Abstract
Background
Urinary incontinence can arise in a woman of any age. Its prevalence is between 10% and 40%. The main clinical problems in urogynecology are stress urinary incontinence (involuntary leakage of urine on exertion, sneezing, or coughing) and the overactive bladder syndrome (nycturia, pollakisuria, and urinary urgency with or without incontinence).
Method
Selective literature search, with special attention to large-scale studies and to the guidelines of the German Society of Obstetrics and Gynecology (Deutsche Gesellschaft für Gynäkologie und Geburtshilfe, DGGG) and its Task Force on Urogynecology and Pelvic Floor Reconstruction (Arbeitsgemeinschaft Urogynäkologie und Beckenbodenrekonstruktion).
Results
There are many diagnostic and therapeutic options, whose use should be based on the degree of the patient’s suffering and on her motivation to be treated. Anticholinergic drugs are of established value in the treatment of overactive bladder. They are used in combination with possible lifestyle changes and bladder training. In some circum-stances, botulinum toxin injections can be considered as well. Stress incontinence should be treated conservatively (with pelvic floor training) before any surgical treatment is provided. The new tension-free vaginal tapes are just as effective as classic treatments, such as colposuspension, while being less invasive and enabling a more rapid recovery.
Conclusion
All women with urinary incontinence should undergo appropriate, specialized diagnostic evaluation and well-founded counseling in order to benefit from individualized treatment.
The term urinary incontinence refers to any type of involuntary urinary loss (International Continence Society, ICS). The problem can occur at any age, but the prevalence and extent of urinary incontinence rise in women with increasing age. For stress incontinence alone, the prevalence in girls and women aged 15 to 64 is 10% to 40% (1). Many studies have shown a reduced quality of life as a result (e1). Appropriate diagnostic evaluation and well founded counseling should be offered to every woman with the problem, providing the opportunity for individualized treatment.
The article aims to explain the current diagnostic and therapeutic options that are available for the treatment of urinary incontinence in women. The authors conducted a selective literature search in PubMed (the database of the US National Library of Medicine, http://www.ncbi.nlm.nih.gov/pubmed/). In particular, they included recent studies that described large populations of patients. The authors also used the guidelines [Frage an die Autoren: ist der Plural hier richtig, d.h. sind alle in diesem Absatz genannten AWMF-Leitlinien von diesen beiden Gesellschaften herausgegeben?) from the German Society of Obstetrics and Gynecology (Deutsche Gesellschaft für Gynäkologie und Geburtshilfe, DGGG) and the working group for urogynecology and reconstructive pelvic surgery within the society, “Belastungsinkontinenz der Frau [stress incontinence in women]” (AWMF [Association of the Scientific Medical Societies in Germany] 015/005), “Descensus genitalis der Frau – Diagnostik und Therapie [genital descent in women—diagnosis and treatment]” (AWMF 015/006), and “Sonographie im Rahmen der urogynäkologischen Diagnostik [sonography in the context of urogynecological diagnostic investigations]” (AWMF 015/055).
Diagnostic investigation
With regard to urinary incontinence in women, the main clinical problems in urogynecology are stress urinary incontinence and the overactive bladder syndrome (nycturia, pollakisuria, and urinary urgency with or without incontinence) (table 1). For the diagnosis, a distinction is made as to whether conservative treatment or surgical treatment is planned. The following examinations and measures are regarded as sufficient before conservative treatment is initiated: a medical history, voiding and fluid intake diary, clinical examination with gynecological findings, urinalysis (urinary tract infection?), measuring of residual urine, and cough test. If symptoms of an overactive bladder are present, urethrocystoscopy is indicated. Before surgical treatment is initiated for stress incontinence, additional recommended investigations include cystometry (assessment of sensory reflexes of the bladder or confirmation [okay fuer Objektivierung?] of detrusor overactivity), uroflowmetry as well as determination of postviod residual urine volume, and documentation of the pathomorphology (for example, by sonography of the introitus or perineum). In terms of imaging, sonographic techniques have pretty much taken over from radiological ones. Sonographic imaging in the sagittal plane allows visualiziation of the urethra, bladder, symphysis pubis, and vagina, and possibly also the uterus and rectum (2). Further investigations may be useful in individual cases (urethral pressure profile/stress profile, measuring pressure transmission, video urodynamics, excretion urography). During the urogynecological history, micturition and defecation impairments, fluid intake habits, comorbidities (neurological disorders, diabetes), use of pads (daytime and nighttime), medication history, and previous surgical procedures should be taken into account. Further, impairments to the patient’s quality of life and sexual activities should be evaluated. In order to assess quality of life, standardized questionnaires are appropriate (for example, the ICIQSF, the International Consultation on Incontinence questionnaire—Short Form [3]). During the history, the severity of stress incontinence is often classified according to Ingelmann-Sundberg (table 2) (e2). The stress or cough test with a full bladder provides clinical confirmation and helps grade the stress incontinence (positive in instantaneous urine leakage upon coughing, Table 3).
