Abstract
To create a scaffold that is suitable for the construction of tissue-engineered skin, a novel asymmetric porous scaffold with different pore sizes on either side was prepared by combining a collagen-chitosan porous membrane with fibrin glue. Tissue-engineered skin was fabricated using this asymmetric scaffold, fibroblasts, and a human keratinocyte line (HaCaT). Epidermal cells could be seen growing easily and achieved confluence on the fibrin glue on the upper surface of the scaffold. Scanning electron microscopy showed typical shuttle-like fibroblasts adhering to the wall of the scaffold and fluorescence microscopy showed them growing in the dermal layer of the scaffold. The constructed composite skin substitute had a histological structure similar to that of normal skin tissue after three weeks of culture. The results of our study suggest that the asymmetric scaffold is a promising biologically functional material for skin tissue engineering, with prospects for clinical applications.
Keywords: Collagen, Chitosan, Fibrin glue, Scaffold, Tissue-engineered skin
1. Introduction
In clinical practice, skin defects are common problems caused by burns, chronic ulcers, or trauma. Application of autogenous skin transplantation, the traditional therapeutic approach for such defects, is restricted in clinics by problems, such as the shortage of supply of normal tissue in extensively burned patients, possible scarring, and pigmentation changes. Fortunately, as a result of developments in biomaterials and life sciences, tissue-engineered skin can now provide an unrestricted supply for patients with skin defects.
Collagen is considered to be the most promising material for skin tissue engineering applications because of its excellent biocompatibility, degradability, low antigenicity, and abundance in mammals. Like collagen, chitosan has been applied in a variety of biomedical fields including skin tissue engineering. A collagen-chitosan porous scaffold with a pore size of 80–150 µm is suitable for dermal fibroblast proliferation and migration (Ma et al., 2003a; 2003b) and has potential as a candidate for skin tissue engineering with enhanced biostability and excellent biocompatibility. However, it is difficult for epidermal cells to grow and achieve confluence on the upper surface.
Asymmetric scaffolds differ from traditional porous scaffolds in that they have a heterogeneous structure, usually comprising two continuous parts with different pore sizes. Previous reports showed that this type of scaffold has better performance in the construction of tissue-engineered skin (Mao et al., 2003; Wang et al., 2006). Fibrin glue is a polymer of fibrinogen that has been reported to promote cell attachment, migration, and proliferation (Ahmed et al., 2000). As a hemostat and tissue adhesive, fibrin glue has been used widely in surgical operations. It is recognized as having good biocompatibility and degradability in vivo because of its porous net-like microstructure, which is associated with the concentration of fibrinogen and thrombin (Karp et al., 2004).
In this study, a novel scaffold with asymmetric structure was prepared by combining a collagen-chitosan porous membrane with fibrin glue and applied in the construction of tissue-engineered skin. The results suggest that asymmetric scaffolds could provide an ideal environment for cell growth and differentiation, and a promising method for the development of tissue-engineered skin.
2. Materials and methods
2.1. Materials
Collagen type I was extracted from fresh bovine tendon by the trypsin digestion and acetic acid dissolution method. Chitosan (viscosity average molecular weight (M η), 1.0×105–1.7×105; degree of deacetylation, 75%–85%), propidium iodide (PI), N,N-(3-dimethylaminopropyl)-N′-ethyl carbodiimide (EDC), N-hydroxysuccinimide (NHS), and 2-(N-morpholino)ethane sulfonic acid (MES) were purchased from Sigma (St. Louis, MO, USA). Fibrinogen and thrombin were purchased from Puji Biotechnique Development Co. (Hangzhou, China). Fluorescein diacetate (FDA) was obtained from Bio Basic Inc. (Ontario, Canada). All other reagents used were of reagent grade.
