Abstract
In laboratory studies of individual differences in stress reactivity, cortisol responses are typically measured by comparing a prestress baseline with values obtained at the end of the stressor. In the present study, we measured cortisol in this manner on a stress day, but we also took samples on a second day when the volunteers rested in the lab at the same time of day and for the same duration. We compared stress responses as the difference from pre- to poststress and also poststress vs. rest-day control. The latter method allowed a greater appreciation of how stress perturbed the underlying diurnal baseline. Although the effect of stress was statistically significant when measured as the change from pre- to poststress, the magnitude of the effect was 54% larger when measured against the time-of-day control from the rest day. In particular the diurnal control method provided a wider range of stress values that potentially provide a greater range of response values in carrying out analyses of individual differences.
Keywords: cortisol, mental stress, reactivity, diurnal cycle, gender
Cortisol is highly reactive to events that may threaten the system's homeostasis, and as such the response of the hypothalamic-pituitary-adrenocortical axis is considered the core of the stress response (Selye, 1936). In keeping with this, a healthy, or normative, stress response is considered essential to homeostasis (de Kloet, 2003), and the integrity of the cortisol response to stress is considered an indicator of a normal state of health (McEwen, 2000). The assessment of cortisol stress responses in the laboratory is a common method for comparing stress reactivity in groups of individuals differing in significant characteristics such as sex, age, and health status (al'Absi et al., 1994; Kirschbaum et al., 1999; Lupien et al., 1999; Cacioppo et al., 2000; Burleson et al., 2003).
Studies examining cortisol stress reactivity usually obtain data on a single occasion in which levels of cortisol prior to a stressor are used as a baseline for levels obtained during or after stress exposure (Kirschbaum et al., 1999; Dickerson and Kemeny, 2004). However, cortisol's well characterized diurnal cycle shows that cortisol levels in blood or saliva are not stable over time, but are high just after awakening and decline over the waking hours (Czeisler and Klerman, 1999; Kunz-Ebrecht et al., 2004). This lack of a stable underlying baseline poses interpretive problems and also presents research opportunities if its effects are taken into account when evaluating stress reactivity effects.
In this paper we present cortisol responses to psychological stress derived from a large data set collected on healthy young adults, and we use these to illustrate the value of comparing data collected on a day of stress compared to a resting control day. The analyses illustrate how the measurement of stress reactivity may benefit from using a diurnal control as the baseline instead of the prestress value. We illustrate this by examining the presence of an anticipatory response on a single stress day and by comparing men and women and the effects of time of day of testing.
Methods
Overview
The present data are derived from the Oklahoma Family Health Patterns Project, an ongoing study of the psychological and physiological characteristics of young adults with and without a family history of alcoholism. The volunteers are screened for present substance use disorders and psychiatric conditions and are in current good health. As part of this study every volunteer who met inclusion criteria, visited the lab on two days, first on a stress day and then on a resting control day. Because of the number of volunteers (N = 324) and due to the use of a consistent protocol, the data set provides a useful resource for assessing the characteristics of the stress response in healthy young adults.
Subjects
The present sample includes 187 females and 137 males, 18 – 30 years of age and in good health, who were recruited through community advertisement from the general population of Oklahoma City, OK, USA. They were 78% European American, 12% African American, 4% Native American, 3% Hispanic, and 3% other race and ethnicity. The participants were (mean ± SEM) 23.7 ± .24 years of age, with males being 24.1 ± 0.43 years and females being 23.4 ± .22 years (p = 0.2, by Student's t test), and as a group had 15 ± 0.3 years of education. All participants signed a consent form approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center and the Veterans Affairs Medical Center in Oklahoma City, OK, USA, and were paid for participating.
Inclusion and exclusion criteria
Prospective volunteers were excluded if they had any of the following: a history of alcohol or drug dependence; met diagnostic criteria for substance abuse within the past 2 months; had current use of any abused drug assessed by a urine drug screen and alcohol breath test on each day of testing; had any current Axis I disorder as defined by the Diagnostic and Statistical Manual of Mental disorders, 4th ed. (Association, 1994) and assessed by Diagnostic Interview Schedule. All participants were in good health as determined by self-report, had a body mass index < 30, had normal hearing assessed by audiologic exam, were taking no prescription medications at the time of testing, and to had no reported history of serious medical disorder, including neurological disorders, cardiovascular diseases, or diabetes.
