SUMMARY
Endocannabinoids are widely regarded as negative modulators of presynaptic release. Here we present evidence that in visual cortex endocannabinoids are crucial for the maturation of GABAergic release. We found that between eye opening and puberty, release changes from an immature state with high release probability, short-term depression (STD) and high release variability during irregular patterned activity, to a mature state with reduced release probability, STD and variability. This transition requires visual experience and stimulation of CB1 cannabinoid receptors as it is mimicked by administration of CB1 agonists, blocked by antagonists and is absent in CB1R KO mice. In immature slices, activation of CB1 receptors induces long-term depression of inhibitory responses (iLTD), and a reduction in STD and response variability. Based on these findings, we propose that visually induced endocannabinoid-dependent iLTD mediates the developmental decrease in release probability, STD and response variability, which are characteristic of maturation of cortical GABAergic inhibition.
INTRODUCTION
Inhibitory GABAergic interneurons comprise 20 % of total cortical neurons (Peters and Kara, 1985), yet they significantly constrain neural activity patterns and propagation, and play a central role in cortical processes including synchrony (Bartos et al., 2007), attention (Mitchell et al., 2007) and plasticity (Jiang et al., 2005; Spolidoro et al., 2009). In sensory cortices, GABAergic circuits are not fully functional at birth, with maturation occurring slowly over development until completion at puberty. Indeed, this protracted maturation is believed to define an early postnatal critical period during which activity-dependent plasticity can be more effectively recruited by sensory experience (Hensch et al., 1998; Huang et al., 1999; Kirkwood and Bear, 1994). Moreover, experience-dependent plasticity might involve changes in GABAer circuitry (Maffei, et al 2006). Hence elucidating the mechanisms that underlie the development of cortical GABAergic circuits is fundamental for understanding cortical processing and synaptic plasticity.
At the synaptic level, the efficacy of fast GABAergic synaptic transmission is dictated by the number of presynaptic release sites, the presynaptic release probability and the postsynaptic response to released GABA. Prominent postsynaptic alterations include a switch in the polarity of the GABAergic response from depolarizing to hyperpolarizing, and a change in the subunit composition of the GABAA receptors (Heinen et al., 2004). However, both of these alterations in rodents are completed in early postnatal development, (Heinen et al., 2004; Luhmann and Prince, 1991) and are therefore unlikely to affect the regulation of GABAergic transmission after eye opening. Indeed, much of the developmental gain in GABAergic strength is attributed to a remarkable 2–3 fold increase in the number of GABAergic synapses from eye opening (circa 15 days in rodents) to puberty (circa 5 weeks). In visual cortex, this has been documented as an increase in the total number of perisomatic GABAergic contacts onto a given pyramidal cell, as well as the number of contacts made by an interneuron (Chattopadhyaya et al., 2004; Huang et al., 1999; Morales et al., 2002). Similar developmental increases in the number of GABAergic synapses have been documented in other sensory cortices and species (Gao et al., 1999; Micheva and Beaulieu, 1995). In visual cortex GABAergic synaptogenesis is triggered by sensory experience (Chattopadhyaya et al., 2004; Huang et al., 1999; Kreczko et al., 2009; Morales et al., 2002) and regulated by several permissive and suppressive mechanisms (Huang and Scheiffele, 2008).
The functional maturation of these newly formed GABAergic synapses is less understood. Curiously, evidence suggests that in visual cortex the probability of release at inhibitory synapses is reduced with postnatal development (Jiang et al., 2005; Morales et al., 2002; Tang et al., 2007) which superficially is at odds with the notion of a developmental increase in GABAergic inhibition. This developmental reduction in the probability of release appears to require normal visual experience, in that it is attenuated by dark rearing (Jiang et al., 2005; Tang et al., 2007). However the exact plasticity mechanisms recruited by visual experience to modify GABAergic release remain unknown.
Synaptic plasticity of the strength of GABAergic transmission has been documented in visual cortex (Komatsu, 1994; Maffei et al., 2006), although the previously described mechanisms do not appear to affect presynaptic release. Research in recent years underscored an essential role for endocannabinoid signaling in the plasticity and regulation of presynaptic release. Endocannabinoids are retrograde messengers that are produced and released postsynaptically in an activity-dependent manner and bind to presynaptic receptors that negatively regulates presynaptic release probability (Chevaleyre et al., 2006; Kano et al., 2009; Lovinger, 2008).
Endocannabinoid-dependent forms of long term depression of inhibitory transmission (termed iLTD) have been described in the amygdala (Marsicano et al., 2002) and in the CA1 region of the hippocampus (Chevaleyre and Castillo, 2003), and similar mechanism may underlie the decrease in release probability at inhibitory synapses over development in the visual cortex. To test this hypothesis we examined iLTD in layer II/III pyramidal cells of the visual cortex. We demonstrate that iLTD is essential for the maturation of presynaptic properties of GABAergic transmission and suggest that induction of iLTD may serve to enhance, rather than decrease, GABAergic function during periods of high neural activity.
RESULTS
Induction of a presynaptic endocannabinoid-dependent form of iLTD in the juvenile visual cortex
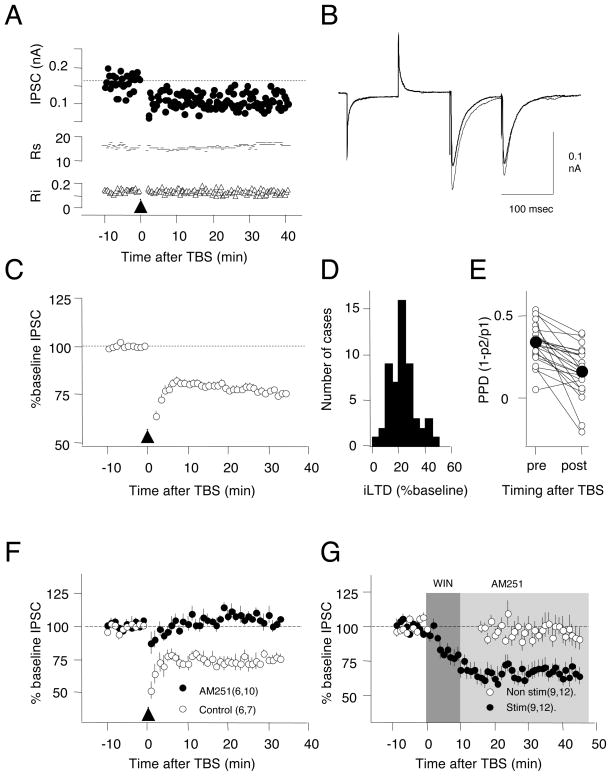
Pharmacologically isolated inhibitory postsynaptic currents (IPSCs) in layer II/III pyramidal cells were evoked by stimulation of layer IV. Theta burst stimulation (TBS, see Methods), previously shown to induce iLTD in the CA1 region of the hippocampus (Chevaleyre and Castillo, 2003), was used to induce iLTD (Figure 1). TBS induced a significant, sustained depression in the IPSC amplitude in cells from 3 week-old rats (77.3±1.3% of baseline at 30 min after TBS, n=53 from 45 rats, paired t-test: p<0.001, Fig 1C) that is robust (66% of the cases showed more that 20% depression, Fig 1D). To examine the locus of expression of iLTD we evaluated changes in paired pulse depression (PPD, inter-stimulus interval= 100 msec, see Methods), which provides a coarse estimate of the probability of release. After iLTD, PPD decreased from 0.23±0.025 to 0.16±0.031 (n=23, p<0.001, Fig 1E), suggesting that a reduction in presynaptic release probability underlies iLTD.
Figure 1. Theta burst stimulation of layer IV induces LTD of pyramidal cell IPSCs (iLTD) in layer II/III.
A) Example experiment showing IPSC amplitude (upper), series resistance (Rs, in MΩ: middle) and input resistance (Ri, in GΩ: lower) following TBS (arrowhead). B) Average of 10 consecutive IPSCs recorded before (thin trace) and 35 minutes after (thick trace) TBS. C) Average iLTD induced by TBS in 53 cells. D) Histogram of the distribution of iLTD magnitude. Bin size: 5% of baseline. E) iLTD is associated with a reduction in paired-pulse depression (PPD). Connected circles indicate values obtained from the same cells. Filled circles indicate mean values. F) Bath application of the CB1 receptor antagonist AM251 (10 μM) blocks iLTD. G) Application of the CB1 receptor agonist WIN55,212-2 (10 μM for 10 min; dark grey; chased with AM251, light grey) induces iLTD in stimulated inputs (filled circles), but not in inputs not stimulated during the drug application (open circles). The number of rats and cells is indicated in parenthesis. All experiments in slices from 3 week-old rats. Error bars in these and subsequent figures represent the standard error of the mean. See also Figure S1.
