Abstract
Carbonylation is a commonly studied form of oxidative modification to proteins which can be conveniently detected using commercially available kits. The most common of these kits is the Oxyblot™ Protein Oxidation Detection Kit (Chemicon/Millipore). Over the past year we have observed severely diminished sensitivity of these kits which was shown to be a result of a change in the formulation of one of the components supplied in the kit. This component, the 10X 2,4-dinitrophenylhydrazine derivatization solution, which had previously been dissolved in 100% trifluoroacetic acid (TFA), was now dissolved in 2N hydrochloric acid, which according to our results is not acid enough. Further, we observed that upon storage even DNPH dissolved in TFA is subject to degradation. Based on these studies, we make recomendations that should improve the sensitivity and reproducibilty of this assay.
Keywords: protein carbonyls, Oxyblot™, method
Introduction
Oxidative modification of proteins can take many forms including, but not limited to: nitrotyrosination, carbonylation, methionine and sulfhydryl oxidation, etc. [reviewed in [1–3] and is thought to be involved in a variety of pathologies. Protein carbonylation is the introduction of a carbonyl group (aldehydes or ketones) into a protein through oxidation of arginine, lysine, threonine, or proline residues through a multi-step series of reactions thought to involve site-directed formation of oxidative species [3]. Owing partly to the convenience of detection, protein carbonylation has become one of the most extensively studied forms of oxidative modification and since 2006 has been included as a MeSH subheading for Medline archiving of published manuscripts. It had been known for some time that exposed carbonyl groups would react with 2,4-dinitrophenylhydrazine (DNPH) to form hydrazones and in 1990, Levine et al [4] published the first spectrophotometric method based on this reaction. However, since excellent antibodies to dinitrophenyl groups had been commercially available for some time, the spectrophotometric method became widely supplanted by Western blot techniques [5–7] which led to the introduction of commercial kits for detection of protein carbonyls.
The most popular of these kits is the Oxyblot™ Protein Oxidation Detection Kit (Product #S7150, Chemicon/Millipore, USA). Since its introduction in the mid 1990s, this kit has been cited in numerous studies, including several of our own [ref [8–10] as examples], that have implicated protein carbonylation in a variety of pathologies. Unfortunately, this technique has also been the subject of primarily anecdotal reports indicating numerous problems with data reproducibility or production of spurious results. Indeed, Levine et al [7] warn that the appearance of carbonyl groups can not be presumed to be evidence for oxidative modification, since glycation can also add these to amino acid residues. Additionally, recent studies point out that measured values of protein carbonylation may be artificially increased by nucleic acid contamination or exposure to thiols [11] or through DNPH reaction with sulfenic acids [12].
Within the last year, we noticed that several Oxyblot™ kits were not yielding reproducible results due to a loss of sensitivity. In discussions with our colleagues at other institutions who also seemed to be having this problem, we have identified lot numbers PS01580868 and PS01596617 associated with this issue, although we do not believe that this list is comprehensive. One of the components supplied in the kit is 10X 2,4-Dinitrophenylhydrazine Derivatization Solution (100mM) which historically was dissolved in 100% trifluoroacteic acid (TFA), [see ref [13]]. In trying to isolate the source of the problem, we noticed that the DNPH solution in the affected kits did not fume or have an acetic acid smell on opening. We discussed this problem with the technical people at Millipore/Chemicon and were informed that due to “regulatory” concerns about shipping of hazardous materials, the 10X DNPH solution had been reformulated to contain 2N hydrochloric acid (HCl) in place of the TFA [see ref [12]. We were concerned that this was the source of the problem and on further investigation determined that dissolution of DNPH in HCl does indeed decrease sensitivity. However, we also observed that even when dissolved in TFA and stored according to package insert instructions (2 to 8°C), reactivity of the DNPH will diminish with time and propose that this may be one source of the anecdotal reports of reproducibility problems with this technique.
Methods
Reagents
The Oxyblot™ kit in its various formulations were obtained from Chemicon/Millipore (USA). Polyclonal anti-DNP antibody raised in rabbit and 2,4-dinitrophenylhydrazine wERE obtained from Sigma-Aldrich (St. Louis, MO). All other reagents were of highest grade and obtained from reputable sources.
Heart tissue lysate
The lysate used for all of these studies was freshly prepared from excess flash frozen tissue derived from an ischemic rat heart and was prepared as previously described [9]. Briefly, flash frozen heart was pulverized, homogenized, and the homogenate centrifuged at 10,000g, at 4°C, to obtain the post-mitochondrial lysate which was then used in the studies.
