed] [Google Scholar]
- 22.Yamada H, Chiba A, Endo T, et al. Characterization of dystroglycan-laminin interaction in peripheral nerve. Journal of Neurochemistry. 1996;66(4):1518–1524. doi: 10.1046/j.1471-4159.1996.66041518.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamada H, Denzer AJ, Hori H, et al. Dystroglycan is a dual receptor for agrin and laminin-2 in Schwann cell membrane. Journal of Biological Chemistry. 1996;271(38):23418–23423. doi: 10.1074/jbc.271.38.23418. [DOI] [PubMed] [Google Scholar]
- 24.Saito F, Masaki T, Kamakura K, et al. Characterization of the transmembrane molecular architecture of the dystroglycan complex in Schwann cells. Journal of Biological Chemistry. 1999;274(12):8240–8246. doi: 10.1074/jbc.274.12.8240. [DOI] [PubMed] [Google Scholar]
- 25.Saito F, Moore SA, Barresi R, et al. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38(5):747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 26.Imamura M, Araishi K, Noguchi S, Ozawa E. A sarcoglycan-dystroglycan complex anchors Dp116 and utrophin in the peripheral nervous system. Human Molecular Genetics. 2000;9(20):3091–3100. doi: 10.1093/hmg/9.20.3091. [DOI] [PubMed] [Google Scholar]
- 27.Sherman DL, Fabrizi C, Gillespie CS, Brophy PJ. Specific disruption of a Schwann cell dystrophin-related protein complex in a demyelinating neuropathy. Neuron. 2001;30(3):677–687. doi: 10.1016/s0896-6273(01)00327-0. [DOI] [PubMed] [Google Scholar]
- 28.Occhi S, Zambroni D, Del Carro U, et al. Both laminin and Schwann cell dystroglycan are necessary for proper clustering of sodium channels at nodes of Ranvier. Journal of Neuroscience. 2005;25(41):9418–9427. doi: 10.1523/JNEUROSCI.2068-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albrecht DE, Sherman DL, Brophy PJ, Froehner SC. The ABCA1 cholesterol transporter associates with one of two distinct dystrophin-based scaffolds in Schwann cells. Glia. 2008;56(6):611–618. doi: 10.1002/glia.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masaki T, Matsumura K, Saito F, et al. Expression of dystroglycan and laminin-2 in peripheral nerve under axonal degeneration and regeneration. Acta Neuropathologica. 2000;99(3):289–295. doi: 10.1007/pl00007440. [DOI] [PubMed] [Google Scholar]
- 31.Masaki T, Matsumura K, Saito F, et al. Association of dystroglycan and laminin-2 coexpression with myelinogenesis in peripheral nerves. Medical Electron Microscopy. 2003;36(4):221–239. doi: 10.1007/s00795-003-0231-2. [DOI] [PubMed] [Google Scholar]
- 32.Masaki T, Matsumura K, Hirata A, et al. Expression of dystroglycan complex in satellite cells of dorsal root ganglia. Acta Neuropathologica. 2001;101(2):174–178. doi: 10.1007/s004010000276. [DOI] [PubMed] [Google Scholar]
- 33.Holt KH, Crosbie RH, Venzke DP, Campbell KP. Biosynthesis of dystroglycan: processing of a precursor propeptide. FEBS Letters. 2000;468(1):79–83. doi: 10.1016/s0014-5793(00)01195-9. [DOI] [PubMed] [Google Scholar]
- 34.Hohenester E, Tisi D, Talts JF, Timpl R. The crystal structure of a laminin G-like module reveals the molecular basis of α-dystroglycan binding to laminins, perlecan, and agrin. Molecular Cell. 1999;4(5):783–792. doi: 10.1016/s1097-2765(00)80388-3. [DOI] [PubMed] [Google Scholar]
- 35.Tisi D, Talts JF, Timpl R, Hohenester E. Structure of the C-terminal laminin G-like domain pair of the laminin α2 chain harbouring binding sites for α-dystroglycan and heparin. The EMBO Journal. 2000;19(7):1432–1440. doi: 10.1093/emboj/19.7.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talts JF, Sasaki T, Miosge N, et al. Structural and functional analysis of the recombinant G domain of the laminin α4 chain and its proteolytic processing in tissues. Journal of Biological Chemistry. 2000;275(45):35192–35199. doi: 10.1074/jbc.M003261200. [DOI] [PubMed] [Google Scholar]
- 37.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. Journal of Cell Biology. 1993;122(4):809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiba A, Matsumura K, Yamada H, et al. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve α-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of α-dystroglycan with laminin. Journal of Biological Chemistry. 1997;272(4):2156–2162. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- 39.Combs AC, Ervasti JM. Enhanced laminin binding by α-dystroglycan after enzymatic deglycosylation. Biochemical Journal. 2005;390(1):303–309. doi: 10.1042/BJ20050375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall H, Bozic D, Michel K, Hubbell JA. N-terminal α-dystroglycan binds to different extracellular matrix molecules expressed in regenerating peripheral nerves in a protein-mediated manner and promotes neurite extension of PC12 cells. Molecular and Cellular Neuroscience. 2003;24(4):1062–1073. doi: 10.1016/j.mcn.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Chung W, Campanelli JT. WW and EF hand domains of dystrophin-family proteins mediate dystroglycan binding. Molecular Cell Biology Research Communications. 1999;2(3):162–171. doi: 10.1006/mcbr.1999.0168. [DOI] [PubMed] [Google Scholar]