In hippocampal CA1 and other systems (Chevaleyre et al., 2006) iLTD is initiated by the binding of endocannabinoids to type 1 cannabinoid receptor (CB1R). The endocannabinoids are produced and released postsynaptically in a process triggered by the activation of the mGluR5 metabotropic glutamate receptor, which is coupled to phospholipase C (PLC) (Chevaleyre and Castillo, 2003). To test whether a similar pathway mediates visual cortical iLTD, we studied the effects of CB1R ligands. Bath application of the CB1R antagonist AM251 prevented the induction of iLTD (10 μM AM251: 102.3±3.4%, 30 min after TBS n=6 rats, 10 cells; Control DMSO: 76.8±2.8%, n=6 rats, 7 cells, t-test: p<0.001, Fig. 1F). The effects of bath application of the CB1R agonist WIN55,212-2 were tested with a two pathway experiment (WIN: 10 μM, 10 min., 9 rats, 12 slices, Fig 1G). Inputs from one pathway were stimulated (paired pulses, 100 msec inter-stimulus interval at 0.03 Hz) during WIN application, and showed a sustained depression of the IPSCs that persisted when the agonist was “chased” with 10 μM AM251. Inputs from the second pathway were not-stimulated, and were not affected by WIN application (stimulated: 65.5±3.9%, 30 min after WIN: non-stimulated: 95.1±7.0%, p=0.004.t-test: p=0.004). The depression induced by 10μM WIN is blocked by AM251 (IPSC after 20 min in WIN: 98.0±6.1%, p=0.250) and absent in a CB1KO mice (Zimmer et al, 1999) line (105.1±3.7, n=3,7. p=0.16. Data not shown). Lower concentration of WIN (1μM) did not affect the evoked IPSCs (101.4±2.5, n=2,12, p=0.701). iLTD was also blocked by bath application of the mGluR5 antagonist MPEP (10 μM) (Control: 76.6±4.2%, n=6,6; MPEP 100.6±3.9%, n=6,10; p=0.001) and by the PLC blocker U73122 (10μM) (DMSO control 80.2±1.8%, n=5,6; U73122=98.3±2.1%, n=5,8; p<0.001. Fig. S1). Together this suggests that in the juvenile visual cortex, like in the CA1 region of the hippocampus, iLTD induction requires stimulation of CB1 receptors in conjunction with presynaptic activity (Heifets et al., 2008).
A critical period for iLTD induction
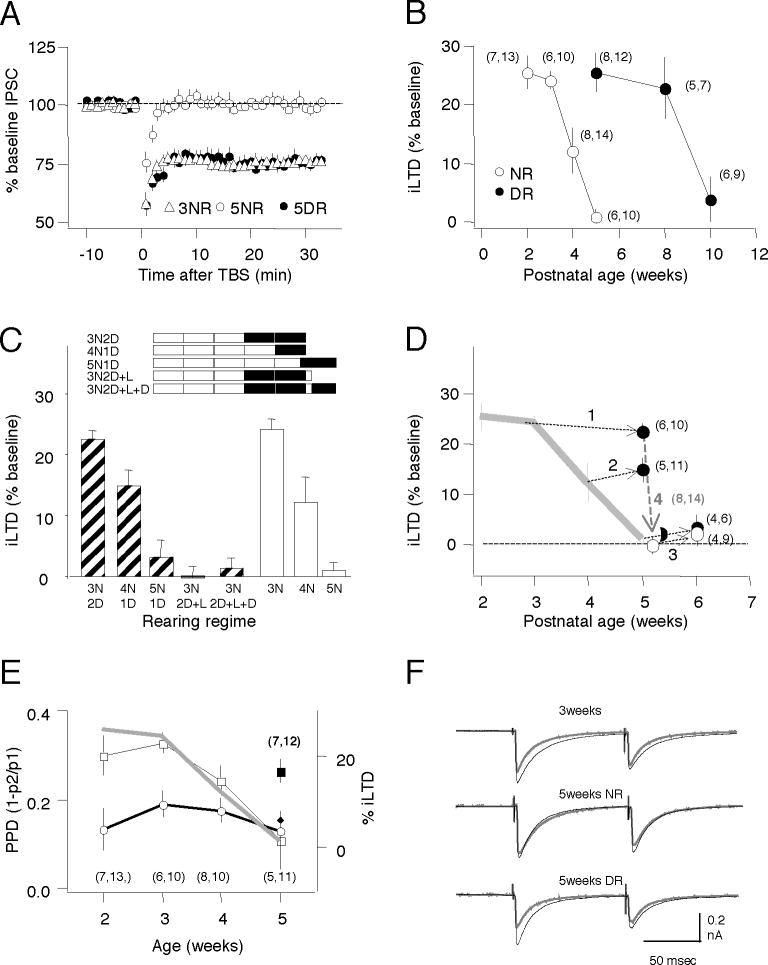
Critical aspects of GABAergic function, such as the number of perisomatic synaptic contacts onto pyramidal cells, continue to mature until puberty under the influence of normal visual experience (Chattopadhyaya et al., 2004; Kreczko et al., 2009; Morales et al., 2002). To examine the role of visual experience in the developmental regulation of iLTD we quantified iLTD in slices of visual cortex prepared from rats aged from 2 (p14–p20) to 10 weeks (p70-p76), reared either in normal light/dark cycles or reared in the dark from birth. In normal reared rats the iLTD magnitude was similar at 2 and 3 weeks of age (2 weeks: 74.6±2.3, n=7,13; 3 weeks: 75.9±1.7%, n=6,10), but was rapidly lost by the 5th week (99.1±1.3%, n=6,10; ANOVA: p<0.001. Fig 2B). In contrast, iLTD was still significant at 5 weeks in dark-reared rats (t-test: p<0.001 versus 5 week-old normal-reared rats. Fig. 2A), but a developmental loss occurred nonetheless, albeit later, between the 8th (77.2±5.5%, n=5,7) and the 10th (96.3±4.1%,n=6,9) week of age (ANOVA: p=0.002. Fig. 2B). These results indicate that in visual cortex iLTD can be induced only during a postnatal critical period with a duration regulated by sensory experience.
Figure 2. Induction of iLTD during a critical period regulated by visual experience.
A) Response to TBS on IPSC amplitudes recorded in pyramidal cells from normal-reared 3 week olds (open diamonds), normal-reared 5 weeks olds (open circles) or dark-reared 5 week olds (filled circles). B) Developmental changes in iLTD magnitude in normal-reared (open circles) and dark-reared rats (filled circles). C) Dark rearing arrests the developmental loss of iLTD. Similar amplitude of iLTD is induced in normal-reared 3 week olds (3N), and in 5 week olds placed in the dark when 3 weeks old(3N2D), or in normal reared 4 week olds (4N), and 5 week olds placed in the dark when 4 weeks old (4N1D). Minimal iLTD induced in normal–reared five week old rats that were exposed to light for 2 days after two weeks of dark-rearing (3N2D+L), or in rats that were reintroduced to the dark for a second duration of dark rearing for 4 – 6 days (3N2D+L+D). D) Same data as in C), expressed as iLTD amplitude versus age. The arrows join the value of iLTD predicted by the duration of light-rearing with the value of iLTD observed following dark rearing for 3N2D (line1), 4N1D (line2), 5N1D (line3), and 3N2D+L (line4). The grey line indicates the changes in iLTD in normal-reared rats (replotted from B). E) Average paired-pulse depression (PPD) before (squares) and 30 min after TBS (circles) (left axis) and changes in iLTD magnitude (grey line; right axis) versus age. Normal-reared animals (open symbols), 5 week-old rats reared in dark from birth (closed symbols). F) Examples traces: averages of 10 consecutive responses (normalized to the first response) recorded before (black) and 30 min after TBS (grey). In parentheses in B,C,E is the number of rats and cells. See also Figure S2.
