Abstract
Background
Caste differentiation in social insects is a type of polyphenism that enables division of labor among members of a colony. This elaborate social integration has attracted broad interest, although little is known about its regulatory mechanisms, especially in Isoptera (termites). In this study, we analyzed soldier differentiation in the damp-wood termite Hodotermopsis sjostedti, focusing on a possible effector gene for caste development. The gene for an actin-binding protein, HsjCib, which shows a high level of expression in developing mandibles during soldier differentiation, is characterized in detail.
Results
To examine the HsjCib gene, full-length cDNAs were obtained by rapid amplification of cDNA ends-polymerase chain reaction (RACE-PCR) and sequencing. Multiple isoforms were identified, and on the basis of the results of northern and Southern hybridization analyses, these isoforms were considered to be transcriptional variants from a single gene. On the basis of their sequence similarity to homologous genes of other organisms, functions in actin assembly were assumed to be different among isoforms. Expression analysis revealed high expression in the head during soldier differentiation, which was consistent with their allometric growth. Although isoform expression was observed in various tissues, different expression levels were observed among tissues, suggesting the possibility of tissue-specific morphogenetic regulation by HsjCib isoforms.
Conclusion
This study revealed the characteristics and dynamics of the HsjCib gene during soldier differentiation as a potential representative of downstream effector genes in caste-specific morphogenesis. From the expression patterns observed, this gene is considered to be involved in cephalic morphogenesis and neural reorganization, resulting in the establishment of caste-specific morphology and behavior.
Background
Social insects constitute complex societies with caste differentiation, and in some cases, they form huge colonies with vast numbers of individuals. The elaborate integration of insect societies has intrigued researchers for many years [1]. Termites (Isoptera, Insecta), which flourish in abundance and diversity in tropical and temperate zones and constitute one of the major groups of social insects, also form complex societies that include various castes [2,3]. The majority of social-insect research has focused primarily on hymenopterans (ants, bees, and wasps). Because the social mode and regulatory mechanisms of termites are different from those of hymenopterans in many respects [4,5], termites can provide an essential source of information for understanding the general and common features of sociality [6-8].
The morphologies of termite soldiers are highly specialized [9] and their differentiation is regulated through mutual interactions with other nest mates [10]. Soldiers are typical examples of the combination of exaggerated morphology [11] and polyphenism [12]. The regulatory mechanisms of polyphenism have been studied in various aspects and such studies have spurred the development of a new research field that spans the boundary between ecology and developmental biology [13]. Recently, the molecular biological approach for the study of social insects such as the ant [14-16], honey bee [17-22] and termite [23-38], has become common. Little is known, however, about the developmental basis of morphological modifications among polyphenic castes. Utilization of the molecular developmental approach with termites, whose caste development involves large morphological modification, has its own advantages and peculiarities [39].
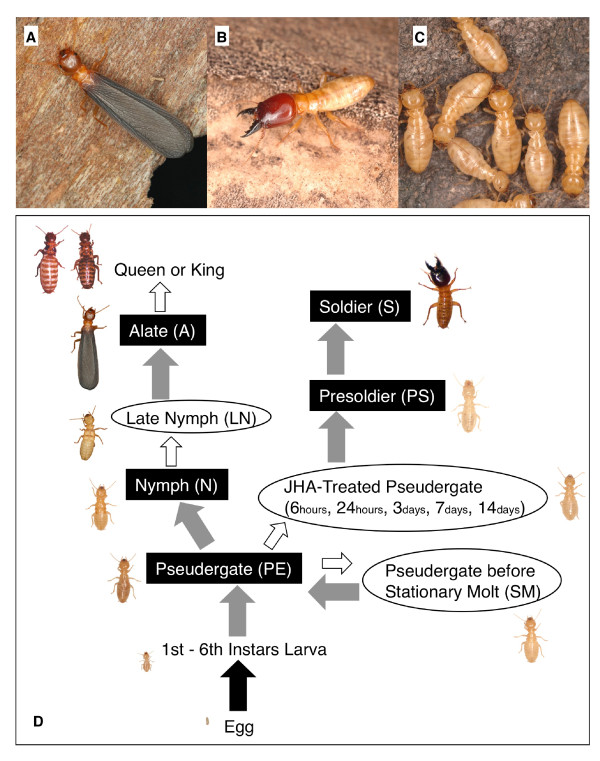
The mode of postembryonic development and caste differentiation varies among termite lineages [5]. In the damp-wood termite Hodotermopsis sjostedti, the pseudergate (seventh or older instar apterous individual) has the potential to develop into one of several castes [6,40,41] (Fig. 1). Although a genetic factor is known to influence caste determination in the termite species Reticulitermes speratus [42], in termites in general it is recognized that environmental factors during larval or pseudergate periods determine the developmental fate of particular castes [10].
Figure 1.
Castes of the damp-wood termite Hodotermopsis sjostedti. A: Alate. B: Soldier. C: Pseudergates. D: Diagram of the caste-differentiation pathway, modified from Miura et al. [41]. The highlighted text denotes the castes used in the present study, and the non-highlighted, parenthesized letters are the short transitional stages used in the study. In the "JHA-Treated Pseudergate" category, we prepared several time stages after JHA application. Gray arrows indicate transitions with molting, white arrows indicate those without molting, and the black arrow indicates hatching.
Gene expression is believed to be altered before the molt in particular castes, in preparation for specific morphologies. Juvenile hormone (JH) has been considered to physiologically control caste differentiation [43]. In many termite species, application of JH (including JH analogs [JHA]) results in differentiation into the soldier form. And thus, the application of JH (or JHA) is a useful mechanism by which to investigate caste differentiation [44,45].
Among termite lineages, a variety of soldier morphologies are known, which can be classified into several types. These types include the biting soldier, reaping soldier, snapping soldier, or nasute soldier [9]. The focal species in the present study, H. sjostedti, has a typical biting soldier morphology which is considered to be the most basic and primitive type among termites. During soldier differentiation, allometric growth of the whole head and mandibles has been observed [46]. Before the molt, a specific morphogenesis causes complex folds to be formed in the epidermal tissues inside the mandibles; this enables rapid expansion of the mandibles at the time of molt into presoldiers [47]. In a previous study, we screened the genes expressed in mandibles during soldier differentiation using the fluorescent differential display method [28]. HsjCib, a homolog of the ciboulot (cib) gene in the fruit fly Drosophila melanogaster, is one of the identified genes thought to be involved in morphogenesis. The cib gene encodes an actin-binding protein and is categorized as a multimeric β-thymosin [48,49]. It is known to be required for the central nervous modification during metamorphosis by genetic and developmental study [48,50] and to regulate actin polymerization by in vitro study [48]. In H. sjostedti, HsjCib in mandibles was the most abundantly expressed at the stage of 14 days after JHA application, which is when specific epidermal morphogenesis occurs.