- 42.Blake DJ, Martin-Rendon E. Intermediate filaments and the function of the dystrophin-protein complex. Trends in Cardiovascular Medicine. 2002;12(5):224–228. doi: 10.1016/s1050-1738(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 43.Albrecht DE, Froehner SC. DAMAGE, a novel α-dystrobrevin-associated MAGE protein in dystrophin complexes. Journal of Biological Chemistry. 2004;279(8):7014–7023. doi: 10.1074/jbc.M312205200. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y-J, Spence HJ, Cameron JM, Jess T, Ilsley JL, Winder SJ. Direct interaction of β-dystroglycan with F-actin. Biochemical Journal. 2003;375(2):329–337. doi: 10.1042/BJ20030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albrecht DE, Froehner SC. Syntrophins and dystrobrevins: defining the dystrophin scaffold at synapses. NeuroSignals. 2002;11(3):123–129. doi: 10.1159/000065053. [DOI] [PubMed] [Google Scholar]
- 46.Hnia K, Hugon G, Masmoudi A, Mercier J, Rivier F, Mornet D. Effect of β-dystroglycan processing on utrophin/Dp116 anchorage in normal and mdx mouse Schwann cell membrane. Neuroscience. 2006;141(2):607–620. doi: 10.1016/j.neuroscience.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada H, Saito F, Fukuta-Ohi H, et al. Processing of β-dystroglycan by matrix metalloproteinase disrupts the link between the extracellular matrix and cell membrane via the dystroglycan complex. Human Molecular Genetics. 2001;10(15):1563–1569. doi: 10.1093/hmg/10.15.1563. [DOI] [PubMed] [Google Scholar]
- 48.Matsumura K, Zhong D, Saito F, et al. Proteolysis of β-dystroglycan in muscular diseases. Neuromuscular Disorders. 2005;15(5):336–341. doi: 10.1016/j.nmd.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Zhong D, Saito F, Saito Y, Nakamura A, Shimizu T, Matsumura K. Characterization of the protease activity that cleaves the extracellular domain of β-dystroglycan. Biochemical and Biophysical Research Communications. 2006;345(2):867–871. doi: 10.1016/j.bbrc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Cai H, Erdman RA, Zweier L, et al. The sarcoglycan complex in Schwann cells and its role in myelin stability. Experimental Neurology. 2007;205(1):257–269. doi: 10.1016/j.expneurol.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Court FA, Sherman DL, Pratt T, et al. Restricted growth of Schwann cells lacking Cajal bands slows conduction in myelinated nerves. Nature. 2004;431(7005):191–195. doi: 10.1038/nature02841. [DOI] [PubMed] [Google Scholar]
- 52.Court FA, Hewitt JE, Davies K, et al. A laminin-2, dystroglycan, utrophin axis is required for compartmentalization and elongation of myelin segments. Journal of Neuroscience. 2009;29(12):3908–3919. doi: 10.1523/JNEUROSCI.5672-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salim C, Boxberg YV, Alterio J, Féréol S, Nothias F. The giant protein AHNAK involved in morphogenesis and laminin substrate adhesion of myelinating Schwann cells. Glia. 2009;57(5):535–549. doi: 10.1002/glia.20782. [DOI] [PubMed] [Google Scholar]
- 54.Nodari A, Previtali SC, Dati G, et al. α6 β4 integrin and dystroglycan cooperate to stabilize the myelin sheath. Journal of Neuroscience. 2008;28(26):6714–6719. doi: 10.1523/JNEUROSCI.0326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michelson AM, Russell ES, Harman PJ. Dystrophia muscularis: a hereditary primary myopathy in the house mouse. Proceedings of the National Academy of Sciences of the United States of America. 1955;41(12):1079–1084. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradley WG, Jenkison M. Neural abnormalities in the dystrophic mouse. Journal of the Neurological Sciences. 1975;25(2):249–255. doi: 10.1016/0022-510x(75)90144-6. [DOI] [PubMed] [Google Scholar]
- 57.Bradley WG, Jaros E, Jenkison M. The nodes of Ranvier in the nerves of mice with muscular dystrophy. Journal of Neuropathology and Experimental Neurology. 1977;36(5):797–806. doi: 10.1097/00005072-197709000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Stirling CA. Abnormalities in Schwann cell sheaths in spinal nerve roots of dystrophic mice. Journal of Anatomy. 1975;119(1):169–180. [PMC free article] [PubMed] [Google Scholar]
