Abstract
Somatosensory deficits have been identified in cerebral palsy (CP), but associated cortical brain activity in CP remains poorly understood. Functional MRI was used to measure blood oxygenation level‐dependent (BOLD) responses during three tactile tasks in 10 participants with spastic diplegia (mean age: 18.70 years, SD: 7.99 years; 5 females) and 10 age‐matched controls (mean age: 18.60 years, SD: 3.86 years; 5 females). Tactile stimulation involved servo‐controlled translation of smooth or embossed surfaces across the right index finger pad; the discrimination tasks with embossed surfaces involved judging whether (1) paired shapes were similar or different, and (2) a rougher set of horizontal gratings preceded or followed a smoother one. Velocity and duration of surface translation was identical across all trials. In addition, an event‐related design revealed response dynamics per trial in both groups. Compared to controls, individuals with spastic diplegia had significantly reduced spatial extents in activated cortical areas and smaller BOLD response magnitudes in cortical areas for somatosensation, motor, and goal‐directed/attention behaviors. These results provide mechanisms for the widespread somatosensory deficits in CP. The reduced activation noted across multiple cortical areas might contribute to motor deficits in CP. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: diplegia, cerebral palsy, magnetic resonance imaging, sensation, human
INTRODUCTION
Cerebral palsy (CP) is a group of motor and postural disorders resulting from a nonprogressive injury to the developing central nervous system [Bax et al., 2005]. CP is also associated with deficits in tactile object and shape recognition and elevated thresholds for two‐point detection and roughness discrimination [Bolanos et al., 1989; Lesny et al., 1993; Sanger and Kukke, 2007; Wingert et al., 2008; Yekutiel et al., 1994]. There has been no investigation involving controlled tactile stimulation in CP. Such information is needed to understand possible mechanisms related to somatosensory and potentially motor deficits in CP.
Individuals with spastic diplegia, a common CP subtype found in low‐birth weight premature infants, typically have periventricular white matter injuries (PWMI) [Volpe, 2009]. Diffusion tensor imaging (DTI) has shown reduced thalamocortical projections is a consequence of PWMI, especially connections to parietal somatosensory areas [Hoon et al., 2009; Nagae et al., 2007; Thomas et al., 2005]. Although structural MRI and DTI reveal anatomical abnormalities related to spastic diplegia, functional MRI (fMRI) can be used to investigate how diminished thalamocortical projections affect cortical activity. The question is whether altered response magnitudes and distributions explain somatosensory psychophysical deficits in individuals with spastic diplegic CP.
Numerous neuroimaging studies have described cortical activity evoked when a normal adult discriminates tactile shapes and surface textures. For example, the primary somatosensory cortex (S1) is activated during textured grating discriminations [Carey et al., 2008] and tactile shape recognition tasks [Bodegard et al., 2000, 2001; Burton et al., 1999, 2006; Servos et al., 2001]. In addition, the secondary somatosensory area (S2), located in the parietal operculum (OP), is activated during tactile shape recognition tasks [Burton et al., 2006, 2008; Ledberg et al., 1995; Reed et al., 2004]. Recently, however, the human OP was divided into four subregions, OP 1–4 [Eickhoff et al., 2006a, b, 2007). OP 1 is likely homologous to the classic S2, responding almost universally during any tactile task; OP 3 and 4 respond especially during tactile tasks requiring attention, cognition, and memory [Burton et al., 2008]. One objective of this study was to compare activity in these traditional somatosensory areas in individuals with diplegia and in normal individuals.
Additional regions outside of traditional somatosensory cortex have been implicated in tactile discrimination. These include a cortical area surrounding the intraparietal sulcal (IPS) cortex, where it is largely coextensive with Brodmann's area 7. There are reports that tactile shape discrimination (TSD) tasks activate an inferior part of this region (iIPS) [Bodegard et al., 2001; Peltier et al., 2007; Roland et al., 1998; Zhang et al., 2004]. However, others described more extensive distributions of somatosensory activation that involve both inferior and superior components of IPS [Jancke et al., 2001; Van de Winckel et al., 2005]. Responses in these regions cannot be strictly interpreted as somatosensory because they are affected by attention to sensory events, either tactile or visual [Burton et al., 2008; Corbetta and Shulman, 2002]. Whether individuals with diplegia show similar tactile responsiveness in these posterior parietal regions is unknown. Other areas involved in somatomotor tasks are premotor cortex (PM) and supplementary motor area (SMA) [Binkofski et al., 2004; Bodegard et al., 2001; Rizzolatti et al., 2002], suggesting these frontal areas may be an important link in the somatosensory‐motor network. A second objective of this study was to determine whether the many cortical regions beyond traditional somatosensory areas are similarly engaged in diplegia and controls during tactile tasks.
Comparing cortical responses to identical tactile stimulation in these different parietal and frontal cortex areas in diplegia and control groups may reveal a neuropathological substrate underlying deficits in spastic diplegia. The general hypothesis underlying the present study was that the cortical areas activated during tactile discrimination tasks in individuals with spastic diplegia resemble the distribution and magnitude of activated regions in age‐matched controls.
METHODS
Participants
We examined 10 individuals with spastic diplegic CP; mean age was 18.70 years, SD 7.99 years; 5 females (Table I). All participants with diplegia ambulated independently, which qualified them for Level I or II of five possible levels on the Gross Motor Function Classification Scale (GMFCS), which indicated that all were able to ambulate independently [Palisano et al., 2000] (Table I). In addition, they were Level I or II (also of five) on the Manual Ability Classification System (MACS), indicating that all participants could handle objects, but with slightly reduced dexterity or speed [Eliasson et al., 2006]. All school‐age participants were in grade‐levels appropriate for their age, with those over 21 years having graduated from college; all followed instructions and responded reliably. Exclusion criteria included individuals with epilepsy, athetoid or quadriplegic CP, a history of selective dorsal rhizotomy, upper or lower extremity surgery in the year prior to testing, botulinum toxin injections in the upper or lower extremities in the 6 months before testing, marked visual or hearing deficits, or currently taking psychotropic medications. Ten age‐matched individuals (mean age: 18.60 years, SD: 3.86 years; 5 females) without epilepsy or other neurologic or orthopedic disabilities served as a control group. All participants had previously been assessed for somatosensory abilities [Wingert et al., 2008] and completed a modified Edinburgh [Raczkowski et al., 1974] handedness inventory to assess proportional hand dominance (a score of 100 indicates complete right handedness; a score of 0 indicates complete left handedness). Participants provided informed consent following guidelines approved by the Human Studies Committee of Washington University.
Table I.
