Abstract
Objective
Compare continence system function of Black and White women in a population-based sample.
Methods
As part of a cross-sectional population-based study Black and White women ages 35–64 years were invited to have pelvic floor testing to achieve pre-specified groups of women with and without urinary incontinence. We analyzed data collected from 335 women classified as continent (n=137) and stress (n=102) and urge incontinent (n=96) based on full bladder stress test and symptoms. Continence system functions were compared across racial and continence groups.
Results
Comparing Black to White women, maximal urethral closure pressure (MUCP) was 22% higher in Blacks than Whites (68.0 vs. 55.8 cm H2O, p<0.0001). White and Black women with stress incontinence had MUCP 19% and 23% lower than continent women. MUCP in urge incontinent White women was as low as stress incontinent Whites, but Blacks with urge had normal urethral function.
Conclusion
Black women have higher urethral closure pressures than White women. White women with urge incontinence, but not Black women, have reduced MUCP.
Keywords: Urinary incontinence, racial differences, urethral closure pressure, urethral axis, epidemiology, prevalence
BACKGROUND AND OBJECTIVE
Urinary incontinence is a common and distressing condition whose care costs $16 billion dollars each year.1 Clinical evaluation has suggested differences in incontinence prevalence between Black and White women.2 Survey-based studies have confirmed these differences, indicating that Black women are less likely than White women to experience urinary incontinence.3–7 In a recently conducted population-based investigation in Southeastern Michigan named Establishing the Prevalence of Incontinence (EPI) Study with adequate sampling of Black women, we found the prevalence of urinary incontinence to be 14.6% for Black women and 33.1% for White women confirming several other reports of lower incontinence rates in Black women.8 A larger proportion of White women with incontinence reported symptoms of pure stress urinary incontinence (UUI;SUI; 39.2%) compared to Black women (25.0%), whereas a larger proportion of Black women (23.8%) reported symptoms of pure urge urinary incontinence (UUI) compared to White women (11.0%), confirming the observations of other studies.9,10 In the EPI study, the distribution of lifestyle and risk factors were generally similar by race.8 Therefore, the reason for higher prevalence of urinary incontinence, especially SUI, in Whites remained unknown. Stress continence depends on the strength of the continence system and the pressures to which it is subjected.11 The continence system consists of the urethral sphincters and their supports, including both endopelvic fascia and the levator ani muscles. In a recent study of stress incontinent women we found that poor urethral sphincteric function was the primary determinant of SUI,12 but the importance of urethral function in determining UUI was not examined. In a prior study we have shown how nulliparous Black women have better urethral function than Whites 13, and this observation may help explain the disparity in SUI symptoms by race.8 But the relative contributions of urethral function, support, and other factors in Black and White continent and incontinent women with either SUI or urge incontinence are not known.
In this study, we compare continence system functions in a population-based sample of continent and incontinent Black and White women to determine the relative contributions of urethral sphincteric function and urethral support to incontinence. Such knowledge should lead to a better understanding of reasons underlying disparities in incontinence and could have important implications not only for more targeted treatment but also to identify potentially modifiable risk factors.
MATERIALS AND METHODS
The EPI study was designed in two phases. As previously described, the first phase of the study involved a telephone interview regarding self-reported incontinence drawn from a community-based sample of women residing in Southeastern Michigan.8 In brief, women ages 35–64 were sampled from telephone records including three Southeast Michigan counties with over-sampling of Black women to ensure adequate representation by race. Of the 12,541 telephone numbers purchased, 9,199 (73.4%) were qualifying households that were contacted and screened. Of these, 3,692 (40.1%) households had an eligible woman resident and 2,814 completed the survey (1,922 Black, 892 White), for a 76.2% response rate. The telephone call was conducted by, trained female interviewers from the Institute for Social Research at the University of Michigan. Women were asked to self-identify their race. If self-identifying as Black or White race the interview progressed to questions about their demographic, health history, lifestyle, and obstetric/gynecologic characteristics as well as their urinary incontinence experience. Those who self-identified as other than of Black or White race were excluded. In the second phase of the EPI study, the focus of this manuscript, a subset of the women who participated in the telephone interview was invited to undergo urodynamic and pelvic floor testing in the clinic.