Multimeric β-thymosins, including Cib, have homology with monomeric vertebrate Thymosin-β. Multimeric β-thymosin includes two-five WH2 domains (Wiskott-Aldrich syndrome protein homology domain 2--one of the actin-binding domains), while Thymosin-β includes only one WH2 domain [51-54]. Although their homology is relatively high, the functions of multimeric β-thymosins and those of Thymosin-β are quite different. Multimeric β-thymosins are thought to promote actin polymerization, whereas Thymosin-β is believed to sequester actin monomers and inhibit their polymerization. Hertzog et al. [55] discussed this distinction, and concluded that the amino acid residues at the N terminus of the WH2 domain are responsible for the different molecular functions. When the amino acid residues at the structurally important site in Thymosin-β were replaced with those of Cib, the molecular function was changed from sequestering to assembly-promoting [55,56]. Hertzog et al. [55] also proposed a model to predict WH2 domain function in many proteins by checking the amino acid residues at the structurally important site. The possible mechanisms by which structural differences translate to functional differences have been discussed previously by several authors [57-59].
Caste differentiation and polyphenism are often regulated through JH signaling, although the mechanisms that link JH signaling and morphological modification remain largely unknown. Characterization of the molecules involved in these mechanisms will contribute to the understanding of the regulation of polyphenism. In the present study, we focused on HsjCib, whose partial sequence was previously identified [28] as a gene responsible for morphogenesis during soldier differentiation in response to JH (JHA). We cloned the full-length cDNA of HsjCib and found multiple isoforms that may serve two different molecular functions. Here, we reveal the expression dynamics of HsjCib using quantitative real-time polymerase chain reaction (qPCR) and in situ hybridization and discuss the possible functional differences among isoforms.
Results
The full-length cDNA sequence and the number of WH2 domains
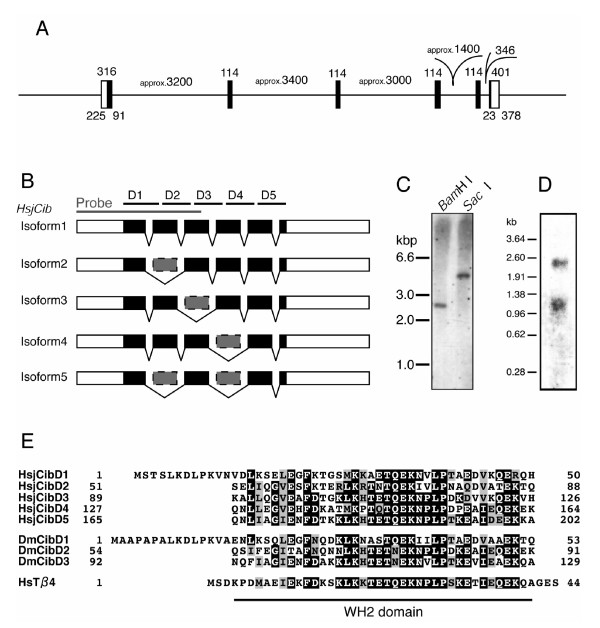
On the basis of the partial sequence of HsjCib previously identified by fluorescent differential display [28], we amplified both ends of the cDNA using the rapid amplification of cDNA ends (RACE) method and sequenced the full-length cDNAs of HsjCib (Fig 2). The predicted amino acid sequence of the longest HsjCib isoform was compared to other organisms with known sequences possessing WH2 domains and found that HsjCib contained five WH2 domains (Fig 2, 3A and 3E). The numbers of WH2 domains in known genes vary among organisms: Drosophila Cib, 3; Caenorhabditis Tetrathymosin, 4; the sea slug Hermissenda CSP24/29, 4/5; and a sea squirt Ciona Multimeric β-thymosin, 5 [48,49,59,60]. The number of WH2 domains in insects varies from three to five, the evolutionary origins and history of duplication/deletion being unclear (see Additional file 1 and 2). The functional significance of the number of WH2 domains is still open to debate [55,57,59].
Figure 2.
cDNA and putative amino acid sequences for the longest isoform of HsjCib. The 1245-bp cDNA, obtained by 5' and 3'-RACE, contains a putative ORF that encodes a polypeptide of 202 amino acid residues. Boxed sequences indicate putative WH2 domains with a different color for each domain. The gray vertical bars indicate positions of introns. The gray shadow indicates a fragment from the original differential screening [28]. A potential polyadenylation signal is underlined.
Figure 3.
Isoform structure of HsjCib gene. A: The gene structure of HsjCib inferred from PCR amplification of genomic DNA. Coding regions are represented in black. Numbers indicate the lengths (bp) of regions. B: Structure of obtained isoforms. Smaller isoforms were thought to skip some exons. The gray bar indicates the fragment used as probes in Southern, northern, and in situ hybridization. D1 to D5 indicates putative WH2 domains. C: Southern hybridization indicates that HsjCib exists as a single-copy gene on the genome. D: Northern hybridization for HsjCib. Several isoforms seem to overlap between the 0.96- and 1.38-kbp markers. The longer fragment around 2.5 kb may be a paralog or an isoform that has not been cloned. E: Alignment of putative amino acid sequences of HsjCib with Drosophila Cib and human Thymosin-β4.
The structure of isoforms
Five HsjCib isoforms were identified from cDNA cloning and sequencing. Four isoforms were truncated compared to the largest isoform (Fig. 3A and 3B). Of the five isoforms, three had the same length: one 1245-bp isoform, three 1131-bp isoforms, and one 1017-bp isoform (accession nos. AB534909, AB534910, AB534911, AB534912, AB534913). Exons 2, 3, 4, and 5 each had a length of 114 bp, which corresponded to 38 amino acid residues and was the same length as a WH2 domain. On the basis of the results of Southern hybridization, we considered the identified isoforms to be splice variants from a single gene (Fig. 3C). The skipping of exons during splicing predicted isoforms with five, four or three WH2 domains. The editing sites of the exons were located in the middle of the WH2 domains, forming a complete WH2 domain when two incomplete portions of WH2 domains were spliced together. By northern hybridization, the splice variants were observed to be poorly separated and a broad band was detected (Fig. 3D). Although another band was observed at around 2.5 kb, we could not conclude whether the band represented an uncloned large isoform, a paralog, or DNA carry-over. The approximate arrangement of exons was estimated using the PCR fragments from the genomic DNA (Fig. 3A). The borders between exons and introns were determined by sequencing both ends of the PCR fragments.