Demographics and task performance accuracy
| Age | Edinburgh | MACS | GMFCS | fMRI Task Performance (mean%) | |||
|---|---|---|---|---|---|---|---|
| Smooth | GD | TSD | |||||
| Diplegia 1 | 12 | 100 | 2 | 1 | 100 | 39.58a | 62.5 |
| Diplegia 2 | 29 | 18 | 2 | 2 | 100 | 97.92 | 93.75 |
| Diplegia 3 | 34 | 23 | 1 | 1 | 100 | 50.00a | 81.25 |
| Diplegia 4 | 18 | 82 | 2 | 2 | 100 | 91.67 | 77.08 |
| Diplegia 5 | 17 | 100 | 2 | 1 | 97.92 | 89.58 | 70.83 |
| Diplegia 6 | 14 | 100 | 1 | 1 | 100 | 97.92 | 89.58 |
| Diplegia 7 | 11 | 100 | 1 | 1 | 93.75 | 89.58 | 77.08 |
| Diplegia 8 | 10 | 5 | 1 | 1 | 100 | 95.83 | 93.75 |
| Diplegia 9 | 24 | 100 | 1 | 2 | 100 | 66.67 | 68.75 |
| Diplegia 10 | 18 | 100 | 2 | 2 | 100 | 64.58 | 66.67 |
| Mean | 18.70 | 72.80 | 99.17 | 86.72 | 78.12 | ||
| SD | 7.99 | 40.28 | 2.01 | 13.45 | 11.29 | ||
| Control 1 | 24 | 95 | — | — | 100 | 100 | 100 |
| Control 2 | 16 | 95 | — | — | 100 | 95.83 | 95.83 |
| Control 3 | 24 | 86 | — | — | 97.92 | 97.92 | 97.92 |
| Control 4 | 23 | 95 | — | — | 100 | 97.92 | 97.92 |
| Control 5 | 17 | 100 | — | — | 100 | 81.25 | 93.75 |
| Control 6 | 18 | 95 | — | — | 89.58 | 93.75 | 95.83 |
| Control 7 | 15 | 90 | — | — | 100 | 100 | 89.58 |
| Control 8 | 13 | 95 | — | — | 100 | 100 | 91.67 |
| Control 9 | 17 | 90 | — | — | 95.83 | 93.75 | 97.92 |
| Control 10 | 19 | 100 | — | — | 100 | 97.92 | 100 |
| Mean | 18.60 | 94.10 | 98.33 | 95.83 | 96.04 | ||
| SD | 3.86 | 4.38 | 3.37 | 5.64 | 3.47 | ||
Data omitted from analyses.
Tactile Stimulation Protocols
Tactile stimulation involved passively translating a 330 cm flexible photopolymer belt across the right index fingertip using a rotating drum device [Burton et al., 2006] Rotation moved the belt surface along the finger pad from proximal to distal, while the finger/hand was passively restrained in a channel that aligned the finger over a task appropriate track on the belt. The belt had three tracks, two of which included 40 mm lengths of raised sections: shapes and horizontal gratings, and one which was continuously smooth. The sequence of embossed patterns on the two tracks with shapes and gratings was random; there were ∼10 cm gaps of smooth surface between the patterns.
The TSD task required participants to judge whether presented shapes matched. Shapes were delivered in sets of two pairs. Shapes within pairs matched (i.e., a shape repeated). The second pair of shapes could be similar (match) or different (no match) from the first pair. Three different embossed 8 mm × 8 mm shapes were used to create the TSD sequences (Fig. 1A: filled circle, three‐sided staple, and a V). All possible combinations of shape sequences were used including both upward and downward oriented staples and V's (e.g., circle vs. circle, circle vs. staple upward, and staple downward vs. V upward, etc.).
Figure 1.
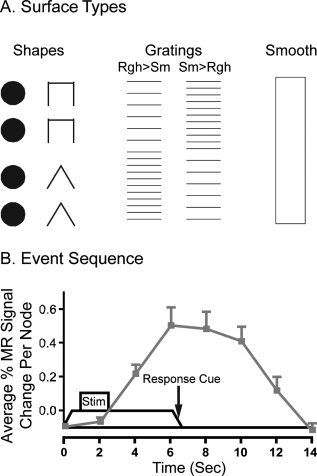
Tactile surface types, event timing, and BOLD response to tactile stimulation. (A) One of three tactile surfaces was rubbed against the right index finger pad per run. Tasks involved detecting matched or unmatched embossed shapes (TSD), determining the direction of roughness change (e.g., rough to smooth: Rgh > Sm) for horizontal gratings (GD), or no discrimination task during stimulation with a smooth surface. Two example tactile strings of each embossed discrimination task are shown. (B) The drum rotated at ∼22 mm/s; the length of each embossed surface was 40 mm. Stimulation with the embossed surface lasted approximately 1.8 s, but the finger was stimulated by the rotating drum for a total duration of 6.6 s per event. A heard tone at 6.4 s (black arrow) cued the motor response, a left 2nd or 3rd finger button push. An example hemodynamic response (gray) is shown overlaid on the task paradigm and timeline.
The grating discrimination (GD) task required participants to judge the direction of roughness change between paired horizontal grating surfaces (Fig. 1A, GD: rough to smooth [Rgh > Sm] or smooth to rough [Sm > Rgh]). Grating strips had constant ridge widths of ∼250 μm and two sections with different groove widths (1,000; 1,800; 2,000; 2,300; 2,500; 2,700; or 2,900 μm). The smoother 1,000 μm standard grating was contrasted with all larger groove widths, which created trials with six groove width differences between 800 and 1,900 μm. The standard surface preceded the wider groove width surfaces on half of the trials; roughness order was reversed for the other trials.
Trials involving the smooth surface required no tactile discrimination. However, drum rotation timing during the smooth task matched that used for the TSD and GD tasks.
A trial involved translating a tactile pattern across the fingerpad at 22 mm/s followed by an overt button‐push response after hearing a tone—Response Cue; rotation paused at the tone. Each trial encompassed a fixed interval of translation that included initial rotary acceleration, constant velocity, deceleration, and a variable interval with no movement with the finger resting on a smooth surface. The duration of the postmovement intervals varied such that timing between trials was jittered between 6 and 11 frames (12.3–22.5 s) and followed a truncated negative exponential distribution. Tactile stimulation from the raised patterns lasted approximately 1.8 s, but the finger was stimulated during drum rotation for 6.6 s per trial (Fig. 1B). A blood oxygenation level‐dependent (BOLD) response was initiated after the tactile stimulation and continued past the termination of drum rotation (Fig. 1B).
Participants responded on every trial with digitally‐recorded left hand button pushes (Lumina, Cedrus Corp., San Pedro, CA) upon hearing the Response Cue tone (Fig. 1B). During the smooth task, participants pushed the button with one finger on all trials within a run (either index or middle finger). The other finger was used for trials in a second smooth task run. Participants were randomly assigned into one of two button‐push paradigms for responses to the TSD and GD tasks. For half of the participants, a button push with the index finger indicated unmatched shapes and, in GD, roughness change from rougher to smoother surfaces; button pushes with the middle finger indicated matching shapes or a roughness change from smoother to rougher. The reverse finger responses applied for the second half of the participants.
Samples of the shapes, gratings, and smooth surfaces were manually presented in five practice trials to familiarize the participants with each task prior to scanning. Participants responded using a mock button‐box and were given immediate feedback. No feedback was provided during imaging.
Each imaging run lasted 193 frames (∼6.6 min), including five frames discarded for magnetization equilibrium, five frames at the beginning and end for baseline measurement, and 24 tactile trials averaging eight frames each. Each task was presented twice and in separate runs (48 events per task). The first and last run involved the smooth task, and runs 2–5 alternately TSD and GD tasks. Participants were instructed about the forthcoming task immediately prior to each run.
All participants wore blindfolds and were instructed to close their eyes during the tasks. All room lights were off during scans. Between scans, however, lights were turned on and participants were instructed to open their eyes.