Table 1. Types of urinary incontinence in women according to the International Continence Society (ICS) (e3).
| Type of incontinence | Definition/symptoms |
| Stress urinary incontinence | Involuntary urine loss during physical exertion/exercise (coughing, sneezing, sports) without urgency |
| Urge incontinence | Involuntary urine loss combined with sudden sensation of urgency
|
| Mixed incontinence | Involuntary urine loss associated with urinary urgency but also with physical exertion |
| Special forms | Among others, neurogenic incontinence, extraurethral incontinence (for example, in the presence of fistula) overflow incontinence, giggle incontinence |
Table 2. Grading of stress urinary incontinence according to Ingelmann-Sundberg (e2).
| Severity | Definition |
| Stress urinary incontinence grade I | Urine loss during coughing, sneezing, pressure, and laughing |
| Stress urinary incontinence grade II | Urine loss during lifting, running, and climbing stairs |
| Stress urinary incontinence grade III | Urine loss during standing without physical activity |
Table 3. Grading of stress urinary incontinence according to the clinical stress or coughing test conducted on a full bladder (about 300 mL) (20).
| Severity | Definition |
| Stress urinary incontinence grade 0 | No urine loss found |
| Stress urinary incontinence grade I | Urine loss in droplets while standing |
| Stress urinary incontinence grade II | Urine loss in a stream while standing |
| Stress urinary incontinence grade III | Urine loss in a stream while lying down |
To determine the extent of incontinence, a pad test may be an option. A standardized short test developed by the German Continence Society can be used for this purpose (1-hour pad test, measuring the volume of urine loss into a pad after specific exercises).
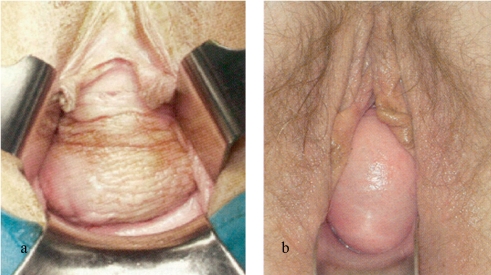
The physical examination includes an inspection of the abdomen and the external genitals as well as a vaginal exam using the speculum, in order to assess a possibly simultaneous genital descent or changes in tissue structure as a result of estrogen deficiency. The vaginal examination is performed at rest and during pressure; the doctor checks for a change to the vaginal walls (descent, prolapse) (figure 1). The pelvic organ prolapse quantification system (POP-Q) has become the established method for classifying genital prolapse in the context of scientific studies, but in clinical practice this has not become established (e3, e4). In accordance with the guidelines, a four-step score is used in this setting (Table 4) (4).