A priori sample size calculations conducted at the outset of the larger EPI Study8 indicated need for 50 to 65 Black and White women in each continence status (continent, SUI, UUI) to achieve power of 0.80 to detect effect sizes of 0.44 to 0.47 in comparing pelvic floor testing parameters. Recruitment was carried out to achieve groups of these sizes. Final group numbers differ somewhat from original targets because it is not possible to completely predict a subject’s continence status on urodynamic testing based on the telephone interview (i.e., some subjects who described themselves as continent during the telephone interview reported being incontinent when they came in for their clinic visit14.
Clinical examinations were performed with women in a semi-recumbent position in a urodynamics chair at a 45° angle. Assessment of vaginal and uterine support was conducted using the Pelvic Organ Prolapse Quantification System (POP-Q), a technique that assesses the downward displacement of specific points along the vagina and cervix at maximal Valsalva.15 Urethral axis inclination measurements were made from the horizontal with a cotton-tipped swab (“Q-tip”) at rest, during maximal Valsalva and during attempt to contract the pelvic floor muscles (maximal contraction).16
Urethral function was assessed with urethral profilometry. For each woman, two or three urethral pressure profile measurements were taken using an 8 Fr. Gaeltec® dual-microtip urodynamics catheter (Medical Measurements Incorporated, Hackensack, NJ) with the transducer laterally oriented and averaged. Post-void residual urine volume was measured by volume obtained during catheterization. First urge to urinate was noted as well as any detrusor contraction during bladder filling through a catheter to cystometric capacity using a medium fill rate. Cough and Valsalva leak point pressures were determined on 300 cc bladder volume. (Bladder volume was reduced to 300 cc through passive catheter drainage if “first urge” occurred at a higher volume during filling). “Load on the system” was quantified as highest cough pressure obtained during the leak point pressure testing. A positive full bladder standing stress test was conducted after removal of the catheter and resulting stress-associated urine leakage with cough or Valsalva was documented. A uroflow was performed after catheter removal. Levator ani muscle function was assessed with an instrumented vaginal speculum designed to measure vaginal closure force both at rest and during maximum voluntary contraction.17
For the purposes of this study, classification of continence status was made using the following definitions.
Stress urinary incontinence (n = 102)
All women that leaked urine during coughing on examination were classified as having the physical finding of SUI. Because the purpose of this portion of the project was to assess the relationship between continence mechanism structures and functional elements, we chose this objective evaluation over self-report of SUI on clinical examination. Thus, all of the SUI women analyzed had demonstrable leakage during cough. None had documented detrusor instability, but some were symptomatically positive for urge.
Urge incontinence (n=96)
Women who were given a final clinical diagnosis of only UUI, without SUI, based on history of symptoms of UUI and negative stress test during examination were classified as having urge incontinence.
Continent (n=137)
Women who denied urinary incontinence 12 times or more per year and who did not demonstrate urinary incontinence during urodynamic testing were classified as continent. Women Excluded from Analysis: Women who self-reported SUI as their only leakage symptoms, but in whom SUI could not be demonstrated on clinical examination (n=22) were excluded from analysis because they could neither be properly classified as having demonstrable SUI, nor could they reasonably be considered continent. Women who demonstrated SUI only on Valsalva maneuver and never during coughing were also excluded (n=29). This decision was made in recognition of the fact that normal women can void by increasing their abdominal pressure while relaxing their pelvic floor muscles. In addition, seven women with other forms of urinary incontinence were excluded; one reported the feeling of moisture but denied urge or stress symptoms, one complained only of urine loss at the end of micturition and five had nocturnal enuresis but denied urge symptoms or demonstrable SUI during a cough.