- 59.Jaros E, Bradley WG. Atypical axon-Schwann cell relationships in the common peroneal nerve of the dystrophic mouse: an ultrastructural study. Neuropathology and Applied Neurobiology. 1979;5(2):133–147. doi: 10.1111/j.1365-2990.1979.tb00666.x. [DOI] [PubMed] [Google Scholar]
- 60.Feltri ML, Wrabetz L. Laminins and their receptors in Schwann cells and hereditary neuropathies. Journal of the Peripheral Nervous System. 2005;10(2):128–143. doi: 10.1111/j.1085-9489.2005.0010204.x. [DOI] [PubMed] [Google Scholar]
- 61.Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. Journal of Cell Biology. 1997;139(6):1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patton BL, Wang B, Tarumi YS, Seburn KL, Burgess RW. A single point mutation in the LN domain of LAMA2 causes muscular dystrophy and peripheral amyelination. Journal of Cell Science. 2008;121(10):1593–1604. doi: 10.1242/jcs.015354. [DOI] [PubMed] [Google Scholar]
- 63.Xu H, Wu X-R, Wewer UM, Engval E. Murine muscular dystrophy caused by a mutation in the laminin α2 (Lama2) gene. Nature Genetics. 1994;8(3):297–302. doi: 10.1038/ng1194-297. [DOI] [PubMed] [Google Scholar]
- 64.Sunada Y, Bernier SM, Utani A, Yamada Y, Campbell KP. Identification of a novel mutant transcript of laminin α2 chain gene responsible for muscular dystrophy and dysmyelination in dy2J mice. Human Molecular Genetics. 1995;4(6):1055–1061. doi: 10.1093/hmg/4.6.1055. [DOI] [PubMed] [Google Scholar]
- 65.Yu W-M, Chen Z-L, North AJ, Strickland S. Laminin is required for Schwann cell morphogenesis. Journal of Cell Science. 2009;122(7):929–936. doi: 10.1242/jcs.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakagawa M, Miyagoe-Suzuki Y, Ikezoe K, et al. Schwann cell myelination occurred without basal lamina formation in laminin α2 chain-null mutant (dy3K/dy3K) mice. Glia. 2001;35(2):101–110. doi: 10.1002/glia.1075. [DOI] [PubMed] [Google Scholar]
- 67.Yang D, Bierman J, Tarumi YS, et al. Coordinate control of axon defasciculation and myelination by laminin-2 and-8. Journal of Cell Biology. 2005;168(4):655–666. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Z-L, Strickland S. Laminin γ1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. Journal of Cell Biology. 2003;163(4):889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gillespie CS, Sherman DL, Fleetwood-Walker SM, et al. Peripheral demyelination and neuropathic pain behavior in periaxin-deficient mice. Neuron. 2000;26(2):523–531. doi: 10.1016/s0896-6273(00)81184-8. [DOI] [PubMed] [Google Scholar]
- 70.Levedakou EN, Chen X-J, Soliven B, Popko B. Disruption of the mouse Large gene in the enr and myd mutants results in nerve, muscle, and neuromuscular junction defects. Molecular and Cellular Neuroscience. 2005;28(4):757–769. doi: 10.1016/j.mcn.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Saito F, Masaki T, Saito Y, et al. Defective peripheral nerve myelination and neuromuscular junction formation in fukutin-deficient chimeric mice. Journal of Neurochemistry. 2007;101(6):1712–1722. doi: 10.1111/j.1471-4159.2007.04462.x. [DOI] [PubMed] [Google Scholar]
- 72.Feltri ML, Porta DG, Previtali SC, et al. Conditional disruption of β1 integrin in Schwann cells impedes interactions with axons. Journal of Cell Biology. 2002;156(1):199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuang W, Xu H, Vachon PH, et al. Merosin-deficient congenital muscular dystrophy: partial genetic correction in two mouse models. Journal of Clinical Investigation. 1998;102(4):844–852. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Previtali SC, Nodari A, Taveggia C, et al. Expression of laminin receptors in Schwann cell differentiation: evidence for distinct roles. Journal of Neuroscience. 2003;23(13):5520–5530. doi: 10.1523/JNEUROSCI.23-13-05520.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gawlik KI, Li J-Y, Petersén A, Durbeej M. Laminin α1 chain improves laminin α2 chain deficient peripheral neuropathy. Human Molecular Genetics. 2006;15(18):2690–2700. doi: 10.1093/hmg/ddl201. [DOI] [PubMed] [Google Scholar]
- 76.Einheber S, Milner TA, Giancotti F, Salzer JL. Axonal regulation of Schwann cell integrin expression suggests a role for α6β4 in myelination. Journal of Cell Biology. 1993;123(5):1223–1236. doi: 10.1083/jcb.123.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fernandez-Valle C, Gwynn L, Wood PM, Carbonetto S, Bunge MB. Anti-β1 integrin antibody inhibits Schwann cell myelination. Journal of Neurobiology. 1994;25(10):1207–1226. doi: 10.1002/neu.480251004. [DOI] [PubMed] [Google Scholar]
