Abstract
The pathogenic bacterium Helicobacter pylori utilizes two essential glutamyl-tRNA synthetases (GluRS1 and GluRS2). These two enzymes are closely related in evolution and yet they aminoacylate contrasting tRNAs. GluRS1 is a canonical discriminating GluRS (D-GluRS) that biosynthesizes Glu-tRNAGlu and cannot make Glu-tRNAGln. In contrast, GluRS2 is non-canonical as it is only essential for production of misacylated Glu-tRNAGln. The co-existence and evident divergence of these two enzymes was capitalized upon to directly examine how GluRS2 acquired tRNAGln specificity. One key feature that distinguishes tRNAGlu from tRNAGln is the third position in the anticodon of each tRNA (C36 vs. G36, respectively). By comparing sequence alignments of different GluRSs, including GluRS1s and GluRS2s, to the crystal structure of the T. thermophilus D-GluRS:tRNAGlu complex, a divergent pattern of conservation in enzymes that aminoacylate tRNAGlu versus those specific for tRNAGln emerged and was experimentally validated. In particular, when an arginine conserved in discriminating GluRSs and GluRS1s was inserted into Hp GluRS2 (Glu334Arg GluRS2), the catalytic efficiency of the mutant enzyme (kcat/KMapp) was reduced by approximately one order of magnitude towards tRNAGln. However, this mutation did not introduce activity towards tRNAGlu. In contrast, disruption of a glycine that is conserved in all GluRS2s but not in other GluRSs (Gly417Thr GluRS2) generated a mutant GluRS2 with weak activity towards tRNAGlu1. Synergy between these two mutations was observed in the double mutant (Glu334Arg/Gly417Thr GluRS2), which specifically and more robustly aminoacylates tRNAGlu1 instead of tRNAGln. As GluRS1 and GluRS2 are related by an apparent gene duplication event, these results demonstrate that we can experimentally map critical evolutionary events in the emergence of new tRNA specificities.
Keywords: Glutamyl-tRNA synthetase, Helicobacter pylori, anticodon recognition, evolution, aminoacylation
The aminoacyl-tRNA synthetases (AARSs) lie at the heart of protein translation. Each of these enzymes catalyzes the attachment of a single cognate amino acid to one or more cognate transfer RNA (tRNAs) with high fidelity - these aminoacyl-tRNAs are subsequently delivered to the ribosome for protein biosynthesis. One of the hallmarks of the AARSs is their ability to discriminate between different tRNAs, rejecting non-cognate tRNAs in nearly all cases.1
In general, one AARS corresponds to each of the twenty encoded amino acids.1 For example, a canonical, discriminating glutamyl-tRNA synthetase (D-GluRS) catalyzes the formation of Glu-tRNAGlu (Eq. 1). Recent efforts have elucidated a few key exceptions to this general rule, via the identification of organisms that generate a complete set of aminoacyl-tRNAs with an incomplete set of AARSs.2, 3 In fact, glutaminyl-tRNA synthetase (GlnRS) is missing from all archaea and most bacteria, organelles and chloroplasts evaluated to date.1 In these organisms, the specificity of GluRS is relaxed or non-discriminatory (ND-GluRS). ND-GluRSs are essential for the biosynthesis of their cognate substrate Glu-tRNAGlu (Eq. 1) and for the misacylation of the non-cognate tRNAGln, to generate Glu-tRNAGln (Eq. 2). Glu-tRNAGln is subsequently transamidatively converted to Gln-tRNAGln by a Glu-tRNAGln glutamine-dependent amidotransferase, Glu-Adt (Eq. 3).4-7
| Eq. 1 |
| Eq. 2 |
| Eq. 3 |
The recent discovery and characterization of pairs of co-existing GluRSs in a few bacteria necessitated the expansion of GluRS types to include GluRS1 and GluRS2.8, 9 In Helicobacter pylori, GluRS2 is unexpectedly non-canonical as its sole catalytic role is to biosynthesize Glu-tRNAGln and it has lost the ability to aminoacylate its “cognate” tRNAGlu. In contrast, Hp GluRS1 is a canonical D-GluRS that aminoacylates the two tRNAGlu isoacceptors of H. pylori (tRNAGlu1 and tRNAGlu2).8, 9 Interestingly, the Acidithiobacillus ferrooxidans (Af) GluRS2 is specific only for tRNAGlnUUG, while Af GluRS1 aminoacylates both tRNAGlu isoacceptors and the remaining tRNAGln isoacceptor, tRNAGlnCUG.9 Soll and coworkers have also demonstrated a correlation between the size of the D-stem helix in tRNAGlu and tRNAGln isoacceptors and the complementary tRNA specificities of GluRS1 and GluRS2 in both H. pylori and in A. ferrooxidans.9 Genes encoding for co-existing GluRS1/GluRS2 pairs have been identified in more than 30 bacterial genomes.