Because cortisol secretion is dependent on the sleep-wake cycle (Czeisler and Klerman, 1999), all volunteers were required to have a normal work or school schedule and to have a nighttime sleep pattern. Also, because acute cortisol secretion is affected by prevailing blood sugar levels (Dallman, 2003), all volunteers ate a standard meal prior to beginning the protocol. This consisted of a small breakfast for morning volunteers and a small lunch for afternoon volunteers prepared by the campus General Clinical Research Center bionutrition core. Women were excluded if they were pregnant based on self-report and were required to have a negative urine pregnancy test on each day of testing. Smoking and smokeless tobacco use were not exclusionary.
Study design and procedure
After screening, subjects visited the lab two more times for behavioral and psychophysiological testing, and each subject was tested at the same time on both days, either in the morning at 0900h (N = 151) or in the afternoon at 1300h (N = 165). In all cases, the first day of the study involved a stress procedure and the second day was designated a resting control day. Subjects were clearly briefed on this order of testing on their screening day in order to maximize the effect of anticipation of the stress procedure on day 1 and to ensure that day 2 was a rest day free of the effects of novelty or anticipation. The description of the stress procedure given on the screening day was that the subject should expect to deliver three short speeches to a member of the lab staff and that they would also undergo a mental arithmetic task. No further detail was provided until the stress day proper.
Stress protocol
The stress protocol consisted of a 30-min prestress baseline, during which time the subject sat quietly and read general interest magazines, followed by 45 min of behavioral stress. Stress included simulated public speaking (Saab et al., 1989) followed by mental arithmetic (al'Absi et al., 1997). The speech task (30 min) included three successive speeches prepared and delivered with no breaks. At the start of each speech period, the subject was given a card with a topic written on it and told that they had 4 min to prepare a speech without making notes and 4-min to deliver it from memory. To increase the sense of realism, the speech was observed by a white-coated experimenter holding a clipboard and with a nearby video camera set to the record mode. The subject was told that his or her speech would be shown to the laboratory staff and that they would judge the subject's fluency of delivery and how convincing their speech was. The speech topics included recounting an article on why hair turns gray, presenting a position for or against whether homosexuals should be allowed to marry, and responding to an accusation that the subject was shoplifting. The order of speech topics was randomly assigned for each participant.
The mental arithmetic task consisted of three 5-min periods with no interruption other than brief instructions. At the start of each period, the subject was given a three-digit number (e.g. 298) and told to add the digits (19) and to add that total to the original number (317), to recite the new number aloud, and to proceed in that fashion for 5 min until told to stop. The experimenter monitored the answers and noted errors by telling the subject when an answer was wrong and to start back with their previous correct answer.
Resting control day
The protocol lasted 75 minutes, during which time the subject sat and read general interest magazines or watched televised video tapes of nature programs or historical documentaries chosen for their interest value and lack of strong emotional content. These cortisol values were therefore considered to represent the portion of the diurnal curve produced by that subject over that time of day.
Saliva collection
Saliva was collected at minutes 15 and 30 of the baseline period and at minutes 30 and 45 of the stress or continued rest period using the Salivette device (Sarstedt, Newton, NC, USA). The subjects were instructed to keep the cellulose absorbent collector in their mouth until it was saturated with saliva and then to replace it in the collection tube.
Saliva assay
After data collection, the Salivettes were centrifuged at 4200 RPM for 20 min. The saliva was transferred to cryogenic storage tubes and placed into a −70° C freezer until shipping. Assays were conducted by Salimetrics (State College, PA, USA) where the saliva free cortisol concentrations were quantified using a competitive enzymatic immunoassay (Salimetrics, 2005). The assay has a sensitivity of < .083 μg/dL and an interassay coefficient of variation of < 6.42%.
Data analysis
Data were analyzed by repeated measures analyses of variance, Student's t test, and Pearson's r using SPSS for Windows (release 18.0, SPSS Chicago, IL, USA). Differences were considered statistically significant if p < 0.05.
Results
Preliminary analyses indicated that body mass index for this sample was 23.9 ± 0.028 and Pearson correlations indicated that body mass was not related to cortisol levels at either baseline period on the stress day or to stress levels or changes from baseline on the stress day, all rs < 0.125, ps > 0.123. The sample had a relatively small number of smokers (N = 40), and cortisol values did not differ as a function of smoker status, t = 0.273, p = 0.79.