Figure 1.
a) Traction cystocele (lateral fascial defect); b) pulsation cystocele (medial fascial defect)
Table 4. Clinical stages of genital descent (4).
| Severity | Definition |
| Grade I | Greatest distal extent to more than 1 cm above the hymenal seam |
| Grade II | Greatest distal extent to the introitus |
| Grade III | Greatest distal extent to a maximum of 2 cm outside the introitus |
| Grade IV | Total prolapse |
In cases of uncomplicated incontinence, urodynamic investigations are not indicated; in the guidelines these are recommended only if an initial therapeutic attempt fails, in recurring infections, and preceding incontinence surgery or for postoperative incontinence (5). Video urodynamic testing is recommended in case of a suspected neurogenic component to the incontinence and in discrepancies to the findings (in that scenario, it is also recommended for stress incontinence and urge incontinence).
If a cystocele is present, the finding can be confirmed additionally by using a cystogram or micturition urethrogram. In order to assess the position of the neck of the bladder, position and shape of the urethra, and the base of the bladder, sonography of the introitus or perineum may be advised. Further, sonography is also the method of choice for assessing residual urine. As a general rule, while investigating possible causes of urinary incontinence, attention should be paid to possibly concomitant pelvic floor defects (rectocele, enterocele).
Cystoscopy is recommended in patients with complicated urinary incontinence, suspected vesicovaginal fistula, or extraurethral incontinence, bladder symptoms with suspected morphological correlates, and (micro-) hematuria.
Dynamic function magnetic resonance imaging of the lesser pelvis enables the exact representation of the dynamic processes in the interaction of all pelvic organs and the pelvic floor. However, this investigation should be used only for specialized problems (rectal prolapse, recurrent incontinence, prolapse after incontinence or descent surgery) and should be performed only in specialized centers.
Treatment
The therapeutic spectrum is wide, enabling individualized treatment. The data situation has improved in recent years thanks to recent studies. For most of the treatment modalities mentioned in this article, high-quality studies exist.
Treating the overactive bladder
The treatment of the overactive bladder (OAB) can be subcategorized into conservative and medication approaches. The basis of conservative treatment consists of a lifestyle change in the sense of fluid intake and bladder training (5). Even only a fluid intake and voiding diary may help to identify factors that cause urinary stress. The diary forms the basis for any therapy; it should be completed by the patient for at least 2 consecutive days in 2 consecutive weeks and then presented to the treating physician. In many cases, the symptoms can be alleviated by slight lifestyle adjustments:
Reducing excessive amounts of fluid intake (drinking 1.5 liters a day is sufficient)
If necessary, no fluid intake within 2 hours before going to sleep
Spread fluid intake evenly throughout the day
Avoid stimulants (nicotine, pepper, chilli, hot spices, fizzy drinks [Ist Kohlensäure oder welche sonstigen Säuren gemeint?])
Avoid constipation.
Bladder training is a supportive measure; extremely short intervals between voiding can be prolonged by means of actively suppressing the urge to urinate.
Local (vaginal) administration of estrogen may significantly improve the symptoms of OAB (6).
Relaxation training of the pelvic floor muscle by means of exercises using biofeedack may achieve a reduction in pelvic floor activity and by reflex alleviate the symptoms of urgency.
In the treatment of OAB, anticholinergic drugs are of central importance (7). Through blocking the muscarinic receptors, these drugs lead to inhibition of involuntary detrusor contractions. Pure anticholinergics include trospium chloride, tolterodine, fesoterodine, solifenacin, and darifenacin. Oxybutinin and propiverine additionally have local anesthetic effects. In prospective randomized studies, the following changes compared with placebo were noted on average:
Reduction in daily episodes of incontinence (0.4–1.1)
Reduction in daily micturition episodes (0.5–1.3)
Reduction in daily episodes of urinary urgency (0.64–1.56), and
Increase in micturition volume (13–40 mL).
Anticholinergics are well tolerated and have few side effects. The most common adverse effects in the studies (anticholinergic versus placebo) included dry mouth (30% versus 8%, mostly only slight), pruritus (15% versus 5%), and constipation (8% versus 4%). Uncontrolled narrow-angle glaucoma and tachyarrhythmia are contraindications to the use of anticholinergics. In order to be able to sufficiently assess the effectiveness of therapy with anticholinergics, a therapeutic interval of 4–6 weeks is required. If this does not have the desired effect, the dosage should be increased (attention needs to be paid to possible residual urine) or another anticholinergic should be selected.
