Abstract
The relation of aerobic fitness to task preparation was examined in a sample of young adults separated into higher- and lower-fit groups according to their maximal oxygen consumption. Participants performed a modified Sternberg working memory task under speed and accuracy instructions, while measures of task performance and contingent negative variation (CNV) were collected. Analyses revealed no significant fitness differences between groups on task performance measures. However, frontal CNV amplitude was significantly larger for lower-fit participants compared to higher-fit participants during the speed instructions; an effect not found for the accuracy instructions. These results suggest that lower-fit individuals may rely to a greater extent on cognitive control processes to respond under speeded conditions, whereas higher-fit individuals may maintain a more constant level of control irrespective of the task instructions.
Keywords: Aerobic fitness, Cognitive control, Task preparation, Contingent negative variation
The relation of aerobic fitness to neuroelectric indices of cognitive and motor task preparation
Evidence for a positive relationship between aerobic fitness and cognitive function has been extensively provided over the last several decades (see Hillman, Erikson, & Kramer, 2008 for review). The beneficial effects of aerobic fitness have been found using a wide variety of tasks that tap a number of psychological processes from simple reaction time (RT; Dustman et al., 1984; Spirduso & Clifford, 1978) to more effortful cognitive control (Hillman, Kramer, Belopolsky, & Smith, 2006; Kramer et al., 1999; but see Colcombe & Kramer, 2003 for review). Although the underlying mechanisms for the beneficial effects of aerobic fitness are not well understood, non-human animal models have suggested that aerobic exercise increases nerve growth factors such as brain-derived neurotrophin factor (Neeper, Gomez-Pinilla, Choi, & Cotman, 1995) and insulin-like growth factor I (Carro, Trejo, Busiguina, & Torres-Aleman, 2001), which may result in an increase in the number of synaptic connections and the development of new neurons that support learning and memory (Lu & Chow, 1999; van Praag, Christie, Sejnowski, & Gage, 1999). Further, recent functional magnetic resonance imaging research has demonstrated that aerobic fitness training increases brain volume including both gray and white matter (Colcombe et al., 2006), and improves functional connectivity between brain regions during a task requiring variable amounts of cognitive control (Colcombe et al., 2004). Thus, of particular interest are recent studies that have indicated a disproportionally larger beneficial relation for tasks or task components requiring extensive cognitive control (Colcombe & Kramer, 2003).
Cognitive control is used to describe the ability to filter and suppress irrelevant information, thoughts, and actions in favor of relevant ones (Casey, Galvan, & Hare, 2005), with working memory, inhibition, and mental flexibility thought to comprise core processes underlying such abilities (Diamond, 2006). Most studies investigating the relation of aerobic fitness to adult cognition have focused on inhibition with considerably fewer studies examining other aspects of cognitive control (i.e., working memory). In addition, a relatively small literature has employed event-related brain potentials (ERPs) to understand this relationship (Hillman et al, 2006; Kamijo & Takeda, 2009; Pontifex, Hillman, & Polich, 2009; Themanson & Hillman, 2006; Themanson, Hillman, & Curtin, 2006; Themanson, Pontifex, & Hillman, 2008). These ERP studies have indicated benefits of aerobic fitness or physical activity on cognitive control in not only older adults, but also in younger adults.
The advantage of the ERP approach is that it provides information regarding discrete cognitive processes that occur between stimulus evaluation and response execution. Two ERP components of interest relative to response processes are the lateralized readiness potential (LRP) and the contingent negative variation (CNV). A previous investigation assessing the relationship between physical activity and the LRP, which reflects preparation for a motor response (Coles, 1989; Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988), has indicated shorter LRP latency (closer to response onset) for active individuals compared to sedentary individuals, suggesting that active individuals prepare their response more rapidly compared to sedentary individuals (Kamijo & Takeda, 2009). Further, Hillman, Weiss, Hagberg, & Hatfield (2002) investigated aerobic fitness effects on response preparation processes using CNV; a negative slow-wave cortical potential of an ERP elicited during the interval between warning (S1) and imperative (S2) stimuli. It is believed that terminal CNV reflects preparatory motor activity when a motor response is required to the S2 (Brunia & van Boxtel, 2001; van Boxtel & Brunia, 1994), which is thought to be superimposed on the readiness potential (Brunia, 1988). Hillman et al. (2002) observed increased CNV amplitude for lower-fit individuals relative to higher-fit individuals, suggesting that lower-fit individuals may allocate more neural resources for response preparation. In other words, response preparation processes of higher-fit individuals may be more efficient than lower-fit individuals. These previous ERP studies (Hillman et al., 2002; Kamijo & Takeda, 2009) imply that aerobic fitness influences not only cognitive processes but also response preparation processes. If this assumption is correct, it stands to reason that aerobic fitness may improve basic motor processes such as simple RT regardless of the cognitive requirements, the latter of which have been extensively studied. Hence, understanding possible differences in fitness effects on cognitive and motor processes may lead to a better understanding of the potential benefits of fitness on brain and cognition.