Visual experience is required for initiating the proliferation of GABAergic synaptic contacts, however, once this process is triggered, it is not stopped or reversed by visual deprivation (Chattopadhyaya et al., 2004; Morales et al., 2002)Therefore, we examined if visual deprivation could reverse the closing of the critical period for iLTD (Fig 2C, D). To avoid the potential confound of incomplete synaptogenesis, experiments were performed in rats older than 5 weeks, and dark rearing was initiated only after at least 3 weeks of age. In rats dark reared for 2 weeks starting at 3 weeks of age, the magnitude of iLTD was substantial (3N2D: 77.6±1.5%, n=6,10), comparable to the robust iLTD observed in normally reared 3 week-old rats (3N: 75.9±1.7%, n=6,10), and significantly larger than iLTD in normally reared 5 week old rats (5N: 99.1±1.3, n=6,10; F[2,27]=76, p<0.001). Similarly, when 4 week-old rats were placed in the dark for 1 week, the magnitude of iLTD measured was comparable to the declining iLTD observed in normally-reared 4 week-old rats (4N1D: 85.2±2.6%, n=5,11; 4N: 87.9±4.1%, n=8,14; p=0.56). If one week of dark rearing was started at 5 weeks of age, the magnitude of iLTD was unchanged, and was comparable to what is seen in 5 week-old rats (5N1D: 96.9±2.6%, n=4,6; p=0.510). These results suggest that visual deprivation can halt the developmental loss of iLTD at any moment during the critical period (Fig 2C,D). Finally, we examined the effects of re-exposure to light in dark-reared animals. We found that in rats dark reared for 2 weeks starting at 3 weeks of age, two days of light exposure was sufficient to “eliminate” iLTD (3N2D+L: 100.3±1.9%, n=8,14 compared with 3N2D: 77.6±1.5%, n=6,10; p<0.001. Columns 4 and 1 in Fig 2C, and downward arrow in Fig 2D). Subsequent reintroduction into dark for 2–6 days did not reactivate iLTD (3N2D+L+1D: 98.7±15,n=4,9, p=0.1. Column 5 in Fig 2C). In sum, the results suggest that visual experience drives the developmental loss of iLTD, which can be accelerated or retarded, but not reversed, by manipulating sensory experience during this period (Fig 2D).
To examine whether the presynaptic release probability and iLTD are co-regulated by age and visual experience, again we probed PPD. We confirmed previous reports (Jiang et al., 2005; Tang et al., 2007) that the magnitude of PPD decreased with age (F[3,36]=4.073; p=0.013) and found that this time course paralleled the developmental loss of iLTD (drawn as a grey line in Fig. 2E). Similar to the loss of iLTD, the developmental reduction in PPD was dependent on visual experience. PPD was larger in 5 week-old rats reared in the dark from birth (filled square in Fig. 2E) than in age-matched reared normal controls (DR: 26.1±2.7%; NR: 11.5±5.6%; p=0.031). Interestingly, despite initial differences in PPD across ages and rearing conditions, following the induction of iLTD with TBS, PPD was reduced to a similar value irrespective of age (thick line in Fig. 2E. F[4,45]=0.478; p=0.75).
The parallel developmental time courses suggest a casual link between the loss of iLTD and PPD. A simple scenario would be that neural activity evoked by vision causes iLTD in vivo, which reduces PPD. On the other hand, if iLTD occurs only in immature naïve synapses, the magnitude of iLTD would reflect the proportion of newly formed GABA synapses, which is high at 3 weeks of age but low at 5 weeks of age. Dark rearing, by preventing iLTD induction in vivo, would maintain the potential for inhibitory synapses to express iLTD.
Loss of sensitivity to endocannabinoids mediates developmental loss of iLTD
To explore the mechanisms involved in the loss of iLTD we set out to investigate which aspects of the CB1R signaling are altered by age. However, first we tested the possibility that the low release probability at 5 weeks (Fig 2E) may obscure the detection of presynaptic iLTD. We asked if enhancing the release probability by elevating extracellular [Ca2+] from 2 to 3.6 mM could rescue iLTD in slices from mature animals. Consistent with an increased release, raising the [Ca2+] increased both the IPSC amplitude (3 weeks: 152.2±12.7%, n=5,8; 5 weeks: 159.7±11.4%, n=6,8) and paired-pulse depression (3 weeks: 15.4±3.6%; 5 weeks: 22.9±3.4%) in cells from both 3 and 5 week-old rats (Fig. S2). However, iLTD was still insignificant in cells from 5 week-old rats (95.2±1.5%), and significant in cells from 3 week-old rats (77.6±2.8%, paired t-test versus baseline: p=0.003), confirming that the loss of iLTD with development is not due to the inability to detect iLTD.
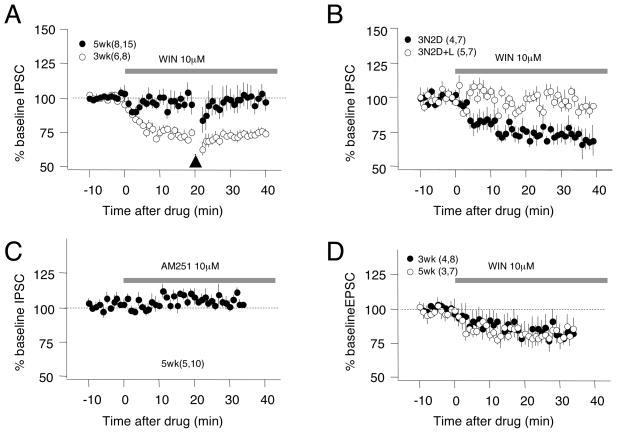
The developmental loss of iLTD could also result from a reduction in the activity-dependent release of endocannabinoids with age. To examine this possibility, we assessed developmental changes in the response to bath application of the CB1R agonist WIN (10 μM). WIN induced a substantial depression in IPSCs recorded in cells from 3 week-old rats, but not from 5 week-old rats (3 weeks: 73.2±2.4% after 30 min in WIN, n=6,8; 5 weeks 96.6±3.6%, n=8,15, p<0.001. Fig 3A). Since iLTD induction requires both endocannabinoids and presynaptic activity (see Fig 1G), we tested the possibility that baseline activation was insufficient to induce iLTD in the older slices. However, even when paired with a much stronger stimulation (TBS), bath application of WIN did not depress the IPSC amplitude in cells from 5 week-old rats (Fig. 3A). Next, we examined the role of visual deprivation on the developmental loss of endocannabinoid sensitivity. We compared the effects of WIN on rats reared in the dark from weeks 3 to 5 (3N2D; which preserves iLTD), with those on similarly dark reared rats re-exposed to light for 2 days (3N2D+L, which eliminates iLTD). WIN depressed the IPSCs in cells from 3N2D rats (77.3±5.7%, n=4,7. p=0.002. Fig. 3B), but not from 3N2D+L rats (98.5±4.3%, n=5,7. p=0.65), demonstrating a co-regulation of iLTD and the response to the CB1R agonist by age and visual experience.
Figure 3. Sensitivity of IPSC to CB1R agonists is regulated by age and experience.
A) Bath application of the CB1R agonist WIN (10 μM) reduces the IPSC, and occludes TBS-induced iLTD (arrowhead), in pyramidal cells from 3 week-old rats (open circles), but not 5 week-old rats (filled circles). B) WIN reduces the IPSCs in 5 week-old rats reared for 2 weeks in dark (3N2D, filled circles), but not in 3N2D animals re-exposed to light the last 2 days (3N2D+L, open circles). C) The CB1R antagonist AM251 (10 μM) does not affect IPSC in cells from 5 week-old rats. D) Bath applied WIN produces an equal reduction in EPSCs in layer II/III pyramidal neurons in 3 week-old and 5 week-old rats. In parentheses is the number of rats and cells. See also Figure S3.
Next we considered the possibility that a developmental increase in the tonic level of endocannabinoids could occlude the effects of exogenous agonist. Such a mechanism might mediate the developmental loss of iLTD in hippocampal CA1, where the CB1R antagonist AM251 increases GABAergic responses in slices from older animals (Kang-Park et al., 2007). However, in layer II/III pyramidal cells from the visual cortex of 5 week-old rats, bath application of AM251 (10μM) did not change IPSC amplitudes (104.4±6.8% at 30 min after AM251, p=0.78. Fig. 3C). In addition, the developmental loss of responsiveness to applied CB1R agonists is restricted to the IPSCs. As shown in Figure 3D, bath application of WIN reduced the glutamatergic EPSC to a similar degree in pyramidal cells from both 3 and 5 week-old rats (3 weeks: 81.8±5.0% at 30 min, n=4,8; 5 weeks: 81.7±6.4, n=3,7; p=0.980). Finally, we evaluated the possibility that changes in CB1 receptors contribute to differences in the responsiveness to endocannabinoids. Quantitative western blot analysis indicated that the CB1R protein content in the visual cortex is higher in 5 week-old rats reared in the dark for two weeks than in age matched normal reared rats (5N: 100.0±3.9%, n=13 rats; 3N2D: 122.3±5.5%, n=15. p=0.004. Fig. S3). Altogether, the results support the idea that the critical period for iLTD induction is mediated by a loss of responsiveness to endocannabinoids, not a change in the supply of endocannabinoids.