We have proposed that GluRS2 could represent a mid-point in an attempt by bacteria to evolve a bacterial GlnRS, unique from known GlnRSs8 (which are all eukaryotic in origin10, 11). This proposal was based on the well-documented ancestral connection between eukaryotic GluRS and GlnRS paralogs10-12 and the fact that GluRS1 and GluRS2 are related by an ancestral gene duplication event.8, 9 Whether or not GluRS2 represents the precursor to a future bacterial GlnRS, the co-existence of pairs of closely related GluRSs offers an unprecedented opportunity to directly probe how subtle divergence in primary sequence can lead to the emergence of new tRNA specificities in evolution. Because species-dependent variations in tRNA sequences are irrelevant in these cases, insight can be gained into the subtle differences between GluRS1 and GluRS2 in a more straightforward manner than when comparing two GluRSs (e.g. a D-GluRS and an ND-GluRS) from different organisms.
In this respect, we are broadly interested in understanding how H. pylori GluRS1 and GluRS2 specifically select only tRNAGlu or tRNAGln, respectively, with the particular goal of using these enzymes to explore specification within the genetic code. In this work, we report evidence for divergent evolution in the anticodon-binding domains (ABD) of GluRS1 versus GluRS2.
Specific residues are differentially conserved in the anticodon-binding domains of GluRS1 versus GluRS2
Identity elements for tRNAGlu and tRNAGln have been determined for E. coli D-GluRS,13, 14 B. subtilis ND-GluRS,15 and E. coli GlnRS.16 In all three cases, the three nucleotides that comprise each tRNA’s anticodon [34YUC36 in tRNAGlu and 34YUG36 in tRNAGln] were identified as identity elements. (U34 is post-transcriptionally modified to mnm5s2U in E. coli;17 the thiouridine modification is also important.18, 19) As these two anticodons differ only in the 3rd position, we asked whether H. pylori GluRS2 might have evolved the unique ability to recognize G36 while rejecting C36 in tRNAGlu.
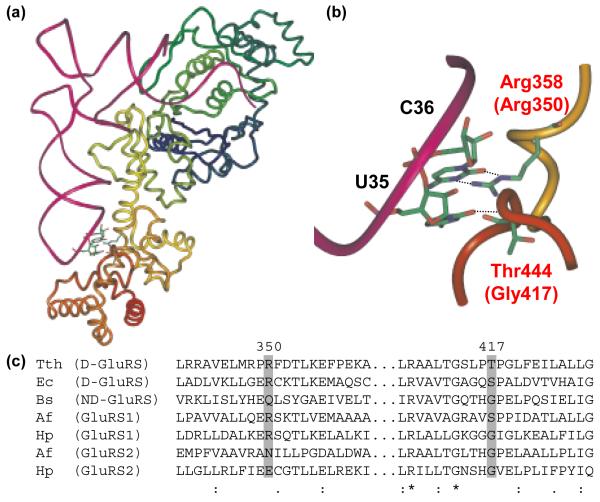
In the crystal structure of Thermus thermophilus (Tth) D-GluRS complexed with tRNAGln (Figure 1a),20 C36 forms two hydrogen bonds with D-GluRS, via bidentate contacts to Arg358 (Figures 1b). Introduction of an Arg358Gln mutation into the Tth GluRS reduced the enzyme’s kcat/KM by a fact or of 25 and activity was slightly recovered by a C36G mutation in tRNAGlu.20 (The activity of this mutant enzyme towards tRNAGln was not reported.)
Figure 1.
Anticodon recognition and conservation in GluRS. (a) The crystal structure of TTh GluRS-D and tRNAGlu (Protein databank ID: 1G59).20 The tRNA is shown as a magenta tube and GluRS is shown in a multicolored ribbon format. Specific interactions between U35 and C36 in the tRNAGlu anticodon with the D-GluRS anticodon-binding domain are shown as ball and stick figures. (b) A close-up of the anticodon loop of tRNAGlu, complexed with Tth GluRS-D, showing the hydrogen bond interactions between Arg358 (Arg 350 in Hp GluRS1) and C36 and the backbone interaction between Thr444 (Gly417 in Hp GluRS2) and U35. Panels A and B were generated using Imol v. 0.30 (www.pirx.com). (c) Representative alignment of several known GluRS sequences, highlighting the distribution of Arg350 and Gly417 amongst the different GluRS categories (Arg350 and Gly417 represent the amino acids from Hp GluRS1 and GluRS2, respectively). The alignment was generated using ClustalX.21 All sequences were obtained from the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov). Abbreviations: TTh - Thermus thermophilus; Ec - Escherichia coli; Bs - Bacillus subtilis; Af - Acidithiobacillus ferrooxidans; Hp - H. pylori. GenBank accession nos. AAS80418 (Tth), AAA65715 (Ec), CAB11868 (Bs), AAD07544 (Hp1), AAD07704 (Hp2). Af GluRS1 and GluRS2 were identified by tBlastN analysis of the incomplete Af genome sequence using the Hp GluRS1 and GluRS2 sequences, respectively (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi); the tRNA specificities of these two enzymes have been reported previously,9 however the genes