2.2. Culture of human dermal fibroblasts and keratinocytes
Isolation of primary human dermal fibroblasts was performed as follows. Fresh adult human foreskin biopsies were washed using phosphate buffered saline (PBS) with 1% (v/v) penicillin and streptomycin and chopped into small pieces, which were immersed in 40 U/mg thermolysin (St. Louis, MO, USA) at 4 °C overnight. The dermis pieces were separated from the epidermis with forceps, and treated with 0.2% (w/v) collagenase (St. Louis, MO, USA) at 37 °C for 1 h to harvest the fibroblasts. The harvested dermal fibroblasts were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified incubator containing 5% CO2 in air at 37 °C.
A human immortalized keratinocyte cell line (HaCaT) was purchased from American Type Culture Collection (ATCC; Rockville, MD, USA) and cultured in high glucose DMEM supplemented with 10% (v/v) FBS and 100 U/ml penicillin-streptomycin in a 5% CO2 incubator at 37 °C.
2.3. Preparation of collagen-chitosan porous scaffold
Collagen and chitosan solutions were dissolved respectively in 0.5 mol/L acetic acid solution to prepare a 0.5% (w/v) solution and then mixed in a mass ratio of 9:1. After deaeration under reduced pressure, the collagen-chitosan mixture was injected into a mold, frozen in a refrigerator at −20 °C for 2 h, and lyophilized for 24 h to obtain a porous collagen-chitosan scaffold, which was cross-linked at 105 °C for 24 h, followed by the treatment with EDC/NHS solution for 24 h. After washing with double-distilled water (10 min×5 times), the scaffolds were freeze-dried again to obtain the EDC/NHS-treated collagen-chitosan scaffolds (Sun, 2007).
2.4. Preparation of asymmetric scaffold
The porous scaffolds fabricated above were immersed in 75% ethanol overnight for sterilization, and then washed in PBS (10 min×5 times). The fibrinogen solution (80 mg/ml) was spread smoothly onto the surface of the collagen-chitosan scaffold with 50 µl/cm2, and then the thrombin solution (600 U/ml) was placed onto the fibrinogen layer in the same quantity. The scaffold was then incubated at 37 °C for 30 min to polymerize the fibrinogen. When fibrin glue formed, a collagen-chitosan/fibrin glue asymmetric scaffold was obtained (Sun, 2007).
2.5. Microstructure observation by scanning electron microscopy
After different experimental manipulations, specimens of the collagen porous scaffold, fibrin glue, and collagen-chitosan/fibrin glue asymmetric scaffold were fixed in 2.5% (w/v) glutaraldehyde for 30 min and 1% (w/v) osmic acid for 20 min, respectively, followed by ethanol gradient dehydration. Specimens were then dried in a critical-point drying apparatus. Finally, the samples were coated with gold-palladium (about 2–5 nm) and analyzed under a scanning electron microscopy (SEM; Stereoscan 260, Cambridge Instruments Ltd., Cambridge, UK).
2.6. Culture of HaCaT cells on collagen-chitosan/fibrin glue asymmetric scaffolds
HaCaT cells were seeded onto the upper surface of the asymmetric scaffolds at a density of 1×106 cells/cm2. After 2 h, HaCaT cells were submerged for 3 to 7 d in DMEM supplemented with 10% (v/v) FBS and 100 U/ml penicillin-streptomycin at 37 °C in a 5% CO2 incubator. These cells were transferred to a stainless steel mesh and cultured in air-liquid interface conditions for 21 d. Calcium chloride was added into the medium at a final concentration of 1.5 mmol/L. HaCaT cells were seeded at an equivalent density onto a scaffold without fibrin glue under the same culture conditions, as a control (Sun, 2007).
2.7. Construction of composite skin substitutes containing fibroblasts
Dermal fibroblasts were isolated from human dermis. After being digested in a flask, fibroblasts were adjusted to a concentration of 2×106 cells/ml and seeded onto the sterilized collagen-chitosan porous scaffold. The scaffold loaded with fibroblasts was incubated in DMEM supplemented with 10% (v/v) FBS and 100 U/ml penicillin-streptomycin at 37 °C in air containing 5% CO2 with medium changes every 3 d. One week later, this porous scaffold was transferred to another 6-well culture plate and incubated at 37 °C for 30 min. When the surface of the porous scaffold was somewhat dry, fibrinogen (80 mg/ml) and then thrombin (600 U/ml) were added onto the surface, each with 50 µl/cm2. Thirty minutes later, when the asymmetric scaffold formed, HaCaT cells were seeded directly onto the upper surface of the scaffold at a density of 1×106 cells/cm2 to construct composite skin. After submersion in the medium for 3 to 7 d, the composite skin substitutes were transferred to a stainless steel mesh, and cultured in air-liquid interface conditions. Paraffin-embedded sections of the composite skin substitutes were prepared after one, two, or three weeks (Sun, 2007).