Systemic injection of CB1R agonists and antagonists respectively mimics and prevent the effects of visual experience on iLTD
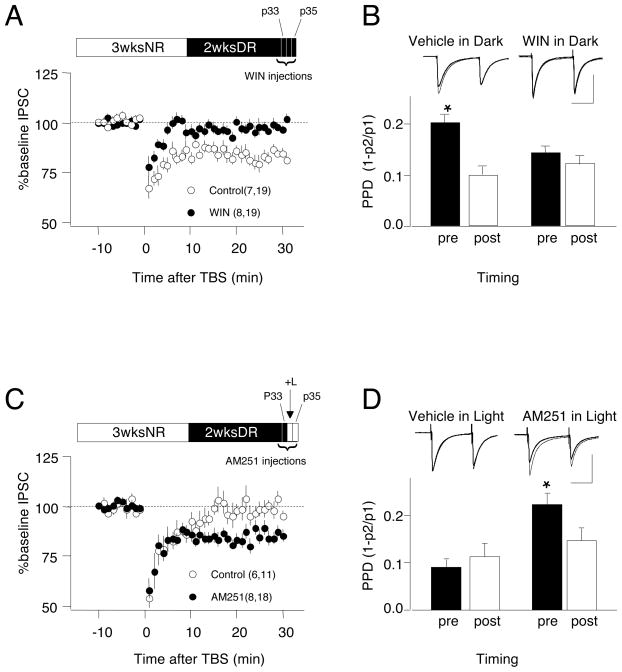
Dark rearing arrests the closure of the critical period for iLTD, while re-exposure to light rapidly stimulates the loss of iLTD (Fig 2). We hypothesized that activity in the visual cortex during light re-exposure releases endocannabinoids, inducing iLTD in vivo and occluding the subsequent induction of iLTD in vitro. Therefore, we tested whether systemic administration of a CB1R agonist during dark rearing occludes the subsequent induction of iLTD in vitro. Experiments were performed in 5 week-old rats that received 3 weeks of normal rearing followed by 2 weeks of dark rearing (3N2D), which express robust iLTD. As shown in Figure 4A, injections of the CB1 agonist WIN (5mg/kg, i.p., twice a day) for 3 days blocked the subsequent induction of iLTD in visual cortical slices (iLTD vehicle-injected controls: 81.6±1.9%, n=7,19; WIN-injected: 99.0±1.2%, n=8,19; t-test: p<0.001). Pre-treatment with the CB1R agonist also depresses PPD to the level seen following iLTD, and occluded a subsequent decrease in PPD following TBS (Figure 4B, PPD in control: 0.20±0.02 pre-TBS and 0.10±0.2 post-TBS, p<0.001; PPD in WIN-treated: 0.14±.0.02 pre-TBS and 0.12±.02 post-TBS, p=0.119). These results indicate that CB1R agonists, like brief light exposure, can reverse the effects of dark rearing and occlude iLTD. We were concerned that residual WIN in the tissue at the time of the experiment might have occluded the induction of iLTD in vitro. Although WIN is rapidly metabolized, with a half-life of about 3 hours in rodents, we tested this possibility by starting the 3-day WIN injection 5 days prior to the experiments, allowing 2 days for clearance of the drug in the dark. The occlusion of iLTD by WIN persisted following this delivery schedule (WIN-injected: 99.7±3.6%, n=6,14; control vehicle injected: 78.0±4.5%; n=5,8; p=0.002), indicating that incomplete clearance of WIN did not occlude the induction of iLTD. These findings are consistent with the idea that dark rearing preserves iLTD in vitro because neural activity in deprived cortex is insufficient to trigger iLTD in vivo.
Figure 4.
Endocannabinoid signaling mediates the effects of visual experience on iLTD. Systemic injection of CB1R agonist (WIN: 5mg/kg, i.p., twice a day for 3 days) prevents preservation of iLTD (A) and the TBS-induced decrease in PPD (B) typically seen in 5 week-old rats dark-reared for 2 weeks. Open circles: vehicle-injected controls; filled circles: WIN-injected. Systemic injection of the CB1R antagonist AM251 (5mg/kg, i.p., twice a day for 3 days) prevents the loss of iLTD (C) and the reduction in paired pulse depression (D) caused by light exposure for 2 days in 5 week-old rats dark-reared for 2 week). The rearing condition is depicted on the top of A and C. Example traces in B and D are averages of 10 responses recorded before (thin trace) and 30 min after TBS (thick trace). The asterisks denotes p<0.05 (t-test). In parentheses in A and C are the numbers of rats and cells.
Next we tested whether blocking endocannabinoid signaling in vivo prevents the loss of iLTD triggered by 2-days light exposure in 3N2D subjects by injecting the antagonist AM251 for 3 days (5mg/kg i.p. twice a day) starting one day before the light exposure. As shown in Figure 4C, iLTD was robust in cells from light exposed rats injected with AM251 and negligible in cells from control rats injected with vehicle (AM251: 80.35±2.5%%, n=8,18, control vehicle: 99.6±0.6%, n=6,11; p<0.001). The “preserved” iLTD is likely mediated by endocannabinoids as it was blocked by bath applied AM251 (DMSO control: 83.6±1.6, n=3, 6; AM251:102:9±2.3, n=3, 6; p<0.001. Data not shown). 3-day AM251 treatment does not restore iLTD in slices from 5 week-old normal reared rats (107±3.3%, n=4,7,p=0.26. data not shown), indicating that the CB1R antagonist alone does not promote iLTD. Finally, we found that TBS changed PPD in cells from AM251-injected 3N2D+L rats (P<0.001) but not in cells from vehicle injected 3N2D+L controls (p=0.229. Fig 4D). These results indicate that injection of AM251 blocks loss of iLTD induced by light exposure of dark-reared rats, supporting the hypothesis that visually induced cortical activity induces an endocannabinoid-dependent iLTD in visual cortex in vivo.
iLTD mediates the maturation of short-term plasticity
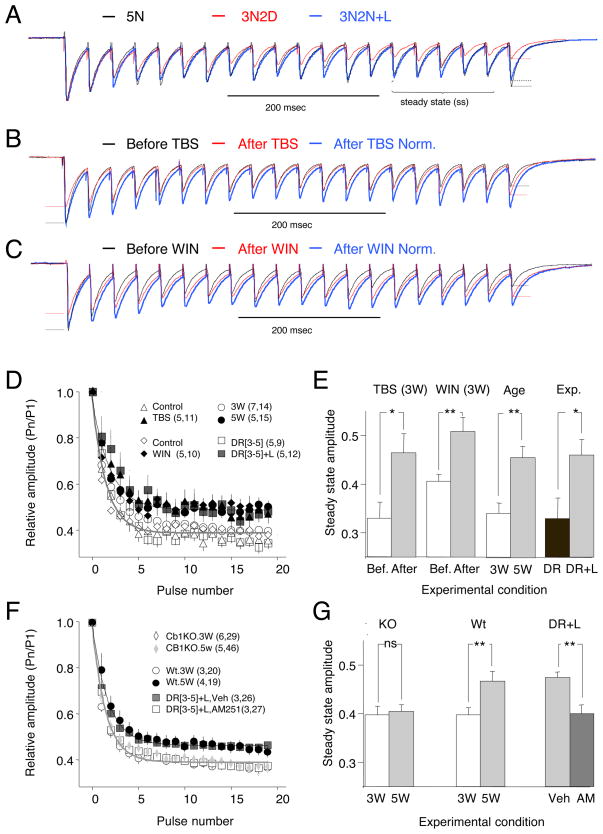
The effectiveness of GABAergic transmission during prolonged stimulation is regulated by short-term synaptic plasticity. IPSCs recorded in layer II/III typically exhibit a short–term depression (STD) evident by a decrease in the amplitude of the IPSC observed during repetitive stimulation until the arrival of a steady state, which depends on stimulation frequency, the release probability and the rate of recovery. STD in visual cortex shares many characteristics with iLTD, including a decrease with age and attenuation of the decrease by dark-rearing from birth (Jiang et al., 2005; Tang et al., 2007). Therefore, we examined whether STD is co-regulated with iLTD by late dark rearing and light exposure. We probed STD with 20 stimulation pulses at 30 Hz because the effects of age and experience on STD are prominent at this frequency (Jiang et al., 2005; Tang et al., 2007). STD was quantified as the amplitude of the IPSC during the steady state (average of the last 5 responses) normalized to the first response. STD was larger in neurons from 5 week-old rats reared in the dark for 2 weeks (3N2D) than in 5 week-old normal-reared rats (5N), or dark-reared re-exposed to light for two days (3N2D+L; Figure 5A,D,E,). Thus, late dark rearing can preserve high levels of STD, which in turn can be reduced by visual experience.
Figure 5. Endocannabinoids, age and visual experience cause parallel reduction in the short-term depression of inhibition.
