Abstract
Cancer metastasis to the thyroid is extremely rare. The more commonly reported primary sites for metastasis to the thyroid are the kidney, breast, lung, colon, esophagus, and uterus. Thyroid metastasis from the stomach has only been reported in three cases. Herein, we report a 71-year-old man presenting with bilateral thyroid multinodular lesions. Bilateral near-total thyroidectomy was performed due to airway compression with related symptoms. Wedge resection of a suspicious pulmonary nodule, detected on CT, was performed for diagnosis. Polypoid lesions in the stomach were examined by trans-scopic biopsy. Poorly differentiated adenocarcinomas with the same histological profiles were noted at these three sites. The immunohistochemical staining for thyroglobulin of these specimens was negative. We conclude that a new thyroid mass appearing in a patient with present or prior malignancies should raise the concern of metastatic disease.
Keywords: Gastric carcinoma, Thyroid metastasis, Lung metastasis
1. Introduction
Metastasis to the thyroid is clinically uncommon, and is typically found only in autopsy cases. Its differentiation from other benign or primary neoplasms of the thyroid is difficult. The more common primary sites for metastasis to the thyroid are the kidney, breast, lung, colon, esophagus, and uterus (Nakhjavani et al., 1997; Wood et al., 2004; Kim et al., 2005; Cichoń et al., 2006; Gerges et al., 2006; Duggal and Horattas, 2008). Thyroid metastasis from the stomach (including carcinoma and sarcoma) has only been reported in three cases in the English-language literature (Chen et al., 1999; Cichoń et al., 2006; Peparini et al., 2008). Herein, a case of proven metastasis of gastric carcinoma to the thyroid and lung is reported.
2. Case report
A 71-year-old man complained of progressive shortness of breath, palpitation, and chest tightness for one week, and was sent to our emergency room for further evaluation. He had ischemic heart disease, hypertension, diabetes mellitus, and chronic liver disease for many years, with relative stability. He had a further history of bilateral pleural empyemas treated six months prior, this having been accompanied by weight loss, anemia with tarry stool passage, and anorexia. On physical examination, he was cachectic, anxious, and dyspneic, but conscious and oriented. The conjunctivae were pale. Firm and large bilateral thyroid masses were noted on neck palpation. Labored breathing with accessory muscles use was noted. Chest auscultation revealed stridor in inspiration due to upper airway compression by the thyroid mass. The lung parenchymal sound was coarse with some dry crackles and friction rubs. The abdomen was slightly protruded with shifting dullness. Laboratory tests revealed mild anemia (hemoglobin level 8.8 g/dl), with 3+ occult blood on stool examination. Chest X-ray revealed the thyroid mass compressing and deviating the tracheal lumen (Fig. 1). Chest and neck computed tomography (CT) revealed that the trachea was compressed by the thyroid mass and that miliary nodules were noted in both lungs (Fig. 2). Ultrasound-guided needle aspiration of the thyroid revealed atypical cells with a high suspicion of malignancy. Esophagogastroduodenoscopy revealed three 1 to 2 cm sessile and polypoid masses at the greater curvature side of the body of stomach (Fig. 3), and pathology taken from trans-endoscopic biopsy revealed poorly differentiated adenocarcinoma (Fig. 4a). The patient underwent bilateral near-total thyroidectomy. Histological examination from the resected specimen revealed poorly-differentiated carcinoma, with characteristics similar to the gastric lesions (Fig. 4b), and negative staining for thyroid transcription factor-1 (TTF-1). The miliary nodules of the lung taken from biopsy through video-assisted thoracoscopic surgery (VATS) were shown to have similar histological profiles as the gastric lesions (Fig. 4c), with negative staining for TTF1. Subsequent evaluation, including a negative I-131 scan, excluded the possibility of lung metastasis from the thyroid, and a Ga-67 tumor scan revealed the stomach as the only possible primary site of cancer. The patient’s postoperative course was uneventful and he was advised to undergo further chemotherapy for the control of his disease. His family, however, refused any type of adjuvant chemotherapy or radiotherapy, and he died four months later.