The terminal CNV reflecting response preparation (Brunia & van Boxtel, 2001; van Boxtel & Brunia, 1994) has typically demonstrated a central topographic maximum (van Boxtel & Brunia, 1994). However, several studies have suggested that frontal CNV is associated with cognitive preparation rather than response preparation processes. That is, whereas predominately motoric tasks present with a central CNV, the frontal CNV is evidenced during the encoding of words (Leynes, Allen, & Marsh, 1998) as well as when tasks are more cognitively demanding (Wild-Wall, Hohnsbein, & Falkenstein, 2007) or require greater amounts of cognitive control (Lorist et al., 2000). Thus, it is plausible that central CNV is associated with response preparation processes and frontal CNV is associated with cognitive preparation processes, which under certain task constraints may reflect cognitive control requirements.
In the present study, we investigated whether aerobic fitness influenced cognitive processes and/or response processes as reflected by CNV amplitude and topography. A modified Sternberg task (Sternberg, 1966) required participants to encode a memory set (S1) containing an array of 3, 5, or 7 letters and decide whether a single probe (S2), presented at a variable delay, appeared in the encoded array. In this task, increased working memory capacity (i.e., necessitating greater amounts of cognitive control) was required to encode and maintain relevant information for larger set sizes, resulting in decreased response speed (Sternberg, 1966) and accuracy (Marshall, Molle, Siebner, & Born, 2005). To our knowledge, previous studies investigating the relation of aerobic fitness or physical activity on cognitive control have only focused on stimulus evaluation (Hillman et al., 2006; Kamijo & Takeda, 2009) and action monitoring (Themanson & Hillman, 2006; Themanson et al., 2006, 2008) processes. Thus, the goal of this study was to better understand the contribution of aerobic fitness to individual aspects (i.e., cognitive, motor) of the stimulus-response relationship. Accordingly, CNV amplitude and topographic distribution were examined to determine whether aerobic fitness differentially influences cognitive and motor processes using the same paradigm.
Lastly, task instructions were varied to emphasize either speed (Speed instructions) or accuracy (Accuracy instructions) to better examine the fitness-cognition relationship. Falkenstein, Hoormann, Hohnsbein, and Kleinsorge (2003) manipulated task instructions and indicated that fronto-central CNV was larger for the speed relative to the accuracy condition, suggesting that the increase in CNV amplitude may reflect increased effort allocation during speeded tasks. In addition, Wild-Wall et al. (2007) showed that increased frontal CNV amplitude was observed for middle-aged individuals compared to young individuals only during a more effortful task condition (i.e., search task vs. non-search task), indicating that middle-aged individuals compensate for age-related decline in cognitive function through the use of a more effortful task preparation strategy. Taken together, more effortful task conditions (e.g., speed instructions) may be more sensitive for detecting subtle changes in cognitive function. Based on previous research, it was hypothesized that smaller CNV amplitude, reflecting increased efficiency, would be observed for the higher-fit group at both frontal and central regions compared to the lower-fit group indicating more efficient cognitive and motor preparation. Further, for the frontal CNV, it was expected that aerobic fitness effects would be selectively greater during more cognitively demanding tasks (i.e., larger encoding requirements), and during task conditions requiring more effortful response preparation (i.e., speed instructions).
Methods
Participants
Seventy-two undergraduate students were recruited from undergraduate kinesiology courses at the University of Illinois at Urbana-Champaign. All participants reported being free of neurological disorders, cardiovascular disease, any medications that influenced central nervous system function, and had (corrected-to-) normal vision. Data from six participants were discarded due to excessive noise in the electroencephalogram (EEG) signal. Further because body mass index (BMI) has been associated with cognitive function (Cournot et al., 2006), two participants with a BMI greater than 3 SD above the mean (M = 23.1 kg/m2, SD = 3.9) were excluded from analyses. Thus, analyses were conducted on 64 participants (39 females, 25 males), and a median split was performed on maximal oxygen consumption (VO2max) values within each sex to divide the participants into higher- and lower-fit groups. Two male participants who exhibited the same median value were classified into the same group (i.e., higher-fit group, VO2max ≥ 50 ml/kg/min), and a female participant who exhibited the median value was classified into the lower-fit group. The demographic and fitness information for both groups are summarized in Table 1. All participants provided informed consent to participate in the experiment, which was approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign.
Table 1.
Group means (± SD) for demographic information categorized by sex.
| Higher-Fit |
Lower-Fit |
|||
|---|---|---|---|---|
| Female |
Male |
Female |
Male |
|
| Sample Size (n) | 19 | 13 | 20 | 12 |
| Age (years) | 19.7 ± 1.5 | 20.2 ± 2.3 | 19.5 ± 1.1 | 21.3 ± 3.4 |
| BMI (kg/m2) | 21.8 ± 2.2 | 22.3 ± 1.6 | 23.0 ± 3.2 | 23.9 ± 3.0 |
| VO2max (ml/kg/min)* | 41.6 ± 2.3 | 56.5 ± 5.3 | 35.0 ± 2.5 | 46.1 ± 2.7 |
| HRmax (bpm) | 190.5 ± 5.7 | 190.8 ± 8.8 | 192.1 ± 7.6 | 193.8 ± 7.6 |
Significant difference, unpaired Student's t-test between groups, p < .05. VO2max norms are available in the American Colledge of Sports Medicine Guidelines for Exercise Testing and Prescription (7th Edition). New York: Lippincott Williams & Wilkins.