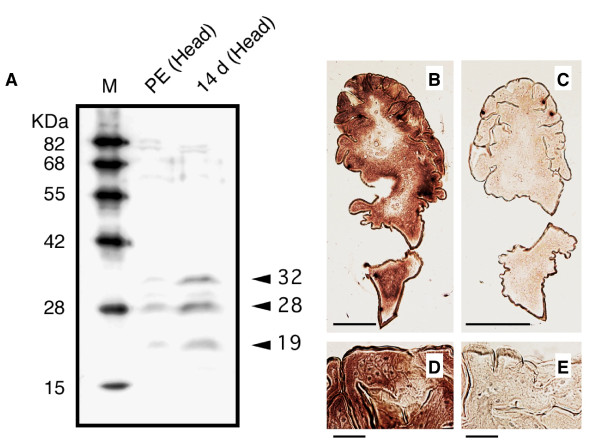
Western blot analysis
Because the expression of mRNA does not provide strict evidence of actual protein expression, protein expression was confirmed by western blot analysis (Fig. 4A). The results were consistent with the expectations for the three isoform sizes from cDNA cloning. However, the molecular weights estimated from relative mobility to markers (32, 28, and 19 kDa) were larger than those estimated from the predicted amino acid sequences (23, 19, and 14 kDa, as computed with the pl/Mw tool in ExPASy, http://ca.expasy.org/tools/). We considered two possibilities for the inconsistency between the electrophoretic mobility and predicted molecular weights. One possibility was that posttranslational modifications caused the difference in mobility. The homolog in Hermissenda, CSP24, is known to be phosphorylated [61], and several possible phosphorylation sites were found in the putative amino acid sequences of HsjCib (Net PhosK 1.0 server, http://www.cbs.dtu.dk/services/NetPhosK). No possible N-glycosylation site [N-X-S(T)] was found in the amino acid sequences (Fig. 2).
Figure 4.
Western blot and in situ hybridization to detect HsjCib expression. A: Western blot analysis detected by anti-Drosophila Cib antibody against head homogenates of the termite. The antibody detected strong bands in the 14 d (14 days after JHA application) lane and weak bands in the PE (normal pseudergate) lane, which were presumed to correspond to isoforms of HsjCib. B-E: In situ hybridization of the HsjCib mRNA in newly formed mandible (14 d stage). Transverse paraffin sections (6 μm) were subjected to in situ hybridization with antisense (B and D) and sense (C and E) DIG-labeled RNA probes. Bars indicate 200 μm (B and C) and 20 μm (D and E), respectively. The expression of HsjCib was observed in the mandibular epidermis.
Although unlikely considering the size relationships, another possibility was that the three isoform sizes did not correspond to the three sizes of bands on the western blot. In that case, the 32-kDa band corresponded to an uncloned isoform and perhaps to the 2.5-kb band observed in northern hybridization. In this possibility, the smallest isoform (isoform 5) derived by cDNA cloning should have been scarcely expressed. However, cDNA of isoform 5 was frequently obtained during the cloning process. Because the isoform sequences resembled each other, it was unlikely that cross-reactivity of the antibody to the different isoforms was very different.
In situ hybridization
Because immunohistochemistry did not produce a distinct signal (the antibody used was known to be ineffective for immunohistochemistry, T. Préat, personal communication), we investigated HsjCib expression in the mandibular tissue by in situ hybridization using 473 bases of the RNA fragment transcribed as a probe in vitro (Fig. 3B). On cross sections of developing mandibles, we identified the HsjCib signal in epidermal tissue (Fig. 4). Although HsjCib was originally observed in the screening for mandibular expression, the expression was also observed in many tissues, as shown in the next section and in Fig. 5.
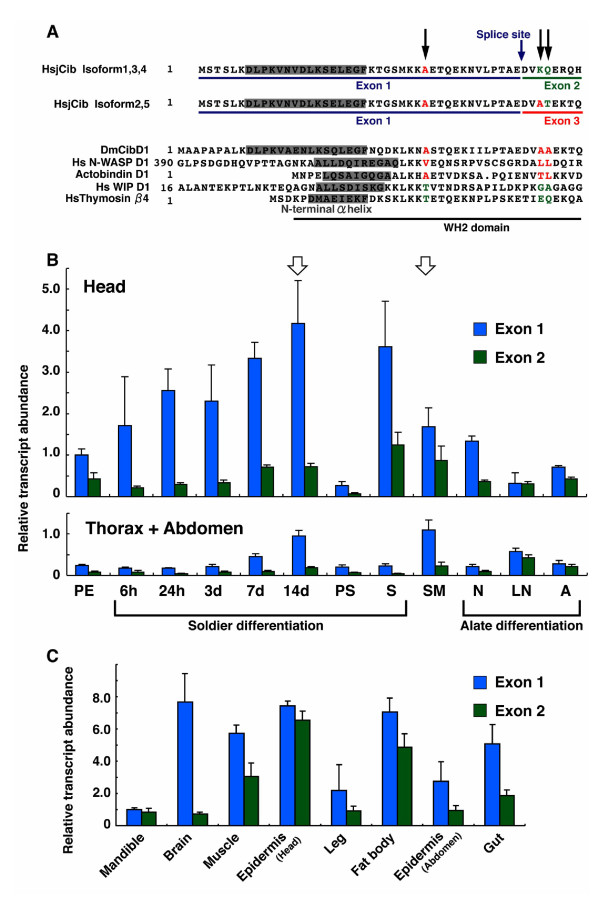
Figure 5.
Expression analyses of HsjCib in various stages and tissues. A: Alignment of WH2 domains in various proteins. Black arrows indicate structurally important amino acid residues as suggested by Hertzog et al. [55]. According to the Hertzog's model, HsjCib isoforms 2 and 5 are supposed to act as assembly-promoting proteins, whereas isoforms 1, 3, and 4 resemble G-actin sequestering proteins. B: Temporal expression pattern of HsjCib during caste differentiation. Exon 1 was contained in all isoforms; consequently, the expression levels of exon 1 indicate the sum of the expressions of all isoforms. The expression levels of exon 2 were thought to indicate G-actin sequestering isoforms. PE: Pseudergate; 6 h-14 d: pseudergate of 6 h to 14 days after JHA application; PS: Presoldier; S: Soldier; SM: Pseudergate before stationary molt; N: Nymph; LN: Late nymph before imaginal molt; A: Alate. White arrows indicate two comparable stages in which the times until the next molt are approximately the same. C: Measurement of expression levels of exon 1 and exon 2 in the indicated tissues at 14 d after JHA application.
Functional differences among HsjCib isoforms
HsjCib isoforms 1, 3, and 4 resembled the "sequestering form," since the functionally important residues in the N-terminal side WH2 domain [55] were hydrophilic K (lysine) and Q (glutamine) (hydropathy index -3.9 and -3.5 according to the calculation method by Kyte and Doolittle [62]). HsjCib isoforms 2 and 5 resembled the "assembly-promoting form" because the functional residues were A (alanine) and T (threonine) (hydropathy index 1.8 and -0.7), which were somewhat hydrophobic (Fig. 5A). These resemblances were not perfect, and we could not assert their precise functions. The features of the functionally important sites were different among HsjCib isoforms, meaning that their properties should have differed from each other. In the sections below, for simplicity, isoforms 1, 3, and 4 will be referred to as "sequestering types," and isoforms 2 and 5 will be designated "assembly-promoting types." This naming convention should aid in understanding the complicated expression analyses.