MRI Acquisition and Reconstruction
Magnetic resonance images were acquired using a Siemens (Erlangen, Germany) 3T Allegra scanner. A vacuum pillow stabilized participants' heads within a standard birdcage headcoil. BOLD contrast (T2*) images [Kwong et al., 1992; Ogawa and Lee, 1990] were acquired with a custom asymmetric spin‐echo, echo‐planar sequence (EPI) (repetition time, TR: 2,048 ms; echo time, TE: 25 ms; flip angle: 90°). Whole brain functional images were acquired in 32 contiguous, interleaved axial 4 mm slices. These were oriented parallel to the bicommissural plane based on registration to an atlas representative target image using a coarse, sagittal magnetization prepared rapid gradient echo (MP‐RAGE) T1‐weighted sequence (TR: 722 ms; TE: 3.93 ms; flip angle: 8°; TI: 380 ms; 2 mm × 2 mm × 2 mm [Mugler and Brookeman, 1990]. In‐plane resolution was 4 mm × 4 mm. Structural data were used for atlas transformation and brain gray/white segmentation in each participant. Detailed structural images were acquired using sagittal, T1‐weighted MP‐RAGE scans (TR: 2,100 ms; TE: 3.93 ms; flip angle: 7°; inversion time [TI]: 1,000 ms; 1 mm × 1 mm × 1.25 mm). Additional axial T2‐weighted (T2W) structural images (TR: 8,430 ms; TE: 98 ms; 1.33 mm × 1.33 mm × 3 mm) were acquired in‐register with the EPI images to facilitate alignment of the EPI images to atlas space [Talairach and Tournoux, 1988].
Prior to statistical analyses, EPI image data were corrected for head motion within and across runs, adjusted for intensity differences due to interleaved slice acquisition, normalized to a global mean signal intensity across EPI runs, compensated for slice‐dependent time shifts using sync interpolation, and aligned to the 711‐2B atlas [Buckner et al., 2004]. The 711‐2B atlas conforms to Talairach and Tournoux atlas space [Talairach and Tournoux, 1988] based on spatial normalization procedures [Lancaster et al., 1995]. Atlas alignment was through 12 parameter affine transforms that linked the first image volume of each EPI run (averaged over all runs after cross‐run realignment) with the MP‐RAGE images [Ojemann et al., 1997] as follows: EPI → T2W → MP‐RAGE → atlas representative target. Atlas transformed images were resampled in atlas space to 2 mm3 isotropic voxels and spatially smoothed (4 mm FWHM).
Statistical Analyses: Volume Based
BOLD responses per participant were analyzed using a general linear model (GLM). The design evaluated each trial of stimulation as a single event with an average duration of eight TR frames per event across the overlap of jittered intervals between trials [Miezin et al., 2000; Ollinger et al., 2001]. The GLM contained regressors for eight time points per event‐type, baseline activity, linear drift, and a high‐pass filter (0.014 Hz). An F‐test per voxel assessed whether the BOLD response variance associated with an event‐type was greater than that due to noise. This test of significance involved no assumptions regarding the hemodynamic response function. The F‐statistics were transformed to equally probable uncorrected z‐scores. In addition, the F‐statistic z‐scores were corrected for multiple comparisons on the basis of Monte Carlo simulations [Forman et al., 1995] at four different thresholds [Burton et al., 2008]. The threshold criteria for corrected z‐scores with P = 0.05 were z = 3.0, 3.5, 4.0, and 4.5 over, respectively, at least 45, 24, 12, and 5 face‐connected voxels. The different thresholds captured higher magnitude/focal and lower magnitude/diffuse response distributions. Activity identified at each of the thresholds was binary coded and logically combined (OR union). In these conjunctions all significant voxels have a value of 1. Thus, the volume‐based analyses in every participant produced two maps for each task from the F‐statistics: unthresholded z‐scores and multiple comparison corrected binary coded maps.
A third volume‐based data value was BOLD response time‐course estimates for each event‐type in each participant. Time‐courses were converted to percent signal change by dividing the difference between baseline and MR signal at each time point (i.e., imaging frame) by baseline signal.
Surface‐based Mapping
Group contrast analyses used volume‐based data that had been individually registered to the cortical surface. Registration of volume‐based data to the cortical surface involved creating participant‐specific cortical surfaces, deforming these individual surfaces to a standard average atlas surface, and registering volume data to the surfaces. Participant‐specific cortical surfaces (fiducial, inflated, flat and spherical) were constructed per hemisphere based on gray/white matter segmentation of the atlas registered 1 mm3 MP‐RAGE along cortical layer four using SureFit software [Van Essen and Dierker, 2007]. Next, the nodes comprising the spherical surface of an individual hemisphere were deformed through spherical alignment of the coordinates for six landmarks (central sulcus, calcarine sulcus, anterior superior temporal gyrus, dorsal and ventral medial wall, and superior circular sulcus within the Sylvian fissure) to the same landmarks in a standard average atlas spherical surface [Van Essen, 2005; Van Essen and Dierker, 2007].
The registration of volume‐based data values proceeded from each participant‐specific fiducial cortical surface to the atlas surface. The three types of volume data (unthresholded z‐scores, binary coded z‐scores, and time‐courses) were first registered to a participant's surfaces by assigning the data value in a voxel to the node(s) enclosed within the coordinates of that voxel. Next, the unique spherical deformation matrix used to map an individual surface to the standard PALS‐B12 atlas was deployed to register the individual surface node values to the PALS‐B12 nodes for each participant. Node values for the uncorrected z‐scores and binary coded‐corrected z‐scores were registered using a nearest‐node‐neighbor algorithm; for response time‐courses, barycentric averaging of node values triangulated around the nearest node was used [Saad et al., 2004].
Regions of interest (ROIs) were created through a multistep procedure involving the intersection of nodes with a specified threshold and nodes associated with previously defined cortical areas [Burton et al., 2008]. Each ROI reflected significant functional activity in any group and task within previously specified cortical subdivisions. The first step in ROI definition involved algebraic summation of individual participant binary coded maps from a task and group to create composite conjunction maps (CMtg). Node values in CMtg maps ranged from 0 to 10, indicating respectively that 0–100% of the participants in a group had multiple‐comparison corrected significant BOLD responses at a given node. Nodes with values ≥6 were coded as 1 and all other nodes as 0. These modified CMtg maps were combined across groups with a logical OR to create an aggregate CM (CMa) with nodes assigned 1 where ≥60% of the participants in either group evoked significant activity in any task [Friston et al., 1999]. An exclusive intersection of CMa and nodes included in previously defined cortical areas specified the ROIs. Previously defined cortical areas deformed to the standard PALS‐B12 atlas included Brodmann areas [Burton et al., 2008; Drury et al., 1999; Van Essen, 2005] and subdivisions of the parietal OP (e.g., OP 1–4) [Burton et al., 2008; Eickhoff et al., 2006a, 2007]. On the basis of prior functional imaging studies, the postcentral gyral BA3 and BA1 ROI were confined to the finger representation [Maldjian et al., 1999]; premotor/precentral gyral BA6 was subdivided into dorsal (PMd) and ventral (PMv) subdivisions at the lateral edge of the superior frontal sulcus [Rizzolatti et al., 2002]; and the IPS cortex (BA7) was subdivided into anterior and posterior regions on the basis of prior functional imaging data [Astafiev et al., 2003; Burton et al., 2008].
Group Analyses: Activity Distribution
The distribution of cortical activity per task for each group was determined by averaging the uncorrected F‐statistic z‐scores per PALS‐B12 node. A t‐test assessed whether average z‐scores significantly differed from a z‐score population mean of zero (i.e., 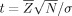 ) [Bosch, 2000]. Bonferroni correction for 69,378 tests (the number of nodes on the PALS‐B12 surface without the medial wall), n – 1 degrees of freedom (i.e., df = 9), and a one‐tailed α = 0.01, required a t‐value of ≥13.45 [Sankoh et al., 1997].
) [Bosch, 2000]. Bonferroni correction for 69,378 tests (the number of nodes on the PALS‐B12 surface without the medial wall), n – 1 degrees of freedom (i.e., df = 9), and a one‐tailed α = 0.01, required a t‐value of ≥13.45 [Sankoh et al., 1997].