Sternberg Working Memory Task
A modified Sternberg task (Sternberg, 1966) asked participants to encode a memory set containing an array of 3, 5, or 7 letters (S1) and decide whether a single probe letter was present in the encoded array (S2). The memory sets were comprised of all capitalized consonants (e.g., RKBXL) and contained no alphabetical consonant strings, whereas the probe letters were lowercase consonants, bilaterally flanked by one, two, or three “?” to match the memory set in physical size and visual content (e.g., ??k??). The participants were instructed to press one of two buttons with their thumbs corresponding to whether the probe was present (right) or absent (left) from the encoded letter array. Probe presence/absence and the three set sizes appeared with equal probability in a random order. The task was performed under the instruction to respond as quickly as possible (Speed instructions) and under the instruction to respond as accurately as possible (Accuracy instructions). After the 20 practice trials, participants completed 208 trials (52 trials × 4 blocks) in the each instruction condition (i.e., speed, accuracy). The order of instructions was counter-balanced among the participants to minimize potential practice effects. The viewing distance was approximately 1 m. All stimuli were 7 cm tall white letters presented on a black background for 2000 ms (encoded array: S1) and 200 ms (probe letter: S2), respectively, with a 1500 ms response window (from S2 offset to S1 onset). An equiprobable, but randomized inter-stimulus interval (ISI; from S1 onset to S2 onset) of 4000, 4500, or 5000 ms was used throughout the task block.
Cardiorespiratory Fitness Assessment
VO2max was measured using a motor-driven treadmill and a modified Balke protocol (American College of Sports Medicine, 2006), which involved walking/running on a treadmill at a constant speed with increasing grade increments of 2% every 2 min until volitional exhaustion occurred. Oxygen consumption was measured using a computerized indirect calorimetry system (ParvoMedics True Max 2400) with averages for VO2 and respiratory exchange ratio (RER) assessed every 30 s. A polar heart rate (HR) monitor (Model A1, Polar Electro, Finland) was used to measure HR throughout the test. VO2max is determined by the highest oxygen consumption that corresponds to at least two of the following criteria: (1) a plateau in VO2 values despite an increase in exercise workload; (2) maximal heart rate (HR) within 10 beats per minute (bpm) of the age-predicted maximum (220 bpm minus age in years); and/or (3) a RER greater than 1.10.
ERP Recording
Electroencephalographic (EEG) activity was measured from 64 electrode sites according to the International 10-10 system (Chatrian, Lettich, & Nelson, 1985), referenced to averaged mastoids, with AFz serving as the ground electrode, and impedances kept below 10 kΩ. To monitor possible artifacts due to eye movements, vertical electrooculogram (EOG) was recorded using electrodes placed above and below the left orbit, and a horizontal EOG was recorded from the outer left and right canthi. Continuous data were digitized at a sampling rate of 500 Hz, amplified 500 times with a DC to 70 Hz filter, and a 60 Hz notch filter using a Neuroscan Synamps amplifier (Neuro, Inc., Charlotte, NC, USA).
Offline EOG correction was applied to the individual trials prior to averaging using a spatial filter (Compumedics Neuroscan, 2003). Data were filtered using a zero phase shift 10 Hz (24 dB/octave) low pass filter. Trials with a response error or artifact exceeding ± 75 μV were rejected. Across groups, a mean of 19 trials (SE = 0.5) were averaged in each task condition. S1-locked epochs were created from −100 to 4000, 4500, or 5000 ms around the stimuli based on ISI duration. CNV amplitudes were measured relative to a 100 ms pre-S1 baseline and the mean amplitude was calculated from the 200 ms period prior to S2 onset.
Procedure
For each participant, testing occurred on two separate days. On the first visit to the laboratory, participants completed an informed consent, health history and demographics questionnaire, a handedness inventory (Chapman and Chapman, 1987), and had their height and weight measured to calculate their BMI. After completing all questionnaires, a cardiorespiratory fitness test was then conducted. On the second visit, the Sternberg task was conducted. Individual participants were prepared for neuroelectric measurement in accordance with the Society for Psychophysiological Research guidelines (Picton et al., 2000). The participant was given the task instructions and allowed practice trials before each experimental task. Upon completion of the last condition, all electrodes were removed and participants were briefed on the purpose of the experiment.
Statistical Analysis
Behavioral data (i.e., RT, response accuracy) were submitted to a 2 (Fitness: higher-fit, lower-fit) × 2 (Instruction: Speed, Accuracy) × 3 (Set Size: 3 letters, 5 letters, 7 letters) × 3 (ISI: short, medium, long) mixed model ANOVA with repeated measures. CNV amplitude was analyzed using a 2 (Fitness) × 2 (Instruction) × 3 (Set Size) × 3 (ISI) × 5 (Region: Fz, FCz, Cz, CPz, Pz) mixed-model ANOVA with repeated measures. Analyses with three or more within-subject levels employed the Greenhouse-Geisser statistic, if the assumption of sphericity was violated. Partial eta squared (η2p) values are reported to demonstrate the effect sizes, with .01-.059 representing a small effect, .06-.139 a medium effect, and > .14 a large effect (Cohen, 1973). Post hoc comparisons were conducted using univariate ANOVAs and Bonferroni corrected t-tests. The significance level was set at .05.