A) Examples of short-term depression of inhibition elicited by repetitive activation (30 Hz, 20 pulses) in neurons from 5 week-old normal reared rats (5N; black), 5 week-old normal reared rats placed in the dark for 2 weeks (3N2D; red), and dark-reared rats re-exposed to light for 2 days (3N2D+L; blue). B,C) Induction of iLTD either by TBS (B) or WIN (C: 10 μM, 10 min, chased with 10μM AM251) depresses the initial responses in the train, but not the steady state response. Example traces (average of 10 consecutive responses) in B and C were recorded before (black) and 10 min after induction of iLTD (red). In each case the renormalized post-iLTD trace is also shown (blue). D) Averaged relative amplitude of the responses to a 30 Hz (20 pulses) stimulation train recorded in neurons from 3 week-old rats before and after TBS (open and filled triangles), before and after WIN (open and filled diamonds), 3 and 5 week-old normal-reared rats (open and filled circles), and 5 week-old rats placed in the dark and those subsequently exposed to light (open and filled squares). E) Response amplitude at the steady state (average of responses 15th to 20th) of cells from animals of the indicated ages and rearing histories. F-G) Short-term depression of inhibition is not affected by age or experience in the absence of CB1R activation. F) STD profiles in dark reared rats exposed to light for 2 days (3N2D+L) injected with AM251 or vehicle (white and black squares), and in 3 and 5 week-old CB1 KO mice (open and filled diamonds) and age matched wild type controls (open and filled circles). E) Steady state response for the experimental conditions described in F. For illustrative purposes data in D and F was arbitrarily fitted with single exponentials: black for data depicted with filled symbols; grey, for data with open symbols. In E and G, * and ** indicate p<0.05 and p<0.001, respectively, t-test. See also Figure S4.
We tested whether we can mimic the developmental reduction in STD by inducing iLTD or WIN application in normal-reared 3 week-old rats. Consistent with induction of iLTD, TBS reduced the response to the first stimulus in the train (69.1±5.2%, p=0.01, n=10, Fig 5B), but did not affect the steady state response (0.327±0.035 of the first pulse before TBS; 0.32±0.029 after TBS, p=0.72). As a result, the relative depression of responses (i.e. STD) was reduced after TBS (p<0.0001 versus non-TBS controls). Similarly, a brief application of WIN (10 min, 10 μM, chased with the antagonist AM251, Fig 5C) reduced the response to the first stimulus in the train (67.7±7.1%, n=11.p<0.001), but had no effect on the steady state response (control: 0.400±0.015; after WIN: 0.388±0.018. p=0.510) and therefore reduced the relative attenuation of the IPSC (p<0.0001 versus DMSO controls). Interestingly, after TBS or WIN was applied to cells from 3 week old rats, the profile of the progressive IPSC attenuation resembled naïve cells in older (5 week-old) rats (Fig. 5D). The TBS- and WIN-induced changes in STD were also comparable to the changes in STD induced by light exposure in dark reared rats (Fig. 5D). As summarized in Figure 5E, the average relative amplitude of the steady state IPSC was equally affected by age (3 weeks: 0.37±0.01,n=7,15; 5 weeks: 0.49±0.02, n=5,15; p<0.001), visual experience (3N2D: 0.35±0.04, n=5,9; 3N2D+L: 0.47±0.004, n=5,12, p=0.02), activity (control: 0.35±0.02; after TBS: 0.47±0.04, n=4,11. p=0.007) and the CB1R agonist (control: 0.39±0.01; after WIN: 0.5 ±0.00; n=5,10. p<0.001). We also examined the effects of age and the CB1R agonist on IPSCs evoked by long and irregular stimulation (160 pulses, with Poisson-distributed inter-stimulus intervals, mean frequency: 30 Hz). Under such regimes, IPSCs are variable because synapses are partially depleted of resources (i.e. synaptic vesicles) and the time for recovering resources varies in each trial. Therefore a reduction in release probability induced by age or CB1R agonists is predicted to reduce vesicle depletion and variability in IPSC amplitude. The results (Fig. S4) indicated that the average relative steady state IPSC amplitude was increased by both age (p=0.002) and bath applied WIN (paired t-test: p=0.011), whereas the coefficient of variation was reduced by both age (p<0.001) and WIN (paired t-test: p=0.009).
Next, we examined how the absence of CB1 signaling would affect the maturation of short-term depression. We found that injection of the CB1R antagonist AM251 prevents the reduction in STP that is normally observed when dark-reared rats are exposed to light (Veh: 0.37±0.02,n=3,26; AM251: 0.46±0.1, n=3,27. p<0.001. Fig 5F,G). In addition, we found that CB1RKO mice do not exhibit a developmental reduction in STD between 3 and 5 weeks of age (WT at 3 weeks: 0.37±0.02, n=3,20;WT at 5 weeks:0.45±0.02,n=4,19; KO at 3weeks: 0.37±0.2,n=6,29; KO at 5weeks: 0.38±0.02, n=5,46. ANOVA: p=0.040. Fig 5F,G). Altogether the results indicate that endocannabinoid activation mimics the effects of age and experience on short-term plasticity at inhibitory synapses, and support the idea the induction of iLTD in vivo is required for the maturation of the properties that govern GABAergic release.
Developmental loss of sensitivity to endocannabinoids in synapses made by fast-spiking interneurons
GABAergic interneurons form a widely diverse group in terms of anatomy, functional properties and molecular composition. To date, CB1 receptors have been detected primarily in CCK-positive interneurons (Katona et al., 1999), and also in somatostatin-positive interneurons (Hill et al., 2007), but not in fast-spiking parvalbumin-positive FS- PV interneurons. However, FS-PV neurons comprise about half of the GABAergic cells in visual cortex (Gonchar and Burkhalter, 1997), provide the bulk of perisomatic inputs, and provide a prominent component of extracellularly evoked IPSCs (Kruglikov and Rudy, 2008; Morales et al., 2002). The high prevalence of iLTD in extracellularly evoked IPSCs (> 70% of cases, see Fig 1D) prompted us to re-examine the possibility that endocannabinoids modulates the synapses made by FS-PV onto pyramidal cells (FS-PV →pyr)
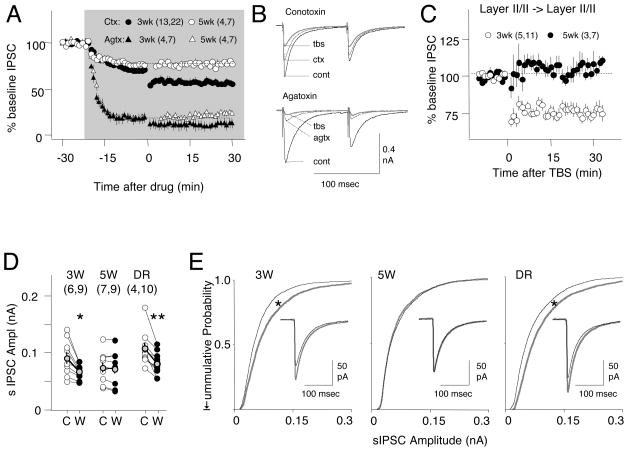
To discriminate between inputs from different interneurons we used Conotoxin (Ctx: 250–300nM) to block the N-type voltage-dependent Ca2+ channels that mediate release in non-FS interneurons, and Agatoxin (Agtx: 500nN) to block the P/Q-type voltage-dependent Ca2+ channels that mediate neurotransmitter release in FS-PV cells, at least in hippocamopus and other neocortical regions (Kruglikov and Rudy, 2008; Wilson et al., 2001; Zaitsev et al., 2007). Each toxin suppressed a complementary fraction of the evoked IPSC in cells from 3 and 5week-old rats (IPSC at 3 weeks in Ctx: 71.3±3.8% of baseline amplitude 20 min after perfusion, n=11,22; in Agtx: 24±7%, n=4,7. At 5 weeks in Ctx: 76.3±1.2%, n=4,7; in Agtx:18.1±5.6, n=4,7, two-way ANOVA: p<0.001. Fig 6A). However, iLTD was induced in each of the toxin-resistant fractions of the IPSC fraction only in cells from 3 week-old, but not from 5 week old rats (iLTD at 3 weeks in Ctxn: 79.8±2.4% of pre-TBS baseline, p<0.001; in Agtx: 68.6±10.1%, p=0.002. At 5 weeks in Ctx: 100.4±2.2% p=0.4; in Agtx127.0±16.8%, p=0.18. Two-way ANOVA: p<0.001). These results suggest that inputs from both FS-PV and nonFS-PV interneuron express developmentally regulated iLTD.
Figure 6. Developmentally regulated iLTD at multiple GABAergic synapses.