are not yet annotated. Hp GluRS1, GluRS2, tRNAGlu1, tRNAGlu2 and tRNAGln were overexpressed or overtranscribed in and purified from E. coli, as described previously.8 Four mutant Hp GluRS variants (Arg350Glu GluRS1, Glu334Arg GluRS2, Gly417Thr GluRS2, and Glu334Arg/Gly417Thr GluRS2) were constructed for comparison to wild-type GluRS1 and GluRS2. These mutations were introduced into pSS001, pSS002 or pJHL003 using the QuikChange Site-Directed® Mutagenesis Kit (Stratagene). The Arg350Glu mutation was inserted into GluRS1 (pSS001) using the two complementary primers JHL#13 (5′-GACGCTCTCAAAGAAGAATCTCAAACAC-3′), and JHL#14 (5′-GTGTTTGAGATTCTTCTTTGAGAGCGTC-3′). Similarly, primer JHL#9 (5′-GCTTTTAAGATTGTTTATAGAAAGATGCGGTACCCTTTTAGAATTG-3′) and its complement JHL #10 (5′-ATTCTAAAAGGGTACCGCATCTTTCTATAAACAATCTTAAAAGC-3′) introduced the Glu334Arg mutation into pSS002, to generate pJHL003. JHL#42 (5′CCGGGAACTCGCATACCGTTGAATTGCC-3′) and JHL#43 (5′GGCAATTCAACGGTATGCGAGTTCCC-3′) were used to introduce the Gly417Thr mutation into pSS002, to generate pJHL019; these primers were also used to generate the double mutant Glu334Arg/Gly417Thr (pJHL020) from pJHL003. Overexpression and purification of each enzyme was accomplished by Ni-NTA affinity as described for GluRS1 and GluRS2.8
We examined conservation patterns for Arg358 (Arg350 in Hp GluRS1) and other amino acids proximal to the anticodon of tRNAGlu. Our working hypothesis was that residues conserved in GluRS1 sequences but not in GluRS2 sequences might be critical for tRNAGlu specificity; similarly, residues conserved in GluRS2 but not GluRS1 could enhance tRNAGln specificity. We included ND-GluRS, D-GluRS, GluRS1 and GluRS2 sequences in our analysis.
To generate alignments for comparison to the crystal structure, completed bacterial genomes were searched for the presence of GluRS, GlnRS and Glu-Adt. The protein product of each gltX gene (the gene encoding GluRS) was categorized in the following manner: If the GluRS was from an organism that encodes GlnRS but not Glu-Adt, then it was categorized as a D-GluRS. ND-GluRSs were from species that do not encode GlnRS but do encode Glu-Adt. GluRS1s and GluRS2s were defined, respectively, based on similarities to the two H. pylori enzymes and by the phylogenetic analyses by us8 and by Soll and coworkers.9 Any GluRS paralogs that didn’t readily fall into one of these categories was omitted from consideration. (For example, it is impossible a priori to predict whether a GluRS in an organism that encodes for both GlnRS and Glu-Adt would be a D-GluRS or an ND-GluRS.) Alignments and phylogenetic trees were generated using ClustalX (version 1.83)21 and were used without further modification. The assignments of GluRS1 versus GluRS2 were further refined based on the phylogenetic tree generated by ClustalX. This analysis led to the identification of 67 ND-GluRS, 31 D-GluRS, 30 GluRS1, and 30 GluRS2 sequences. Each GluRS group was aligned using ClustalX so that sequence variations in the ABD could be examined.
These alignments show that this arginine (referred to as Arg350 from here on, according to Hp GluRS1 numbering, Figure 1C, Arg358 in Tth D-GluRS) is invariant in known GluRS1s and nearly invariant in D-GluRSs (with one conservative lysine substitution amongst 31 sequences). In contrast, Arg350 is prohibited in known GluRS2 sequences where it is always replaced by either a small hydrophobic residue (83% of surveyed sequences) or glutamic acid (17%). Arg350 is highly variable in ND-GluRSs, with 8 different amino acids found in this position including Arg (43.5% of the time). Our alignment analysis revealed a second residue of interest, Gly417 (Hp GluRS2 numbering, Gly417 corresponds to Thr444 in Tth D-GluRS). This position is an invariant glycine in all known GluRS2 sequences; in other GluRS categories, it is less conserved but typically occupied by small residues like threonine, serine or alanine. In the crystal structure of Tth D-GluRS:tRNAGlu,20 the backbone carbonyl of this Thr444 forms a hydrogen bond with U35 in the center of the tRNAGlu anticodon (Figure 1b).