- 78.Feltri ML, Scherer SS, Nemni R, et al. β4 integrin expression in myelinating Schwann cells is polarized, developmentally regulated and axonally dependent. Development. 1994;120(5):1287–1301. doi: 10.1242/dev.120.5.1287. [DOI] [PubMed] [Google Scholar]
- 79.Michele DE, Barresi R, Kanagawa M, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418(6896):417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 80.Kim D-S, Hayashi YK, Matsumoto H, et al. POMT1 mutation results in defective glycosylation and loss of laminin-binding activity in α-DG. Neurology. 2004;62(6):1009–1011. doi: 10.1212/01.wnl.0000115386.28769.65. [DOI] [PubMed] [Google Scholar]
- 81.Toda T, Kobayashi K, Takeda S, et al. Fukuyama-type congenital muscular dystrophy (FCMD) and alpha-dystroglycanopathy. Congenital Anomalies. 2003;43(2):97–104. doi: 10.1111/j.1741-4520.2003.tb01033.x. [DOI] [PubMed] [Google Scholar]
- 82.Toda T, Chiyonobu T, Xiong H, et al. Fukutin and α-dystroglycanopahties. Acta Myologica. 2005;24(2):60–63. [PubMed] [Google Scholar]
- 83.Mercuri E, Messina S, Bruno C, et al. Congenital muscular dystrophies with defective glycosylation of dystroglycan: a population study. Neurology. 2009;72(21):1802–1809. doi: 10.1212/01.wnl.0000346518.68110.60. [DOI] [PubMed] [Google Scholar]
- 84.Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annual Review of Neuroscience. 1986;9:305–328. doi: 10.1146/annurev.ne.09.030186.001513. [DOI] [PubMed] [Google Scholar]
- 85.Eldridge CF, Bunge MB, Bunge RP. Differentiation of axon-related Schwann cells in vitro: II. Control of myelin formation by basal lamina. Journal of Neuroscience. 1989;9(2):625–638. doi: 10.1523/JNEUROSCI.09-02-00625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matsumura K, Yamada H, Saito F, Sunada Y, Shimizu T. Peripheral nerve involvement in merosin-deficient congenital muscular dystrophy and dy mouse. Neuromuscular Disorders. 1997;7(1):7–12. doi: 10.1016/s0960-8966(96)00402-6. [DOI] [PubMed] [Google Scholar]
- 87.Masaki T, Matsumura K, Hirata A, et al. Expression of dystroglycan and the laminin-α2 chain in the rat peripheral nerve during development. Experimental Neurology. 2002;174(1):109–117. doi: 10.1006/exnr.2001.7856. [DOI] [PubMed] [Google Scholar]
- 88.Rambukkana A, Salzer JL, Yurchenco PD, Tuomanen EI. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-α2 chain. Cell. 1997;88(6):811–821. doi: 10.1016/s0092-8674(00)81927-3. [DOI] [PubMed] [Google Scholar]
- 89.Kunz S, Sevilla N, McGavern DB, Campbell KP, Oldstone MBA. Molecular analysis of the interaction of LCMV with its cellular receptor α-dystroglycan. Journal of Cell Biology. 2001;155(2):301–310. doi: 10.1083/jcb.200104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kunz S, Rojek JM, Perez M, Spiropoulou CF, Oldstone MBA. Characterization of the interaction of lassa fever virus with its cellular receptor α-dystroglycan. Journal of Virology. 2005;79(10):5979–5987. doi: 10.1128/JVI.79.10.5979-5987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rambukkana A, Zanazzi G, Tapinos N, Salzer JL. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science. 2002;296(5569):927–931. doi: 10.1126/science.1067631. [DOI] [PubMed] [Google Scholar]
- 92.Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nature Medicine. 2006;12(8):961–966. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- 93.Colognato H, Galvin J, Wang Z, et al. Identification of dystroglycan as a second laminin receptor in oligodendrocytes, with a role in myelination. Development. 2007;134(9):1723–1736. doi: 10.1242/dev.02819. [DOI] [PubMed] [Google Scholar]
- 94.Miyagoe-Suzuki Y, Nakagawa M, Takeda S. Merosin and congenital muscular dystrophy. Microscopy Research and Technique. 2000;48(3-4):181–191. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<181::AID-JEMT6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 95.Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. Journal of Cell Biology. 2003;163(2):397–408. doi: 10.1083/jcb.200304154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bunge RP, Bunge MB, Bates M. Movements of the Schwann cell nucleus implicate progression of the inner (axon-related) Schwann cell process during myelination. Journal of Cell Biology. 1989;109(1):273–284. doi: 10.1083/jcb.109.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han R, Kanagawa M, Yoshida-Moriguchi T, et al. Basal lamina strengthens cell membrane integrity via the laminin G domain-binding motif of α-dystroglycan. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12573–12579. doi: 10.1073/pnas.0906545106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsumura K, Chiba A, Yamada H, et al. A role of dystroglycan in schwannoma cell adhesion to laminin. Journal of Biological Chemistry. 1997;272(21):13904–13910. doi: 10.1074/jbc.272.21.13904. [DOI] [PubMed] [Google Scholar]