Protein Carbonyl Assay
All procedures for derivatization of protein carbonyls with DNPH and subsequent detection using the Oxyblot™ kit have been extensively described previously [8–10] and basically follow the procedure as outlined in the kit brochures. Where indicated, 10X-DNPH derivatization solution was prepared as 100mM in 100% TFA, or 2 or 10N HC1, which was diluted to 1X with 9 volumes of water just prior to use. A non-commercial neutralization solution was prepared as 2M TRIS/30% Glycerol (same as in kit). In all experiments, 20 μg protein was derivatized and separated on a 4–20% pre-cast gel (BioRad, USA) using standard SDS polyacrylamide electrophoresis under reducing conditions. Following separation, the gels were transferred to a PVDF membrane and probed with the primary and secondary antibodies provided in the kit or with polyclonal anti-DNP (Sigma) and anti-rabbit IgG (Santa Cruz Biotechnology, USA) and developed using enhanced chemiluminescence (Perkin Elmer, USA).
Results and Discussion
Reactivity of DNPH for protein carbonyls is altered with changes in the acidity of the DNPH solution
Our initial contact with Millipore was to provide them with the image presented in figure 1A which is a comparison of the same heart lysates treated with 1X DNPH (10mM) from an older kit that still contained 100% TFA (10X), or with a kit that was obtained in May, 2009, containing the new formulation in 2N HCl (10X). As is obvious, the intensity of the DNPH signal was severely diminished. We were concerned that the decreased acidity of the DNPH derivatization solution was the basis for the diminished reactivity. To confirm this heart lysates were derivatized with 1X DNPH (10mM) prepared as 10X in 2 or 10N HCl, and 100% TFA. The most intense signal was observed in the DNPH:TFA derivatized sample, the least in the DNPH:2N HCl (figure 1b). Increasing the acidity to 10N HCl, improved the sensitivity of the reaction. So that all reaction conditions would be the same, with the exception of the DNPH derivatization solution, the separated proteins were detected with polyclonal anti-DNP (1:2000) obtained from Sigma-Aldrich (St. Louis, MO). It was surprising to us that the kit contained 10X DNPH in 2N HC1, when earlier studies clearly indicated that the diluted 1X DNPH should have a final concentration of 2N [4–6]. In late 2009, the DNPH was again reformulated so that it contained 1X DNPH (10mM) in 2N HCl which was not to be diluted [see ref [14] which was the exact conditions suggested in the original study published by Levine et al [4]. In late December, 2009, we obtained a reformulated Oxyblot™ kit (Lot #PS01648355) which was tested in mid-January, 2010. We again compared same heart lysates derivatized with DNPH from the three kits (final 1X acid concentration; 10% TFA; 0.2N HCl; 2N HCl) with freshly made 10X DNPH:TFA diluted to 1X (10mM DNPH:10% TFA). Again, so that all reaction conditions would be the same, the separated derivatized proteins were detected with the Sigma polyclonal anti-DNP (1:1500). The increased acidity in the newly reformulated kit dramatically increased the sensitivity of the assay so that it was at least equal to that of freshly made DNPH:TFA (figure 1c). We suspect that changes in acidity affect the sensitivity of the assay through changes in protein solubility, as suggested by Levine et al [7], or even solubility of DNPH which is known to be more soluble and stable in strong acids [15]. However, what was disconcerting was the apparent loss of sensitivity observed between the old TFA containing kit, obtained in October 2008, and the freshly made DNPH in TFA. This becomes an issue because the commercially available Oxyblot™ kits do not have an expiration date and the kit brochure indicate that the diluted 1X DNPH solution is stable for up to 6 months.
Figure 1. Dissolution of DNPH in hydrochloric acid decreases intensity of Oxyblot signal.

Panel A presents a comparison of the same heart cell lysate reacted with DNPH from an older kit (Lot #PS01514715) that still contained DNPH:TFA versus a new kit (Lot #PS01580868) that contained DNPH:HCl. In this experiment the primary antibody used was supplied in the new kit. This experiment was only performed one time and the result transmitted to the company. Panel B presents a comparison of the same heart cell lysate reacted with DNPH 100 mM dissolved in 10N and 2N HCl and 100% TFA. In this experiment, the concentrations are the 10X and are diluted to 1X with water (final; 10mM DNPH; 1N and 0.2N HCl; 10% TFA). The primary antibody was polyclonal anti-DNP (Sigma, D9656) raised in rabbit. The depicted membrane is representative of 2 separate experiments. Panel C presents a comparison of the same heart cell lysates reacted with 1X 10 mM DNPH in (final): TFA (10%) (Lot #PS01514715 - obtained 10/2008); HCl (0.2N) (Lot #PS01580868 - obtained 05/2009); HCl (2N) (Lot #PS01648355 - obtained 12/2009); and freshly prepared in TFA (10%). The primary antibody was the same polyclonal anti-DNP used in Panel B. The depicted membrane is representative of 2 to 3 separate experiments.