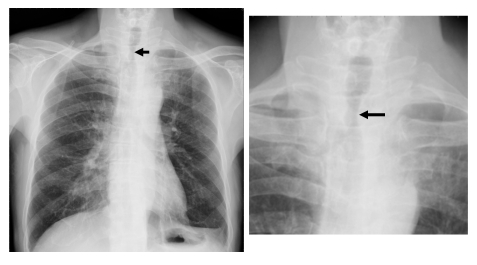
Fig. 1.
Chest X-ray examination revealing the thyroid mass compressing and deviating the tracheal lumen (arrows)
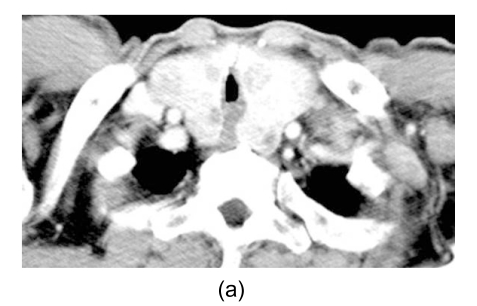
Fig. 2.
Computed tomography scan revealing the trachea being compressed by the thyroid mass (a) and miliary nodules in both lungs (b)
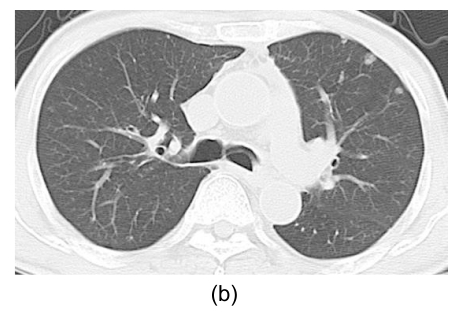
Fig. 3.
PES revealing sessile and polypoid masses at the greater curvature side of upper body of the stomach
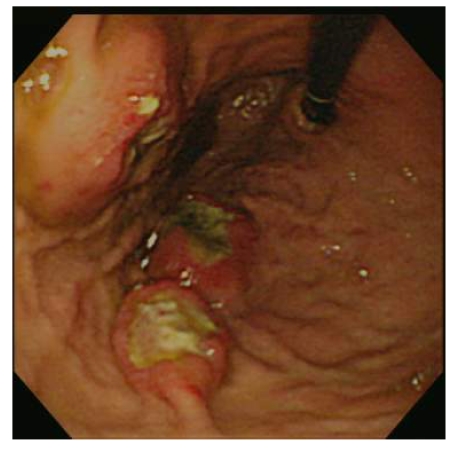
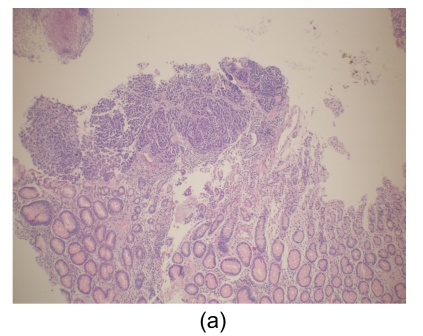
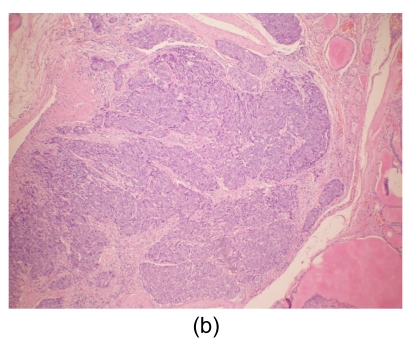
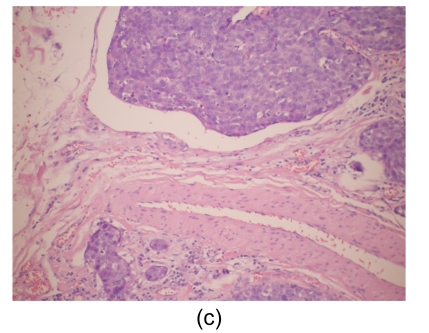
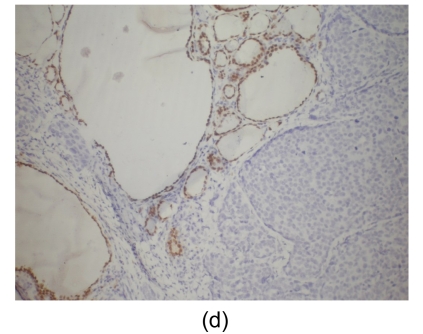
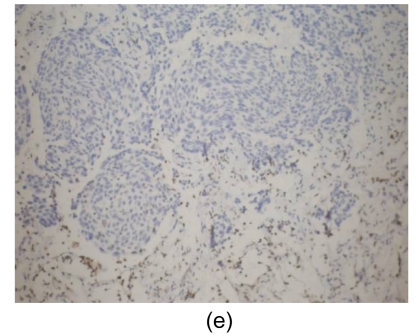
Fig. 4.