A quantitative examination of the spatial distributions of activity utilized the binary coded z‐scores registered onto the CARET PALS‐B12 average fiducial surface [Van Essen, 2005]. For this analysis, a spatial metric was computed by task for each participant that was an area‐proportion index [Burton et al., 2008]. The index was the ratio of two fiducial surface areas. One area reflected the nodes with a value of 1 from the binary coded z‐scores that were located within a specified ROI. The second area was of the total extent of the same ROI. All computed area measures manifested the unique anatomy of a selected ROI in each individual, but in atlas space. A larger index indicated that more of a particular ROI was significantly activated. A random effects ANOVA examined the contribution of task, group, and interactions between these factors on the magnitude of the indices in selected ROI. Post‐hoc t‐tests evaluated the null hypothesis of similar spatial distributions (equal magnitude indices) between groups for selected tasks; significance levels were Bonferroni corrected for multiple comparisons.
Group Analyses: Response Magnitude
The analysis of group differences in response magnitudes per task was based on individual participant response time‐courses registered to nodes within ROI. An average BOLD response time‐course for each participant and selected ROI was computed by averaging the time‐course data registered to all the nodes within the ROI. An average response magnitude per participant and task was computed across the percent MR signal changes that spanned ∼4–8 s (This time interval corresponded to imaging frames 3–5) because this segment of the hemodynamic response most probably reflected the 1.8‐s interval of tactile stimulation from the raised surface patterns (Fig. 1B). Group differences in these averaged responses were assessed by ROI and task using a repeated‐measures, random effects one‐way ANOVA (PROC GLM, Statistical Analysis System version 9.1, SAS Institute, Carey, NC). Between‐group sphericity was assessed with Levene's test. The significance threshold for Levene's test and each region‐wise by task ANOVA of the group main effect was P ≤ 0.001 (adjusted with Bonferroni's correction based on three tests for 16 ROIs).
RESULTS
Age, gender, and handedness between groups did not differ significantly and were therefore not a probable contributor to the group effects (Table I).
Task Performance During Scans
Diplegia and control groups performed similarly on the smooth task (P = 0.99), with mean accuracies of 99.17% (2.01% SD) and 98.33% (3.37% SD), respectively (Table I). On the TSD task, the diplegia group was less accurate than controls (P < 0.01), with performance means of 78.12% (11.29% SD) compared to 96.04% (3.47% SD) in controls. There were no significant performance differences between groups on the gratings task (P = 0.14); mean accuracies were 86.72% (13.45% SD) and 95.83% (5.64% SD) for diplegia and control groups, respectively. Gratings disrimination (GD) data from two participants with diplegia were omitted from analyses of BOLD time‐courses because they performed with accuracy ≤50%.
Group Regional Activation Patterns: t‐statistic of Average z‐Score Surface Maps
All tactile tasks activated similar regions in both groups and there were no uniquely activated regions in the diplegia group.
Parietal Cortex
TSD and GD tasks activated the digit representation of the postcentral gyrus (S1) bilaterally in both groups (Fig. 2). Activity was greatest in BA1 and BA2, and less in BA3. All tasks also activated the posterior parietal OP bilaterally in both groups. However, activation of OP subdivisions varied amongst tasks and between groups. All tasks evoked OP 1 activity bilaterally in both groups. In the control group, OP 3 was extensively activated bilaterally by the TSD and GD tasks, and minimally by the smooth task. In diplegia, OP 3 had no significant activity. OP 4 was activated bilaterally by all tasks in both groups, with the exception of absent ipsilateral OP 4 activity during the smooth task in diplegia.
Figure 2.
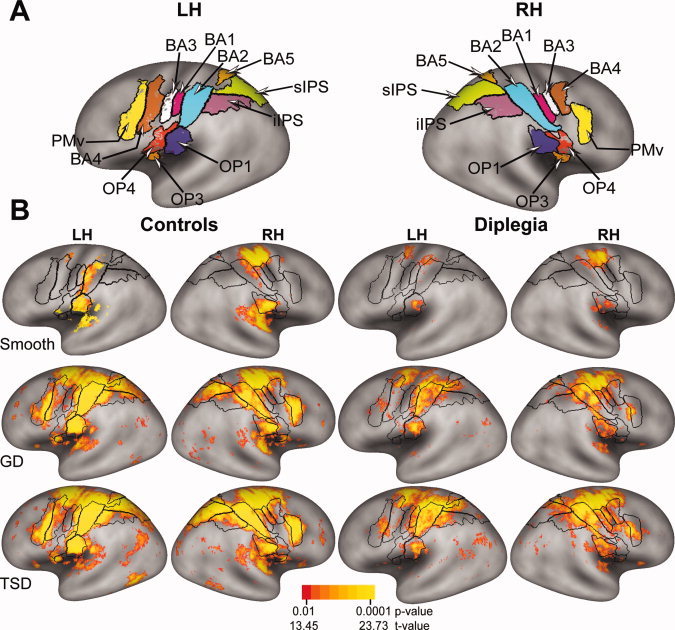
(A) Defined ROI painted onto a standard very inflated average cortical surface from the PALS‐B12 atlas [Van Essen, 2005]. Borders (black lines) of Brodmann areas and cytoarchitectonically defined parietal opercular subregions [Burton et al., 2008; Eickhoff et al., 2006a, b] are shown for left (LH) and right (RH) hemispheres. The painted areas inside the black borders indicate defined ROI that were determined from conjunction analyses, which were based on binary‐coded, multiple‐comparison corrected z‐scores across groups and tasks (see Methods). (B) Group‐level cortical activation maps per task were created using the mean z‐scores from the unthresholded z‐scores registered per node. The images show random effect t‐statistics [Bosch, 2000; Holmes and Friston, 1998] that were multiple‐comparison corrected for the number of cortical surface nodes in the PALS‐B12 atlas. The scale shows the range of associated p‐values. Black borders mark ROI shown in (A).
The anterior aspect of the superior parietal lobule (BA5) was activated bilaterally in both groups during the TSD and GD tasks (Fig. 2). In diplegia, only the inferior‐most aspect of BA5 was activated during these tasks. The smooth task activated ipsilateral BA5 only in the control group. Contralateral BA5 activation was reduced in diplegia compared to controls, but similar between groups ipsilaterally (analysis of time‐course data in Fig. 5).
Figure 5.
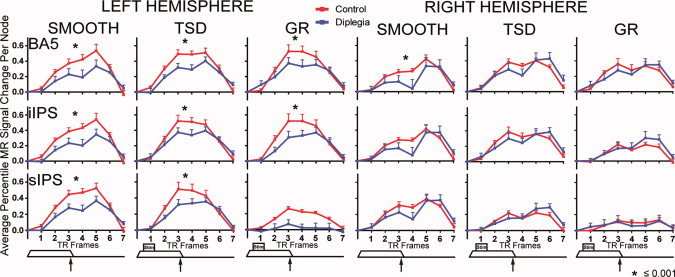
Hemodynamic response time‐courses in posterior parietal cortex ROI (BA5, Brodmann area 5; iIPS, inferior intraparietal sulcus; sIPS, superior intraparietal sulcus) in left and right hemisphere ROI. Control (gray) and diplegia (black) group time‐courses are shown for each task (smooth; TSD, tactile shapes discrimination; GD, gratings discrimination) in average percent signal change per node. Data at each TR frame represent the group mean and SEM. Tactile stimulus paradigm timing (Fig. 1) is shown below each column. Asterisk indicates significant group differences as in Figure 4.
The superior and inferior parts of the intraparietal sulcus (sIPS and iIPS, respectively) were activated bilaterally in both groups by TSD and GD tasks (Fig. 2). The distribution of activated cortex was greater in iIPS and sIPS in controls.