Reactivity of DNPH for protein carbonyls declines with storage
To determine if storage of dissolved DNPH has any effect on the reactivity of the DNPH, heart lysate was reacted with 1X DNPH:TFA diluted with water from 10X that had been stored for up to 9 days at 2 to 8° C as indicated in the Oxyblot™ kit documentation. An additional sample was reacted with 1X DNPH:TFA diluted from the stock 10X bottle from a kit (Lot #PS01514715) received in October, 2008, yet stored as indicated in the documentation. All derivatized samples were then treated in the same manner and separated using standard SDS PAGE under reducing conditions. Following transfer, the derivatized proteins were probed with the Sigma anti-DNP antibody. Peak reactivity was observed in the lysates derivatized with freshly made TFA:DNPH (figure 2). Following 2 days of storage reactivity declines and after 9 days is diminished by over 50% (P<0.05, ANOVA, Tukeys). The signal intensity of the lysate derivatized with the DNPH:TFA from the kit was noticeably less than even the 9 day old DNPH indicating marked loss of reactivity. It is not obvious why this occurs. A survey of the pertinent literature does not reveal an obvious problem associated with storage of DNPH:TFA [4,7,16] and the Oxyblot™ kit documentation [14] indicates that these solutions can be stored for up to 6 months. From our own experiences we have observed differences in signal intensity between fresh Oxyblot kits and those that have been stored for an extended period of time. Because we have limited experience with the newly reformulated product (1X 10mM DNPH:2N HCl) it is not clear that these will suffer from a similar problem. Nonetheless, this result does suggest that loss of reactivity with storage may be partly responsible for some of the anecdotal reports of reproducibility problems associated with this assay and should be considered when interpreting results from studies conducted at different times.
Figure 2. Decay of sensitivity with increasing storage time of DNPH:TFA.
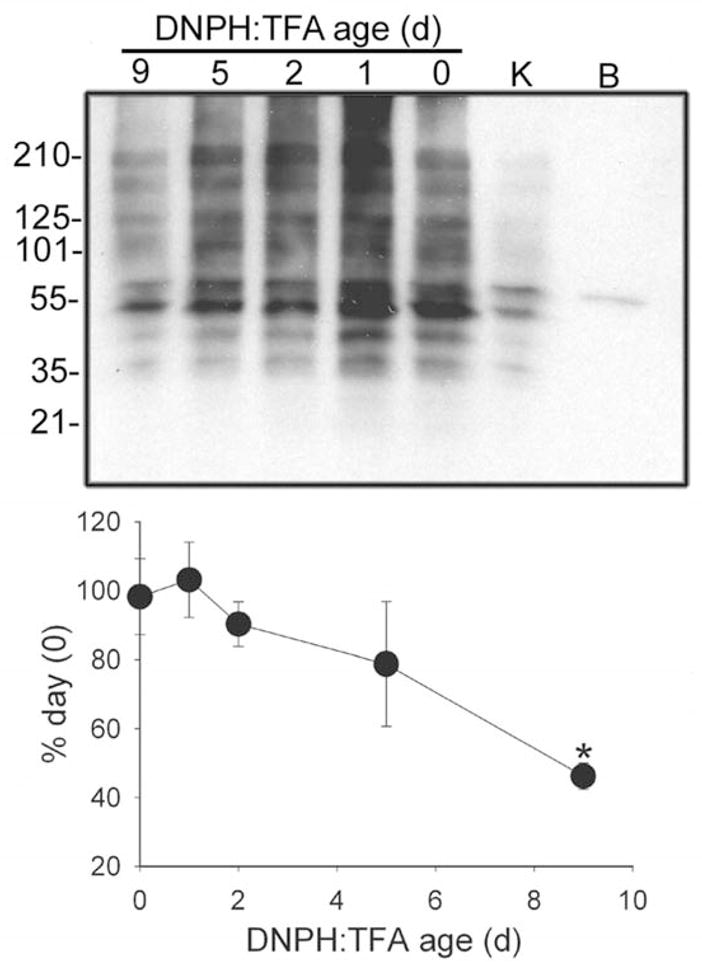
The top panel presents a representative immunoblot depicting loss of reactivity of DNPH with protein carbonyls with time of storage. The “K” lane contains lysate reacted with DNPH:TFA from a kit in excess of 6 months of age. The “B” lane is a blank derived from lysate reacted with TFA only (no DNPH) and then treated the same as the other samples. The bottom panel presents the densitometric analysis of the DNPH:TFA lanes with values expressed as the percent intensity of freshly prepared DNPH:TFA (day0). All values represent the mean ( ± SEM) of 3 separate determinations.