Results
Given the number of variables included in the study design, not all findings are reported in the Results section. With regard to the purpose of the study, only those findings that involve Fitness, Instruction, and Set Size factors are presented. Briefly though, the scalp topography of the CNV component indicated continuity of amplitude from frontal to parietal midline sites with the largest amplitude noted at the FCz site (Fz: M = −2.4 μV, SE = 0.6; FCz: M = −3.0 μV, SE = 0.5; Cz: M = −2.6 μV, SE = 0.5; CPz: M = −2.7 μV, SE = 0.5; Pz: M = −1.0 μV, SE = 0.6). Further, CNV amplitude differed as a function of ISI duration with larger amplitude for longer ISIs (short: M = −1.2 μV, SE = 0.4; medium: M = −2.4 μV, SE = 0.4; Cz: M = −3.4 μV, SE = 0.5), t(1, 63) ≥ 4.2, p < .001. Finally, a Task Order × Instruction interaction, F(1, 59) = 6.5, p = .014, η2p = .09, indicated larger CNV amplitude for the speed instruction when that condition preceded accuracy (M = −4.5 μV, SE = 0.7) compared to when accuracy preceded speed (M = −1.5 μV, SE = 0.6).
Task Performance
RT analysis revealed main effects for Instruction, F(1, 62) = 204.1, p < .001, η2p = .77, with shorter RT latency during the Speed compared to the Accuracy instruction, and Set Size, F(1.5, 92.3) = 217.7, p < .001, η2p = .78, with follow up Bonferroni-corrected t-tests indicating significantly longer RTs from 3 letters to 7 letters, t(1, 63) ≥ 6.6, p < .001. These main effects were superseded by a Instruction × Set Size interaction, F(2, 124) = 16.4, p < .001, η2p = .21. However, post hoc analyses revealed Instruction differences for each Set Size, t(1, 63) ≥ 13.0, p < .001. This interaction indicated that the Instruction differences were larger for larger set sizes (3 letters: M = 169.8 ms, SE = 11.5; 5 letters: M = 197.1 ms, SE = 15.2; 7 letters: M = 220.0 ms, SE = 16.4). No significant Fitness effect was observed on RT.
Response accuracy analysis revealed main effects for Instruction, F(1, 62) = 28.2, p < .001, η2p = .31, with higher response accuracy during the Accuracy compared to the Speed instruction, and Set Size, F(1.5, 96.1) = 200.7, p < .001, η2p = .76, with follow up Bonferroni-corrected t-tests indicating significant decreases in response accuracy from 3 letters to 7 letters (3 letters: M = 89.2%, SE = 0.8; 5 letters: M = 85.3%, SE = 1.0; 7 letters: M = 74.3%, SE = 1.3), t(1, 63) ≥ 6.9, p < .001. No significant Fitness effect was observed on response accuracy.
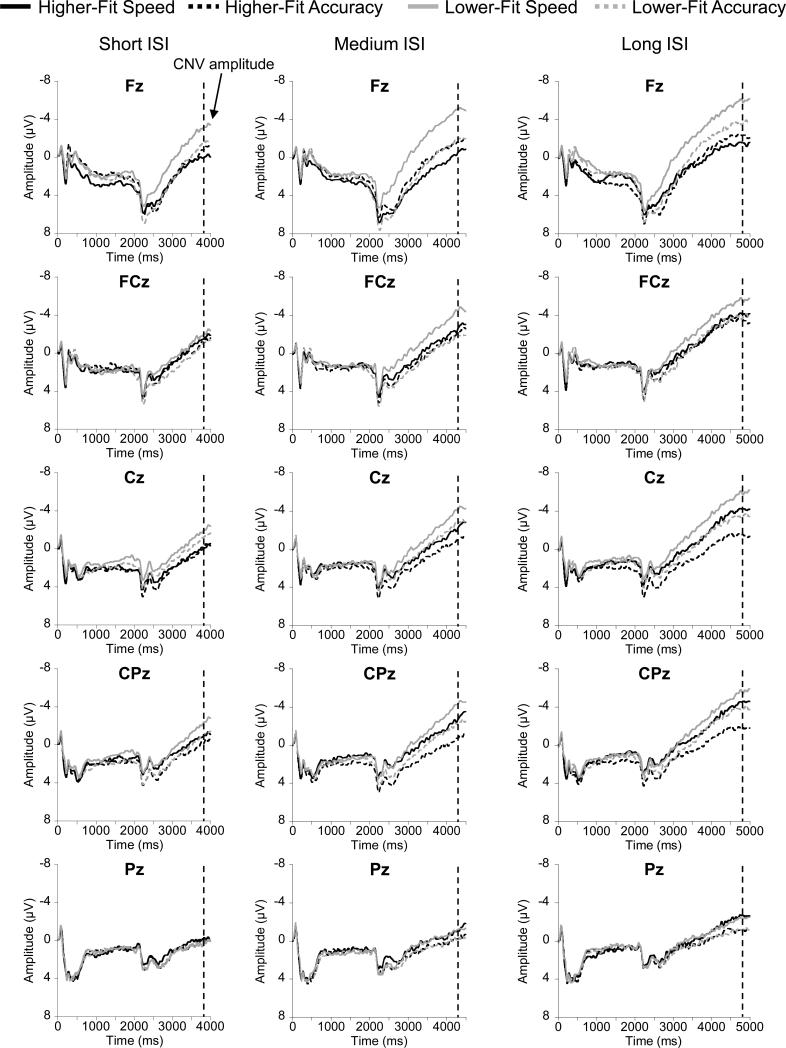
CNV
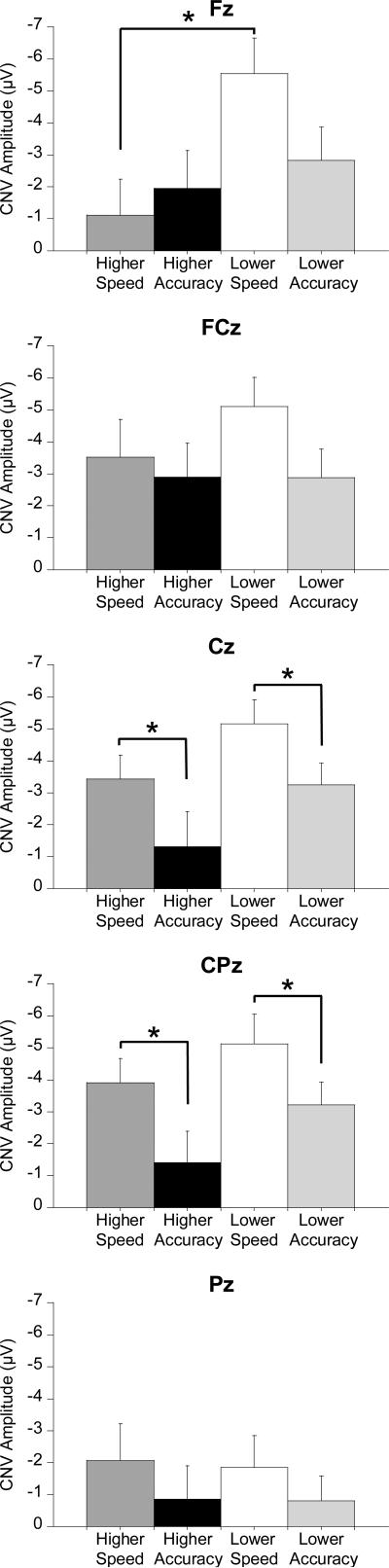