Arg350 is an anti-determinant for tRNAGln aminoacylation
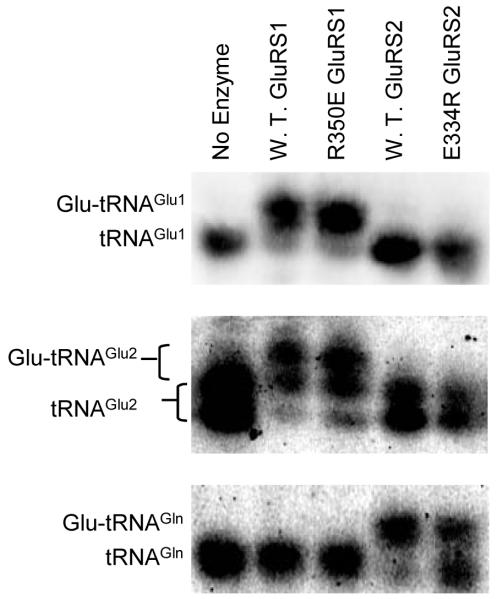
To determine the contribution of Arg350 in tRNA recognition, we constructed two mutant H. pylori GluRS variants - Arg350Glu GluRS1 and Glu334Arg GluRS2. The tRNA specificities of these four enzymes (wild-type GluRS1 and GluRS2 and the two mutants, Arg350Glu GluRS1, and Glu334Arg GluRS2) were examined by acid-gel electrophoresis and Northern Blot analyses22 (Figure 2) with Hp tRNAGlu1, tRNAGlu2, and tRNAGln. In these gels, aminoacylated tRNA migrates more slowly, due to the positively charged α-amine on the amino acid; each aa-tRNA can consequently be quantified via Northern blot, using tRNA isoacceptor specific oligonucleotides.8, 22 The top two panels demonstrate that the two mutations (Arg350Glu and Glu334Arg) do not alter the tRNAGlu isoacceptor specificities of GluRS1 and GluRS2 under the conditions of our assays. GluRS1 (lane 2) and Arg350Glu (lane 3) GluRS1 each aminoacylate tRNAGlu1 and tRNAGlu2, whereas neither GluRS2 (lane 4) nor Glu334Arg (lane 5) GluRS2 aminoacylates either of these tRNAs. [Note: tRNAGlu2 migrates as two distinct bands, presumably due to incomplete introduction of a post-transcriptional modification - previous work has demonstrated that this difference is non-essential.8, 9] In fact, Arg350Glu GluRS1 appears to still have wild-type tRNA propensities in that the introduction of this mutation does not confer tRNAGln aminoacylation activity onto GluRS1 (Compare lanes 2 and 3 in the bottom panel).
Figure 2.
In GluRS2, the Glu334Arg mutation reduces aminoacylation of tRNAGln, without increasing tRNAGlu specificity; Arg350Glu GluRS1 still readily aminoacylates tRNAGlu but not tRNAGln. Acid gel electrophoresis was used to separate aminoacylated-tRNA from deacylated tRNA; each tRNA isoacceptor was visualized by Northern Blot as previously described.8, 22 In each case, aminoacylation is indicated by an upward shift of the observed band. The three species of Hp tRNAs, tRNAGlu1 (top), tRNAGlu2 (middle), and tRNAGln (bottom) were separately aminoacylated by wild-type GluRS1 (lane 2), Arg350Glu GluRS1 (lane 3), wild-type GluRS2 (lane 4), or Glu334Arg GluRS2 (lane 5). Lane 1 shows deacylated tRNA as a control.
Preparation of glutamyl-tRNAs for Acid-Urea Gels: Aminoacylation reactions were conducted at 37°C, in 50 mM HEPES-OH (pH 7.2), 4 mM MgCl2, 2 mM ATP, 100 uM glutamate, and 300 nM of enzyme. Reactions (50 uL) were quenched after 90′ with phenol (pH 4.5) and two volumes of 10 mM NaOAc (pH 4.4). Each tRNA was ethanol-precipitated and redissolved in 10mM NaOAc (pH4.4) with 1 unit/μL of RNAsin (Promega).
Acid-Urea Gel (pH 4.0) Electrophoresis and Northern Blotting. A 40 cm polyacrylamide gel (pH 4.0) containing 8% bis-acrylamide (19:1), 0.2M NaOAc and 8M urea, was pre-run at 400V, 4°C, for 30 mins. Typically each redissolved aminoacylation timepoint was adjusted to an OD260= 50, in a final volume of 4uL. Migration of the tRNAs proceeded under the same conditions as the pre-run, for 20∼24 hrs. Nucleotides samples were transferred to Immobilon Ny+ membrane (Millipore) at 320mA for 90′ using a semi-dry electrophoresis apparatus (Fisher Biosciences). Membranes were baked, prehybridized and hybridized by 32P-labeled probes as previously described, using hybridization probes designed to specific for a single Hp tRNA.8 Hybridized membranes were exposed to a PhosphorImager screen (Molecular Dynamics) and scanned using ImageQuant software (Molecular Dynamics). Each gel is representative of experiments run in triplicate.
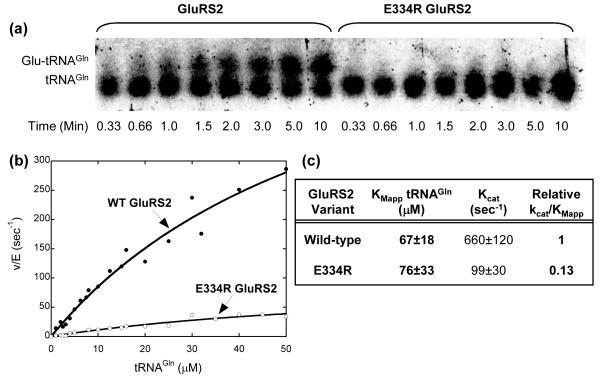
In contrast, the Glu334Arg GluRS2 mutation has a dramatic effect on the extent of tRNAGln aminoacylation. After 90 minutes, the reaction catalyzed by wild-type GluRS2 is complete and all tRNAGln has been converted into Glu-tRNAGln (Figure 2, bottom panel, lane 4). In contrast, only about 50% of the tRNAGln has been aminoacylated by Glu334Arg GluRS2 (Figure 2, bottom panel, lane 5). When shorter time points are considered, the diminished efficiency of the Glu334Arg GluRS2 mutant becomes more apparent (Figure 3a). In 10 minutes, wild-type GluRS2 has aminoacylated approximately 50% of available tRNAGln, whereas Glu-tRNAGln formation remains undetectable when treated with the same concentration of the Glu334Arg mutant enzyme under the same conditions. This difference is due to a reduction in kcat/KMApp for the Glu334Arg GluRS2 mutant by nearly one order of magnitude, as determined using standard aminoacylation assay conditions19 (Figures 3b and 3c).