Frontal Cortex
The precentral gyrus, especially the ventral premotor cortex (PMv), and the anterolateral part of primary motor cortex (BA4) were activated bilaterally by the TSD and GD tasks in controls. Activation in these regions was greatly reduced in diplegia (Fig. 2).
Spatial Extent
The diplegia group compared to controls showed attenuated spatial extent of activation during the TSD and GD tasks in many cortical regions. Representative examples were significantly smaller spatial extents for the diplegia group in left BA2 and OP 1 and right BA 2 during the TSD task (Fig. 3).
Figure 3.
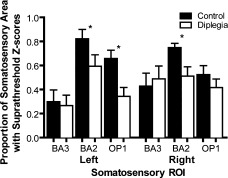
Area proportion indices (API) for activity evoked during the TSD task in selected primary (BA3 and BA2) and secondary (OP1) somatosensory regions. Bars show means and SEM. Asterisk marks ROI with significant group differences in API.
Time‐Course Analyses
BOLD response shape was indicative of three factors within each event: (1) 6.6 s of stimulation from a moving surface, (2) 1.8 s of additional tactile stimulation from an embossed surface, but only during the discrimination tasks, and (3) the motor response cued at 6.4 s (Fig. 1B). Components of the BOLD response in the left hemisphere ROI that extended between TR frames 2–6 presumably reflected tactile stimulation from the moving surface, with an additional contribution from pushing a response key in TR frames 5–6. In the right hemisphere, BOLD response peaks at TRs 5 and 6 probably reflected button pushes. BOLD response components during TR 2–4 most probably indicated tactile stimulation from the embossed surfaces, given the timing of the tactile stimulation and hemodynamic delays [Boynton et al., 1996]. In subsequent analyses, only magnitude data from TRs 2‐4 were considered.
Nearly all ROI showed smaller MR signal magnitudes in diplegia compared to controls. Assessments of between‐group differences were based on the ANOVA and a highly conservative significance threshold (P ≤ 0.001) to correct for multiple comparisons across three tasks and 12 regions (Table II). The ANOVA sphericity assumption (equal variance) was violated in one between‐group comparison, ipsilateral OP 3 on TSD. Therefore, the significance threshold was adjusted to P ≤ 0.0001 to safeguard against potential Type I error rate inflation for this comparison.
Table II.
Region analyses: group comparisons by task
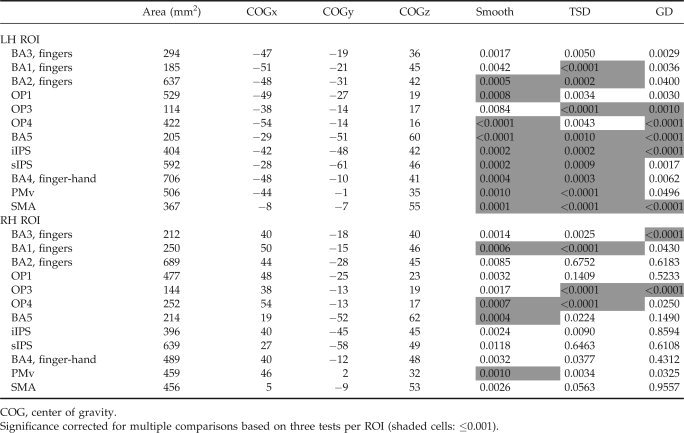
|
Despite a conservative threshold, time‐courses in most contralateral regions were significantly different between‐groups (Figs. 4, 5, 6, Table II). BA3 was the only contralateral region (i.e., left hemisphere) not significantly different between groups for any task (Fig. 4). Group‐wise time‐course comparisons that did not reach significance followed the general trend of lower MR signal magnitudes in diplegia, nearly always approaching P ≤ 0.001. On smooth and TSD tasks, significant differences were observed in S1, OP subdivisions, BA5, sIPS, iIPS, and most frontal cortex ROIs (Figs. 4, 5, 6). Fewer regions were significantly different on GD, a likely consequence of decreased statistical power secondary to unequal samples. However, responses in OP 3 and OP 4, BA5, sIPS, and SMA were significantly diminished in diplegia compared to controls on GD.
Figure 4.
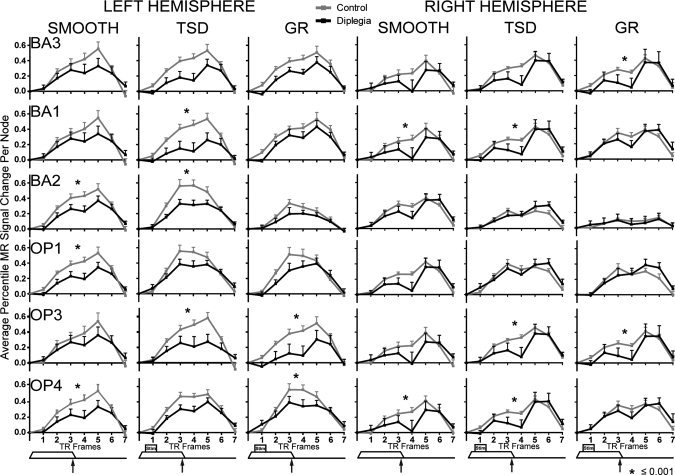
Hemodynamic response time‐courses in parietal cortex somatosensory areas: BA 3, 1, and 2, and parietal opercular (OP) cortex subregions OP 1, 3, and 4 in left and right hemisphere. Control (gray) and diplegia (black) group time‐courses are shown for each task (smooth; TSD, tactile shapes discrimination; GD, gratings discrimination) in average percent signal change per node. Data at each TR frame represent the group mean and SEM. Tactile stimulus paradigm timing (Fig. 1) is shown below each column. Asterisk indicates significant group differences from ANOVA of responses averaged over frames 2–4.
Figure 6.
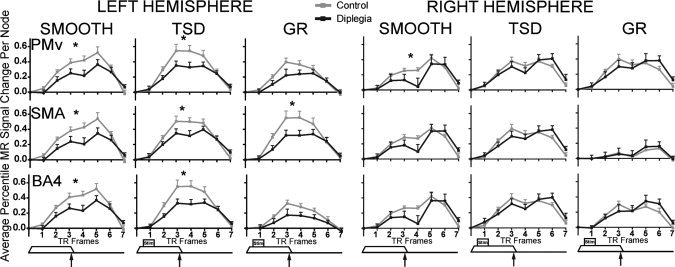
Hemodynamic response time‐courses in frontal cortex ROI (PMv, ventral premotor cortex; SMA, supplementary motor area; BA4, Brodmann area 4) in left and right hemisphere ROI. Control (gray) and diplegia (black) group time‐courses are shown for each task (smooth; TSD, tactile shapes discrimination; GD, gratings discrimination) in average percent signal change per node. Data at each TR frame represent the group mean and SEM. Tactile stimulus paradigm timing (Fig. 1) is shown below each column. Asterisk indicates significant group differences as in Figure 4.
Fewer regions ipsilateral to stimulation had significant group differences in BOLD responses. Exceptions included significant group differences in OP 3 and OP 4, consistent with known bilateral OP function in tactile discrimination (Fig. 4). Other ipsilateral regions with significant group differences included BA1, BA5, and PMv, on the smooth task, and BA1 on TSD (Table II).
DISCUSSION
The group with diplegia showed significantly less activity in most cortical regions known to be responsive to tactile stimulation. Consequently, diminished tactile discrimination abilities in spastic diplegia [Wingert et al., 2008] possibly involve smaller responses within the affected cortical regions. Additionally, several of the studied areas contribute to cognitive processing of somatosensory input. Thus, smaller responses in these regions possibly contribute to or reflect the clinical characteristics of spastic diplegia, as discussed below.