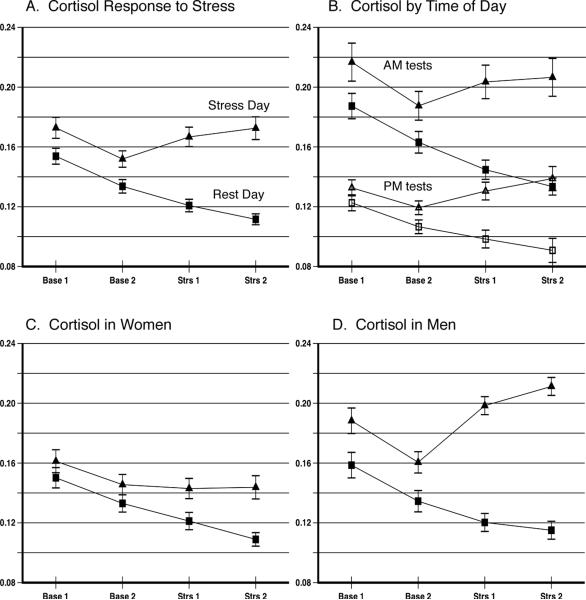
Figure 1 shows cortisol values across the four sampling times on the resting control day and the stress day. Panel A shows cortisol values for all subjects. The data were tested using an analysis of variance for repeated measures with 2 Days and 4 Time Periods. This showed that there was a significant effect of Day with the stress day producing higher cortisol concentrations than the rest day (F1, 322 = 48.90, p < .0001) and a significant Day by Period interaction (F1, 322 = 35.68, p < .0001). Inspection of Panel A reveals two important features of the data. The rest day shows a pronounced reduction in cortisol concentrations across the four samples taken during the 75 min protocol, with concentrations declining from 0.173 μg/dL to 0.112 μg/dL, for a decline of 29%. The stress day shows a variation in cortisol from 0.151 at the end of baseline and a rise to 0.172 μg/dL at the end of the stress period, a rise of 14%. The effect size of this comparison is very small (partial eta2 = .03). In contrast, the comparison of the cortisol concentrations at the end of the stress period versus the corresponding control day period is 0.172 ± 0.008 μg/dL vs. 0.112 ± 0.004 μg/dL, for a difference of 54%, with a correspondingly large effect size (partial eta2 = .17). The 54% difference between these values at the end of the stress vs. rest periods is a more realistic measure of the influence of the stressors than is the 14% rise during the stress period itself because the control-day value at that time represents the true unperturbed baseline for the specific time period of comparison. The availability of the rest day data therefore shows features of the data not apparent from the stress data alone.
Figure 1.
Saliva free cortisol values on days of mental stress and days of rest. A. Data from the full sample; B. Morning and afternoon values on rest and stress days; C. Data from women; D. Data from men. Entries show M ± SEM in μg/dL.
Panel B in Figure 1 presents data for subjects tested in the morning (N = 155) vs. afternoon (N = 169) hours. These data were subjected to a 2 Day by 2 Times of Day by 4 Time Periods repeated measures analysis of variance. The effects of Day and Period were highly significant as described above. As expected, the morning values were higher than the afternoon values, F1, 322 = 65.39, p < .0001. The effect of stress was compared for the morning vs. afternoon hours using the Day by Time of Day interaction term. Stress in the afternoon was less effective at elevating cortisol than stress exposure in the morning hours, F1, 322 = 4.58, p = .033. It is noteworthy that if only stress day data were available, the effect of stress measured as absolute change from Baseline 2 to the end of stress at Stress 2 would appear to be nearly identical at the morning and afternoon time periods, 0.0190 μg/dL and 0.0195 μg/dL, respectively.
Panels C and D in Figure 1 illustrate the effects of the stress protocol in women and men, respectively. To emphasize the effect of gender on stress reactivity, these data were analyzed using 2 Gender by 4 Time Periods analyses of variance done separately for rest day and stress day. On the rest day, there was no significant effect of gender, F1, 322 = 0.194, p = .660, indicating that the basal levels of cortisol did not differ in men and women, an effect that would be obscured by examining only stress days, although there was a modest effect of Gender by Time period under resting conditions, F3, 320 = 3.56, p = .015, indicating somewhat greater variation over time among the men. The effect of stress was greater among men than women, as seen in a significant Gender by Time Period interaction, F3, 320 = 7.56, p < .0001, indicating that stress produced a larger cortisol rise in men than in women. This difference is supported by a main effect of Gender on the stress day, F1, 322 = 12.58, p < .0001. As for the full sample, the two methods of determining stress reactivity yield very different pictures for both men and women. Among women, the prestress vs. poststress values on the stress day actually show a numerical decline (.133 μg/dL vs. .109 μg/dL) with an effect size that is nil (partial eta2 = .000). In contrast, the effect size for the rest day vs. stress day poststress values (.109 μg/dL vs. .144 μg/dL) has a larger effect size (partial eta2 = .104). A similar pattern holds for the men, although both effect sizes are larger: prestress vs. poststress partial eta2 = .113 and poststress on rest day vs. stress day has a partial eta2 = .26.