2.8. Cell viability
FDA/PI staining was used to determine cell viability. A stock solution of FDA was made by mixing 10 mg of FDA with 1 ml of acetone. A working solution of FDA was freshly prepared by adding 0.04 ml of stock to 10 ml of Dulbecco’s phosphate buffered saline (DPBS). A solution of PI was prepared with 1 mg of PI and 50 ml of DPBS. The stock solution of FDA and the PI solution were stored in the dark at 4 °C, and 0.1 ml (4 µg) of FDA working solution and 0.03 ml (0.6 µg) of PI solution were added directly to the samples which were incubated for 10 min at room temperature, and then placed on ice. Cells were observed using a Zeiss Axiophot fluorescence microscope (AxioCam MRc, Zeiss, Germany). Microphotographs were acquired using a digital video camera (AxioCam MRc, Zeiss, Germany) and AxioVision Zeiss software.
2.9. Histological observation and immunohistochemistry
At the specified time intervals, the samples of tissue-engineered skin were fixed in 4% (w/v) formaldehyde overnight, dehydrated in a series of increasing ethanol concentrations, and embedded in paraffin. Sections (5 µm) were stained with haematoxylin and eosin (HE) for histological observations. Immunostaining was performed using primary mouse anti-human monoclonal antibody specific for pan-CK, CK-10 (Maixin_Bio, Fuzhou, China), and normal host serum which was regarded as a negative control, followed by staining with appropriate horse radish peroxidase (HRP)-conjugated secondary antibodies. The