In the absence of a satisfactory therapeutic effect of the anticholinergics or if the patient does not tolerate these, intravesical injection of botulinum toxin A may be considered (9). Botulinum toxin is injected directly into the vesical detrusor muscle, resulting in a reduced number of episodes of incontinence, increase in micturition volume, and a reduction in the frequency of micturition. The side effects are usually negligible, but cases of temporary residual urine—including urinary retention—after the intervention have been observed. The procedure is effective for an average of 9–12 months (9). The effect does not decrease after repeated injections. Since botulinum toxin has thus far not been licensed for use in urinary incontinence (off-label use), this therapeutic option should be offered by specialist centers only.
Electromotive drug administration (EMDA) offers an alternative therapeutic option (1). This entails targeted administration of medications (for example, a combination of lidocaine, epinephrine, buscopan, dexamethasone, and pentosan) through a transurethral catheter directly into the bladder tissue. Through an electrical field the substances penetrate into the deeper tissue layers of the bladder (active diffusion).
Sacral neuromodulation can stimulate the sacral nerves through weak electrical impulses; this may also reduce urgency and episodes of incontinence, as well as increase bladder capacity (11). This approach is offered to therapy resistant patients in specialized centers.
Treating stress incontinence
The following recommendations for the treatment of stress urinary incontinence follow the S2k guideline of the German Society of Obstetrics and Gynecology (DGGG) and the working group for urogynecology and reconstructive pelvic surgery within the society, entitled “Belastungsinkontinenz der Frau [stress incontinence in women]” (AWMF guideline registry No 015/005).
The two treatment modalities for stress incontinence are conservative management or surgery. As a rule, a conservative approach should be tried first. However, in patients with a concomitant descent or prolapse, immediate surgery may be indicated.
Conservative treatment
The conservative treatment of stress urinary incontinence entails recommendations for lifestyle adjustments (weight loss), physiotherapy, medication treatment, and use of aids.
Weight loss
With regard to lifestyle changes, weight loss in obese woman often suffices to achieve the desired therapeutic success. A randomized study showed that weight loss of 5% to 10% was associated with a 60% decrease in episodes of incontinence (12).
Physiotherapy
Physiotherapy for stress urinary incontinence involves several forms of pelvic floor training (with or without biofeedback), electrostimulation treatment, training with vaginal cones, and other therapeutic concepts (for example, vibration therapy, high-energy magnetic field treatment). Physiotherapy aims to strengthen the pelvic floor muscles (primarily the levator ani muscle) and to optimize the coordination of the intentional pelvic floor contractions (timing), so that in case of impending urinary loss because of an increase in intraabdominal pressure, the urethra can be closed tightly. According to a meta-analysis by the Cochrane Library, pelvic floor training is effective (13). Response rates (cure/improvement) of 46% to 75% have been published. It has thus far not been confirmed whether additional biofeedback improves the effectiveness of the training.
Electrostimulation of the pelvic floor with vaginal electrodes that affect passively a contraction of the levator ani muscle may improve patients’ awareness of their pelvic floor in women with stress urinary incontinence; whether electrostimulation as an add-on to pelvic floor training leads to improved results has not been confirmed.
There is insufficient data to support a recommendation of treatment with vaginal cones, perineal vibration therapy, and other therapeutic options (14). Extracorporeal magnetic innervation therapy has not been found to be effective (15, 16).
Medication treatment
Duloxetine is a serotonin/noradrenaline reuptake inhibitor that is licensed for 3 indications in Germany: depression, peripheral diabetic neuropathic pain, and stress urinary incontinence. Large randomized controlled trials have shown a response rate (subjective improvement of stress incontinence) of 62% (placebo 40%) for duloxetine 2×40 mg/day.
However, under “everyday” conditions, duloxetine has shown a notably worse effectiveness (only 37%) and a high rate of adverse effects (71%, nausea [24%]), both of which resulted in a high proportion of people dropping out of therapy (66%) (17). Duloxetine can be used to treat stress incontinence but in everyday clinical practice it is indicated to a very limited degree. To reduce adverse effects, a gradually increasing dosage (2×20 mg over two weeks) is recommended.