Discussion
The foregoing presentation of stress cortisol responses in a large sample of healthy young adults is intended to emphasize the value added to the understanding of stress responses when resting time-of-day control data are used as the reference baseline. The results illustrate three useful features of the data. First, the effect of stress on cortisol is much less apparent if only stress day data are available. While the effect of stress was significant compared against the baseline data on the stress day, the magnitude of the response to stress can be appreciated more thoroughly when the resting control day data are available for comparison.
Second, the influence of time of day on stress reactivity is pronounced when stress values are compared against the corresponding rest day values. In this case the effect of stress is much larger during the morning hours as revealed by inspection of Panel B in Figure 1 (see Dickerson and Kemeney, 2004). In contrast, stress day changes from baseline are indistinguishable at the two times of day.
Third, the effect of gender on stress responses has a different appearance when rest day data are considered. The cortisol values on rest days show that the circulating free cortisol levels do not differ between men and women under basal conditions. More importantly, data from the women on the stress day, as measured against the prestress value, would suggest the women had, a minimal stress response at best. However, this view changes when stress day is measured against the diurnal background. In this comparison, the women have substantial cortisol elevations in comparison to their time-of-day control values. Among the men, the stress response was numerically large comparing stress vs. baseline values, but the difference from the rest day data was virtually twice as large as within day change on the stress day.
Finally, the present data show that there is an anticipatory effect on cortisol when the volunteers are confronted with a novel set of procedures that they expect is likely to be stressful or challenging. The effect of this anticipation is clearly illustrated in the elevated prestress values on the stress day relative to the time-of-day control values on the rest day. Our purpose in using this design was to maximize the stressfulness of the procedures by putting them first and maximizing the accuracy of the diurnal values on the control day, when the subject was familiar with the lab and not anticipating any stress exposure. We expect that counterbalancing stress and rest days may have yielded averaged baseline values that would be similar on the rest and stress days. We note that presenting stress on the first day mimics the typical experimental design in which the subject visits the lab on a single day, has samples taken prestress and poststress and then is dismissed. The results provide a revealing view of how novelty and anticipation of acute stress is capable of perturbing the normal diurnal secretion of this critical stress hormone.
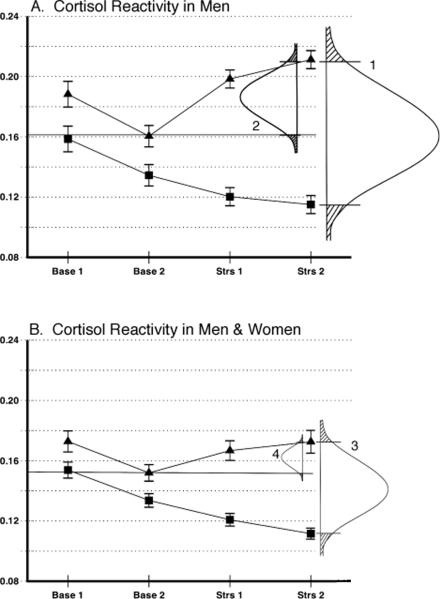
Perhaps the most common use of reactivity data is to compare variations in stress responses across persons to answer questions about individual differences. A practical consequence of using a resting control day as the point of reference for determining reactivity is illustrated in Figure 2. In this case we have displayed the data from panels A and D from Figure 1 along with the sizes of the hypothetical reactivity distributions obtained when the baseline is the resting control day compared with the case in which the baseline is the prestress value. In addressing questions such as, “How does reactivity in Group A compare with Group B?” it is desirable to have the greatest spread among persons representing these subgroups in order to increase individual differences estimates in relation to error variance. In Figure 2, the reactivity data from the men are shown with hypothetical response distributions drawn in showing the spread of data when the rest day is used as the baseline (1) and when the prestress value is used as the baseline (2). The lower panel shows the same distributions for the full sample of men and women. If the goal is to find the clearest distinction between subgroups, curve 1 provides more resolution than curve 2, and curve 3 provides more resolution than curve 4. A similar analysis in women would allow almost no spread of the data if the prestress value were used as the baseline for assessing reactivity. The use of a design as advocated here calls for two days of testing for each subject. The value of the additional experimental effort must be weighed against the goals of the study. However, the use of only a stress day may result in particularly large underestimates of stress reactivity, as shown in the data from the women.
Figure 2.