Quantification of expressions by qPCR
To confirm the gene expression pattern, we performed qPCR for "head" tissues and "thorax + abdomen" tissues over the time course (temporal pattern) and for several tissues at the 14 d (14 days after JHA application) stage (spatial pattern). To detect the isoform joining of exons, we initially tried to identify every isoform separately by making primers on the editing points between exons and introns (see also Fig. 3B). This procedure, however, failed to discriminate each isoform separately in the preliminary experiments. Consequently, we discontinued quantifying all the isoforms separately, and instead designed primers to quantify exons 1 and 2 separately. Exon 1 was contained in all the isoforms, and its expression amounts were equal to those of all HsjCib isoforms (sequestering + assembly promoting). Exon 2 was contained in isoforms 1, 3, and 4. Since the existence of exon 2 makes a sequestering isoform, its expression amount apparently represents the amount of sequestering isoforms. With this experiment, note that castes (PE, PS, S, N and A) were not necessarily in a same intermolt stage, but use of pooled samples could average their expression levels.
Quantification of exon 1 in termite heads demonstrated that the expression level gradually increased during soldier differentiation; the highest expression was observed at the 14 d stage, just prior to the molt to presoldier (Fig. 5B). The expression level was subsequently reduced in the presoldier, but was again increased in the soldier. Exon 1 expression was also increased in the SM stage (pseudergate before the stationary molt), although the level was lower than that observed at 14 d. A comparison indicated that the days to molt were approximately the same between the SM and 14 d stages [28]. Expression level was not high before the imaginal molt.
Exon 1 expression levels can be correlated to allometrical changes associated with prospective growth [46,47]. It is notable that expression level of exon 1 was highest in the head at 14 d when morphogenesis was taking place. Thorax + abdomen exon 1-expression was generally lower than that of the head, although at 14 d and in the SM stage it was higher than expected. During soldier differentiation, expression of exon 1 was much higher in the head samples compared to thorax + abdomen. Expression levels were similar in both tissues while in stationary molt (Fig. 5B white arrows). The expression of exon 2 was much less than that of exon 1 during soldier differentiation, which meant that HsjCib isoforms without exon 2 (assembly-promoting type) were primarily expressed.
Future wing tissue rapidly grows inside the wing bud in the late nymph stage (LN) [41], but considering the active morphogenesis taking place, expression levels of exon 1 was not observed to be high. In LN, exon expression was not very different, which meant that HsjCib isoforms with exon 2 (sequestering type) were mainly expressed.
Quantification in various tissues at 14 d revealed HsjCib expression in every tissue that was examined (Fig. 5C). The exon transcript amounts indicated that the proportions of assembly-promoting type splice variant (amounts of exon 1 minus amounts of exon 2) and sequestering type splice variant (amounts of exon 2) were different among tissues. The largest proportion of assembly-promoting type splice variant was observed in the brain. This corresponded well to Drosophila, in which Cib is required in the brain during metamorphosis and for which only the assembly-promoting type is known. In Hodotermopsis, the behavioral pattern is greatly altered during soldier differentiation, where HsjCib is possibly involved in neuronal modification with axon growth. In mandible and head epidermis, the proportions of sequestering type variants were higher. Isoforms with prospective functions were expressed differentially among tissues, suggesting that HsjCib regulates cellular morphogenesis in diverse ways among various tissues.
Discussion
This study demonstrates that the expression profiles of HsjCib, acts as a downstream gene in a regulatory pathway which correlates with tissue morphogenesis occurring in termite soldier differentiation. The genes underlying caste differentiation of social insects have been extensively studied, but the present study is the first report on the detailed expression properties of a possible effector gene for caste-specific morphogenesis.
Putative function in soldier differentiation
Previously, we demonstrated the expression of HsjCib in mandibles during soldier development was approximately 20-fold higher than that in an intermolt (pseudergate) stage [28]. Recent experiments show an almost ubiquitous expression of the gene throughout the body. HsjCib expression levels in the thorax + abdomen during stationary molt was unexpectedly equivalent to that in soldier differentiation. Although it is not a specific gene to soldier differentiation, HsjCib is required for every molt.
Conspicuous morphogenesis in the epidermis and fat bodies before the presoldier molt of Hodotermopsis correlates closely to elevated HsjCib expression in the head as well as the thorax + abdomen at the 14 d stage [47,63]. In Drosophila, Cib is involved in neuronal modification during metamorphosis [48]. During developmental modification in the brain of Hodotermopsis, neuronal changes required for soldier behavior occur [64], implicating high HsjCib expression levels in the axonal growth, retraction or synaptic development during this stage.
Soldier differentiation is a peculiar developmental pathway which was acquired only once in the ancestral lineage of termites [5,39]. The present study showed that HsjCib has ubiquitous expression, but when considering its responsiveness to JHA treatment and linkage to presoldier-dependent morphogenesis, the JH-dependent elevation of Cib expression seems to have evolved in the lineage from an ancestral termite to Hodotermopsis. This supports the idea that a preexisting gene recruited for exploitation in soldier differentiation acquired a new regulatory mechanism and novel way of morphogenesis.
Relationship between functions and sequence difference among isoforms
The expression level of the assembly-promoting type exon tended to be higher than the sequestering type. These proportional differences imply the control mechanism of these two types. Further molecular functional analysis of HsjCib would require protein purification or recombinant protein synthesis as well as subsequent in vitro analysis with actin molecules.
RNA interference (RNAi) is also a way to test the function of HsjCib. However, in our preliminary RNAi experiment did not provide clear results of phenotypic impacts (see Additional file 1).
The role of HsjCib among cytoskeleton-regulating molecules
Actin is one of the fundamental cytoskeletal components; its assembly and disassembly are the most important processes of morphogenesis at the cellular level [65]. This mechanism was thought to be regulated by various actin-binding proteins [51]. In the present study, we analyzed one gene, HsjCib, as a downstream gene in caste differentiation in the termite. Multiple actin-binding proteins have been shown to regulate cellular morphogenesis in animals [51,66,67]. In the termite, other proteins, such as Profilin, ADF/Cofilin and CAP [51,67] in addition to HsjCib could be cooperatively involved in cellular morphogenesis.
Morphogenetic regulation during soldier differentiation
Cytoskeletal regulators are influenced at multiple levels by various molecules. In our previous work, regulatory molecules such as G-protein-related factor RhoGEF and splicing factor U2AF were revealed to be upregulated in developing mandibles during soldier differentiation [28]. Considering that soldier differentiation in the termite is triggered by a high JH titer, these genes would be regulated downstream of the JH-signaling cascade. The intrinsic JH titer and histological aspects of hormone-producing glands have been studied extensively in the focal species [68]. Because the expression of HsjCib was already altered at 6 h after JHA application, it is conceivable that the expression was directly controlled by JH or through a small number of regulatory factors. Further analysis will shed light on the mutual relationships among these factors and the overall picture of soldier differentiation regulation.