- 99.Podratz JL, Rodriguez E, Windebank AJ. Role of the extracellular matrix in myelination of peripheral nerve. Glia. 2001;35(1):35–40. doi: 10.1002/glia.1068. [DOI] [PubMed] [Google Scholar]
- 100.Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56(14):1498–1507. doi: 10.1002/glia.20740. [DOI] [PubMed] [Google Scholar]
- 101.James M, Nuttall A, Ilsley JL, et al. Adhesion-dependent tyrosine phosphorylation of β-dystroglycan regulates its interaction with utrophin. Journal of Cell Science. 2000;113(10):1717–1726. doi: 10.1242/jcs.113.10.1717. [DOI] [PubMed] [Google Scholar]
- 102.Ilsley JL, Sudol M, Winder SJ. The interaction of dystrophin with β-dystroglycan is regulated by tyrosine phosphorylation. Cellular Signalling. 2001;13(9):625–632. doi: 10.1016/s0898-6568(01)00188-7. [DOI] [PubMed] [Google Scholar]
- 103.Yang B, Jung D, Motto D, Meyer J, Koretzky G, Campbell KP. SH3 domain-mediated interaction of dystroglycan and Grb2. Journal of Biological Chemistry. 1995;270(20):11711–11714. doi: 10.1074/jbc.270.20.11711. [DOI] [PubMed] [Google Scholar]
- 104.Cavaldesi M, Macchia G, Barca S, Defilippi P, Tarone G, Petrucci TC. Association of the dystroglycan complex isolated from bovine brain synaptosomes with proteins involved in signal transduction. Journal of Neurochemistry. 1999;72(4):1648–1655. doi: 10.1046/j.1471-4159.1999.721648.x. [DOI] [PubMed] [Google Scholar]
- 105.Russo K, Di Stasio E, Macchia G, Rosa G, Brancaccio A, Petrucci TC. Characterization of the β-dystroglycan-growth factor receptor 2 (Grb2) interaction. Biochemical and Biophysical Research Communications. 2000;274(1):93–98. doi: 10.1006/bbrc.2000.3103. [DOI] [PubMed] [Google Scholar]
- 106.Sotgia F, Lee H, Bedford MT, Petrucci T, Sudol M, Lisanti MP. Tyrosine phosphorylation of β-dystroglycan at its WW domain binding motif, PPxY, recruits SH2 domain containing proteins. Biochemistry. 2001;40(48):14585–14592. doi: 10.1021/bi011247r. [DOI] [PubMed] [Google Scholar]
- 107.Spence HJ, Dhillon AS, James M, Winder S. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Reports. 2004;5(5):484–489. doi: 10.1038/sj.embor.7400140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Batchelor CL, Winder SJ. Sparks, signals and shock absorbers: how dystrophin loss causes muscular dystrophy. Trends in Cell Biology. 2006;16(4):198–205. doi: 10.1016/j.tcb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 109.Batchelor CL, Higginson JR, Chen Y-J, Vanni C, Eva A, Winder SJ. Recruitment of Dbl by ezrin and dystroglycan drives membrane proximal Cdc42 activation and filopodia formation. Cell Cycle. 2007;6(3):353–363. doi: 10.4161/cc.6.3.3819. [DOI] [PubMed] [Google Scholar]
- 110.Vogtländer NPJ, Visch HJ, Bakker MAH, Berden JHM, van der Vlag J. Ligation of α-dystroglycan on podocytes induces intracellular signaling: a new mechanism for podocyte effacement? PLoS ONE. 2009;4(6, article e5979) doi: 10.1371/journal.pone.0005979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou YW, Oak SA, Senogles SE, Jarrett HW. Laminin-alpha1 globular domains 3 and 4 induce heterotrimeric G protein binding to alpha-syntrophin’s PDZ domain and alter intracellular Ca2+ in muscle. American Journal of Physiology. 2005;288(2):C377–C388. doi: 10.1152/ajpcell.00279.2004. [DOI] [PubMed] [Google Scholar]
- 112.Langenbach KJ, Rando TA. Inhibition of dystroglycan binding to laminin disrupts the PI3K/AKT pathway and survival signaling in muscle cells. Muscle and Nerve. 2002;26(5):644–653. doi: 10.1002/mus.10258. [DOI] [PubMed] [Google Scholar]
- 113.Zhou Y, Jiang D, Thomason DB, Jarrett HW. Laminin-induced activation of Rac1 and JNKp46 is initiated by Src family kinases and mimics the effects of skeletal muscle contraction. Biochemistry. 2007;46(51):14907–14916. doi: 10.1021/bi701384k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xiong Y, Zhou Y, Jarrett HW. Dystrophin glycoprotein complex-associated Gβγ subunits activate phosphatidylinositol-3-kinase/akt signaling in skeletal muscle in a laminin-dependent manner. Journal of Cellular Physiology. 2009;219(2):402–414. doi: 10.1002/jcp.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oak SA, Zhou YW, Jarrett HW. Skeletal muscle signaling pathway through the dystrophin glycoprotein complex and Rac1. Journal of Biological Chemistry. 2003;278(41):39287–39295. doi: 10.1074/jbc.M305551200. [DOI] [PubMed] [Google Scholar]