Postcentral gyrus regions are noted for involvement in tactile discrimination of objects [Bodegard et al., 2001, 2006; Reed et al., 2004] and roughness [Carey et al., 2008; Kitada et al., 2005; Zhang et al., 2005]. Lesions of different subparts of the postcentral gyrus impair discrimination of different tactile attributes: texture and shape for damage to BA3, texture for BA1, and shape for BA2 [Randolph and Semmes, 1974]. Therefore, as in the lesion‐behavioral studies in monkeys, inferior discrimination abilities on textures and shapes in diplegia [Wingert et al., 2008] are plausibly related to reduced responses to roughness and shapes especially in contralateral BA1 and BA2.
Another parietal somatosensory region, the parietal OP, has been subdivided into four regions: OP 1–4 [Eickhoff et al., 2006b, 2007). OP 1 is most probably homologous to S2 [Burton et al., 2008; Eickhoff et al., 2006a, 2007]. OP 3 and OP 4, also responsive during tactile tasks, particularly require more cognitive involvement with tactile inputs [Burton et al., 2008]. Lesions in these opercular regions disrupt a range of somatosensory discrimination abilities [Carlson and Burton, 1988; Murray and Mishkin, 1984]. The tactile discrimination tasks in this study required processing and remembering tactile features, which possibly explains widespread activity especially in OP 3 and OP 4 [Burton et al., 2008], but only in controls. Inferior tactile discrimination abilities observed in diplegia, especially for more cognitively demanding embossed surface discrimination tasks [Wingert et al., 2008], might relate to this reduced activation of OP 3 and 4.
Tactile activation in IPS corroborate earlier studies involving iIPS in roughness discriminations [Kitada et al., 2005] and object identifications [Bodegard et al., 2001; Roland et al., 1998], and sIPS in object identifications [Jancke et al., 2001; Van de Winckel et al., 2005]. IPS also generally contributes to goal‐directed, selective search of and attention to stimulus attributes [Fox et al., 2006], whether tasks are purely somatosensory [Burton et al., 2008] or somatomotor [Binkofski et al., 1999a, b; Reed et al., 2005; Roland et al., 1998]. BA5 also has a role in task‐related attention in nonhuman primates [Mountcastle et al., 1975] and humans [Astafiev et al., 2003] and integrates sensory information for motor planning [Andersen et al., 1997; Kalaska and Crammond, 1995]. All current tasks followed tactile stimulation with a goal and motor response. Consequently, reduced activity in BA5 and IPS in diplegia suggests possible trouble attending to tactile stimulation and deficient integration of attended somatosensory inputs for goal‐directed motor outputs.
The present results confirm somatosensory activation of primary motor (M1, BA4), ventral premotor (PMv), and the medial frontal SMA during tactile object [Bodegard et al., 2001; Reed et al., 2004; Stoeckel et al., 2003] and roughness discriminations [Kitada et al., 2005; Zhang et al., 2005]. These frontal cortical regions receive somatosensory information from parietal cortex [Jones and Powell, 1969] and thalamus [Huffman and Krubitzer, 2001; Jones et al., 1979; Rouiller et al., 1999]. They especially contribute to cognitive processing of an associated sensorimotor output [Naito et al., 2000; Rizzolatti et al., 2002] and integration of sensorimotor processing [Binkofski et al., 1999a, b; Rizzolatti et al., 2002] particularly during goal‐directed behavior [Fox et al., 2005]. Reduced activity in these frontal cortex areas in diplegia suggests deficient integration of somatic sensations in guiding motor behavior (i.e., a potential for abnormal motor coordination).
Somatosensory deficits in spastic diplegia probably result from decreased thalamocortical projections due to damaged posterior thalamic radiations in white matter subsequent to PWMI [Nagae et al., 2007]. These lesions affect inputs to somatosensory parietal cortex and thalamic projections to parietal areas like BA 5 and IPS. Another important factor, possibly secondary to diminished input to parietal somatosensory areas, is decreased cognitive processing associated with somatosensory‐linked goal‐directed behavior. These effects possibly were expressed by reduced activity in OP 3, OP 4, BA5, iIPS and sIPS and frontal cortex SMA, PMv, M1. A lesion in any of these areas disrupts tactile discrimination [Binkofski et al., 1998; Caselli1991, 1993; De Renzi et al., 1987; Rapcsak et al., 1987; Reed et al., 1996].
In animal studies, early sensory deprivation caused decreased axonal and dendritic branches between functionally connected neurons [Bryan and Riesen, 1989], decreased discriminative acuity [Murphy and Mitchell, 1991; Tusa et al., 1991], and decreased complex sensory‐processing [Tees and Midgley, 1978; Tees and Symons, 1987]. Thus, sensory inputs typically impact development of cortical function and structure. Individuals with PWMI and damaged thalamocortical projections have reduced somatosensory input to cortical areas, and consequently, reduced input‐driven functional connections with cortical areas involved in complex processing of somatosensory information, whether cognitive or motor. Therefore, diminished connections may be important negative contributors to delayed motor development and decreased activity in individuals with spastic diplegia.
There was no evidence of adaptive plasticity in the diplegia group presenting as activity in unique cortical regions or varied temporal response dynamics. The same regions were activated in both groups. Possibly the surface‐based conjunction map methods employed here overlooked individual variability in activation patterns. However, a volume‐based ANOVA, devoid of any significance thresholding or corrections for multiple comparisons, showed only two areas of activation outside defined ROI. Those two exceptions were in auditory cortex, attributable to hearing the tone, and BA22, which had similar activity between groups on post‐hoc t‐tests.
The finding of no uniquely activated regions on these tactile tasks, is surprising given that cortical reorganization has been shown in other studies involving early life neurological injuries [Fair et al., 2006; Guzzetta et al., 2007; Staudt et al., 2002, 2006; Thickbroom et al., 2001]. These earlier studies investigated individuals with unilateral injuries, and thus involved a presumably less affected or intact hemisphere. Two studies of people with bilateral CP showed some evidence of expansion of cortical representation following intervention, either bilateral somatosensory expansion following selective dorsal rhizotomy [Ojemann et al., 2005] or contralateral sensorimotor and bilateral SMA expansion after 2 weeks of body‐weight supported treadmill training [Phillips et al., 2007].
Clinical Implications
Symptomatic of spastic diplegia is impaired motor coordination of the lower limbs to a greater extent than the upper limbs. Diminished cortical activity in parietal and frontal cortical somatosensory regions suggests that deficient processing and integration of tactile inputs might impact the coordination of motor abilities. Thus, motor planning might be disrupted because the cortical areas crucial to processing, attending and utilizing somatosensory input in parietal cortex send an insufficient amount of information to the motor areas in frontal cortex and thereby contribute to the motor disorders of CP. Questions regarding the importance of intact somatosensory processing are especially timely given DTI data showing that corticospinal pathways are less extensively damaged than somatosensory pathways in diplegia [Hoon et al., 2009; Nagae et al., 2007]. Interestingly, BA5 and inferior and superior IPS activation contralateral to the button push (i.e., ipsilateral to tactile stimulation) was similar between groups, suggesting that both groups comparably attended to spatial localization and motor coordination of the button‐push response. However, this was a simple and limited movement; the impacts of such system‐wide somatosensory deficits on purposeful and more complex movements are unknown, and warrant further study.
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Additional support was provided by the Foundation for Physical Therapy PODS Scholarship. The authors would like to acknowledge Dr. Janice E. Brunstrom‐Hernandez for her numerous contributions to this research.