Figure 3.
The Glu334Arg mutation in GluRS2 causes a reduction in catalytic efficiency. (a) Time course comparison of wild-type and Glu334Arg GluRS2s. (b) Determination of kinetic constants. (c) The kinetic constants for GluRS2 and Glu334Arg GluRS2 with tRNAGln. Aliquots of a standard aminoacylation reaction were quenched at the times indicated and analyzed by acid gel electrophoresis and Northern blot as described in Figure 2. For kinetic constant determination, aminoacylation reactions19 were carried out at 37 °C in 50 mM HEPES-OH (pH 7.2), 4 mM MgCl2, 4 mM ATP, 500 uM glutamate and L-[3,4-3H] glutamate (100∼150 dpm/pmol) with various concentration of tRNA (0.56uM ∼ 50uM) and enzymes (220nM ∼ 1.05uM). Aliquots were precipitated on filter paper pre-soaked with a 5% trichloroacetic acid (TCA) solution and washed three times with 5% TCA. Data was fit to the Michaelis-Menten Equation, using Kaleidagraph v. 3.6.2 (Synergy Software). (Note: Because of the high KMApp value for tRNAGln, these kinetic constants were determined using sub-saturating concentrations of tRNAGln.)
Interestingly, Glu334Arg GluRS2’s diminished catalytic efficiency is primarily due to a drop in kcat, as the KM for tRNAGln remains unaffected (within the experimental error of our assay). Only a few aminoacyl-tRNA synthetases, including GluRS, require bound cognate tRNA for amino acid activation (forming an aminoacyl adenylate, AA-AMP).23-28 In the absence of tRNAGlu, structural analyses have shown that ATP binds to the Tth D-GluRS active site in a non-productive manner.29 When tRNAGlu binds, the enzyme undergoes extensive structural perturbations that induce productive ATP binding and amino acid activation. These movements include rotation of domains four and five in the anticodon-binding domain as they twist to contact the tRNA anticodon (by 6° and 8°, respectively).29 The mechanisms by which these ABD structural changes are communicated to and impact the conformation of the enzyme active site are not well understood. It is intriguing to speculate, however, that our Glu334Arg mutation has a diminished kcat because it disrupts this communication pathway between the tRNA anticodon and the active site of the enzyme. Conclusive proof of such inter-domain communication awaits a more detailed kinetic analysis of these mutations and a crystal structure of a representative GluRS2.
Gly417 is an antideterminant for tRNAGlu1 aminoacylation in GluRS2
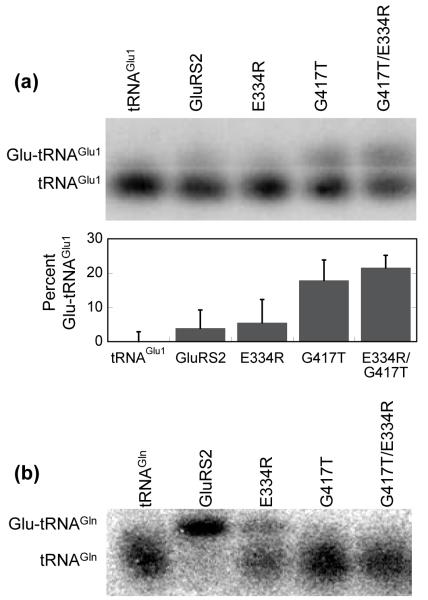
Because the Glu334Arg GluRS2 mutation did not introduce detectible tRNAGlu aminoacylation, the presence or absence of this arginine is not the sole determinant in tRNAGlu versus tRNAGln specificity, a result that is not unexpected given the overall complexity of tRNA recognition. For this reason, we also examined the role of Gly417 (Thr444 in Tth D-GluRS). This glycine is invariant in GluRS2s, but is less conserved and is typically a serine in other classes of GluRS (Figure 1c). We constructed a Gly417Thr GluRS2 single mutant and a Glu334Arg/Gly417Thr GluRS2 double mutant and evaluated each mutant enzyme’s tRNA specificities in comparison to wild-type GluRS2 (Figure 4a).
Figure 4.
Disruption of Gly417 in Hp GluRS2 leads to tRNAGlu aminoacylation and eliminates tRNAGln aminoacylation. (a) Aminoacylation of tRNAGlu1 by Gly417Thr and Glu334Arg/Gly417Thr GluRS2. Transfer RNAGlu1 was separately aminoacylated by wild-type GluRS2 (lane 2), Glu334Arg GluRS2 (lane 3), Gly417Thr GluRS2 (lane 4), or the double mutant Glu334Arg/Gly417Thr GluRS2 (lane 5), as described for Figure 2. Lane 1 shows deacylated tRNAGlu1 as a control. Acid gels and Northern blots were performed as described in Figure 2. Gel shown is representative of experiments run in triplicate. The percent Glu-tRNAGlu1 formed was determined using ImageQuant software (Molecular Dynamics) to quantify the ratio of the upper and lower bands. The bar graph shows the results of this analysis; Lane 1 (deacylated tRNAGlu1) was used as a background control and subtracted from lanes 2-5. (b) Aminoacylation of tRNAGln by Hp GluRS2 and various mutants. Transfer RNAGln was separately aminoacylated as in Figure 4a. Lane 1 shows deacylated tRNAGln as a control.