Histological findings
(a) Stomach. Tumor cells arise from the gastric mucosa (hematoxylin & eosin (H & E) staining, magnification 40×); (b) & (c) Thyroid. Multi-focal nests of tumor cells are distributed between the follicles (b) (H & E staining, 40×) and in the vascular lumen (c) (H & E staining, 200×); (d) & (e) Lung. Negative staining for thyroid transcription factor-1 (TTF-1) (d) and thyroglobulin (e) in tumor cells (200×)
Microscopically, tumor cells seen in the gastric mucosa, thyroid, and lung revealed the same histological features. Factors that led us to believe the tumor cells in the gastric mucosa were primary and those in the thyroid and lung metastatic are as follows. First, tumor cells in the gastric mucosa were seen to arise from the mucosa glands. Second, tumor cells in the thyroid were multifocal and were seen in the stroma, mostly in between the follicular cells, and several tumor cells were seen within the blood vessel lumen. Third, tumor cells in the lungs were multifocal and several were also seen within blood vessel lumen. Furthermore, immunohistochemical studies were negative for thyroglobulin and TTF-1 (Figs. 4d and 4e).
3. Discussion
Metastasis to the thyroid may be less rare than previously described because the clinical findings are far less common than the postmortem findings. The incidence in autopsy series has varied, ranging from 1.25% in unselected cases to 24% in patients with widespread malignant neoplasms (Abrams et al., 1950; Berge and Lundberg, 1977; Ivy, 1984; Parker et al., 1996; Nakhjavani et al., 1997). Metastasis to the thyroid, however, only accounts for 2%–3% of all clinical cases with malignant tumors of the thyroid (Cichoń et al., 2006). Breast and lung carcinomas have been reported as the most common two metastatic diseases to the thyroid in autopsy series (Abrams et al., 1950; Berge and Lundberg, 1977; Ivy, 1984; Parker et al., 1996; Nakhjavani et al., 1997). In contrast, renal cell carcinoma has been found to be the most common source of metastasis in clinical cases, followed by lung and breast (Nakhjavani et al., 1997; Chen et al., 1999). Esophageal and colon carcinomas are the two most frequently reported gastrointestinal sources of metastasis to the thyroid (Chen et al., 1999; Cichoń et al., 2006). Thyroid metastasis from gastric neoplasms, including carcinomas and sarcomas, is extremely rare, and only three cases have been reported previously (Chen et al., 1999; Cichoń et al., 2006; Peparini et al., 2008). In our reported case, lung metastasis was also noted. Thyroid and lung metastases have never been reported in gastric carcinoma, but have been reported in cases of malignancy in other regions of the alimentary tract, such as the colon (Hanna et al., 2006).
Accurate diagnosis of a thyroid lesion as a benign, primary, or metastatic neoplasm is a significant challenge; primary and secondary lesions do not show specific features on imaging studies, such as computed tomography or ultrasound (Hurlimann et al., 1987; Nakhjavani et al., 1997; Cheng et al., 2008; Peparini et al., 2008). Furthermore, a very long time interval (from 5 years to more than 20 years) between the diagnosis of primary malignancy (usually renal cell carcinoma) and the thyroid metastasis is not uncommon. This results in difficulty in diagnosis (Nakhjavani et al., 1997; Peparini et al., 2008).