Figure 1 shows the grand averaged ERP waveforms for each fitness group, instruction as a function of ISI duration and electrode site. CNV analyses revealed main effects for Instruction, F(1, 62) = 5.0, p = .03, η2p = .08, with larger CNV amplitude during the Speed compared to the Accuracy instruction, and Set Size, F(2, 124) = 9.6, p < .001, η2p = .13, with follow up Bonferroni-corrected t-tests indicating CNV amplitude for the 7 letters set size were larger than the other set sizes, t(1, 63) ≥ 2.7, p ≤ .01. A Fitness × Instruction × Region interaction was also observed, F(3.2, 200.6) = 2.6, p = .046, η2p = .04. Breaking down the 3-way interaction by examining Fitness × Instruction at each Region revealed a significant interaction only at Fz, F(1, 62) = 5.4, p = .02, η2p = .08. Post hoc analyses indicated that CNV amplitude was significantly larger for lower-fit participants compared to higher-fit participants during the Speed instructions, t(1, 62) = 2.8, p = .007, with no such effect observed for the Accuracy instructions, t(1, 62) = 0.6, p = .58, (Figure 2). No interaction was observed at other regions, F(1, 62) ≤ 0.8, p ≥ .37, η2p ≤ .01. Further, the two-way ANOVAs (Fitness × Instruction at each Region) revealed a main effect for Instruction at the Cz and CPz sites, F(1, 62) ≥ 8.9, p ≤ .004, η2p ≥ .13, with larger CNV amplitude during the Speed compared to the Accuracy instruction (Figure 2). Lastly, the two-way ANOVA also indicated a marginal Fitness effect at Cz, F(1, 62) = 3.5, p = .066, η2p =.05, with larger CNV amplitude for the lower-fit compared to the higher-fit group.
Figure 1.
Grand averaged ERP waveforms for each fitness group, instruction as a function of ISI duration and electrode site.
Figure 2.
Mean (± SE) CNV amplitude (μV) for each region across fitness groups and instructions. The Set size and ISI factors were collapsed in this figure.
Discussion
In this study, the relation of aerobic fitness to task preparation processes was investigated to understand whether a specific relation of fitness to either cognitive or motor processes existed, or whether the relationship was generalized across both components of preparatory response processing. We used two task instructions that emphasized either speed or accuracy and manipulated encoding requirements (3, 5, 7 letters) to vary cognitive control demands. Analyses revealed that participants responded more slowly and accurately during the accuracy instructions relative to the speed instructions and more slowly and less accurately from 3 letters to 7 letters. Although group difference in behavioral measures were not observed, selective effects of aerobic fitness based on task instructions were found for frontal CNV. Alternatively, encoding set size was not associated with aerobic fitness for either frontal or central CNV amplitude, suggesting that the processes reflected by the CNV are not sensitive to this cognitive manipulation.