slides were processed in diaminobenzidine and redyed with haematoxylin solution, dehydrated and mounted in Permount. Images were visualized on an Olympus microscope (Olympus, Japan) and captured with an attached camera linked to a computer.
3. Results
3.1. Morphology of collagen-chitosan/fibrin glue asymmetric scaffold
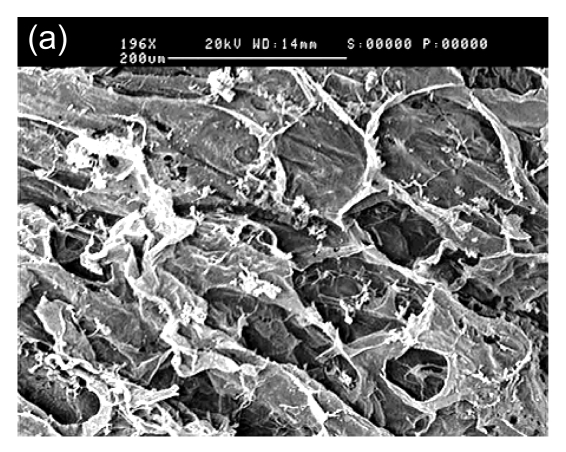
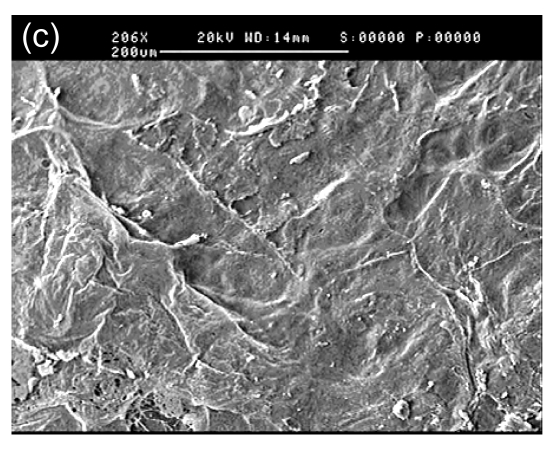
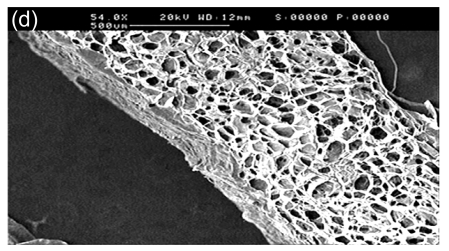
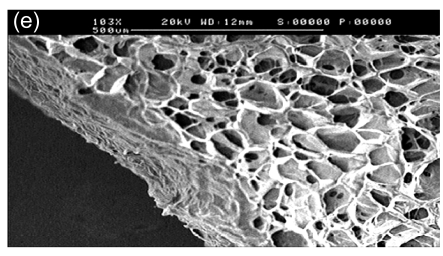
In SEM images, the collagen-chitosan porous scaffold showed a porous structure with a pore size from 80 to 150 µm (Fig. 1a). Fibrin glue polymerized by fibrinogen (80 mg/ml) in the presence of thrombin (600 U/ml) also had a porous and net-like structure (Fig. 1b). A smooth interface was revealed by SEM at the upper surface of the more compacted collagen-chitosan/fibrin glue asymmetric scaffold (Fig. 1c), which had an asymmetric bilayer structure in cross-section. The bottom layer composed of the collagen-chitosan porous scaffold was thicker with a loose and porous structure, while the top layer composed of fibrin glue was a thin layer with a tight structure. The range of pore size in the bottom layer and the top layer was 80–150 µm and 5–20 µm, respectively (Figs. 1d and 1e).
Fig. 1.
Surfaces of collagen-chitosan porous scaffold (a), fibrin glue (b), and asymmetric scaffold (c) scanned by SEM; Cross-section SEM images of the collagen-chitosan/fibrin glue asymmetric scaffold (d, e)