- 116.Kucherenko MM, Pantoja M, Yatsenko AS, et al. Genetic modifier screens reveal new components that interact with the Drosophila dystroglycan-dystrophin complex. PLoS ONE. 2008;3(6, article e2418) doi: 10.1371/journal.pone.0002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li S, Liquari P, McKee KK, et al. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. Journal of Cell Biology. 2005;169(1):179–189. doi: 10.1083/jcb.200501098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guicheney P, Vignier N, Helbling-Leclerc A, et al. Genetics of laminin α2 chain (or merosin) deficient congenital muscular dystrophy: from identification of mutations to prenatal diagnosis. Neuromuscular Disorders. 1997;7(3):180–186. doi: 10.1016/s0960-8966(97)00460-4. [DOI] [PubMed] [Google Scholar]
- 119.Shorer Z, Philpot J, Muntoni F, Sewry C, Dubowitz V. Demyelinating peripheral neuropathy in merosin-deficient congenital muscular dystrophy. Journal of Child Neurology. 1995;10(6):472–475. doi: 10.1177/088307389501000610. [DOI] [PubMed] [Google Scholar]
- 120.Mora M, Moroni I, Uziel G, et al. Mild clinical phenotype in a 12-year-old boy with partial merosin deficiency and central and peripheral nervous system abnormalities. Neuromuscular Disorders. 1996;6(5):377–381. doi: 10.1016/0960-8966(96)00359-8. [DOI] [PubMed] [Google Scholar]
- 121.Di Muzio A, De Angelis MV, Di Fulvio P, et al. Dysmyelinating sensory-motor neuropathy in merosin-deficient congenital muscular dystrophy. Muscle and Nerve. 2003;27(4):500–506. doi: 10.1002/mus.10326. [DOI] [PubMed] [Google Scholar]
- 122.Quijano-Roy S, Renault F, Romero N, Guicheney P, Fardeau M, Estournet B. EMG and nerve conduction studies in children with congenital muscular dystrophy. Muscle and Nerve. 2004;29(2):292–299. doi: 10.1002/mus.10544. [DOI] [PubMed] [Google Scholar]
- 123.Habeeb YKR, Al-Bloushi M, Al-Jumah ES, De Souza TM, Moosa A. Congenital muscular dystrophy in Arab children. Journal of Child Neurology. 2006;21(5):400–405. doi: 10.1177/08830738060210050701. [DOI] [PubMed] [Google Scholar]
- 124.Deodato F, Sabatelli M, Ricci E, et al. Hypermyelinating neuropathy, mental retardation and epilepsy in a case of merosin deficiency. Neuromuscular Disorders. 2002;12(4):392–398. doi: 10.1016/s0960-8966(01)00312-1. [DOI] [PubMed] [Google Scholar]
- 125.Vainzof M, Marie SKN, Reed UC, et al. Deficiency of merosin (laminin M or α2) in congenital muscular dystrophy associated with cerebral white matter alterations. Neuropediatrics. 1995;26(6):293–297. doi: 10.1055/s-2007-979777. [DOI] [PubMed] [Google Scholar]
- 126.Trevisan CP, Martinello F, Ferruzza E, Fanin M, Chevallay M, Tomé FMS. Brain alterations in the classical form of congenital muscular dystrophy. Clinical and neuroimaging follow-up of 12 cases and correlation with the expression of merosin in muscle. Child’s Nervous System. 1996;12(10):604–610. doi: 10.1007/BF00261655. [DOI] [PubMed] [Google Scholar]
- 127.Haenggi T, Fritschy J-M. Role of dystrophin and utrophin for assembly and function of the dystrophin glycoprotein complex in non-muscle tissue. Cellular and Molecular Life Sciences. 2006;63(14):1614–1631. doi: 10.1007/s00018-005-5461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Muntoni F, Torelli S, Brockington M. Muscular dystrophies due to glycosylation defects. Neurotherapeutics. 2008;5(4):627–632. doi: 10.1016/j.nurt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamamoto T, Kato Y, Kawaguchi-Niida M, et al. Characteristics of neurons and glia in the brain of Fukuyama type congenital muscular dystrophy. Acta Myologica. 2008;27:9–13. [PMC free article] [PubMed] [Google Scholar]
- 130.Waite A, Tinsley CL, Locke M, Blake DJ. The neurobiology of the dystrophin-associated glycoprotein complex. Annals of Medicine. 2009;41(5):344–359. doi: 10.1080/07853890802668522. [DOI] [PubMed] [Google Scholar]
- 131.Delague V, Bareil C, Tuffery S, et al. Mapping of a new locus for autosomal recessive demyelinating charcot- marie-tooth disease to 19q13.1-13.3 in a large consanguineous Lebanese family: exclusion of MAG as a candidate gene. American Journal of Human Genetics. 2000;67(1):236–243. doi: 10.1086/302980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boerkoel CF, Takashima H, Stankiewicz P, et al. Periaxin mutations cause recessive dejerine-sottas neuropathy. American Journal of Human Genetics. 2001;68(2):325–333. doi: 10.1086/318208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guilbot A, Williams A, Ravisé N, et al. A mutation in periaxin is responsible for CMT4F, an autosomal recessive form of Charcot-Marie-Tooth disease. Human Molecular Genetics. 2001;10(4):415–421. doi: 10.1093/hmg/10.4.415. [DOI] [PubMed] [Google Scholar]