A) Conotoxin (250–300 nM)-resistant IPSCs (putative FS-inputs: circles) and Agatoxin (500nM)-resistant IPSCs (putative non FS-cells: triangles) express iLTD in cells from 3 week-old (black symbols) but not from 5 week-old rats (open symbols). The grey box denotes the duration of bath application of toxins. B) Example experiments with average responses recorded before (grey line), 20 min after the toxin application (black line), and after TBS (dotted line). C) TBS induces iLTD of inputs from layer II/III in cells from 3 week-old (open circles) but not from 5 wee-k rats (filled circles). Stimulation was applied ~ 200 microns laterally from the recorded pyramidal cell. D,E) Modulation of the spontaneous IPSCs by WIN depends on age and visual experience. (D) Changes in the average sIPSC amplitude induced by WIN (C: Control; W: after 10 min in 10 μMWIN) in cells from rats aged 3 weeks (3W), 5 weeks (5W), dark-reared from the 3rd to the 5th week of age (3N2D) Connected open circles represent individual cells; black circles, averages across cells. * = p<0.05, ** p<0.001, paired t-test. E) Cumulative probability distributions for sIPSCs (300 events per cell) recorded before (grey) and after WIN (black). Inset: isolated sIPSCs (averaged across cells) recorded before (grey) and after WIN (black). Calibration: 20 msec, 100 pA. * indicates p<0.05. Kolmogorov-Smirnov.
We also explored whether the developmental loss of iLTD is restricted to inputs recruited with layer IV stimulation. Layer IV and layer II/III stimulation recruit from the same population of GABAergic inputs (Choi et al., 2002; Morales et al., 2002). Therefore, as predicted we found that IPSCs recruited by stimulation of layer II/III also exhibit a loss of TBS-induced iLTD between 3 and 5 weeks of age (iLTD at 3 weeks: 73.6±3.5%, n=5,11; at 5 weeks: 105.7±3.2%. p<0.001. Fig 6B ). In addition we examined developmental changes in the response to application of the CB1R agonist WIN on spontaneously occurring IPSCs (sIPSC), which likely reflect activation of FS-PV since connotoxin did not affect the rate (control: 5.4±1.0; in ctx: 5.3±1.3 Hz; n=2,4. p=0.86, data not shown) or the amplitude (control: 91.1±12.8 pA; in ctx: 84.2±10.4 pA, p=0.31. data not shown). Bath applied WIN (10μM, 15 min) significantly reduced the sIPSC amplitudes in cells from 3 week-old (3W control: 88.6±8.9 pA; WIN: 65.0±4.4; n=6,9. p=0.006 ), has little effect on cells from 5 week-old rats (5W, control:74.6±9.2 pA; WIN: 72.8±10.0,. n=7,9. p=0.29), and reduced the amplitude in cells from 5 week-old rat dark reared for two weeks (NRDR, 103.1±8.9 pA; WIN: 78.4±6.1; n=5,10. p=0.001). The sIPSC frequency was not affected by WIN (3W: p=0.19; 5W: p=0.1; NRDR: p=0.89). These results indicate that the developmental loss of iLTD is not restricted to inputs activated by layer IV stimulation.
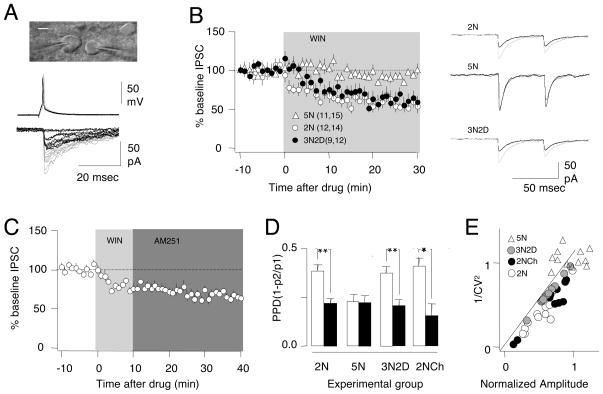
The possible endocannabinoid modulation of release in FS-PV cells was intriguing. Therefore, we performed paired recordings in slices prepared from transgenic mice that express GFP in PV-positive interneurons (Goldberg et al., 2005) as model to study unitary IPSCs (uIPSCs) in connected FS-PV→pyr pairs (Fig 7A). We confirmed that the developmental decrease in PPD and CV that we observed in Long Evans rats was also present in the PV-GFP mice (PPD at 2 weeks: 0.36 ± 0.02, n=40,68; PPD at 5 weeks 0.23±0.02, n=25,59. p=0.016, Figure 7D. CV at 2 weeks: 0.56±0.04; CV at 5 weeks: 0.44±0.03, p=0.004). As predicted, bath application of the CB1R agonist WIN (10 μM) modulates the uIPSC’s of FS-PV→pyr in an age- and experience-dependent manner (Fig 7B). In cells from 2 week-old mice WIN induced a rapid and stable depression of the uIPSCs (55.4±0.9% measured at 25–30 min after wash-in, n=13,14). WIN-induced depression of the uIPSCs did not occur when WIN was applied in the presence of the CB1R antagonist AM251 (10 μM, 98.2±3.9%, n=9,10. p=0.52, data not shown) or when the WIN concentration was reduced to 1 μM (1 μM: 91.2±9.6%, 10 μM 40.2±5/8 %, n=4. p=0.013, data not shown). In contrast, WIN did not affect uIPSCs recorded from 5 week-old normally reared mice (94.6%, n=11,14, open triangles in Fig 7B), but induced a significant depression in uIPSCs in slices from 5-week old animals reared in dark for two weeks (57.1±2.1%, n=4,9; filled circles in Fig 7B, p<0.001). Thus, like the extracellularly evoked compound IPSCs, uIPSCs from FS-PV→pyr pairs were modulated by CB1R agonists in an age- and experience-dependent manner.
Figure 7. A critical period for endocannabinoid modulation of synapses between PV fast-spiking interneurons and pyramidal cells in layer II/III of mouse visual cortex.
A) Example of WIN-induced depression of uIPSC recorded in a pyramidal neuron (right neuron in the upper image. Scale bar: 20 μm) connected to a fast-spiking interneuron identified by PV-GFP fluorescence (left cell). Top: interneuron action potentials; bottom, corresponding pyramidal cell IPSCs. Traces were recorded before (grey) and 20 min after (black) bath application of WIN (10 μM). B) Effects of 10 μM WIN on uIPSC’s recorded in cells pairs from 2 week-old mice (open circles), 5 week-old normal-reared mice (open triangles) or 5 week-old mice placed in the dark for 2 weeks (filled circles). Right: average of ten responses recorded before (grey) and after 20 min in WIN (black). In parenthesis is the number of mice and cells. C) The depression of the uIPSC’s persists when WIN (light grey) is chased with AM251 (10 μM; dark grey). D) Changes in paired pulse depression (PPD) induced by WIN application in the indicated experimental groups. ** indicates p < 0.001, paired t-test. E) CV analysis. For each cell the normalized change in 1/CV2 induced by WIN is plotted against the corresponding normalized changes in uIPSC amplitude. Data from normal-reared 2 week-old mice during continuous superfusion with WIN (2NR; open circles), normal-reared 5 week-old mice (5NR), 5 week-old placed in the dark for 2 weeks mice (3N2D, filled circles), and 2 week-old mice, whose slices being chased with AM251 (open triangles). See also Figure S5.
The CB1R-mediated depression of the uIPSCs recorded from FS-PV→pyr pairs was similar to CB1R-mediated depression of the extracellularly evoked IPSCs in additional important aspects. First, depression of the uIPSCs persisted when WIN was applied for 10 min and chased with 10 μM AM251 (67.4±1.2%, n=6,11; p<0.001, Fig 7C). Second, in all experimental groups that responded to WIN, the depression of the uIPSC was associated with a significant (p<0.001) decrease in PPD (Fig 7D), consistent with a reduced probability of release. We further examined the locus of the depression by performing a CV analysis of the uIPSC amplitude. Assuming a binomial model of release, theory predicts that if the mechanism of plasticity has a postsynaptic locus, the changes in CV2 should be constant; if the mechanism has a presynaptic locus, the changes in CV2 should be ≥ the changes in the mean (Faber and Korn, 1991; Sjostrom et al., 2003). In a plot of the normalized 1/CV2 vs normalized changes in the mean amplitude of uIPSCs, the data points locate on or under the diagonal (Fig 7E), consistent with a presynaptic locus.