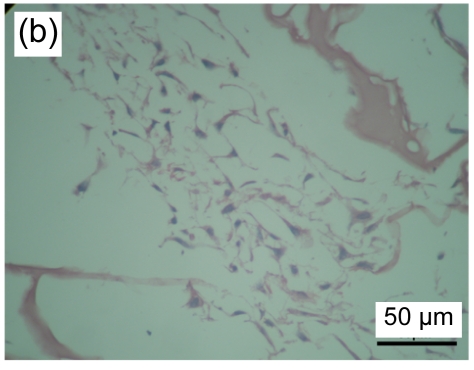
3.2. Cultured HaCaT cells in collagen-chitosan/fibrin glue asymmetric scaffold
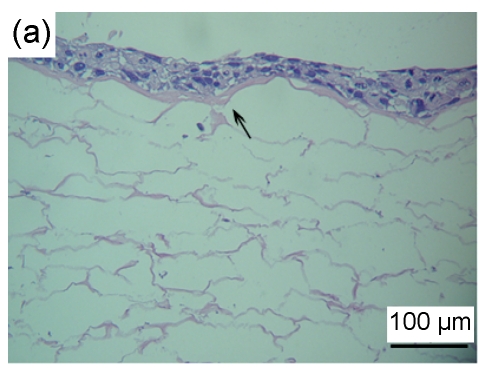
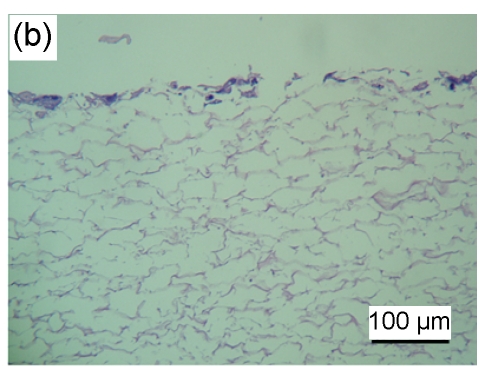
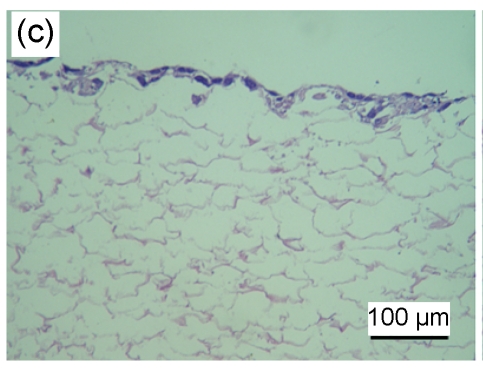
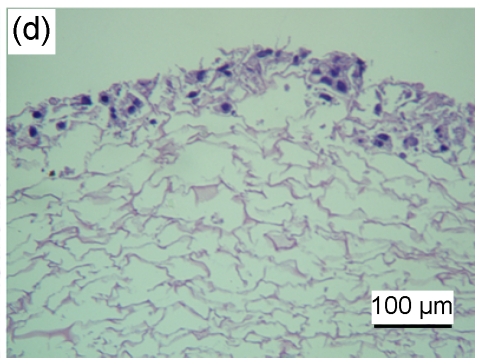
HaCaT cells of 3 to 4 layers were observed on the surface of the asymmetric scaffold after being cultured on an air-liquid interface for two weeks (Fig. 2a). Only a few cells were observed on the surface of the scaffold without the fibrin glue layer even though after three weeks of culture (Figs. 2b–2d).
Fig. 2.
HE staining of HaCaT cells cultured on the surfaces of collagen-chitosan/fibrin glue asymmetric scaffold (a) and collagen-chitosan porous scaffold without fibrin glue (b, c, d)
Black arrow indicates fibrin glue. HaCaT cells were cultured on an air-liquid interface for two weeks (a), one week (b), two weeks (c), and three weeks (d), respectively
3.3. Cell viability of fibroblasts in collagen-chitosan/fibrin glue asymmetric scaffold
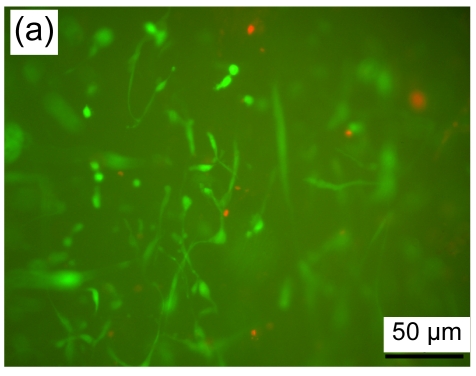
Cell viability of fibroblasts seeded in a collagen-chitosan/fibrin glue asymmetric scaffold was determined using FDA/PI staining. A large number of cells (green fluorescence) grew in the dermal layer of the asymmetric scaffold with shuttle-like or irregular morphology in fluorescence images (Fig. 3a). In concert with these results, fibroblasts and an abundance of extracellular matrix were found in SEM images (Fig. 3b).
Fig. 3.