Frontal CNV
The current findings indicated that frontal CNV amplitude was larger for the lower-fit relative to the higher-fit participants during the speed instruction condition. Recent studies have suggested that frontal CNV increases when greater amounts of cognitive control are required to act correctly within the stimulus environment (Lorist et al. 2000; Wild-Wall et al., 2007) or when an increase in resource allocation is required by effortful demands associated with task instruction (i.e., speed instructions; Falkenstein et al., 2003). Based on these findings, and the known beneficial relation of fitness to other cognitive processes (Hillman et al, 2006; Kamijo & Takeda, 2009; Pontifex et al., 2009; Themanson & Hillman, 2006; Themanson et al., 2006, 2008), it is plausible that the increased frontal CNV amplitude evidenced by the lower-fit participants was associated with the necessity to allocate greater amounts of cognitive control to respond within the more demanding task environment when instructions required speeded responding. Such a relationship is supported by the literature indicating that CNV amplitude is augmented under speeded task instruction resulting from increased task demands (Falkenstein et al., 2003). The current results suggest that such a task environment places disproportionately larger demands upon lower-fit individuals, necessitating larger CNV amplitude, which likely reflects greater preparation for action. Alternatively, higher-fit individuals appear to maintain constant control over the relevant cognitive processes irrespective of the task instructions, resulting in more efficient cognitive preparation during the speed condition. This efficiency is especially apparent given the lack of fitness-related differences in task performance.
The current findings also support previous studies examining fitness effects on the error-related negativity potential (ERN; Themanson & Hillman, 2006; Themanson et al., 2006). The ERN is maximal over fronto-central electrode sites, and is considered a neuroelectric correlate of a subset of cognitive control processes involved in action monitoring (Holroyd & Coles, 2002). Previous ERN studies have indicated smaller amplitude for higher-fit relative to lower-fit individuals during task with speeded response task instructions, suggesting that increased top-down control among higher-fit individuals decreased activation of action monitoring processes (Themanson & Hillman, 2006; Themanson et al., 2006); or in other words, the cognitive control of action. In a similar manner, the present findings provide additional evidence that higher-fit individuals may increase top-down cognitive control to efficiently allocate task preparation processes.
It is noteworthy that a previous ERN study manipulating task instructions indicated that aerobic fitness effects were only observed for the accuracy instructions (Themanson et al., 2008). Such a finding on the surface may appear to conflict with the present findings. However, ERN amplitude is increased when accuracy is emphasized over speed due to changes in the action monitoring system (Gehring, Goss, Coles, Meyer, & Donchin, 1993). That is, when accuracy is stressed the monitoring system is more sensitive due to the increased salience of the error (Gehring et al., 1993) or increased attentional focus (Yeung, Botvinick, & Cohen., 2004). Thus, it appears that greater amounts of cognitive control are required for the accuracy condition relative to the speed condition during action monitoring processes, with aerobic fitness enhancing ERN amplitude during this condition. Alternatively, in the current study, frontal CNV amplitude was larger for the speed condition compared to the accuracy condition, which is consistent with the findings of Falkenstein and colleagues (2003). These conflicting findings may be the result of the differential processes reflected by the two components (i.e., ERN, CNV). In the S1-S2 task, participants may need to allocate more resources due to the effortful demands of the speed condition (Falkenstein et al., 2003) to prepare a response as quickly as possible. In contrast, less resource allocation may be needed during task preparation processes for the accuracy instructions, but more cognitive control would be required after S2 presentation (i.e., stimulus evaluation processes and action monitoring processes). Thus, changes in cognitive control requirements resulting from task instructions may differ depending on the aspect of cognition studied. Accordingly, the ERP components (i.e., ERN or CNV) would be expected to modulate differentially by fitness based on the cognitive processes reflected by each component. Taken together though, they provide convergent evidence for a beneficial relationship of aerobic fitness on the cognitive control of action.
In the context of the relationship of fitness to cognitive control, we hypothesized that group differences in frontal CNV amplitude would increase with the set size required at encoding, because larger set sizes are thought to place greater demands upon working memory to encode and to maintain relevant information (Marshall et al., 2005; Sternberg, 1966). However, our data did not support this hypothesis. One possible explanation for the non-selective relationship based on encoding set size is that working memory demands were relatively high even in the smallest set size (i.e., 3 letters). That is, participants had to hold 3 letters in their memory store to response to S2. Indeed, no differences in CNV amplitudes were observed between the 3 and 5 letter set sizes. Thus, aerobic fitness effects on frontal CNV amplitude might not differ based on this variable due to relatively high working memory demands across set sizes. A second explanation may be that encoding processes were completed prior to response preparation processes. Ruchkin et al. (Ruchkin, Johnson, Grafman, Canoune, & Ritter, 1992) observed differences in a parietal slow wave between 1000 and 3000 ms after S1 onset as a function of working memory load (3, 4, and 5 letter set sizes), and suggested that this activity reflected long duration encoding processes. The present ISI's were between 4000 and 5000 ms, and thus encoding processes may have been completed prior to the late CNV component. Further investigation into these possibilities is needed to clarify this issue.