REFERENCES
- Andersen RA, Snyder LH, Bradley DC, Xing J ( 1997): Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 303–330. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M ( 2003): Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci 23: 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, Jacobsson B, Damiano D ( 2005): Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol 47: 571–576. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ ( 1998): Human anterior intraparietal area subserves prehension: A combined lesion and functional MRI activation study. Neurology 50: 1253–1259. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H ( 1999a): A fronto‐parietal circuit for object manipulation in man: Evidence from an fMRI‐study. Eur J Neurosci 11: 3276–3286. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Stephan KM, Rizzolatti G, Seitz RJ, Freund HJ ( 1999b): A parieto‐premotor network for object manipulation: Evidence from neuroimaging. Exp Brain Res 128: 210–213. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Zilles K, Fink GR ( 2004): Supramodal representation of objects and actions in the human inferior temporal and ventral premotor cortex. Cortex 40: 159–161. [DOI] [PubMed] [Google Scholar]
- Bodegard A, Ledberg A, Geyer S, Naito E, Zilles K, Roland PE ( 2000): Object shape differences reflected by somatosensory cortical activation. J Neurosci 20: RC51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodegard A, Geyer S, Grefkes C, Zilles K, Roland PE ( 2001): Hierarchical processing of tactile shape in the human brain. Neuron 31: 317–328. [DOI] [PubMed] [Google Scholar]
- Bolanos AA, Bleck EE, Firestone P, Young L ( 1989): Comparison of stereognosis and two‐point discrimination testing of the hands of children with cerebral palsy. Dev Med Child Neurol 31: 371–376. [DOI] [PubMed] [Google Scholar]
- Bosch V ( 2000): Statistical analysis of multi‐subject fMRI data: Assessment of focal activations. J Magn Reson Imaging 11: 61–64. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ ( 1996): Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16: 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan GK, Riesen AH ( 1989): Deprived somatosensory‐motor experience in stumptailed monkey neocortex: Dendritic spine density and dendritic branching of layer IIIB pyramidal cells. J Comp Neurol 286: 208–217. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ ( 2004): A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas‐based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage 23: 724–738. [DOI] [PubMed] [Google Scholar]
- Burton H, Abend NS, MacLeod AM, Sinclair RJ, Snyder AZ, Raichle ME ( 1999): Tactile attention tasks enhance activation in somatosensory regions of parietal cortex: A positron emission tomography study. Cereb Cortex 9: 662–674. [DOI] [PubMed] [Google Scholar]
- Burton H, McLaren DG, Sinclair RJ ( 2006): Reading embossed capital letters: An fMRI study in blind and sighted individuals. Hum Brain Mapp 27: 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, McLaren DG ( 2008): Cortical network for vibrotactile attention: A fMRI study. Hum Brain Mapp 29: 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LM, Abbott DF, Egan GF, Donnan GA ( 2008): Reproducible activation in BA2, 1 and 3b associated with texture discrimination in healthy volunteers over time. Neuroimage 39: 40–51. [DOI] [PubMed] [Google Scholar]
- Carlson M, Burton H ( 1988): Recovery of tactile function after damage to primary or secondary somatic sensory cortex in infant Macaca mulatta . J Neurosci 8: 833–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ ( 1991): Rediscovering tactile agnosia. Mayo Clin Proc 66: 129–142. [DOI] [PubMed] [Google Scholar]
- Caselli RJ ( 1993): Ventrolateral and dorsomedial somatosensory association cortex damage produces distinct somesthetic syndromes in humans. Neurology 43: 762–771. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Zambolin A, Crisi G ( 1987): The pattern of neuropsychological impairment associated with left posterior cerebral artery infarcts. Brain 110: 1099–1116. [DOI] [PubMed] [Google Scholar]
- Drury HA, Van Essen DC, Corbetta M, Snyder AZ ( 1999): Surface‐based analyses of the human visual cortex Brain Warping, In: Toga A, editor. New York: Academic Press; pp 337–363. [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K ( 2006a): The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16: 268–279. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR ( 2007): The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex 17: 1800–1811. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K ( 2006b): The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex 16: 254–267. [DOI] [PubMed] [Google Scholar]
- Eliasson AC, Krumlinde‐Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, Rosenbaum P ( 2006): The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol 48: 549–554. [DOI] [PubMed] [Google Scholar]
- Fair DA, Brown TT, Petersen SE, Schlaggar BL ( 2006): fMRI reveals novel functional neuroanatomy in a child with perinatal stroke. Neurology 67: 2246–2249. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME ( 2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103: 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ ( 1999): Multisubject fMRI studies and conjunction analyses. Neuroimage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- Guzzetta A, Bonanni P, Biagi L, Tosetti M, Montanaro D, Guerrini R, Cioni G ( 2007): Reorganisation of the somatosensory system after early brain damage. Clin Neurophysiol 118: 1110–1121. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ ( 1998): Generalisability, random effects and population inference. Neuroimage 7: S754. [Google Scholar]
- Hoon AH Jr, Stashinko E, Nagae LM, Lin D, Keller J, Bastian A, Campbell ML, Levey E, Mori S, Johnston MV ( 2009): Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol 51: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L ( 2001): Thalamo‐cortical connections of areas 3a and M1 in marmoset monkeys. J Comp Neurol 435: 291–310. [DOI] [PubMed] [Google Scholar]
- Jancke L, Kleinschmidt A, Mirzazade S, Shah NJ, Freund HJ ( 2001): The role of the inferior parietal cortex in linking the tactile perception and manual construction of object shapes. Cereb Cortex 11: 114–121. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP ( 1969): Connexions of the somatic sensory cortex of the rhesus monkey. I. Ipsilateral cortical connexions. Brain 92: 477–502. [DOI] [PubMed] [Google Scholar]
- Jones EG, Wise SP, Coulter JD ( 1979): Differential thalamic relationships of sensory‐motor and parietal cortical fields in monkeys. J Comp Neurol 183: 833–881. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ ( 1995): Deciding not to GO: neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cereb Cortex 5: 410–428. [DOI] [PubMed] [Google Scholar]
- Kitada R, Hashimoto T, Kochiyama T, Kito T, Okada T, Matsumura M, Lederman SJ, Sadato N ( 2005): Tactile estimation of the roughness of gratings yields a graded response in the human brain: An fMRI study. Neuroimage 25: 90–100. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng HM, Brady TJ, Rosen BR ( 1992): Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 89: 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT ( 1995): A modality‐independent approach to spatial normalization of tomographic images of the human brain. Hum Brain Mapp 3: 209–223. [Google Scholar]
- Ledberg A, O'Sullivan BT, Kinomura S, Roland PE ( 1995): Somatosensory activations of the parietal operculum of man. A PET study. Eur J Neurosci 7: 1934–1941. [DOI] [PubMed] [Google Scholar]
- Lesny I, Stehlik A, Tomasek J, Tomankova A, Havlicek I ( 1993): Sensory disorders in cerebral palsy: two‐point discrimination. Dev Med Child Neurol 35: 402–405. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Gottschalk A, Patel RS, Detre JA, Alsop DC ( 1999): The sensory somatotopic map of the human hand demonstrated at 4 Tesla. Neuroimage 10: 55–62. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL ( 2000): Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage 11( 6 Pt 1): 735–759. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C ( 1975): Posterior parietal association cortex of the monkey: Command functions for operations within extrapersonal space. J Neurophysiol 38: 871–908. [DOI] [PubMed] [Google Scholar]