Fibroblasts seeded in the asymmetric scaffold
(a) FDA/PI staining, cultured for one week (fluorescence microscope); (b) SEM micrograph, cultured for two weeks. Black arrows indicate extracellular matrix
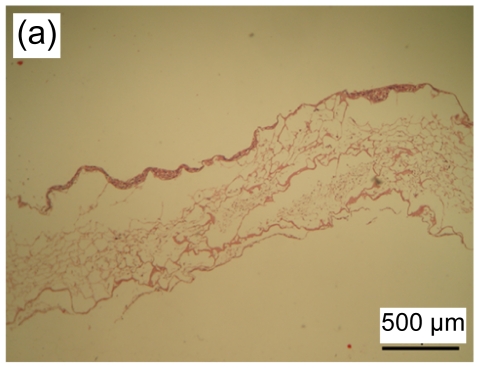
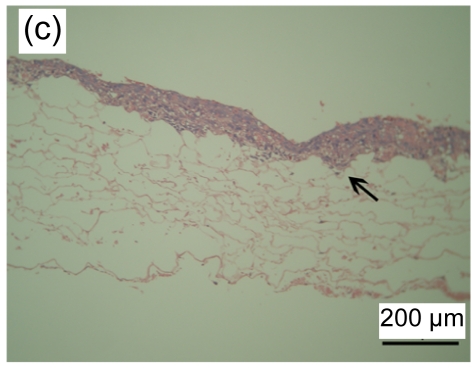
3.4. Histological observation of tissue-engineered skin
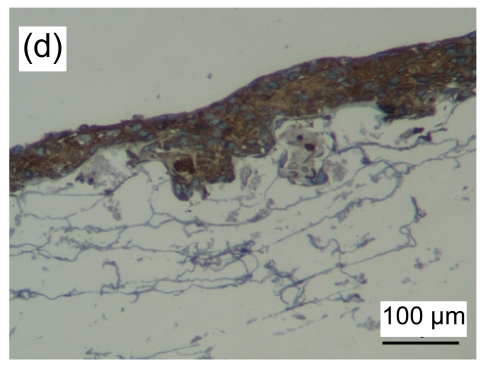
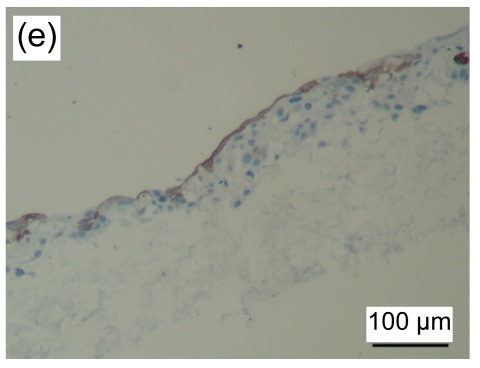
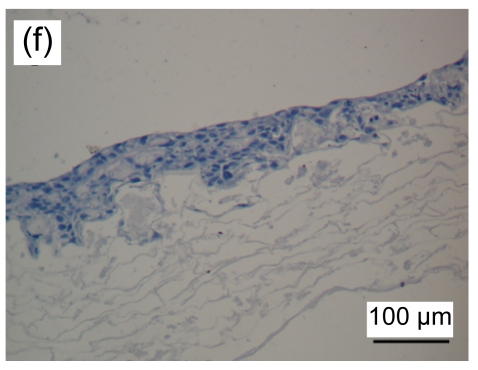
HE stained images showed that a large number of fibroblasts grew in the bottom layer of the asymmetric scaffold after cultivation in air-liquid interface conditions for one week. HaCaT cells began to differentiate into a multilayer structure on the top layer (Figs. 4a and 4b) after one week of culture and developed into an epidermis-like structure with 7 to 10 layers of cells (Fig. 4c) after three weeks. Immunohistochemical examination revealed that pan-CK positive cells were in the entire layer of the epidermis, but CK-10 positive cells were only on the upper part after two weeks of cultivation (Figs. 4d–4f). The results showed that the constructed composite skin substitute of multilayer epidermis had a histological structure similar to that of normal skin.
Fig. 4.
Histology of composite tissue-engineered skin when cultured for different time on the air-liquid interface
(a, b) one week, HE staining; (c) three weeks, HE staining. Black arrow indicates the epidermal layer which developed a rete ridge-like structure inserted into the dermal layer in some areas. Immunohistochemical examination of tissue-engineered skin cultured for two weeks on an air-liquid interface: pan-CK (d), CK-10 (e), and control groups (f)
4. Discussion
Biomaterial is very important in the field of tissue engineering. As a vehicle for seeding cells, it provides a three-dimensional (3D) space for cell proliferation, nutrition metabolism, and extracellular matrix secretion. An ideal scaffold for use in skin tissue engineering should have a suitable microstructure, porosity, controllable biodegradability, good biocompatibility, and suitable mechanical properties (Mao et al., 2003).
Fibrin glue is a polymer (fibrinogen-thrombin) with a 3D net-like structure (Blomback and Bark, 2004) and has good compatibility, biodegradation, and plasticity (Ye et al., 2000). It has been applied widely in the field of tissue engineering and combined with seed cells or growth factors, has been used as a carrier to repair skin defects (Wechselberger et al., 2002; Kneser et al., 2005).