- 134.Takashima H, Boerkoel CF, De Jonghe P, et al. Periaxin mutations cause a broad spectrum of demyelinating neuropathies. Annals of Neurology. 2002;51(6):709–715. doi: 10.1002/ana.10213. [DOI] [PubMed] [Google Scholar]
- 135.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56(14):1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 136.Mirsky R, Woodhoo A, Parkinson DB, Arthur-Farraj P, Bhaskaran A, Jessen KR. Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation. Journal of the Peripheral Nervous System. 2008;13(2):122–135. doi: 10.1111/j.1529-8027.2008.00168.x. [DOI] [PubMed] [Google Scholar]
- 137.Chen X, Xu H, Yuan P, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133(6):1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 138.Müller F-J, Laurent LC, Kostka D, et al. Regulatory networks define phenotypic classes of human stem cell lines. Nature. 2008;455(7211):401–405. doi: 10.1038/nature07213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gstaiger M, Aebersold R. Applying mass spectrometry-based proteomics to genetics, genomics and network biology. Nature Reviews Genetics. 2009;10(9):617–627. doi: 10.1038/nrg2633. [DOI] [PubMed] [Google Scholar]
- 140.Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40(2):297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- 141.Salzer JL, Brophy PJ, Peles E. Molecular domains of myelinated axons in the peripheral nervous system. Glia. 2008;56(14):1532–1540. doi: 10.1002/glia.20750. [DOI] [PubMed] [Google Scholar]
- 142.Bowe MA, Mendis DB, Fallon JR. The small leucine-rich repeat proteoglycan biglycan binds to α-dystroglycan and is upregulated in dystrophic muscle. Journal of Cell Biology. 2000;148(4):801–810. doi: 10.1083/jcb.148.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Talts JF, Andac Z, Göhring W, Brancaccio A, Timpl R. Binding of the G domains of laminin α1 and α2 chains and perlecan to heparin, sulfatides, α-dystroglycan and several extracellular matrix proteins. The EMBO Journal. 1999;18(4):863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Henry MD, Satz JS, Brakebusch C, et al. Distinct roles for dystroglycan, β1 integrin and perlecan in cell surface laminin organization. Journal of Cell Science. 2001;114(6):1137–1144. doi: 10.1242/jcs.114.6.1137. [DOI] [PubMed] [Google Scholar]
- 145.Mirouse V, Christoforou CP, Fritsch C, St. Johnston D, Ray RP. Dystroglycan and perlecan provide a basal cue required for epithelial polarity during energetic stress. Developmental Cell. 2009;16(1):83–92. doi: 10.1016/j.devcel.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146.Sato S, Omori Y, Katoh K, et al. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nature Neuroscience. 2008;11(8):923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- 147.Côté PD, Moukhles H, Carbonetto S. Dystroglycan is not required for localization of dystrophin, syntrophin, and neuronal nitric-oxide synthase at the sarcolemma but regulates integrin α7B expression and caveolin-3 distribution. Journal of Biological Chemistry. 2002;277(7):4672–4679. doi: 10.1074/jbc.M106879200. [DOI] [PubMed] [Google Scholar]
- 148.Driss A, Charrier L, Yan Y, Nduati V, Sitaraman S, Merlin D. Dystroglycan receptor is involved in integrin activation in intestinal epithelia. American Journal of Physiology. 2006;290(6):G1228–G1242. doi: 10.1152/ajpgi.00378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guadagno E, Moukhles H. Laminin-induced aggregation of the inwardly rectifying potassium channel, Kir4.1, and the water-permeable channel, AQP4, via a dystroglycan-containing complex in astrocytes. Glia. 2004;47(2):138–149. doi: 10.1002/glia.20039. [DOI] [PubMed] [Google Scholar]
- 150.Nicchia GP, Cogotzi L, Rossi A, et al. Expression of multiple AQP4 pools in the plasma membrane and their association with the dystrophin complex. Journal of Neurochemistry. 2008;105(6):2156–2165. doi: 10.1111/j.1471-4159.2008.05302.x. [DOI] [PubMed] [Google Scholar]