Central CNV
At central and centro-parietal regions, CNV amplitude during speed instructions was larger than during the accuracy instructions. It is well established that central CNV is associated with response preparation processes (Leynes et al., 1998; van Boxtel & Brunia, 1994) with larger CNV amplitude observed with increases in time pressure (Falkenstein, Hohnsbein, & Hoormann, 1994; Wascher, Verleger, Jaskowski, & Wauschkuhn, 1996). The current data suggest that participants required more resources in preparation to act under conditions emphasizing speed relative to conditions emphasizing response accuracy. More importantly, a trend for larger central CNV amplitude was observed for lower-fit compared to higher-fit participants (although only marginally significant), indicating that lower-fit individuals might require more neural resources to prepare for action compared to higher-fit individuals. Similar to cognitive preparation (i.e., frontal CNV), these findings suggest that the response preparation processes of higher-fit individuals may be more efficient than lower-fit individuals, indicating that aerobic fitness influences both cognitive and motor preparation processes, albeit to a different extent.
Interestingly, aerobic fitness trends observed for central CNV amplitude were found across task instructions, which contrast with the frontal CNV findings. Colcombe and Kramer (2003) conducted a meta-analysis using only randomized clinical trials to clarify which aspects of cognition are most susceptible to the influence of aerobic fitness. Their findings indicated that aerobic fitness had the greatest effect on cognitive control processes relative to all other aspects of cognition. In the present study, the selective effects for frontal CNV based on the task instructions support this notion, as the largest fitness effects were observed for task conditions requiring the most extensive amount of cognitive control. Importantly, Colcombe and Kramer (2003) also indicated that fitness had a general effect across multiple aspects of cognition with disproportionately smaller effects on other processes, including simple RT. The present data support this notion as selectively larger effects were noted for cognitive relative to motor processes as a function of fitness. Additionally, the fitness trend observed for central CNV provides some convergence across these relatively diverse aspects of cognition. That is, fitness trends observed for simple RT might result from more efficient motor preparation in higher-fit compared to lower-fit individuals. The present central CNV findings are also consistent with those of Hillman et al. (2002) and Kamijo and Takeda (2009), suggesting that the beneficial relation of aerobic fitness to response preparation may be independent of task conditions. Thus, the aerobic fitness effects on motor preparation processes appear to be independent of cognitive control requirements.
Limitations
In this study, a median split was performed on VO2max values to bifurcate the participants into higher- and lower-fitness groupings. According to the American College of Sports Medicine guidelines (American College of Sports Medicine, 2006), the participants who comprised the higher-fit group are indeed considered high-fit, with mean VO2max values above the 70th percentile for females and above the 90th percentile for males. However, mean VO2max values of the lower-fit group corresponded to around the 40th percentile for females and the 50th percentile for males, indicating that this group may be considered moderately-fit, rather than low-fit. Thus, the beneficial relation of aerobic fitness to task preparation processes may have been attenuated in the present study due to the relatively smaller fitness difference between groups. In this context, no group differences were observed for behavioral measures and aerobic fitness effects were marginal for central CNV amplitude. However, a lack of fitness group differences for behavioral measures in the present study are consonant with a previous CNV study (Hillman et al., 2002) in which higher-fit older and younger adults demonstrated smaller CNV amplitude than their lower-fit peers with no differences in behavioral measures. Thus, one might speculate that when a warning stimulus (S1) precedes an imperative stimulus (S2), fitness effects on task performance may be attenuated relative to other experimental designs that do not employ a warning stimulus (Hillman et al, 2006; Kamijo & Takeda, 2009; Pontifex et al., 2009; Themanson & Hillman, 2006; Themanson et al., 2006, 2008). As such, future research should examine truly lower-fit participants and continue to manipulate experimental parameters to further investigate task performance relative to aerobic fitness.
Summary
In sum, aerobic fitness selectively influenced frontal CNV as a function on task demands, whereas more general fitness effects were observed for central CNV. These alterations in frontal CNV amplitudes support previous findings that aerobic fitness may exert its most beneficial effects on cognitive control operations (Colcombe & Kramer, 2003). The present study also provides new insight suggesting that aerobic fitness may influence cognitive preparation processes reflected by CNV, extending the fitness and cognitive control database. Additional evidence is also provided suggesting that aerobic fitness influences motor preparation processes and that this relationship may be independent of cognitive control requirements. Accordingly, aerobic fitness appears to exert differential benefits to processes underlying cognitive and motor preparation.
Acknowledgements
Support for our research and the preparation of this manuscript were provided by the Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowships for Research Abroad to Keita Kamijo, grants from the National Institute of Mental Health (F31 MH076463) to Jason Themanson, as well as the National Institute of Child Health and Human Development (RO1 HD060385) to Charles Hillman.