Cytological or histological examination of thyroid specimens from fine needle aspiration (FNA) can aid in the differentiation between benign and malignant thyroid diseases (Kim et al., 2005; Peparini et al., 2008). Its value, however, in the discrimination between primary and metastatic thyroid malignancies is still controversial. It is very difficult to differentiate between high grade metastatic and primary anaplastic carcinomas of thyroid gland by cytological or histological examination from FNA specimens (Kim et al., 2005). A pathological picture of the thyroid gland being compatible with the primary tumor and the negative result of immunohistochemical staining for thyroglobulin or TTF-1 suggest, but cannot confirm, the diagnosis of metastatic lesions because 20%–30% of primary anaplastic carcinomas of thyroid are also negative for the above staining (Abrams et al., 1950; Hurlimann et al., 1987; Peparini et al., 2008). Other diagnostic procedures, such as tru-cut biopsy or surgery (total thyroidectomy, subtotal thyroidectomy, or lobectomy) can be used for either diagnosis or treatment (Wood et al., 2004). In our reported case the possibility of primary anaplastic carcinoma of thyroid cannot be excluded because of its rapid growing with airway compression, and thus surgical resection was required. Pathological findings revealed multi-focal lesions with similar pathologies to the gastric lesions (poor-differentiated adenocarcinomas, instead of anaplastic type) and negative TTF-1 staining. The lung lesions revealed similar pathological profiles to the thyroid and gastric ones. Thus we consider that in this case the lesions in the stomach are the primary ones, and metastases to the thyroid and lung occurred.
The appearance of metastasis in the thyroid gland presenting with widespread localization in several organs (miliary nodules in the case reported) indicates poor prognosis and an oncological indication for urgent thyroidectomy. In this case, surgery was necessary to relieve threatening airway obstruction due to tracheal compression. Nonetheless, it has been demonstrated that metastatic involvement of the thyroid has the same impact on prognosis as nonthyroidal metastases. Indeed, if the thyroid represents the only secondary localization of a primary tumor (thyroid localization of clear cell renal carcinoma is often unique), surgical resection with total thyroidectomy is indicated to avoid further dissemination of the primary tumor, especially if the primary carcinoma has been resected (Wood et al., 2004).
Methods of tissue harvesting for the diagnosis of undetermined lung lesions include FNA or tru-cut biopsy under the guide of ultrasound or computed tomography (Libby et al., 2004). These methods, however, are very challenging in terms of accurate sampling sub-centimeter or non-subpleural lesions, as in on our presented case. Video-assisted thoracoscopic surgery has been widely used in many procedures for the diagnosis or treatment of intrathoracic diseases, including the diagnosis of undetermined lung lesions (Luh et al., 2005; Luh and Liu, 2006; Hsu et al., 2009). Lung metastasis from the stomach is a reasonable diagnosis in the presented case because his lung lesions were multiple and small, unlike the single location of his gastric neoplasm.
In conclusion, a new thyroid mass appearing in a patient with a present or past history of malignancy should raise the possibility of metastasis. Although the prognosis of metastasis in the thyroid is usually poor, aggressive medical or surgical treatment is still required to relieve the acute life threatening conditions (airway obstruction), and may provide the opportunity for other treatments affecting overall survival.