The hypothetical spread of individual differences in four possible data comparisons. A. The distribution of stress cortisol responsivity in males. 1. Shows the stress response when the rest day time-linked control period (Strs 2) is used as the baseline for the stress day Strs 2 time point. 2. Shows the stress response when the prestress period (Base 2) is the point of reference. B. 3 and 4 show the same reactivity distributions in the full sample.
A limitation of the present data is that they represent a single stress paradigm, namely public speaking followed by mental arithmetic. This protocol represents one commonly used in assessing psychological stress in the laboratory with human volunteers. However, the diurnal pattern seen on the rest day is not dependent on the stress paradigm, and we believe that the comparisons would be qualitatively similar if other stressors were used. A second limitation is that the present data do not address the effect of menstrual cycle phase on stress responses in women (Kirschbaum et al., 1999; Symonds et al., 2004). To address this question would require a specifically structured study design that necessarily would differ from the one used here. Given the large sample of women in this study, we suspect that the effects of oral contraceptive use and of menstrual cycle phase should all be represented in the stress day data, indicating that on average women have smaller responses to public speaking and mental arithmetic than do men. However, a potential hormonal source of the difference cannot be determined from the present results. With that limitation in mind, the basic message holds equally well for women as for men that using a resting control day produces a larger estimate of cortisol stress reactivity.
The present data indicate that the effects of an acute stressor on cortisol secretion appear very different when comparison data are collected on a resting control day. This yields a larger estimate of the effects of the stressor. Perhaps most importantly for understanding the glucocorticoid response to stress, the availability of resting control data provides a greater appreciation for the extent to which an acute stressor can perturb the underlying diurnal cycle. In the absence of the control day data, this impact of the stressor would not have been apparent in comparing morning vs. afternoon responses. The responses of males would appear greater than those of females in either case, however the effect of the stressor on women would be significantly underestimated using pre- to poststress comparisons. The results presented here suggest that the additional time and effort to measure cortisol on both a rest day and a stress day may prove useful given the needs of a specific research question.
Acknowledgements
Supported by the Department of Veterans Affairs Medical Research Service, the National Institutes of Health, NIAAA, grant AA12207, and NIRR, grant M01 RR14467.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest The authors have no financial interest in any product associated with this research.
References
- al'Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Lovallo WR, McKey BS, Pincomb GA. Borderline hypertensives produce exaggerated adrenocortical responses to mental stress. Psychosom Med. 1994;56:245–250. doi: 10.1097/00006842-199405000-00011. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Burleson MH, Poehlmann KM, Hawkley LC, Ernst JM, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Glaser R, Cacioppo JT. Neuroendocrine and cardiovascular reactivity to stress in mid-aged and older women: long-term temporal consistency of individual differences. Psychophysiology. 2003;40:358–369. doi: 10.1111/1469-8986.00039. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Burleson MH, Poehlmann KM, Malarkey WB, Kiecolt-Glaser JK, Berntson GG, Uchino BN, Glaser R. Autonomic and neuroendocrine responses to mild psychological stressors: effects of chronic stress on older women. Ann Behav Med. 2000;22:140–148. doi: 10.1007/BF02895778. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–130. discussion 130–132. [PubMed] [Google Scholar]
- Dallman MF. Fast glucocorticoid feedback favors `the munchies'. Trends Endocrinol Metab. 2003;14:394–396. doi: 10.1016/j.tem.2003.09.005. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Hormones, brain and stress. Endocr Regul. 2003;37:51–68. [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Nair NP, Briere S, Maheu F, Tu MT, Lemay M, McEwen BS, Meaney MJ. Increased cortisol levels and impaired cognition in human aging: implication for depression and dementia in later life. Rev Neurosci. 1999;10:117–139. doi: 10.1515/revneuro.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Saab PG, Matthews KA, Stoney CM, McDonald RH. Premenopausal and postmenopausal women differ in their cardiovascular and neuroendocrine responses to behavioral stressors. Psychophysiology. 1989;26:270–280. doi: 10.1111/j.1469-8986.1989.tb01917.x. [DOI] [PubMed] [Google Scholar]
- Salimetrics High sensitivity salivary cortisol enzyme immunoassay kit. 2005.
- Selye H. Thymus and adrenals in the response of the organism to injuries and intoxications. Br J Exp Pathol. 1936;17:234–248. [Google Scholar]
- Symonds CS, Gallagher P, Thompson JM, Young AH. Effects of the menstrual cycle on mood, neurocognitive and neuroendocrine function in healthy premenopausal women. Psychol Med. 2004;34:93–102. doi: 10.1017/s0033291703008535. [DOI] [PubMed] [Google Scholar]