Use of aids
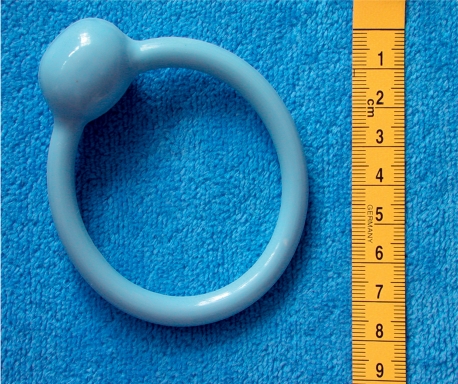
Special incontinence pads are sensible supportive measures. Regarding intravaginal aids, special urethral pessaries/shields (figure 2) and hydrophilic polyurethane tampons (figure 3) are commonly used. The ring-shaped, elastic urethral pessary has a ring shaped dome that is intended to shift the transition between bladder and urethra upwards and to thus prevent the upper urethra from opening in stress situations (coughing, movement). This pessary is suitable for (daily) independent exchange by patients themselves; this helps avoid pressure ulcers. Foam rubber pessaries are to be discarded after use; they should be worn only temporarily during certain activities (walking, jogging).
Figure 2.
Urethral pessary after Arabin
Figure 3.
Polyurethane tampons
Surgical therapy for stress urinary incontinence
Numerous surgical options are available to treat stress incontinence in women. The following are but the most important ones:
Colposuspension
Tension-free transvaginal tape (TFT sling; mid-urethral position)
“Traditional” tape (near the neck of the bladder)
Intraurethral injections.
Implantation of an artificial sphincter is the treatment of last resort for stress urinary incontinence, if all other approaches have remained unsuccessful.
Colposuspension
Colposuspension in its original form was published by Marshall, Marchhetti, und Krantz in 1949 and has undergone many modifications to-date. The best known is Burch colposuspension. The procedure aims to lift the neck of the bladder, which results in tight closure of the urethra once intraabdominal pressure rises. Originally, the vaginal fascia was attached to the periosteum of the posterior wall of the symphysis pubis. In Burch colposuspension, however, the vaginal fascia is elevated to the Cooper ligaments on both sides of the bladder neck by means of two or three suspending sutures each. By means of this procedure, any lateral defects can be leveled out simultaneously. Colposuspension can be done as an open procedure (incision across the lower abdomen) or laparoscopically. Good data exist for open colposuspension, especially long term data (up to 20 years’ follow-up). The success rates analyzed in a Cochrane review came to 85% to 90% after 1 year and about 70% after 5 years (18). Alcalay et al. have reported success rates of as high as 78% after 20 years (19). Adverse effects associated with the surgery include impaired bladder voiding (12.5%; persistent in 3.5%), development of urge incontinence (de novo 6.6%), and genital descent (22%, especially rectocele/enterocele); however, only 5% of patients required a second surgical procedure to correct the descent. Possible adverse effects should be included in any information given to patients preoperatively. According to current recommendations, colposuspension is indicated in the context of the following interventions (20):
Incontinence surgery during laparotomy for a different indication (for example, abdominal hysterectomy)
During abdominal surgery for genital descent (for example, correction of paravaginal defects)
During surgery for a recurrence (for example, after unsuccessful application of a tension-free sling).