Comments and recommendations
Much of the confusion associated with the Oxyblot™ kit over the past year is the result of the company’s failure to adequately inform investigators of the changes in the formulations of the DNPH derivatization solution. Confusion was magnified by the presence of the January 2006 kit brochure on the company’s website (www.millipore.com) during this time which has only recently been updated to the September 2009 kit brochure. As late as January 15, 2010, when queried, the company still responded that “Component 90448: 10X 2,4-Dinitrophenylhydrazine (DNPH) Solution contains trifluoroacetic acid” which is obviously incorrect. Because of the confusion and the possibility that the DNPH derivatization solution may degrade with prolonged storage, we no longer use the kit and suggest the following. First, to improve reproducibility between assays, and to insure optimal sensitivity, prepare the 10X DNPH:TFA fresh and dilute and use within 72 hours. It is not clear to us that 1X DNPH prepared in 2N HCl, has this issue, nonetheless we would still suggest not storing this beyond 30 days. Second, consider using one of the many excellent commercially available anti-DNP antibodies. We have the most experience with the Sigma antibody (D9656) raised in rabbit which can be used at a dilution of 1:1000 to 1:2000 as opposed to the primary antibody provided in the kit which we routinely used at a dilution of 1:150. Also, it should be pointed that unlike the Oxyblot™ kit brochure, we do not recommend adding extraneous thiol to samples which is in agreement with Luo and Wehr [11] who observed artifactual increases in protein carbonylation upon addition of thiol. Since making these changes, we have found that both reproducibility and sensitivity are improved and are now able to observe even very subtle increases in protein carbonylation.
Acknowledgments
Sources of funding This study was supported by NIH HL68936 (to SRP).
Footnotes
Conflicts of interest. None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stadtman ER, Moskovitz J, Levine RL. Oxidation of methionine residues of proteins: biological consequences. Antioxid Redox Signal. 2003;5:577–582. doi: 10.1089/152308603770310239. [DOI] [PubMed] [Google Scholar]
- 2.Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324:1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stadtman ER. Metal-ion catalyzed oxidation of proteins: biochemical mechanism and consequences. Free Radic Biol Med. 1990;9:315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura A, Goto S. Analysis of protein carbonyls with 2,4-dinitrophenyl hydrazine and its antibodies by immunoblot in two-dimensional gel electrophoresis. J Biochem (Tokyo) 1996;119:768–774. doi: 10.1093/oxfordjournals.jbchem.a021306. [DOI] [PubMed] [Google Scholar]
- 6.Yan LJ, Orr WC, Sohal RS. Identification of oxidized proteins based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunochemical detection, isoelectric focusing, and microsequencing. Anal Biochem. 1998;263:67–71. doi: 10.1006/abio.1998.2799. [DOI] [PubMed] [Google Scholar]
- 7.Levine RL, Wehr N, Williams JA, Stadtman ER, Shacter E. Determination of carbonyl groups in oxidized proteins. Methods Mol Biol. 2000;99:15–24. doi: 10.1385/1-59259-054-3:15. [DOI] [PubMed] [Google Scholar]
- 8.Powell SR, Wang P, Katzeff HL, Shringarpure R, Teoh C, Khaliulin I, Das DK, Schwalb H. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function. Essential role of the proteasome. Antioxid Redox Signal. 2005;7:538–5. doi: 10.1089/ars.2005.7.538. [DOI] [PubMed] [Google Scholar]
- 9.Powell SR, Gurzenda EM, Wahezi SE. Actin is oxidized during myocardial ischemia. Free Radic Biol Med. 2001;30:1171–1176. doi: 10.1016/s0891-5849(01)00514-7. [DOI] [PubMed] [Google Scholar]
- 10.Schwalb H, Olivson A, Li J, Houminer E, Wahezi SE, Opie LH, Maulik D, Borman JB, Powell SR. Nicorandil decreases postischemic actin oxidation. Free Radic Biol Med. 2001;31:607–614. doi: 10.1016/s0891-5849(01)00620-7. [DOI] [PubMed] [Google Scholar]
- 11.Luo S, Wehr NB. Protein carbonylation: avoiding pitfalls in the 2,4-dinitrophenylhydrazine assay. Redox Rep. 2009;14:159–166. doi: 10.1179/135100009X392601. [DOI] [PubMed] [Google Scholar]
- 12.Oxyblot™ protein oxidation kit (catalog No. S7150). Revision F. Millipore Corporation; 2009. [Google Scholar]
- 13.Oxyblot™ protein oxidation kit (catalog No. S7150). Revision E:41418. Millipore corporation; 2006. [Google Scholar]
- 14.Oxyblot™ protein oxidation kit (catalog No. S7150). Revision G. Millipore corporation; 2009. [Google Scholar]
- 15.Johnson GD. A new preparation of 2,4-Dinitrophenylhydrazine. J Am Chem Soc. 1951;73:5888–5889. [Google Scholar]
- 16.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]