In the black marching termite Hospitalitermes, which is phylogenetically distant from Hodotermopsis, soldier mandibles do not develop; instead, a horn-like structure called a "nasus" is formed during soldier differentiation [69]. Folding structures of the epidermis and their subsequent unfolding and growth after the molt into presoldier are common mechanisms in biting soldiers (e.g., Hodotermopsis) and nasute soldiers (e.g., Hospitalitermes) [47,69], suggesting that functions of various genes (including cib homolog) would be conserved. Extensive search with expressed sequence tag (EST) analysis, which has already been performed in Reticulitermes [31,38], will help to obtain a more comprehensive picture of this unique morphogenesis.
There is limited knowledge regarding the regulatory mechanism of the exaggerated morphology and phenotypic plasticity of insects [12,70-73]. The generalized question would be if there is a common molecular bases among independent lineages of species with exaggerated morphologies. The regulatory mechanism for phenotype and their connection to the subsequent processes of morphogenesis are important for understanding phenotypic evolution.
Conclusion
The present study documents the characteristics of the termite homolog of an actin-binding protein gene, HsjCib, which is one of the downstream genes for soldier differentiation. HsjCib includes up to five WH2 actin-binding domains, and functional differences in actin polymerization among isoforms were predicted on the basis of their similarity to homologous genes of other organisms. During caste differentiation, multiple isoforms were expressed with different quantitative ratios in various tissues. We propose the high probability that the functions of these isoforms during soldier differentiation involve cephalic morphogenesis and neural rearrangement, enabling soldier-specific exaggerated morphology and defensive behavior.
Methods
Insects and RNA extraction
Colonies of H. sjostedti Holmgren (Isoptera, Termopsidae) were sampled from rotten wood in evergreen forests on Yakushima Island, Kagoshima Prefecture, Japan, from May 2003 to 2007. They were kept in the laboratory as stock colonies at approximately 25˚C under constant darkness. Total RNA was extracted from various castes and stages (Fig. 1D) determined by their morphology [28,40,41]. Soldier differentiation was induced by the ingestion method of a juvenile hormone analog (JHA, pyriproxyfen, Sumitomo chemicals) [45], and tissues were analyzed by RACE, northern hybridization, western blot and in situ hybridization. The topical application method of JHA [23] was used for tissue examination via qPCR. Individuals from one or two colonies were used for each experiment utilizing seven colonies in total.
Cloning of full-length cDNA by RACE
Total RNA was extracted from the mandibles of 14 d pseudergates (14 days after JHA application) using an RNAgents Total RNA Isolation System (Promega). Total RNA was reverse-transcribed with oligo(dT) primer, and RACE-PCR was performed using a SMART-RACE Kit (BD Bioscience). The PCR product was subcloned into pGEM vector with a pGEM-TA Cloning Kit (Promega). Nucleotide sequence was determined with a BigDye Terminator v3.0 Kit and an ABI 310 Genetic Analyzer (Applied Biosystems).
Southern hybridization
Genomic DNA was extracted from whole insects (without guts) using a Wizard Genomic DNA Extraction System (Promega). Extracted DNA was digested with restriction enzymes (BamHI or SacI) for 48 h, separated in an agarose gel, and transferred to a positive-charge membrane, Hybond N+ (Amersham). The genomic HsjCib allele was detected by an Alkphos Direct Labelling and Detection System (Amersham). The 473-bp DNA fragment was used as a probe, as indicated in Fig. 3B.
Northern hybridization
Total RNA was extracted from 160 individuals of 14d, using an RNAgents Total RNA Isolation System (Promega). Poly(A) RNA was isolated from total RNA using a PolyATract mRNA Isolation System (Promega). Poly(A) RNA (23 μg) was separated in an agarose gel, and transferred to a positive-charge membrane, Hybond N+ (Amersham). HsjCib mRNAs were detected by an Alkphos Direct Labeling and Detection System (Amersham). The 473-bp DNA fragment was used as a probe, similar to Southern hybridization.
Western blot analysis
The heads of each of two normal pseudergates and 14 d pseudergates were homogenized in lysis buffer, and the protein quantities were calibrated by optical density (OD) at 280 nm using a protein quantification mode of a NanoDrop ND-1000 spectrophotometer. Proteins were separated in a sodium dodecyl sulfate (SDS)-polyacrilamide gel and electroblotted onto a polyvinyl difluoride membrane (Cosmo Bio). The proteins bound to the membrane were probed with anti-Drosophila Cib rabbit antiserum at 1:2000 dilution [48] followed by an anti-mouse IgG conjugated with horseradish peroxidase at 1:2000 dilution. Bands were visualized by fluorography with an ECL Western Blotting Detection System (Amersham).
In situ hybridization
The mandibles were dissected from 14 d pseudergates and embedded in paraffin, after which cross sections were made, as described by Koshikawa et al. [47], except that 4% paraformaldehyde was used instead of FAA for fixation and all steps were performed under RNase-free conditions. The target gene in the sections was detected using an ISHR Starting Kit (Nippon Gene). Briefly, the DIG-labeled RNA probe was prepared by in vitro transcription from a DNA fragment (473-bp DNA, as indicated in Fig. 3B, plus a T7 or SP6 promoter sequence) and hybridized to rehydrated sections. The signal was detected by an alkaline phosphatase-labeled anti-DIG antibody with NBT/BCIP solution as substrate.
Quantification of expression levels among developmental stages
Total RNA was extracted from 14-20 heads and thoraxes + abdomens of various castes and transitional stages (Fig. 1D) with an RNAgents Total RNA Isolation Systems (Promega). Individuals used were essentially from a same colony to unify the genetic background. Reverse transcription was performed with random hexamers. The expression levels at different stages were quantified by qPCR with a Power SYBR Green PCR Master Mix and an ABI Prism 7000 Sequence Detection System (Applied Biosystems). As an endogenous control of constitutive expression, we used 18S rRNA gene sequences (AF220567). When β-actin was used as an endogenous control, results were similar to 18S rRNA (data not shown), but due to high expression in the soldier caste and inconsistent expression, 18S rRNA was the preferred control [74]. Primer sequences for qPCR were as follows: HsjCib-Exon1F, AGGACCTGCCCAAGGTGAA; HsjCib-Exon1R, CTTCCTGTCTTGAATCCTTCCAA; HsjCib-Exon2F, GACAACACAGCGAGCTTATTCAA; HsjCib-Exon2R, GAGTGTTGGTCCGCTTTAACCT; Hsj18S-F, CTTGCAATTGTTCCCCATGA; and Hsj18S-R, ACGTAATCAACGCGAGCTTATG. The baseline and threshold for the Ct (cycle threshold) were set automatically. Each category was tested in triplicate, and standard errors were calculated by the relative standard curve method as described in User Bulletin 2 for the ABI Prism 7700 Sequence Detection System (Applied Biosystems). Statistic significances were analyzed by one-way ANOVA and Tukey's HSD test (see Additional file 1 and 3).