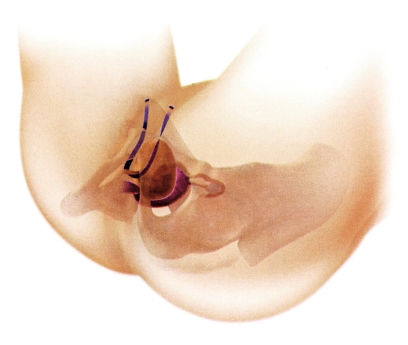
Tension-free vaginal tape
By contrast to traditional vaginal slings and colposuspension, newer vaginal slings avoid elevation of the bladder neck. A polypropylene tape is placed, tension-free, under the middle part of the urethra and pulled out either retropubically (figure 4) or bilaterally through the obturator foramen (figure 5). An abdominal incision is not required. Success rates over time are between 73% and 95% and are thus comparable with those associated with colposuspension; this has been shown in prospective randomized studies (21– 23). Postoperative genital descent in the posterior compartment (rectocele) has been observed less often than after colposuspension (18). The minimal invasiveness compared with colposuspension, the more rapid reconvalescence, and the safety of the procedure explain the success of the modern slings. However, some adverse effects have been observed; these include intraoperative bladder perforation (4%), retropubic hemorrhage (1%), impaired bladder voiding (4% to 11%; in 3% of cases this has necessitated severing the tape); injuries to the urethra, intestine, or vasculature have been described in individual case scenarios, as have some deaths. Patients should receive information about the risk of dyspareunia associated with the placement of tension-free vaginal tape (e6, e7).
Figure 4.
Retropubic tension-free vaginal tape (TVT). The mesh implant is placed under the urethra and exits directly above the symphysis pubis (with kind permission from ETHICON Women’s Health & Urology)
Figure 5.
Transobturator TVT. The mesh implant is placed under the urethra and exits in the vicinity of the genito-femoral fold. (with kind permission from ETHICON Women’s Health & Urology)
Traditional slings
In the traditional, autologous, abdominovaginal slings (mostly rectal fascia or fascia lata), the sling is placed around the bladder neck. The procedure is rarely used these days. Indications exist, however, in patients whose functional urethra is very short and in recurrent incontinence. Reported success rates are 87% for a follow-up period of 10 years (20). Long term problems may include persistent bladder voiding problems and de novo urgency (imperative urgency to urinate).
Injection techniques (24)
The aim of periurethral placement of bulking agents (bovine collagen, silicon-carbon spheres, dextranomer/hyaluronic acid [Dx/HA], copolymers, polycrylamide hydrogels) is to plump up the urethral mucosa so that the urethra remains closed under stress. Response rates after 1 to 2 years are 50% to 80%, but they are much lower in the long term (20% to 30%). Repeated injection is often required (25). The following adverse effects have been observed: local immune reactions to foreign substances (granuloma), allergies, urinary tract infection, hematuria, impaired bladder voiding (mostly reversible), urgency, arrosion [okay?], abscesses, and paraurethral cysts. Altogether, injection techniques have worse long term results than other types of incontinence surgery. Because data are limited the technique should not be used in patients in whom more effective approaches can be used.
Key Messages.
Urinary incontinence in women is common and can occur at any age; the prevalence is 10% to 40%.
Diagnostic evaluation and treatment need to be based on patients’ subjective experience of their condition. Individualized approaches are possible thanks to numerous conservative and surgical therapeutic options.
In treating the overactive bladder, anticholinergic drugs have gained an important role.
Before surgical treatment of stress urinary incontinence is being considered, conservative measures (pelvic floor training) should be tried.
The new tension-free vaginal slings (for example, TVT) have the same therapeutic efficacy as traditional approaches (for example, colposuspension) but are less invasive and patients convalesce more rapidly.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
Ms Bauer has received lecture honoraria from Astellas Pharma GmbH, PD Dr Danneker has received honoraria from Ethikon. Professor Stief and Professor Friese declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Hunskaar S, Lose G, Sykes D, Voss S. The prevalence of urinary incontinence in women in four european countries. BJU international. 2004;93:324–330. doi: 10.1111/j.1464-410x.2003.04609.x. [DOI] [PubMed] [Google Scholar]
- 2.Leitlinien der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG) gemeinsam mit der AG Urogynäkologie und Plastische Beckenbodenrekonstruktion (AGUB) dAUurBA, Österreich), der Österr. Ges. für Urologie, und der AG Urogynäkologie (AUG, Schweiz) Sonographie im Rahmen der urogynäkologischen Diagnostik 1996 (letzte Überarbeitung 8/2008) http://leitlinien.net/
- 3.Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. Iciq: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourology and urodynamics. 2004;23:322–330. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 4.Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (DGGG), Arbeitsgemeinschaft Urogynäkologie und Plastische Beckenbodenrekonstruktion (AGUB), Deutsche Gesellschaft für Urologie (DGU), Arbeitsgemeinschaft Urogynäkologie und rekonstruktive Beckenbodenchirurgie (AUB Ö, Österreichische Gesellschaft für Urologie, Arbeitsgemeinschaft Urogynäkologie (AUG S Descensus genitalis der Frau - Diagnositk und Therapie, 2008, AWMF 015/006 (s2k) http://www.agub.de/download/g_01_03_02_genitalis_frau_diagnostik_therapie.pdf.