Unlike the Glu334Arg GluRS2 mutant, introduction of the Gly417Thr mutation converts GluRS2 into an enzyme that aminoacylates tRNAGlu1 (compare lanes 2 and 4 in Figure 4a, top panel). Furthermore, the extent of aminoacylation is increased when the Gly417Thr and Glu334Arg mutations are combined (lane 5). In this case, as much as 30% of the tRNAGlu1 is converted to Glu-tRNAGlu1 (quantification shown in bottom panel); this synergy occurs despite the fact that the Glu334Arg GluRS2 point mutant does not aminoacylate tRNAGlu1 at detectible levels. Like the Glu334Arg mutation, these two new mutations are both deficient in tRNAGln aminoacylation (Figure 4b, lanes 4 and 5).
In the crystal structure of D-GluRS, the Thr444 backbone carbonyl contacts U35 in tRNAGlu.20 Why then does Gly417 prevent tRNAGlu recognition? Perhaps this glycine induces a perturbation into the structure of the ABD of GluRS2s that is distinct from that observed in D-GluRSs. Gly417 is almost always immediately followed by a proline in GluRS2s (91% of surveyed sequences had Pro418). This proline is also highly conserved in other GluRS categories. Perhaps the Gly-Pro sequence unique to GluRS2 paralogs induces a GluRS2-specific β-turn that directs the backbone carbonyl of Gly417 away from U35, diminishing anticodon recognition. Alternatively, this turn could align and consequently strengthen other more specific contacts with G36 (recall that U35 is conserved in the anticodons of both tRNAGlu and tRNAGln). The precise impact of this conserved glycine won’t be completely understood until a crystal structure of a representative GluRS2 becomes available.
A few key mutations lead to divergence in tRNA specificity
The 20 canonical AARSs are subdivided into two unrelated classes, Class I and Class II, based on commonalities in the active sites of enzymes within a given class.20 The modern synthetases arguably emerged from two precursors (one for each class) via a series of gene duplications and subsequent divergence of function.30 As most of the aminoacyl-tRNA synthetases are uniformly conserved across the three domains of life,1 our ability to directly and experimentally assess the evolutionary events leading to each enzyme’s emergence are generally limited. GlnRS is one of the few exceptions to the universality of aminoacyl-tRNA biosynthesis and consequently comparisons between the different mechanisms for Gln-tRNAGln biosynthesis can be considered in an evolutionarily relevant framework but in a modern context with sequences and enzymes from living organisms.
It is the breadth of different tRNA specificities utilized by different GluRSs in bacteria that separates it from other AARSs. (Aspartyl-tRNA synthetase can also be discriminating or non-discriminating31 but an AspRS analogous to GluRS2 has not yet been discovered.) This uniqueness makes GluRS an ideal case study to experimentally examine the emergence of new tRNA aminoacylation substrate specificities. Alignments of each individual category of GluRS can be compared to highlight correlations between conserved amino acids and tRNAGlu and/or tRNAGln specificity. By experimentally testing these patterns, we can verify evolutionary permutations that differentiate GluRS2 and its ability to uniquely aminoacylate tRNAGln from other GluRSs that have retained tRNAGlu activity. In other words, we can now directly examine the mutagenesis events that led GluRS2 to gain exclusive tRNAGln specificity, much in the way that each of the other AARSs probably emerged and acquired their own unique substrate patterns.
In this work, we focused on divergence between the anticodon-binding domains of GluRS1 versus GluRS2. We have identified complementary conservation patterns that correlate with tRNAGlu versus tRNAGln specificity. Thus, as GluRS2 diverged from GluRS1, we can imagine that it lost Arg350 (replaced by Glu334 in Hp) in order to optimize tRNAGln aminoacylation activity. At the same time, evolutionary pressure would have led to the fixation of Gly417 in Hp GluRS2 paralogs in order to eliminate tRNAGlu recognition. Thus, in general, the absence of Arg350 and the presence of Gly417 can be considered a signature sequence to identify GluRS2 sequences and preferential activity for tRNAGln. Interestingly, a few GluRS1 paralogs, including Hp GluRS1, have both Arg350 and Gly417, despite the fact that Hp GluRS1 only aminoacylates tRNAGlu isoacceptors. 8 Thus, it would appear that it is only the combination of the presence of Gly417 and the absence of Arg350 that leads to tRNAGln specificity, consistent with the synergy we see when these two positions are simultaneously mutagenized (Figure 4a).