Quantification of expression level among tissues
Each of seven normal pseudergates and 14 d pseudergates was dissected for various specific tissues (mandibles, brain, mandible closer/opener muscle, head epidermis, leg, fat body, body epidermis, and gut), and total RNA was extracted from them with an SV Total RNA Extraction System (Promega). The quantification methods used were the same as those described above.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SK carried out most of the experiments in this study. RC participated in experimental design and carried out some experiments. TMa participated in experimental design and coordination. SK and TMi were responsible for overall experiment design, analysis of data, and writing of the manuscript. All authors have read and approved the final manuscript.
Supplementary Material
Supplemental results. RNAi experiments, phylogenetic analysis of cib homologs and statistic analysis of qPCR.
Supplemental Fig. 1. Phylogenetic analysis of HsjCib and ciboulot homologs from other animals.
Supplemental Table 1. Summary of Tukey's HSD test for qPCR results.
Contributor Information
Shigeyuki Koshikawa, Email: skoshikawa@wisc.edu.
Richard Cornette, Email: cornette@affrc.go.jp.
Tadao Matsumoto, Email: cmatsu@u-air.ac.jp.
Toru Miura, Email: miu@ees.hokudai.ac.jp.
Acknowledgements
We are grateful to three anonymous reviewers for their helpful comments and suggestions. We thank J. Selegue for improving the manuscript; S. Sameshima and M. Hojo for assistance in experiments; K. Maekawa, M. Machida, A. Fujita, A. Gotoh, S. Hongo, A. Ishikawa, Y. Ishikawa, H. Gotoh, A. Hattori, and K. Nagata for field sampling, maintenance of colonies, and advice during the study; T. Préat for anti-Drosophila Cib antibody; Sumitomo Chemicals (Ōsaka) for pyriproxyfen. This work was supported by KAKENHI (Nos. 18047002, 18370007, 20033002, and 21677001). The first author was supported by a Japan Society for the Promotion of Science Research Fellowship for Young Scientists. All research reported in this paper complies with the present laws of Japan.
References
- Wilson EO. The insect societies. Cambridge: Harvard University Press; 1971. [Google Scholar]
- Bignell DE, Eggleton P. In: Termites: Evolution, Sociality, Symbioses, Ecology. Abe T, Bignell DE, Higashi M, editor. Vol. 2000. Dordrecht, The Netherlands: Kluwer Academic Publishers; Termites in ecosystems; pp. 363–387. [Google Scholar]
- Eggleton P. In: Termites: Evolution, Sociality, Symbioses, Ecology. Abe T, Bignell DE, Higashi M, editor. Vol. 2000. Dordrecht, The Netherlands: Kluwer Academic Publishers; Global patterns of termite diversity; pp. 25–51. [Google Scholar]
- Thorne BL, Traniello JF. Comparative social biology of basal taxa of ants and termites. Annu Rev Entomol. 2003;48:283–306. doi: 10.1146/annurev.ento.48.091801.112611. [DOI] [PubMed] [Google Scholar]
- Roisin Y. In: Termites: Evolution, Sociality, Symbioses, Ecology. Abe T, Bignell DE, Higashi M, editor. Vol. 2000. Dordrecht, The Netherlands: Kluwer Academic Publishers; Diversity and evolution of caste patterns; pp. 95–119. [Google Scholar]
- Miura T. Proximate mechanisms and evolution of caste polyphenism in social insects: From sociality to genes. Ecol Res. 2004;19:141–148. doi: 10.1111/j.1440-1703.2003.00618.x. [DOI] [Google Scholar]
- Miura T. Developmental regulation of caste-specific characters in social-insect polyphenism. Evol Dev. 2005;7:122–129. doi: 10.1111/j.1525-142X.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- Scharf ME, Zhou X. Termite sociogenomics: a growing field. Nat Rev Genet. 2005;6 doi:10.1038/nrg1575-c1031. [Google Scholar]
- Deligne J, Quennedey A, Blum MS. In: Social Insects. Hermann HR, editor. Vol. 2. New York: Academic Press; 1981. The enemies and defense mechanisms of termites; pp. 1–76. [Google Scholar]
- Scharf ME, Buckspan CE, Grzymala TL, Zhou X. Regulation of polyphenic caste differentiation in the termite Reticulitermes flavipes by interaction of intrinsic and extrinsic factors. J Exp Biol. 2007;210:4390–4398. doi: 10.1242/jeb.010876. [DOI] [PubMed] [Google Scholar]
- Emlen DJ, Nijhout HF. The development and evolution of exaggerated morphologies in insects. Annu Rev Entomol. 2000;45:661–708. doi: 10.1146/annurev.ento.45.1.661. [DOI] [PubMed] [Google Scholar]
- Nijhout HF. Development and evolution of adaptive polyphenisms. Evol Dev. 2003;5:9–18. doi: 10.1046/j.1525-142X.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Ecological developmental biology: Developmental biology meets the real world. Dev Biol. 2001;233:1–12. doi: 10.1006/dbio.2001.0210. [DOI] [PubMed] [Google Scholar]
- Goodisman MAD, Isoe J, Wheeler DE, Wells MA. Evolution of insect metamorphosis: A microarray-based study of larval and adult gene expression in the ant Camponotus festinatus. Evolution. 2005;59:858–870. [PubMed] [Google Scholar]
- Graff J, Jemielity S, Parker JD, Parker KM, Keller L. Differential gene expression between adult queens and workers in the ant Lasius niger. Mol Ecol. 2007;16:675–683. doi: 10.1111/j.1365-294X.2007.03162.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Jemielity S, Uva P, Wurm Y, Graff J, Keller L. An annotated cDNA library and microarray for large-scale gene-expression studies in the ant Solenopsis invicta. Genome Biol. 2007;8:R9. doi: 10.1186/gb-2007-8-1-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Wheeler DE. Differential gene expression between developing queens and workers in the honey bee, Apis mellifera. Proc Natl Acad Sci USA. 1999;96:5575–5580. doi: 10.1073/pnas.96.10.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Wheeler DE. Expression profiles during honeybee caste determination. Genome Biol. 2000;2:research0001. doi: 10.1186/gb-2000-2-1-research0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Wheeler DE. Gene expression and the evolution of insect polyphenisms. BioEssays. 2001;23:62–68. doi: 10.1002/1521-1878(200101)23:1<62::AID-BIES1008>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Grozinger CM, Whitfield CW. Sociogenomics: Social life in molecular terms. Nat Rev Genet. 2005;6:257–270. doi: 10.1038/nrg1575. [DOI] [PubMed] [Google Scholar]