- 5.Thüroff J, Abrams P, Andersson K, Artibani W, Chartier-Kastler E, Hampel C, Van Kerrebroeck P. Guidelines on urinary incontinence, European Association of Urology, 2006. http://www.uroweb.org/fileadmin/user_upload/Guidelines/16%20Urinary%20Incontinence.pdf.
- 6.Cardozo L, Lose G, McClish D, Versi E. A systematic review of the effects of estrogens for symptoms suggestive of overactive bladder. Acta obstetricia et gynecologica Scandinavica. 2004;83:892–897. doi: 10.1111/j.0001-6349.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 7.Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3:46–53. doi: 10.1016/s1474-4422(03)00622-7. [DOI] [PubMed] [Google Scholar]
- 8.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: An update of a systematic review and meta-analysis. European urology. 2008;54:543–562. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 9.Seif C, Boy S, Wefer B, Dmochowski R, Braun P, Junemann K. Botulinumtoxin in der Therapie der überaktiven Blase - ein Überlick. Urologe. 2008;47:46–53. doi: 10.1007/s00120-007-1592-2. [DOI] [PubMed] [Google Scholar]
- 10.Haferkamp A, Hohenfellner M. Intravesikale Therapie des overactive-bladder-syndroms. Urologe. 2008;47:46–53. doi: 10.1007/s00120-006-1178-4. [DOI] [PubMed] [Google Scholar]
- 11.Chartier-Kastler E. Sacral neuromodulation for treating the symptoms of overactive bladder syndrome and non-obstructive urinary retention: >10 years of clinical experience. BJU international. 2008;101:417–423. doi: 10.1111/j.1464-410X.2007.07233.x. [DOI] [PubMed] [Google Scholar]
- 12.Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. Weight loss: a novel and effective treatment for urinary incontinence. J Urol. 2005;174:190–195. doi: 10.1097/01.ju.0000162056.30326.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay-Smith EJ, Dumoulin C. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane database of systematic reviews (online) 2006 doi: 10.1002/14651858.CD005654. CD005654. [DOI] [PubMed] [Google Scholar]
- 14.Sonksen J, Ohl DA, Bonde B, Laessoe L, McGuire EJ. Transcutaneous mechanical nerve stimulation using perineal vibration: a novel method for the treatment of female stress urinary incontinence. J Urol. 2007;178:2025–2028. doi: 10.1016/j.juro.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Gilling PJ, Wilson LC, Westenberg AM, et al. A double-blind randomized controlled trial of electromagnetic stimulation of the pelvic floor vs sham therapy in the treatment of women with stress urinary incontinence. BJU international. 2009;103:1386–1390. doi: 10.1111/j.1464-410X.2008.08329.x. [DOI] [PubMed] [Google Scholar]
- 16.Ismail SI, Forward G, Bastin L, Wareham K, Emery SJ, Lucas M. Extracorporeal magnetic energy stimulation of pelvic floor muscles for urodynamic stress incontinence of urine in women. J Obstet Gynaecol. 2009;29:35–39. doi: 10.1080/01443610802484393. [DOI] [PubMed] [Google Scholar]
- 17.Duckett JR, Vella M, Kavalakuntla G, Basu M. Tolerability and efficacy of duloxetine in a nontrial situation. Bjog. 2007;114:543–547. doi: 10.1111/j.1471-0528.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 18.Lapitan MC, Cody DJ, Grant AM. Open retropubic colposuspension for urinary incontinence in women. Cochrane database of systematic reviews (online) 2003 doi: 10.1002/14651858.CD002912. CD002912. [DOI] [PubMed] [Google Scholar]
- 19.Alcalay M, Monga A, Stanton SL. Burch colposuspension: a 10-20 year follow up. BJOG. 1995;102:740–745. doi: 10.1111/j.1471-0528.1995.tb11434.x. [DOI] [PubMed] [Google Scholar]
- 20.Leitlinien der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG), DGGG AUuBid. Belastungsinkontinenz der Frau AWMF-Leitlinien-Register Nr. 015/005 2008. http://www.agub.de/download/g_01_03_01_belastungsinkontinenz_frau.pdf.