Finally, we checked the modulation of the FS-PV→pyr connections in slices prepared from the rat visual cortex to confirm that the data obtained from the transgenic mice are consistent with those obtained from the rats. In rats the FS-PV interneurons were identified electrophysiologically with firing rates higher than 100 Hz and thin action potentials. We found that bath application of WIN (10 μM, 10 min) induced a lasting depression of uIPSCs (60.8±6.0%, n=11,14; p<0.001) that was associated with a decrease in paired-pulse depression (p<0.001) and increases in the CV2 (Suppl. Fig 3). Altogether, the results obtained with paired recordings suggest that CB1R-mediated iLTD regulated the inputs of FS-PV inputs onto layer II/III pyramidal cells.
DISCUSSION
In GABAergic synapses in layer II/III of the immature visual cortex the probability of release is high, resulting in pronounced short-term depression (Jiang et al., 2005; Tang et al., 2007). Here we present evidence that the transition to a mature state with low release probability and reduced STD is an experience-dependent process that requires the recruitment of endocannabinoid-dependent mechanisms similar to those that subserve iLTD in vitro. In support of this proposition we demonstrate that 1) endocannabinoid-dependent presynaptic iLTD is expressed in pyramidal neurons of layer II/III only during a critical period, which can be arrested but not reversed by visual deprivation, 2) synapses made by fast-spiking interneurons exhibit developmentally constrained iLTD and 3) although iLTD reduces the probability of GABA release, it also reduces short-term depression and the variability of synaptic transmission during irregular patterns of stimulation, similar to the changes we observe in response to visual experience. Together this suggests a novel role of endocannabinoid-mediated synaptic plasticity in the maturation of GABAergic transmission in the mammalian visual cortex, which may serve to shift the relative efficacy of GABAergic transmission to higher frequencies.
Endocannabinoid-dependent forms of presynaptic LTD have been described at both excitatory and inhibitory synapses in multiple brain regions, and a diversity of mechanisms appears to link synaptic activity to endocannabinoid synthesis and LTD induction (Chevaleyre et al., 2006; Hashimotodani et al., 2007; Lovinger, 2008). The rules of induction of iLTD in layer II/III pyramidal neurons appear to be similar to those described in hippocampal CA1 neurons (Chevaleyre and Castillo, 2003), although we have not identified which endocannabinoid species is involved in layer II/III iLTD. In both cases iLTD is only induced in active GABAergic inputs, and glutamatergic release is required to activate mGluR5 receptors and PLC, suggesting a heterosynaptic induction mechanism. However, there are seemingly mechanistic differences: CA1 iLTD requires spontaneous firing in the interneurons to activate calcineurin (Heifets et al., 2008) in the axon terminals, whereas FS(PV) cells rarely fire in cortical slices (Maffei et al., 2004). It is unclear whether in visual cortex calcineurin is not necessary, or if it is sufficiently activated by baseline stimulation alone.
The mechanisms for the developmental loss of iLTD are more difficult to compare due the scarcity of data. Prior to our study, developmental changes in endocannabinoid-mediated LTD have only been reported in inhibitory synapses in CA1 (Corlew et al., 2007; Kang-Park et al., 2007) and in excitatory synapses in layer II/III of visual cortex (Huang et al., 2008). The developmental reduction of iLTD in CA1 has been attributed to an increase in the tonic level of endogenous agonists around puberty (Kang-Park et al., 2007). In contrast, we demonstrate that the developmental loss of iLTD in layer II/III pyramidal neurons is associated with the loss of response to endocannabinoid agonists. In FS-PV→pyr synapses, this loss of responsiveness coincides with the end of synaptogenesis. The relationship between synaptogenesis and iLTD is unclear, especially given that at this stage visual experience drives many unrelated changes in synaptic function. Nevertheless, a simple scenario to consider is that iLTD is induced in vivo by visually evoked activity and somehow drives the loss of responsiveness to endocannabinoids. The target of CB1R agonists would therefore be only naïve synapses; consequently, the response to endocannabinoids disappears with the end of GABAergic synaptogenesis. Whether the CB1Rs remain in FS(PV) axon terminals after the end of the critical period, and whether iLTD becomes irreversible once induced remain open questions.
Our study is the first to report endocannabinoid modulation of synaptic function made by FS-PV interneurons in the mammalian cortex, although such modulation has been documented in the amygdala (McDonald and Mascagni, 2001) and basal ganglia (Freiman et al., 2006; Narushima et al., 2006; Uchigashima et al., 2007). In cortex it was reported that FS-PV neurons were immunocytochemically negative for CB1 receptors (Katona et al., 1999). However, it is possible that immunocytochemistry is not sensitive enough to detect CB1Rs in PV-positive interneurons. There are several examples in which immunocytochemical methods did not detect the presence of CB1Rs, but cannabinoid modulation was confirmed with other methods (Hill et al., 2007; Sjostrom et al., 2003). Our finding that WIN does not affect the FS(PV)-mediated uIPSC at 1 μM but it depresses them at 10 μM suggest a low content of CB1 receptors in PV containing axon terminals. A relative lower CB1 content at the PV-axon terminal could also explain why 1 μM WIN depresses the CCK-mediated, but not the FS(PV)-mediated uIPSC (Galarreta et al., 2008). Alternatively, higher concentrations of WIN might recruit additional processes (other than CB1Rs) that are also required for this form of iLTD. Regardless of the mechanism, the requirement for a high dose of agonist predicts that the threshold for iLTD induction would be rarely achieved in vivo in the absence of visual experience.
Recent work has shown that presynaptic forms of iLTD and iLTP coexist at the same synapses (Nugent et al., 2007; Pan et al., 2008). Postsynaptic form of LTP and LTD are generally regarded as activity-dependent mechanisms that allow bi-directional modification of synaptic strength. Interestingly, the reduction in inhibitory function after iLTD in CA1 pyramidal neurons also lowers the threshold for activity-dependent postsynaptic LTP at excitatory synapses (Chevaleyre and Castillo, 2004). Here we demonstrate that iLTD plays additional roles in the visual cortex. Our finding that iLTD reduces the responses evoked at low frequencies (0.03 to 0.05 Hz) but not at high frequencies (30 Hz) (Fig 5B) is consistent with a redistribution model of presynaptic plasticity in which changes in synaptic strength depend on the stimulation frequency (Abbott et al., 1997; Tsodyks and Markram, 1997). Thus, iLTD might enhance rather than reduce GABAergic function during high firing rates. One proposed role of fast-spiking interneurons is to integrate network activity and to broadcast an inhibitory signal that confers homeostatic stability to the network. Such a negative feedback function would be better served by synapses with low probability of release that attenuate less during sustained firing than by synapses with high probability of release that rapidly deplete synaptic resources.
It is interesting to note that in parallel with the developmental decrease in the probability of release, fast-spiking GABAergic cells also increase the expression of potassium channels responsible for the repolarization of the action potential (Goldberg et al., 2005). This shortens the duration of the action potential, thereby reducing the probability of the release per single action potential, but allows higher rates of firing and release. This suggests a developmental orchestration of mechanisms aimed at enhancing GABAergic performance during high frequency activation, at the expense of low frequency performance. During maturation, the reduction in the probability of release at low frequencies may be compensated by the 2 to 3-fold increase in the number of release sites. Whether such a developmental increase in the capacity fast transmission contributes to the closure of critical period remains to be determined. Finally, the principles we describe for the maturation of GABAergic inputs to layer II/III pyramidal cells might be more general. In all cases examined, glutamatergic synapses in sensory cortices undergo a developmental reduction in paired-pulse depression (Feldmeyer and Radnikow, 2009; Frick et al., 2007; Reyes and Sakmann, 1999; Yanagisawa et al., 2004) and also deprivation-induced increases in short-term depression (Finnerty et al., 1999), similar to what we describe for GABAergic synapses. It is possible that endocannabinoid-mediated regulation of synaptic transmission may be a ubiquitous mechanism in the developmental maturation of synaptic transmission at both GABAergic and glutamatergic synapses.
EXPERIMENTAL PROCEDURES
Visual cortical slices (300 μm) from Long-Evans rats and C57BL/6 mice reared in normal light/dark 12 hour cycles, or in the dark for defined periods with care provided under infrared illumination. Slices were cut as described (Kirkwood and Bear, 1994) in ice-cold dissection buffer containing (in mM): 212.7 sucrose, 5 KCl, 1.25 NaH2PO4, 10 MgCl2, 0.5 CaCl2, 26 NaHCO3, 10 dextrose, bubbled with 95% O2/5% CO2 (pH 7.4). Slices were transferred to normal artificial cerebrospinal fluid (ACSF) for at least an hour prior to recording. Normal ACSF was similar to the dissection buffer except that sucrose was replaced by 124 mM NaCl, MgCl2 was lowered to 1 mM, and CaCl2 was raised to 2 mM.