- Whitfield CW, Band MR, Bonaldo MF, Kumar CG, Liu L, Pardinas JR, Robertson HM, Soares MB, Robinson GE. Annotated expressed sequence tags and cDNA microarrays for studies of brain and behavior in the honey bee. Genome Res. 2002;12:555–566. doi: 10.1101/gr.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield CW, Cziko AM, Robinson GE. Gene expression profiles in the brain predict behavior in individual honey bees. Science. 2003;302:296–299. doi: 10.1126/science.1086807. [DOI] [PubMed] [Google Scholar]
- Cornette R, Koshikawa S, Hojo M, Matsumoto T, Miura T. Caste-specific cytochrome P450 in the damp-wood termite Hodotermopsis sjostedti (Isoptera, Termopsidae) Insect Mol Biol. 2006;15:235–244. doi: 10.1111/j.1365-2583.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- Hojo M, Koshikawa S, Cornette R, Matsumoto T, Miura T. Identification of soldier-specific genes in the nasute termite Nasutitermes takasagoensis (Isoptera: Termitidae) Entomol Sci. 2005;8:379–387. doi: 10.1111/j.1479-8298.2005.00138.x. [DOI] [Google Scholar]
- Hojo M, Matsumoto T, Miura T. Cloning and expression of a geranylgeranyl diphosphate synthase gene: Insights into the synthesis of termite defence secretion. Insect Mol Biol. 2007;16:121–131. doi: 10.1111/j.1365-2583.2007.00709.x. [DOI] [PubMed] [Google Scholar]
- Hojo M, Morioka M, Matsumoto T, Miura T. Identification of soldier caste-specific protein in the frontal gland of nasute termite Nasutitermes takasagoensis (Isoptera: Termitidae) Insect Biochem Mol Biol. 2005;35:347–354. doi: 10.1016/j.ibmb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Korb J, Weil T, Hoffmann K, Foster KR, Rehli M. A Gene Necessary for Reproductive Suppression in Termites. Science. 2009;324:758–758. doi: 10.1126/science.1170660. [DOI] [PubMed] [Google Scholar]
- Koshikawa S, Cornette R, Hojo M, Maekawa K, Matsumoto T, Miura T. Screening of genes expressed in developing mandibles during soldier differentiation in the termite Hodotermopsis sjostedti. FEBS Lett. 2005;579:1365–1370. doi: 10.1016/j.febslet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Miura T, Kamikouchi A, Sawata M, Takeuchi H, Natori S, Kubo T, Matsumoto T. Soldier caste-specific gene expression in the mandibular glands of Hodotermopsis japonica (Isoptera: Termopsidae) Proc Natl Acad Sci USA. 1999;96:13874–13879. doi: 10.1073/pnas.96.24.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf ME, Ratliff CR, Wu-Scharf D, Zhou X, Pittendrigh BR, Bennett GW. Effects of juvenile hormone III on Reticulitermes flavipes: changes in hemolymph protein composition and gene expression. Insect Biochem Mol Biol. 2005;35:207–215. doi: 10.1016/j.ibmb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Scharf ME, Wu-Scharf D, Pittendrigh BR, Bennett GW. Caste- and development-associated gene expression in a lower termite. Genome Biol. 2003;4:R62. doi: 10.1186/gb-2003-4-10-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf ME, Wu-Scharf D, Zhou X, Pittendrigh BR, Bennett GW. Gene expression profiles among immature and adult reproductive castes of the termite Reticulitermes flavipes. Insect Mol Biol. 2005;14:31–44. doi: 10.1111/j.1365-2583.2004.00527.x. [DOI] [PubMed] [Google Scholar]
- Weil T, Rehli M, Korb J. Molecular basis for the reproductive division of labour in a lower termite. BMC Genomics. 2007;8:198. doi: 10.1186/1471-2164-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Oi FM, Scharf ME. Social exploitation of hexamerin: RNAi reveals a major caste-regulatory factor in termites. Proc Natl Acad Sci USA. 2006;103:4499–4504. doi: 10.1073/pnas.0508866103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Smith JA, Oi FM, Koehler PG, Bennett GW, Scharf ME. Correlation of cellulase gene expression and cellulolytic activity throughout the gut of the termite Reticulitermes flavipes. Gene. 2007;395:29–39. doi: 10.1016/j.gene.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Zhou X, Song C, Grzymala TL, Oi FM, Scharf ME. Juvenile hormone and colony conditions differentially influence cytochrome P450 gene expression in the termite Reticulitermes flavipes. Insect Mol Biol. 2006;15:749–761. doi: 10.1111/j.1365-2583.2006.00675.x. [DOI] [PubMed] [Google Scholar]
- Zhou X, Tarver MR, Bennett GW, Oi FM, Scharf ME. Two hexamerin genes from the termite Reticulitermes flavipes: Sequence, expression, and proposed functions in caste regulation. Gene. 2006;376:47–58. doi: 10.1016/j.gene.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Zhou X, Tarver MR, Scharf ME. Hexamerin-based regulation of juvenile hormone-dependent gene expression underlies phenotypic plasticity in a social insect. Development. 2007;134:601–610. doi: 10.1242/dev.02755. [DOI] [PubMed] [Google Scholar]
- Miura T. Morphogenesis and gene expression in the soldier-caste differentiation of termites. Insectes Soc. 2001;48:216–223. doi: 10.1007/PL00001769. [DOI] [Google Scholar]
- Miura T, Hirono Y, Machida M, Kitade O, Matsumoto T. Caste developmental system of the Japanese damp-wood termite Hodotermopsis japonica (Isoptera: Termopsidae) Ecol Res. 2000;15:83–92. doi: 10.1046/j.1440-1703.2000.00320.x. [DOI] [Google Scholar]
- Miura T, Koshikawa S, Machida M, Matsumoto T. Comparative studies on alate wing formation in two related species of rotten-wood termites: Hodotermopsis sjostedti and Zootermopsis nevadensis (Isoptera, Termopsidae) Insectes Soc. 2004;51:247–252. doi: 10.1007/s00040-003-0736-2. [DOI] [Google Scholar]
- Hayashi Y, Lo N, Miyata H, Kitade O. Sex-linked genetic influence on caste determination in a termite. Science. 2007;318:985–987. doi: 10.1126/science.1146711. [DOI] [PubMed] [Google Scholar]
- Nijhout HF, Wheeler DE. Juvenile hormone and the physiological basis of insect polymorphisms. Quart Rev Biol. 1982;57:109–133. doi: 10.1086/412671. [DOI] [Google Scholar]
- Howard RW, Haverty MI. Termites and juvenile hormone analogues: A review of methodology and observed effects. Sociobiology. 1979;4:269–278. [Google Scholar]
- Ogino K, Hirono Y, Matsumoto T, Ishikawa H. Juvenile hormone analogue, S-31183, causes a high level induction of presoldier differentiation in the Japanese damp-wood termite. Zool Sci. 1993;10:361–366. [Google Scholar]
- Koshikawa S, Matsumoto T, Miura T. Morphometric changes during soldier differentiation of the damp-wood termite Hodotermopsis japonica (Isoptera, Termopsidae) Insectes Soc. 2002;49:245–250. doi: 10.1007/s00040-002-8309-8. [DOI] [Google Scholar]