- 21.El-Barky E, El-Shazly A, El-Wahab OA, Kehinde EO, Al-Hunayan A, Al-Awadi KA. Tension free vaginal tape versus burch colposuspension for treatment of female stress urinary incontinence. Int Urol Nephrol. 2005;37:277–281. doi: 10.1007/s11255-004-6101-6. [DOI] [PubMed] [Google Scholar]
- 22.Jelovsek JE, Barber MD, Karram MM, Walters MD, Paraiso MF. Randomised trial of laparoscopic burch colposuspension versus tension-free vaginal tape: long-term follow up. Bjog. 2008;115:219–225. doi: 10.1111/j.1471-0528.2007.01592.x. discussion 225. [DOI] [PubMed] [Google Scholar]
- 23.Ward KL, Hilton P. Tension-free vaginal tape versus colposuspension for primary urodynamic stress incontinence: 5-year follow up. Bjog. 2008;115:226–233. doi: 10.1111/j.1471-0528.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 24.Keegan PE, Atiemo K, Cody J, McClinton S, Pickard R. Periurethral injection therapy for urinary incontinence in women. Cochrane database of systematic reviews (online) 2007 doi: 10.1002/14651858.CD003881.pub2. CD003881. [DOI] [PubMed] [Google Scholar]
- 25.Dannecker C, Strauss A, Deppe C, Hepp H. Intraurethrale Injektionstechniken bei der Behandlung der Belastungsharninkontinenz. Gynäkologe. 2004;73:1004–1011. [Google Scholar]
- e1.Lasserre A, Pelat C, Gueroult V, Hanslik T, Chartier-Kastler E, Blanchon T, Ciofu C, Montefiore ED, Alvarez FP, Bloch J. Urinary incontinence in french women: prevalence, risk factors, and impact on quality of life. Eur Urol. 2009;56(1):177–183. doi: 10.1016/j.eururo.2009.04.006. [DOI] [PubMed] [Google Scholar]
- e2.Fischer W. Fischer W, Kölbl H, editors. Epidemiologie der Harninkontinenz. Urogynäkologie in Praxis und Klinik. Berlin. Walter de Gruyter. 1995 [Google Scholar]
- e3.Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, Shull BL, Smith AR. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- e4.Auwad W, Freeman RM, Swift S. Is the pelvic organ prolapse quantification system (popq) being used? A survey of members of the international continence society (ics) and the american urogynecologic society (augs) Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:324–327. doi: 10.1007/s00192-004-1175-3. [DOI] [PubMed] [Google Scholar]
- e5.Demirkesen O, Onal B, Tunc B, Alici B, Cetinele B. Does vaginal anti-incontinence surgery affect sexual satisfaction? A comparison of tvt and burch-colposuspension. Int Braz J Urol. 2008;34:214–219. doi: 10.1590/s1677-55382008000200012. [DOI] [PubMed] [Google Scholar]
- e6.Marszalek M, Roehlich M, Racz U, Metzenbauer M, Ponholzer A, Rauchenwald M, Madersbacher S. Sexual function after tension-free vaginal tape procedure. Urologia internationalis. 2007;78:126–129. doi: 10.1159/000098069. [DOI] [PubMed] [Google Scholar]