As we have shown here, mutagenesis of only two residues in Hp GluRS2 is sufficient to convert this enzyme into one that aminoacylates tRNAGlu1 instead of tRNAGln. A similar result has been observed between isoleucyl-tRNA synthetase (IleRS) and methionyl-tRNA synthetase (MetRS), two aminoacyl-tRNA synthetases that are less closely related than our GluRS1 and GluRS2 pairs. Using a combinatorial selection method, a single Arg to Trp mutation in the anticodon-binding domain of a modified IleRS was sufficient to convert the tRNA specificities of IleRS into those of MetRS.32 When combined, this previous work and the present study highlight the importance of single tRNA-protein contacts in tRNA recognition and selection.
Importantly, we have not completed this interconversion because our Glu334Arg/Gly417Thr GluRS2 double mutant does not aminoacylate tRNAGlu1 at levels comparable to wild-type GluRS1 nor does it yet aminoacylate the Hp tRNAGlu2 isoacceptor (Lee and Hendrickson, unpublished). This partial conversion is not surprising because GluRS recognizes identity elements in the D-loop and acceptor stem of tRNAGlu as well as the anticodon.13 Thus, we expect that it will be necessary to expand the scope of our mutagenesis efforts if we are to truly convert GluRS2 into an enzyme with efficient GluRS1-like activity.
Supplementary Material
Acknowledgements
The authors thank the Research Corporation for a Research Innovation Award (RI0500) and Johns Hopkins University for funding. We also thank Prof. Marc Greenberg and Prof. Sarah Woodson for providing Phosphorimager time.
Abreviations used
- Af
Acidithiobacillus ferrooxidans
- Bs
Bacillus subtilis
- C
cytidine
- D-GluRS
discriminating glutamyl-tRNA synthetase
- Ec
Escherichia coli
- G
guanidine
- GlnRS
glutaminyl-tRNA synthetase
- Hp
Helicobacter pylori
- ND-GluRS
non-discriminating glutamyl-tRNA synthetase
- Tth
Thermus thermophilus
References
- 1.Ibba M, Soll D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000;69:617–50. doi: 10.1146/annurev.biochem.69.1.617. [DOI] [PubMed] [Google Scholar]
- 2.Tumbula D, Vothknecht UC, Kim HS, Ibba M, Min B, Li T, Pelaschier J, Stathopoulos C, Becker H, Soll D. Archaeal aminoacyl-tRNA synthesis: diversity replaces dogma. Genetics. 1999;152:1269–76. doi: 10.1093/genetics/152.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibba M, Soll D. Aminoacyl-tRNAs: setting the limits of the genetic code. Genes Dev. 2004;18:731–8. doi: 10.1101/gad.1187404. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox M, Nirenberg M. Transfer RNA as a cofactor coupling amino acid synthesis with that of protein. Proc. Natl. Acad. Sci. U. S. A. 1968;61:229–36. doi: 10.1073/pnas.61.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcox M. Gamma-phosphoryl ester of glu-tRNA-GLN as an intermediate in Bacillus subtilis glutaminyl-tRNA synthesis. Cold Spring Harb. Symp. Quant. Biol. 1969;34:521–8. doi: 10.1101/sqb.1969.034.01.059. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox M. Gamma-glutamyl phosphate attached to glutamine-specific tRNA. A precursor of glutaminyl-tRNA in Bacillus subtilis. Eur. J. Biochem. 1969;11:405–12. doi: 10.1111/j.1432-1033.1969.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 7.Curnow AW, Hong KW, Yuan R, Kim SI, Martins O, Winkler W, Henkin TM, Soll D. Glu-tRNAGln amidotransferase: a novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc. Natl. Acad. Sci. U. S. A. 1997;94:11819–26. doi: 10.1073/pnas.94.22.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skouloubris S, Ribas de Pouplana L, De Reuse H, Hendrickson TL. A noncognate aminoacyl-tRNA synthetase that may resolve a missing link in protein evolution. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11297–302. doi: 10.1073/pnas.1932482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salazar JC, Ahel I, Orellana O, Tumbula-Hansen D, Krieger R, Daniels L, Soll D. Coevolution of an aminoacyl-tRNA synthetase with its tRNA substrates. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13863–8. doi: 10.1073/pnas.1936123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamour V, Quevillon S, Diriong S, N’Guyen VC, Lipinski M, Mirande M. Evolution of the Glx-tRNA synthetase family: the glutaminyl enzyme as a case of horizontal gene transfer. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8670–4. doi: 10.1073/pnas.91.18.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JR, Doolittle WF. Gene descent, duplication, and horizontal transfer in the evolution of glutamyl- and glutaminyl-tRNA synthetases. J. Mol. Evol. 1999;49:485–95. doi: 10.1007/pl00006571. [DOI] [PubMed] [Google Scholar]
- 12.Siatecka M, Rozek M, Barciszewski J, Mirande M. Modular evolution of the Glx-tRNA synthetase family--rooting of the evolutionary tree between the bacteria and archaea/eukarya branches. Eur. J. Biochem. 1998;256:80–7. doi: 10.1046/j.1432-1327.1998.2560080.x. [DOI] [PubMed] [Google Scholar]