While studies have indicated that the porous structure of a collagen-chitosan scaffold is suitable for fibroblast growth, proliferation, and migration, it has been difficult for epidermal cells to grow and achieve confluence on a collagen-chitosan scaffold porous surface. Thus, different types of asymmetric bilayer scaffolds have been developed for artificial skin (Mao et al., 2003; Wang et al., 2006). These asymmetric bilayer scaffolds showed superiority in the construction of tissue-engineered skin, but it was still hard to effectively inoculate epidermal cells into the small-pore layer and fibroblasts into the large-pore layer using a “one-step shaping” method. In this study, to improve the effectiveness of inoculation and the adhesion of epidermal cells, a collagen-chitosan/fibrin glue asymmetric scaffold was prepared using a “two-step shaping” method and was used to construct tissue-engineered skin. Epidermal cells grew and achieved confluence easily on the fibrin glue (i.e., the upper surface of the asymmetric scaffold). Therefore, we theorized that a collagen-chitosan porous scaffold with a rough surface structure of large pore size was not suitable for the adhesion, growth, confluence, and differentiation of epidermal cells, while the smooth interface supplied by fibrin glue in the asymmetric scaffold was suitable. Moreover, in an asymmetric scaffold, fibrin glue can isolate epidermal cells and fibroblasts and make them grow in their respective regions without affecting the other side. Meanwhile, the micropores of fibrin glue permit the communication of nutrition and growth factors between the two layers.
It has been reported that cell viability is related to aspects of the microstructure of scaffolds, such as surface property, pore size, porosity, and connectivity of pores (Sechriest et al., 2000; Wang et al., 2003). In this study, cell viability was evaluated by FDA/PI fluorescence staining and SEM. Under a fluorescence microscope, most living cells emitted green fluorescence with shuttle-like or irregular morphology, while a few necrotic cells emitted red fluorescence. SEM images revealed that the fibroblasts adhered to the walls of the scaffold and an abundance of extracellular matrix was secreted in this area, suggesting fibroblasts had good growth and the asymmetric scaffold had excellent cell compatibility.
Regarding culture conditions, several reports demonstrated that the concentration of calcium ions in the medium has an influence on the proliferation and differentiation of keratinocytes: a low concentration (<0.3 mmol/L) promotes proliferation of the cells, while a high concentration (>0.6 mmol/L) promotes terminal differentiation (Boyce and Ham, 1983; Yu et al., 2002). Hence, in our experiments, 1.5 mmol/L of calcium chloride was supplemented in the medium after the cell-scaffold constructs were shifted onto the air-liquid interface. A multiple layered pan-CK positive epithelium structure was observed after one week of cultivation on an air-liquid interface and became more apparent after two weeks. In the top area of the epidermis in the constructed skin, positive cell staining by the CK-10 antibody was noted, which indicated that HaCaT cells had begun to differentiate into terminal cells, but no complete corneous layer was observed. Nevertheless, no distinct differentiation was observed in the basal, spinous, or granular layers. This may have been because the seeding cells we used in the study were of a cell lineage rather than normal keratinocytes.
Currently, gel and porous materials are used widely in the construction of tissue-engineered skin. In this study, we made the best of the advantages of both materials and successfully developed a composite asymmetric scaffold. Our results showed that cells could be seeded in different layers and grew easily in the collagen-chitosan/fibrin glue asymmetric scaffold. Although further studies are necessary, our study on tissue-engineered skin suggests that such scaffolds may have potential for clinical applications.