Footnotes
This manuscript was presented as a poster at the Asian Pacific Digestive Week in Taipei, Taiwan, China (Sept. 27–30, 2009), and the abstract has been accepted for publication in Journal of Gastroenterology and Hepatology, 2009, 24(Suppl. 1):A2
References
- 1.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3(1):74–85. doi: 10.1002/1097-0142(1950)3:1<74::AID-CNCR2820030111>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Berge T, Lundberg S. Cancer in Malmo 1958–1969. an autopsy study. Acta Pathologica et Microbiologica Scandinavica Supplement. 1977;(260):1–235. [PubMed] [Google Scholar]
- 3.Chen H, Nicol TL, Udelsman R. Clinically significant, isolated metastatic disease to the thyroid gland. World Journal of Surgery. 1999;23(2):177–180. doi: 10.1007/PL00013162. [DOI] [PubMed] [Google Scholar]
- 4.Cheng JH, Huang YC, Kuo C, Lai YS, Wu TC, Tsao TC, Luh SP, Tsai CB. Double primary bronchogenic carcinoma of the lung and papillary thyroid carcinoma: a case report. Journal of Medical Case Reports. 2008;2:309. doi: 10.1186/1752-1947-2-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cichoń S, Anielski R, Konturek A, Barczynski M, Cichon W. Metastases to the thyroid gland: seventeen cases operated on in a single clinical center. Langenbeck’s Archives of Surgery. 2006;391(6):581–587. doi: 10.1007/s00423-006-0081-1. [DOI] [PubMed] [Google Scholar]
- 6.Duggal NM, Horattas MC. Metastatic renal cell carcinoma to the thyroid gland. Endocrine Practice. 2008;14(8):1040–1046. doi: 10.4158/EP.14.8.1040. [DOI] [PubMed] [Google Scholar]
- 7.Gerges AS, Shehata SR, Gouda IA. Metastasis to the thyroid gland; unusual site of metastasis. Journal of the Egyptian National Cancer Institute. 2006;18(1):67–72. [PubMed] [Google Scholar]
- 8.Hanna WC, Ponsky TA, Trachiotis GD, Knoll SM. Colon cancer metastatic to the lung and the thyroid gland. Archives of Surgery. 2006;141(1):93–96. doi: 10.1001/archsurg.141.1.93. [DOI] [PubMed] [Google Scholar]
- 9.Hsu KY, Lee HC, Ou CC, Luh SP. Value of video-assisted thoracoscopic surgery in the diagnosis and treatment of pulmonary tuberculoma: 53 cases analysis and review of literature. Journal of Zhejiang University SCIENCE B. 2009;10(5):375–379. doi: 10.1631/jzus.B0820368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurlimann J, Gardiol D, Scazziga B. Immunohistology of anaplastic thyroid carcinoma. A study of 43 cases. Histopathology. 1987;11(6):567–580. doi: 10.1111/j.1365-2559.1987.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 11.Ivy HK. Cancer metastatic to the thyroid: a diagnostic problem. Mayo Clinic Proceedings. 1984;59(12):856–859. doi: 10.1016/s0025-6196(12)65622-5. [DOI] [PubMed] [Google Scholar]
- 12.Kim TY, Kim WB, Gong G, Hong SJ, Shong YK. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clinical Endocrinology. 2005;62(2):236–241. doi: 10.1111/j.1365-2265.2005.02206.x. [DOI] [PubMed] [Google Scholar]
- 13.Libby DM, Smith JP, Altorki NK, Pasmantier MW, Yankelevitz D, Henschke CI. Managing the small pulmonary nodule discovered by CT. Chest. 2004;125(4):1522–1529. doi: 10.1378/chest.125.4.1522. [DOI] [PubMed] [Google Scholar]
- 14.Luh SP, Liu HP. Video-assisted thoracic surgery—the past, present status and the future. Journal of Zhejiang University-SCIENCE B. 2006;7(2):118–128. doi: 10.1631/jzus.2006.B0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luh SP, Chou MC, Wang LS, Chen JY, Tsai TP. Video-assisted thoracoscopic surgery in the treatment of complicated parapneumonic effusions or empyemas: outcome of 234 patients. Chest. 2005;127(4):1427–1432. doi: 10.1378/chest.127.4.1427. [DOI] [PubMed] [Google Scholar]
- 16.Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79(3):574–578. doi: 10.1002/(SICI)1097-0142(19970201)79:3<574::AID-CNCR21>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA: A Cancer Journal for Clinicians. 1996;46(1):5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- 18.Peparini N, Di Matteo FM, Maturo A, Marzullo A, Campana FP. Unusual metastasis of gastrointestinal stromal tumor misdiagnosed as anaplastic thyroid carcinoma. International Journal of Surgery. 2008;6(5):415–417. doi: 10.1016/j.ijsu.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Wood K, Vini L, Harmer C. Metastases to the thyroid gland: the Royal Marsden experience. European Journal of Surgical Oncology. 2004;30(6):583–588. doi: 10.1016/j.ejso.2004.03.012. [DOI] [PubMed] [Google Scholar]