- 151.Ambrosini E, Serafini B, Lanciotti A, et al. Biochemical characterization of MLC1 protein in astrocytes and its association with the dystrophin-glycoprotein complex. Molecular and Cellular Neuroscience. 2008;37(3):480–493. doi: 10.1016/j.mcn.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 152.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiological Reviews. 2002;82(2):291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 153.Eshed Y, Feinberg K, Poliak S, et al. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47(2):215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 154.Noël G, Belda M, Guadagno E, Micoud J, Klöcker N, Moukhles H. Dystroglycan and Kir4.1 coclustering in retinal Müller glia is regulated by laminin-1 and requires the PDZ-ligand domain of Kir4.1. Journal of Neurochemistry. 2005;94(3):691–702. doi: 10.1111/j.1471-4159.2005.03191.x. [DOI] [PubMed] [Google Scholar]
- 155.Prins KW, Humston JL, Mehta A, Tate V, Ralston E, Ervasti JM. Dystrophin is a microtubule-associated protein. Journal of Cell Biology. 2009;186(3):363–369. doi: 10.1083/jcb.200905048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hall A. Rho GTPases and the control of cell behaviour. Biochemical Society Transactions. 2005;33(5):891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 157.Rosenbaum T, Kim HA, Boissy YL, Ling B, Ratner N. Neurofibromin, the neurofibromatosis type 1 RaS-GAP, is required for appropriate P0 expression and myelination. Annals of the New York Academy of Sciences. 1999;883:203–214. [PubMed] [Google Scholar]
- 158.Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. The EMBO Journal. 2004;23(15):3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Woodhoo A, Alonso MBD, Droggiti A, et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nature Neuroscience. 2009;12(7):839–847. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ye F, Chen Y, Hoang T, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the β-catenin-TCF interaction. Nature Neuroscience. 2009;12(7):829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li H, Richardson WD. Genetics meets epigenetics: HDACs and Wnt signaling in myelin development and regeneration. Nature Neuroscience. 2009;12(7):815–817. doi: 10.1038/nn0709-815. [DOI] [PubMed] [Google Scholar]
- 162.Topilko P, Schneider-Maunoury S, Levi G, et al. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371(6500):796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- 163.Yu W-M, Feltri ML, Wrabetz L, Strickland S, Chen Z-L. Schwann cell-specific ablation of laminin γ1 causes apoptosis and prevents proliferation. Journal of Neuroscience. 2005;25(18):4463–4472. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Parkinson DB, Bhaskaran A, Droggiti A, et al. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. Journal of Cell Biology. 2004;164(3):385–394. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ilić D, Damsky CH, Yamamoto T. Focal adhesion kinase: at the crossroads of signal transduction. Journal of Cell Science. 1997;110(4):401–407. doi: 10.1242/jcs.110.4.401. [DOI] [PubMed] [Google Scholar]
- 166.Grove M, Komiyama NH, Nave K-A, Grant SG, Sherman DL, Brophy PJ. FAK is required for axonal sorting by Schwann cells. Journal of Cell Biology. 2007;176(3):277–282. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vartanian T, Goodearl A, Lefebvre S, Park S-K, Fischbach G. Neuregulin induces the rapid association of focal adhesion kinase with the erbB2-erbB3 receptor complex in Schwann cells. Biochemical and Biophysical Research Communications. 2000;271(2):414–417. doi: 10.1006/bbrc.2000.2624. [DOI] [PubMed] [Google Scholar]
- 168.Rettino A, Rafaneli F, Genovese G, et al. Identification of SP1 and GC-boxes as transcriptional regulators of mouse Dag-1 gene promoter. American Journal of Physiology Cell Physiology. 2009;297(5):C1113–1123. doi: 10.1152/ajpcell.00189.2009. [DOI] [PubMed] [Google Scholar]
- 169.Miura P, Chakkalakal JV, Boudreault L, et al. Pharmacological activation of PPARβ/δ stimulates utrophin A expression in skeletal muscle fibers and restores sarcolemmal integrity in mature mdx mice. Human Molecular Genetics. 2009;18(23):4640–4649. doi: 10.1093/hmg/ddp431. [DOI] [PubMed] [Google Scholar]
- 170.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128(4):669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 171.Melendez-Vasquez CV, Rios JC, Zanazzi G, Lambert S, Bretscher A, Salzer JL. Nodes of Ranvier form in association with ezrin-radixin-moesin (ERM)-positive Schwann cell processes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(3):1235–1240. doi: 10.1073/pnas.98.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Etienne-Manneville S. Polarity proteins in glial cell functions. Current Opinion in Neurobiology. 2008;18(5):488–494. doi: 10.1016/j.conb.2008.09.014. [DOI] [PubMed] [Google Scholar]