Visualized whole-cell voltage-clamp recordings were made from layer II/III pyramidal glass pipettes filled with intracellular solution containing (in mM: CsCl 140, CaCl2 0.2, NaCl 8, EGTA 2, NaGTP 0.5, MgATP 4, and HEPES 10, pH 7.2). Only cells with membrane potentials < −65 mV, series resistance <20 MΩ, and input resistance >100 MΩ were studied. Cells were excluded if input resistance changed > 15% over the experiment. Data were filtered at 5 kHz and digitized at 10 kHz using Igor Pro (WaveMetrics Inc., Lake Oswego, Oregon). Synaptic currents were recorded at −60 mV in the presence 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 100 μM 2-amino-5-phosphonovaleric acid (APV), and evoked every 20 sec by stimulating layer IV with 0.2 ms pulses delivered in pairs (inter-stimulus interval: i.s.i= 100 msec) to compute paired-pulse depression (PPD=1-p2/p1, where p1 and p2 are the amplitude of the response to the first and second stimulation, respectively). Stimulation was delivered through concentric bipolar stimulating electrodes (FHC, Bowdoin, ME) with intensity adjusted to evoke 100–300 pA responses. In dual-cell recordings, the interneuron was recorded under current clamp with a pipette containing (in mM): 130 K-gluconate, 10 KCl, 10 HEPES, 0.5 Na3GTP4, MgATP and 10 Na-phosphocreatine. Fast-spiking cells were identified under fluorescence, in the case of the GFP mouse line, or by their fast spiking capabilities. Synaptic strength was quantified as the IPSC amplitude and 10 min of stable baseline (less than 10% change) was allowed before any manipulation. ILTD was induced with theta burst stimulation (TBS), consisting on 4 theta burst epochs delivered at 0.1 Hz. Each TBS epoch consisted of 10 trains of 4 pulses (100 Hz) delivered at 5 Hz. See supplementary methods for details of the analysis of spontaneous IPSCs
Drug solutions
: For systematic injection of CB1 receptor antagonist and agonist, AM251[1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-(1-piperidyl)pyrazole-3-carboxamide] and WIN55,212-2 {(R)-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone} were dissolved in vehicle (composed of 10% Tween 80, 20% DMSO and 70% saline (0.9% NaCl)) to a final concentration of 1mg/mL. Each rat was intraperitoneally injected (5mg/kg) twice a day (12 h interval) for 3 days. For in vitro experiments, AM251 or WIN were dissolved in DMSO in stock solution and diluted it just before use in ACSF to the indicated final concentration. AM251, Agatoxin, WIN, U-73122 and MPEP were purchased from Tocris; CNQX, APV, Tween 80, ω-Conotoxin and DMSO from Sigma.
Supplementary Material
Acknowledgments
We thank Dr. E Quinlan for insightful comments, Drs J. Pickel and J.Z. Huang for the CB1R KO and the GFP(FS) mice, and Dr K Mackey for the CB1R antibody. Supported by RO1 EY12124.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Choi SY, Morales B, Lee HK, Kirkwood A. Absence of long-term depression in the visual cortex of glutamic Acid decarboxylase-65 knock-out mice. J Neurosci. 2002;22:5271–5276. doi: 10.1523/JNEUROSCI.22-13-05271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991;60:1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Radnikow G. Developmental alterations in the functional properties of excitatory neocortical synapses. J Physiol. 2009;587:1889–1896. doi: 10.1113/jphysiol.2009.169458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty GT, Roberts LS, Connors BW. Sensory experience modifies the short-term dynamics of neocortical synapses. Nature. 1999;400:367–371. doi: 10.1038/22553. [DOI] [PubMed] [Google Scholar]
- Freiman I, Anton A, Monyer H, Urbanski MJ, Szabo B. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. J Physiol. 2006;575:789–806. doi: 10.1113/jphysiol.2006.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Feldmeyer D, Sakmann B. Postnatal development of synaptic transmission in local networks of L5A pyramidal neurons in rat somatosensory cortex. J Physiol. 2007;585:103–116. doi: 10.1113/jphysiol.2007.141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Erdelyi F, Szabo G, Hestrin S. Cannabinoid sensitivity and synaptic properties of 2 GABAergic networks in the neocortex. Cereb Cortex. 2008;18:2296–2305. doi: 10.1093/cercor/bhm253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Newman DE, Wormington AB, Pallas SL. Development of inhibitory circuitry in visual and auditory cortex of postnatal ferrets: immunocytochemical localization of GABAergic neurons. J Comp Neurol. 1999;409:261–273. doi: 10.1002/(sici)1096-9861(19990628)409:2<261::aid-cne7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Goldberg EM, Watanabe S, Chang SY, Joho RH, Huang ZJ, Leonard CS, Rudy B. Specific functions of synaptically localized potassium channels in synaptic transmission at the neocortical GABAergic fast-spiking cell synapse. J Neurosci. 2005;25:5230–5235. doi: 10.1523/JNEUROSCI.0722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Ca(2+)-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol. 2007;17:360–365. doi: 10.1016/j.conb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci U S A. 2008;105:10250–10255. doi: 10.1073/pnas.0711880105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen K, Bosman LW, Spijker S, van Pelt J, Smit AB, Voorn P, Baker RE, Brussaard AB. GABAA receptor maturation in relation to eye opening in the rat visual cortex. Neuroscience. 2004;124:161–171. doi: 10.1016/j.neuroscience.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Faglioni M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of expereince-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Ferezou I, Cauli B, Rossier J, Schweitzer P, Lambolez B. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007;97:2580–2589. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- Huang JZ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF Regulates the Maturation of Inhibition and the Critical Period of Plasticity in Mouse Visual Cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yasuda H, Sarihi A, Tsumoto T. Roles of endocannabinoids in heterosynaptic long-term depression of excitatory synaptic transmission in visual cortex of young mice. J Neurosci. 2008;28:7074–7083. doi: 10.1523/JNEUROSCI.0899-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Scheiffele P. GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development. Curr Opin Neurobiol. 2008;18:77–83. doi: 10.1016/j.conb.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev. 2005;50:126–133. doi: 10.1016/j.brainresrev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Kang-Park MH, Wilson WA, Kuhn CM, Moore SD, Swartzwelder HS. Differential sensitivity of GABA A receptor-mediated IPSCs to cannabinoids in hippocampal slices from adolescent and adult rats. J Neurophysiol. 2007;98:1223–1230. doi: 10.1152/jn.00091.2007. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y. Age-dependent long-term potentiation of inhibitory synaptic transmission in rat visual cortex. J Neurosci. 1994;14:6488–6499. doi: 10.1523/JNEUROSCI.14-11-06488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreczko A, Goel A, Song L, Lee KK. Visual Deprivation Decreases Somatic GAD65 Puncta Number on Layer 2/3 Pyramidal Neurons in Mouse Visual Cortex. Neural Plasticity. 2009 doi: 10.1155/2009/415135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001;107:641–652. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. An anatomical substrate for experience-dependent plasticity of the rat barrel field cortex. Proc Natl Acad Sci (USA) 1995;92:11834–11838. doi: 10.1073/pnas.92.25.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur J Neurosci. 2006;24:2246–2252. doi: 10.1111/j.1460-9568.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008;28:14018–14030. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Kara DA. The neuronal composition of area 17 of rat visual cortex. II. The nonpyramidal cells. J Comp Neurol. 1985;234:242–263. doi: 10.1002/cne.902340209. [DOI] [PubMed] [Google Scholar]
- Reyes A, Sakmann B. Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. J Neurosci. 1999;19:3827–3835. doi: 10.1523/JNEUROSCI.19-10-03827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Spolidoro M, Sale A, Berardi N, Maffei L. Plasticity in the adult brain: lessons from the visual system. Exp Brain Res. 2009;192:335–341. doi: 10.1007/s00221-008-1509-3. [DOI] [PubMed] [Google Scholar]
- Tang AH, Chai Z, Wang SQ. Dark rearing alters the short-term synaptic plasticity in visual cortex. Neurosci Lett. 2007;422:49–53. doi: 10.1016/j.neulet.2007.05.053. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci U S A. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Yanagisawa T, Tsumoto T, Kimura F. Transiently higher release probability during critical period at thalamocortical synapses in the mouse barrel cortex: relevance to differential short-term plasticity of AMPA and NMDA EPSCs and possible involvement of silent synapses. Eur J Neurosci. 2004;20:3006–3018. doi: 10.1111/j.1460-9568.2004.03756.x. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Lewis DA, Krimer LS. P/Q-type, but not N-type, calcium channels mediate GABA release from fast-spiking interneurons to pyramidal cells in rat prefrontal cortex. J Neurophysiol. 2007;97:3567–3573. doi: 10.1152/jn.01293.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.