References
- American College of Sports Medicine . ACSM's guidelines for exercise testing and prescription. 7th ed. Lippincott Williams & Wilkins; New York: 2006. [Google Scholar]
- Brunia CH. Movement and stimulus preceding negativity. Biological Psychology. 1988;26:165–178. doi: 10.1016/0301-0511(88)90018-x. [DOI] [PubMed] [Google Scholar]
- Brunia CH, van Boxtel GJ. Wait and see. International Journal of Psychophysiology. 2001;43:59–75. doi: 10.1016/s0167-8760(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. Journal of Neuroscience. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain and Cognition. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. American Journal of EEG Technology. 1985;25:83–92. [Google Scholar]
- Cohen J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educational and Psychological Measurement. 1973;33:107–112. [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. Journal of Gerontology: Medical Sciences. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles MG. Modern mind-brain reading: psychophysiology, physiology, and cognition. Psychophysiology. 1989;26:251–269. doi: 10.1111/j.1469-8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Compumedics Neuroscan . Offline analysis of acquired data (SCAN 4.3 - Vol. II, EDIT 4.3). [Software Manual] El Paso, TX: 2003. [Google Scholar]
- Cournot M, Marquie JC, Ansiau D, Martinaud C, Fonds H, Ferrieres J, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67:1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50. [DOI] [PubMed] [Google Scholar]
- Diamond A. The early development of executive functions. In: Bialystok E, Craik FIM, editors. Lifespan cognition: Mechanisms of change. Oxford University Press; New York: 2006. pp. 70–95. [Google Scholar]
- Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, et al. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiology of Aging. 1984;5:35–42. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Time pressure effects on late components of the event-related potential (ERP). Journal of Psychophysiology. 1994;8:22–30. [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J, Kleinsorge T. Short-term mobilization of processing resources is revealed in the event-related potential. Psychophysiology. 2003;40:914–923. doi: 10.1111/1469-8986.00109. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gratton G, Coles MG, Sirevaag EJ, Eriksen CW, Donchin E. Pre- and poststimulus activation of response channels: a psychophysiological analysis. Journal of Experimental Psychology: Human Perception and Performance. 1988;14:331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Kramer AF, Belopolsky AV, Smith DP. A cross-sectional examination of age and physical activity on performance and event-related brain potentials in a task switching paradigm. International Journal of Psychophysiology. 2006;59:30–39. doi: 10.1016/j.ijpsycho.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Weiss EP, Hagberg JM, Hatfield BD. The relationship of age and cardiovascular fitness to cognitive and motor processes. Psychophysiology. 2002;39:303–312. doi: 10.1017/s0048577201393058. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Kamijo K, Takeda Y. General physical activity levels influence positive and negative priming effects in young adults. Clinical Neurophysiology. 2009;120:511–519. doi: 10.1016/j.clinph.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Leynes PA, Allen JD, Marsh RL. Topographic differences in CNV amplitude reflect different preparatory processes. International Journal of Psychophysiology. 1998;31:33–44. doi: 10.1016/s0167-8760(98)00032-4. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Klein M, Nieuwenhuis S, De Jong R, Mulder G, Meijman TF. Mental fatigue and task control: planning and preparation. Psychophysiology. 2000;37:614–625. [PubMed] [Google Scholar]
- Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. Journal of Neuroscience Research. 1999;58:76–87. [PubMed] [Google Scholar]
- Marshall L, Molle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neuroscience. 2005;6:23. doi: 10.1186/1471-2202-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr., et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pontifex MB, Hillman CH, Polich J. Age, physical fitness, and attention: P3a and P3b. Psychophysiology. 2009;46:379–387. doi: 10.1111/j.1469-8986.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchkin DS, Johnson R, Jr., Grafman J, Canoune H, Ritter W. Distinctions and similarities among working memory processes: An event-related potential study. Cognitive Brain Research. 1992;1:53–66. doi: 10.1016/0926-6410(92)90005-c. [DOI] [PubMed] [Google Scholar]
- Spirduso WW, Clifford P. Replication of age and physical activity effects on reaction and movement time. Journal of Gerontology. 1978;33:26–30. doi: 10.1093/geronj/33.1.26. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Hillman CH. Cardiorespiratory fitness and acute aerobic exercise effects on neuroelectric and behavioral measures of action monitoring. Neuroscience. 2006;141:757–767. doi: 10.1016/j.neuroscience.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Hillman CH, Curtin JJ. Age and physical activity influences on action monitoring during task switching. Neurobiology of Aging. 2006;27:1335–1345. doi: 10.1016/j.neurobiolaging.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Pontifex MB, Hillman CH. Fitness and action monitoring: evidence for improved cognitive flexibility in young adults. Neuroscience. 2008;157:319–328. doi: 10.1016/j.neuroscience.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel GJ, Brunia CH. Motor and non-motor aspects of slow brain potentials. Biological Psychology. 1994;38:37–51. doi: 10.1016/0301-0511(94)90048-5. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wascher E, Verleger R, Jaskowski P, Wauschkuhn B. Preparation for action: an ERP study about two tasks provoking variability in response speed. Psychophysiology. 1996;33:262–272. doi: 10.1111/j.1469-8986.1996.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Wild-Wall N, Hohnsbein J, Falkenstein M. Effects of ageing on cognitive task preparation as reflected by event-related potentials. Clinical Neurophysiology. 2007;118:558–569. doi: 10.1016/j.clinph.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]