- 13.Sekine S, Nureki O, Sakamoto K, Niimi T, Tateno M, Go M, Kohno T, Brisson A, Lapointe J, Yokoyama S. Major identity determinants in the “augmented D helix” of tRNA(Glu) from Escherichia coli. J. Mol. Biol. 1996;256:685–700. doi: 10.1006/jmbi.1996.0118. [DOI] [PubMed] [Google Scholar]
- 14.Sekine S, Nureki O, Tateno M, Yokoyama S. The identity determinants required for the discrimination between tRNAGlu and tRNAAsp by glutamyl-tRNA synthetase from Escherichia coli. Eur. J. Biochem. 1999;261:354–60. doi: 10.1046/j.1432-1327.1999.00293.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim SI, Soll D. Major identity element of glutamine tRNAs from Bacillus subtilis and Escherichia coli in the reaction with B. subtilis glutamyl-tRNA synthetase. Mol Cells. 1998;8:459–65. [PubMed] [Google Scholar]
- 16.Hayase Y, Jahn M, Rogers MJ, Sylvers LA, Koizumi M, Inoue H, Ohtsuka E, Soll D. Recognition of bases in Escherichia coli tRNA(Gln) by glutaminyl-tRNA synthetase: a complete identity set. EMBO J. 1992;11:4159–65. doi: 10.1002/j.1460-2075.1992.tb05509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kambampati R, Lauhon CT. MnmA and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry. 2003;42:1109–17. doi: 10.1021/bi026536+. [DOI] [PubMed] [Google Scholar]
- 18.Sylvers LA, Rogers KC, Shimizu M, Ohtsuka E, Soll D. A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry. 1993;32:3836–41. doi: 10.1021/bi00066a002. [DOI] [PubMed] [Google Scholar]
- 19.Madore E, Florentz C, Giege R, Sekine S, Yokoyama S, Lapointe J. Effect of modified nucleotides on Escherichia coli tRNAGlu structure and on its aminoacylation by glutamyl-tRNA synthetase. Predominant and distinct roles of the mnm5 and s2 modifications of U34. Eur. J. Biochem. 1999;266:1128–35. doi: 10.1046/j.1432-1327.1999.00965.x. [DOI] [PubMed] [Google Scholar]
- 20.Sekine S, Nureki O, Shimada A, Vassylyev DG, Yokoyama S. Structural basis for anticodon recognition by discriminating glutamyl-tRNA synthetase. Nat. Struct. Biol. 2001;8:203–6. doi: 10.1038/84927. [DOI] [PubMed] [Google Scholar]
- 21.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–82. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varshney U, Lee CP, RajBhandary UL. Direct analysis of aminoacylation levels of tRNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J. Biol. Chem. 1991;266:24712–8. [PubMed] [Google Scholar]
- 23.Ravel JM, Wang SF, Heinemeyer C, Shive W. Glutamyl and Glutaminyl Ribonucleic Acid Synthetases of Escherichia Coli W. Separation, Properties, and Stimulation of Adenosine Triphosphate-Pyrophosphate Exchange by Acceptor Ribonucleic Acid. J. Biol. Chem. 1965;240:432–8. [PubMed] [Google Scholar]
- 24.Mitra K, Mehler AH. The role of transfer ribonucleic acid in the pyrophsphate exchange reaction of arginine-transfer ribonucleic acid synthetase. J. Biol. Chem. 1966;241:5161–2. [PubMed] [Google Scholar]
- 25.Mehler AH, Mitra SK. The activation of arginyl transfer ribonucleic acid synthetase by transfer ribonucleic acid. J. Biol. Chem. 1967;242:5495–9. [PubMed] [Google Scholar]
- 26.Lee LW, Ravel JM, Shive W. A general involvement of acceptor ribonucleic acid in the initial activation step of glutamic acid and glutamine. Arch. Biochem. Biophys. 1967;121:614–8. doi: 10.1016/0003-9861(67)90045-8. [DOI] [PubMed] [Google Scholar]
- 27.Deutscher MP. Rat liver glutamyl ribonucleic acid synthetase. II. Further properties and anomalous pyrophosphate exchange. J. Biol. Chem. 1967;242:1132–9. [PubMed] [Google Scholar]
- 28.Lapointe J, Soll D. Glutamyl transfer ribonucleic acid synthetase of Escherichia coli. II. Interaction with intact glutamyl transfer ribonucleic acid. J. Biol. Chem. 1972;247:4975–81. [PubMed] [Google Scholar]
- 29.Sekine S, Nureki O, Dubois DY, Bernier S, Chenevert R, Lapointe J, Vassylyev DG, Yokoyama S. ATP binding by glutamyl-tRNA synthetase is switched to the productive mode by tRNA binding. EMBO J. 2003;22:676–88. doi: 10.1093/emboj/cdg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagel GM, Doolittle RF. Phylogenetic analysis of the aminoacyl-tRNA synthetases. J. Mol. Evol. 1995;40:487–98. doi: 10.1007/BF00166617. [DOI] [PubMed] [Google Scholar]
- 31.Charron C, Roy H, Blaise M, Giege R, Kern D. Non-discriminating and discriminating aspartyl-tRNA synthetases differ in the anticodon-binding domain. EMBO J. 2003;22:1632–43. doi: 10.1093/emboj/cdg148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auld DS, Schimmel P. Switching recognition of two tRNA synthetases with an amino acid swap in a designed peptide. Science. 1995;267:1994–6. doi: 10.1126/science.7701322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.