Footnotes
Project supported by the National Basic Research Program (973) of China (No. 2005CB623902-1) and the Science Research Foundation of the Ministry of Health of China (No. WKJ2006-2-2007)
References
- 1.Ahmed Z, Underwood S, Brown RA. Low concentrations of fibrinogen increase cell migration speed on fibronectin/fibrinogen composite cables. Cell Motility and the Cytoskeleton. 2000;46(1):6–16. doi: 10.1002/(SICI)1097-0169(200005)46:1<6::AID-CM2>3.3.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 2.Blomback B, Bark N. Fibrinopeptides and fibrin gel structure. Biophysical Chemistry. 2004;112(2-3):147–151. doi: 10.1016/j.bpc.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. Journal of Investigative Dermatology. 1983;8l(Suppl. 1):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 4.Karp JM, Sarraf F, Shoichet MS, Davies JE. Fibrin-filled scaffolds for bone tissue engineering: an in vivo study. Journal of Biomedical Materials Research Part A. 2004;71(1):162–171. doi: 10.1002/jbm.a.30147. [DOI] [PubMed] [Google Scholar]
- 5.Kneser U, Voogd A, Ohnolz J, Buettner O, Stangenberg L, Zhang YH, Zhang YH, Stark GB, Schaefer DJ. Fibrin gel-immobilized primary osteoblasts in calcium phosphate bone cement: in vivo evaluation with regard to application as injectable biological bone substitute. Cells Tissues Organs. 2005;179(4):158–169. doi: 10.1159/000085951. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, Gao C, Zhou J, Shen J, Hu X, Han C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 2003;24(26):4833–4841. doi: 10.1016/S0142-9612(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 7.Ma L, Gao CY, Mao ZW, Shen J, Hu X, Han CM. Thermal dehydration treatment and glutaraldehyde cross-linking to increase the biostability of collagen-chitosan porous scaffolds used as dermal equivalent. Journal of Biomaterials Science, Polymer Edition. 2003;14(8):861–874. doi: 10.1163/156856203768366576. [DOI] [PubMed] [Google Scholar]
- 8.Mao J, Zhao L, de Yao K, Shang Q, Yang G, Cao Y. Study of novel chitosan-gelatin artificial skin in vitro. Journal of Biomedical Materials Research Part A. 2003;64(2):301–308. doi: 10.1002/jbm.a.10223. [DOI] [PubMed] [Google Scholar]
- 9.Sechriest VF, Miao YJ, Niyibizi C, Westerhausen-Larson A, Matthew HW, Evans CH, Fu FH, Suh JK. GAG-augmented polysaccharide hydroglue: a novel biocompatible and biodegradable material to support chondrogenesis. Journal of Biomedical Materials Research. 2000;49(4):534–541. doi: 10.1002/(SICI)1097-4636(20000315)49:4<534::AID-JBM12>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Sun JZ. Preparation of Collagen-Chitosan/Fibrin Glue Asymmetric Scaffold and Its Application in Construction of Tissue Engineered Skin. Hangzhou, China: Zhejiang University; 2007. (MS Thesis) [Google Scholar]
- 11.Wang TW, Huang YC, Sun JS, Lin FH. Organotypic keratinocyte fibroblast cocultures on a bilayer gelatin scaffold as a model of skin equivalent. Biomedical Sciences Instrumentation. 2003;39:523–528. [PubMed] [Google Scholar]
- 12.Wang TW, Wu HC, Huang YC, Sun JS, Lin FH. Biomimetic bilayered gelatin-chondroitin 6 sulfate-hyaluronic acid biopolymer as a scaffold for skin equivalent tissue engineering. Artificial Organs. 2006;30(3):141–149. doi: 10.1111/j.1525-1594.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 13.Wechselberger G, Russell RC, Neumeister MW, Schoeller T, Piza-Katzer H, Rainer C. Successful transplantation of three tissue-engineered cell types using capsule induction technique and fibrin glue as a delivery vehicle. Plastic and Reconstructive Surgery. 2002;110(1):123–129. doi: 10.1097/00006534-200207000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Ye Q, Zünd G, Benedikt P, Jockenhoevel S, Hoerstrup SP, Sakyama S, Hubbell JA, Turina M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. European Journal of Cardio-Thoracic Surgery. 2000;17(5):587–591. doi: 10.1016/S1010-7940(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 15.Yu HQ, Zhou Y, Hua P, Tan WS. Calcium-regulated growth and differentiation of the mouse epidermal keratinocytes. Sheng Wu Gong Cheng Xu Bao. 2002;18:626–629. (in Chinese) [PubMed] [Google Scholar]