- Mugler JP III, Brookeman JR ( 1990): Three‐dimensional magnetization‐prepared rapid gradient‐echo imaging (3D MP RAGE). Magn Reson Med 15: 152–157. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Mitchell DE ( 1991): Vernier acuity of normal and visually deprived cats. Vision Res 31: 253–266. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M ( 1984): Relative contributions of SII and area 5 to tactile discrimination in monkeys. Behav Brain Res 11: 67–83. [DOI] [PubMed] [Google Scholar]
- Nagae LM, Hoon AH Jr, Stashinko E, Lin D, Zhang W, Levey E, Wakana S, Jiang H, Leite CC, Lucato LT, van Zijl PC, Johnston MV, Mori S ( 2007): Diffusion tensor imaging in children with periventricular leukomalacia: Variability of injuries to white matter tracts. Am J Neuroradiol 28: 1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K ( 2000): Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J Neurophysiol 83: 1701–1709. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM ( 1990): Magnetic resonance imaging of blood vessels at high fields: In vivo and in vitro measurements and image simulation. Magn Reson Med 16: 9–18. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE ( 1997): Anatomic localization and quantitative analysis of gradient refocused echo‐planar fMRI susceptibility artifacts. Neuroimage 6: 156–167. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, McKinstry RC, Mukherjee P, Park TS, Burton H ( 2005): Hand somatosensory cortex activity following selective dorsal rhizotomy: Report of three cases with fMRI. Childs Nerv Syst 21: 115–121. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL ( 2001): Separating processes within a trial in event‐related functional MRI. Neuroimage 13: 218–229. [DOI] [PubMed] [Google Scholar]
- Palisano RJ, Hanna SE, Rosenbaum PL, Russell DJ, Walter SD, Wood EP, Raina PS, Galuppi BE ( 2000): Validation of a model of gross motor function for children with cerebral palsy. Phys Ther 80: 974–985. [PubMed] [Google Scholar]
- Peltier S, Stilla R, Mariola E, LaConte S, Hu X, Sathian K ( 2007): Activity and effective connectivity of parietal and occipital cortical regions during haptic shape perception. Neuropsychologia 45: 476–483. [DOI] [PubMed] [Google Scholar]
- Phillips JP, Sullivan KJ, Burtner PA, Caprihan A, Provost B, Bernitsky‐Beddingfield A ( 2007): Ankle dorsiflexion fMRI in children with cerebral palsy undergoing intensive body‐weight‐supported treadmill training: A pilot study. Dev Med Child Neurol 49: 39–44. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat JW, Nebes R ( 1974): Reliability and validity of some handedness questionnaire items. Neuropsychologia 12: 43–47. [DOI] [PubMed] [Google Scholar]
- Randolph M, Semmes J ( 1974): Behavioral consequences of selective subtotal ablations in the postcentral gyrus of Macaca mulatta . Brain Res 70: 55–70. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Gonzalez Rothi LJ, Heilman KM ( 1987): Phonological alexia with optic and tactile anomia: A neuropsychological and anatomical study. Brain Lang 31: 109–121. [DOI] [PubMed] [Google Scholar]
- Reed CL, Caselli RJ, Farah MJ ( 1996): Tactile agnosia. Underlying impairment and implications for normal tactile object recognition. Brain 119 ( Part 3): 875–888. [DOI] [PubMed] [Google Scholar]
- Reed CL, Shoham S, Halgren E ( 2004): Neural substrates of tactile object recognition: An fMRI study. Hum Brain Mapp 21: 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CL, Klatzky RL, Halgren E ( 2005): What vs. where in touch: An fMRI study. Neuroimage 25: 718–726. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V ( 2002): Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol 12: 149–154. [DOI] [PubMed] [Google Scholar]
- Roland PE, O'Sullivan B, Kawashima R ( 1998): Shape and roughness activate different somatosensory areas in the human brain. Proc Natl Acad Sci USA 95: 3295–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM, Tanne J, Moret V, Boussaoud D ( 1999): Origin of thalamic inputs to the primary, premotor, and supplementary motor cortical areas and to area 46 in macaque monkeys: A multiple retrograde tracing study. J Comp Neurol 409: 131–152. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, Argall B, Japee S, Cox RW ( 2004): SUMA: An interface for surface‐based intra‐ and inter‐subject analysis with AFNI. 1510–1513.
- Sanger TD, Kukke SN ( 2007): Abnormalities of tactile sensory function in children with dystonic and diplegic cerebral palsy. J Child Neurol 22: 289–293. [DOI] [PubMed] [Google Scholar]
- Servos P, Lederman S, Wilson D, Gati J ( 2001): fMRI‐derived cortical maps for haptic shape, texture, and hardness. Brain Res Cogn Brain Res 12: 307–313. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh‐Mann I ( 2002): Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain 125( Part 10): 2222–2237. [DOI] [PubMed] [Google Scholar]
- Staudt M, Braun C, Gerloff C, Erb M, Grodd W, Krageloh‐Mann I ( 2006): Developing somatosensory projections bypass periventricular brain lesions. Neurology 67: 522–525. [DOI] [PubMed] [Google Scholar]
- Stoeckel MC, Weder B, Binkofski F, Buccino G, Shah NJ, Seitz RJ ( 2003): A fronto‐parietal circuit for tactile object discrimination: An event‐related fMRI study. Neuroimage 19: 1103–1114. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Coplanar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical. [Google Scholar]
- Tees RC, Midgley G ( 1978): Extent of recovery of function after early sensory deprivation in the rat. J Comp Physiol Psychol 92: 768–777. [DOI] [PubMed] [Google Scholar]
- Tees RC, Symons LA ( 1987): Intersensory coordination and the effects of early sensory deprivation. Dev Psychobiol 20: 497–507. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Archer SA, Nagarajan L, Mastaglia FL ( 2001): Differences in sensory and motor cortical organization following brain injury early in life. Ann Neurol 49: 320–327. [PubMed] [Google Scholar]
- Thomas B, Eyssen M, Peeters R, Molenaers G, Van Hecke P, De Cock P, Sunaert S ( 2005): Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain 128( Part 11): 2562–2577. [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Repka MX, Smith CB, Herdman SJ ( 1991): Early visual deprivation results in persistent strabismus and nystagmus in monkeys. Invest Ophthalmol Vis Sci 32: 134–141. [PubMed] [Google Scholar]
- Van de Winckel A, Sunaert S, Wenderoth N, Peeters R, Van Hecke P, Feys H, Horemans E, Marchal G, Swinnen SP, Perfetti C, De Weerdt W ( 2005): Passive somatosensory discrimination tasks in healthy volunteers: differential networks involved in familiar versus unfamiliar shape and length discrimination. Neuroimage 26: 441–453. [DOI] [PubMed] [Google Scholar]
- Van Essen DC ( 2005): A Population‐Average, Landmark‐ and Surface‐based (PALS) atlas of human cerebral cortex. Neuroimage 28: 635–662. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker DL ( 2007): Surface‐based and probabilistic atlases of primate cerebral cortex. Neuron 56: 209–225. [DOI] [PubMed] [Google Scholar]
- Volpe JJ ( 2009): Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8: 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert JR, Burton H, Sinclair RJ, Brunstrom JE, Damiano DL ( 2008): Tactile sensory abilities in cerebral palsy: deficits in roughness and object discrimination. Dev Med Child Neurol 50: 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekutiel M, Jariwala M, Stretch P ( 1994): Sensory deficit in the hands of children with cerebral palsy: A new look at assessment and prevalence. Dev Med Child Neurol 36: 619–624. [DOI] [PubMed] [Google Scholar]
- Zhang M, Weisser VD, Stilla R, Prather SC, Sathian K ( 2004): Multisensory cortical processing of object shape and its relation to mental imagery. Cogn Affect Behav Neurosci 4: 251–259. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mariola E, Stilla R, Stoesz M, Mao H, Hu X, Sathian K ( 2005): Tactile discrimination of grating orientation: fMRI activation patterns. Hum Brain Mapp 25: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]