- Koshikawa S, Matsumoto T, Miura T. Mandibular morphogenesis during soldier differentiation in the damp-wood termite Hodotermopsis sjoestedti (Isoptera: Termopsidae) Naturwissenschaften. 2003;90:180–184. doi: 10.1007/s00114-003-0408-5. [DOI] [PubMed] [Google Scholar]
- Boquet I, Boujemaa R, Carlier MF, Préat T. Ciboulot regulates actin assembly during Drosophila brain metamorphosis. Cell. 2000;102:797–808. doi: 10.1016/S0092-8674(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Telford MJ. The multimeric beta-thymosin found in nematodes and arthropods is not a synapomorphy of the Ecdysozoa. Evol Dev. 2004;6:90–94. doi: 10.1111/j.1525-142X.2004.04013.x. [DOI] [PubMed] [Google Scholar]
- Boquet I, Hitier R, Dumas M, Chaminade M, Préat T. Central brain postembryonic development in Drosophila: implication of genes expressed at the interhemispheric junction. J Neurobiol. 2000;42:33–48. doi: 10.1002/(SICI)1097-4695(200001)42:1<33::AID-NEU4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Dominguez R. Actin-binding proteins: A unifying hypothesis. Trends Biochem Sci. 2004;29:572–578. doi: 10.1016/j.tibs.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Edwards J. Are beta-thymosins WH2 domains? FEBS Lett. 2004;573:231–232. doi: 10.1016/j.febslet.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Lappalainen P, Mattila P. Reply to: Are beta-thymosins WH2 domains? FEBS Lett. 2004;573:233–233. doi: 10.1016/j.febslet.2004.06.099. [DOI] [PubMed] [Google Scholar]
- Paunola E, Mattila PK, Lappalainen P. WH2 domain: A small, versatile adapter for actin monomers. FEBS Lett. 2002;513:92–97. doi: 10.1016/S0014-5793(01)03242-2. [DOI] [PubMed] [Google Scholar]
- Hertzog M, van Heijenoort C, Didry D, Gaudier M, Coutant J, Gigant B, Didelot G, Préat T, Knossow M, Guittet E. The beta-thymosin/WH2 domain; structural basis for the switch from inhibition to promotion of actin assembly. Cell. 2004;117:611–623. doi: 10.1016/S0092-8674(04)00403-9. [DOI] [PubMed] [Google Scholar]
- Domanski M, Hertzog M, Coutant J, Gutsche-Perelroizen I, Bontems F, Carlier MF, Guittet E, van Heijenoort C. Coupling of folding and binding of thymosin beta4 upon interaction with monomeric actin monitored by nuclear magnetic resonance. J Biol Chem. 2004;279:23637–23645. doi: 10.1074/jbc.M311413200. [DOI] [PubMed] [Google Scholar]
- Aguda AH, Xue B, Irobi E, Preat T, Robinson RC. The structural basis of actin interaction with multiple WH2/beta-thymosin motif-containing proteins. Structure. 2006;14:469–476. doi: 10.1016/j.str.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Irobi E, Aguda AH, Larsson M, Guerin C, Yin HL, Burtnick LD, Blanchoin L, Robinson RC. Structural basis of actin sequestration by thymosin-beta4: Implications for WH2 proteins. EMBO J. 2004;23:3599–3608. doi: 10.1038/sj.emboj.7600372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Ono K, Dewitte D, Jonckheere V, De Ruyck N, Vandekerckhove J, Ono S, Ampe C. TetraThymosin beta is required for actin dynamics in Caenorhabditis elegans and acts via functionally different actin-binding repeats. Mol Biol Cell. 2004;15:4735–4748. doi: 10.1091/mbc.E04-03-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Redell JB, Tian LM, Xue-Bian J, Dash PK. Inhibition of conditioned stimulus pathway phosphoprotein 24 expression blocks the development of intermediate-term memory in Hermissenda. J Neurosci. 2003;23:3415–3422. doi: 10.1523/JNEUROSCI.23-08-03415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Xue-Bian JJ. Identification of a 24 kDa phosphoprotein associated with an intermediate stage of memory in Hermissenda. J Neurosci. 2000;20:RC74. doi: 10.1523/JNEUROSCI.20-10-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Cornette R, Matsumoto T, Miura T. Histological analysis on fat body development and molting events during soldier differentiation in the damp-wood termite Hodotermopsis sjostedti (Isoptera, Termopsidae) Zool Sci. 2007;24:1066–1074. doi: 10.2108/zsj.24.1066. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Aonuma H, Miura T. Soldier-specific modification of the mandibular motor neurons in termites. PLoS One. 2008;3:e2617. doi: 10.1371/journal.pone.0002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 5. New York: Garland Science; 2007. [Google Scholar]
- Van Troys M, Vandekerckhove J, Ampe C. Structural modules in actin-binding proteins: towards a new classification. Biochim Biophys Acta. 1999;1448:323–348. doi: 10.1016/S0167-4889(98)00152-9. [DOI] [PubMed] [Google Scholar]
- Dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: Regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Cornette R, Gotoh H, Koshikawa S, Miura T. Juvenile hormone titers and caste differentiation in the damp-wood termite Hodotermopsis sjostedti (Isoptera, Termopsidae) J Insect Physiol. 2008;54:922–930. doi: 10.1016/j.jinsphys.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Miura T, Matsumoto T. Soldier morphogenesis in a nasute termite: discovery of a disc-like structure forming a soldier nasus. Proc Biol Sci. 2000;267:1185–1189. doi: 10.1098/rspb.2000.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen DJ, Allen CE. Genotype to phenotype: Physiological control of trait size and scaling in insects. Integr Comp Biol. 2003;43:617–634. doi: 10.1093/icb/43.5.617. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. New York: Oxford University Press; 2003. [Google Scholar]
- Moczek AP, Rose D, Sewell W, Kesselring BR. Conservation, innovation, and the evolution of horned beetle diversity. Dev Genes Evol. 2006;216:655–665. doi: 10.1007/s00427-006-0087-2. [DOI] [PubMed] [Google Scholar]
- Emlen DJ, Szafran Q, Corley LS, Dworkin I. Insulin signaling and limb-patterning: candidate pathways for the origin and evolutionary diversification of beetle 'horns'. Heredity. 2006;97:179–191. doi: 10.1038/sj.hdy.6800868. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Okada Y, Ishikawa A, Miyakawa H, Koshikawa S, Miura T. Gene expression changes during caste-specific neuronal development in the damp-wood termite Hodotermopsis sjostedti. BMC Genomics. 2010;11:314. doi: 10.1186/1471-2164-11-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental results. RNAi experiments, phylogenetic analysis of cib homologs and statistic analysis of qPCR.
Supplemental Fig. 1. Phylogenetic analysis of HsjCib and ciboulot homologs from other animals.
Supplemental Table 1. Summary of Tukey's HSD test for qPCR results.