Statistical Methods
Sampling weights were applied to the data to adjust for over-sampling for urinary incontinence and Black race, for the purpose of projecting the clinical sample to the population from which the survey sample was drawn (i.e., source population). Demographic characteristics, health history, lifestyle factors, and obstetric/gynecologic history were compared between Black and White subjects within each of the continence status groups using Chi-square tests (Table 1). Least squares mean measures of urethral function, urethrovaginal supports, and urodynamics were compared between White and Black women, adjusted for age (continuous), body mass index (continuous), diabetes (yes, no) and vaginal parity (0, 1–2, ≥3) (Table 2). Multivariable logistic regression analyses were conducted to determine factors best explaining SUI or UUI, separately for Black and White women (Table 3). All logistic regression models were adjusted for age (continuous), body mass index (continuous), diabetes (yes, no) and vaginal parity (0, 1–2, ≥3), and weighted to reflect the overall source population. For each model, goodness of fit was assessed using the Max re-scaled R2, and the area under the OROC curve. Within each racial group, pairwise comparisons of pelvic floor measures across continence groups were calculated using t-tests, with an indication if the comparison remained statistically significant after Bonferroni adjustment for multiple inferences (Appendix A, Figures). P-values less than 0.05 were considered statistically significant. All analyses were conducted using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Table 1.
Demographic, health history, lifestyle, and obstetric/gynecologic characteristics of the EPI Study sample, overall and by continence status and race1.
| Overall % | Black % | Continent White % | p-value | Stress Urinary Incontinent | Urge Urinary Incontinent | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Black % | White % | p-value | Black % | White % | p-value | |||||
| Demographics | ||||||||||
| Age (years) | ||||||||||
| 35–44 | 37.9 | 36.7 | 52.7 | 0.11 | 23.5 | 19.6 | 0.40 | 35.3 | 36.4 | 0.45 |
| 45–54 | 34.9 | 41.5 | 27.6 | 55.3 | 42.5 | 37.9 | 24.5 | |||
| ≥55 | 27.2 | 21.8 | 19.7 | 21.2 | 37.9 | 26.8 | 39.1 | |||
| Education level (years completed) | ||||||||||
| <12 | 6.6 | 7.3 | 0.0 | 0.003 | 8.5 | 8.1 | 0.68 | 3.8 | 16.8 | 0.006 |
| 12 | 21.4 | 18.0 | 11.0 | 36.5 | 32.5 | 17.3 | 28.3 | |||
| 13–15 | 34.2 | 42.3 | 34.3 | 37.4 | 28.4 | 57.0 | 18.6 | |||
| ≥16 | 37.8 | 32.5 | 54.7 | 17.6 | 31.0 | 21.9 | 36.2 | |||
| Currently working for pay | 66.1 | 62.0 | 71.3 | 0.20 | 74.0 | 60.8 | 0.27 | 59.1 | 68.0 | 0.44 |
| Household income ($) | ||||||||||
| <35,000 | 28.1 | 27.1 | 14.9 | 0.14 | 42.9 | 36.4 | 0.75 | 21.6 | 43.1 | 0.15 |
| 35,000–69,999 | 18.5 | 17.9 | 17.7 | 26.3 | 22.9 | 21.9 | 11.1 | |||
| ≥70,000 | 53.4 | 55.0 | 67.4 | 30.8 | 40.7 | 56.5 | 45.8 | |||
| Marital status | ||||||||||
| Married/Living together | 51.1 | 44.5 | 55.6 | 0.02 | 26.9 | 54.2 | 0.19 | 39.6 | 62.3 | 0.09 |
| Never Married | 14.8 | 17.0 | 20.3 | 14.8 | 10.8 | 15.3 | 6.1 | |||
| Divorced/Separated | 31.6 | 30.7 | 24.1 | 55.3 | 33.8 | 39.4 | 31.6 | |||
| Widowed | 2.5 | 7.8 | 0.0 | 2.9 | 1.1 | 5.7 | 0.0 | |||
| Health history | ||||||||||
| Diabetes | 13.0 | 19.7 | 1.9 | <0.0001 | 9.7 | 17.0 | 0.42 | 19.3 | 18.7 | 0.95 |
| Mobility impairment | 11.4 | 9.5 | 5.9 | 0.36 | 18.0 | 19.1 | 0.91 | 19.9 | 8.6 | 0.15 |
| Constipation | 89.3 | 93.0 | 97.1 | 0.19 | 88.0 | 83.4 | 0.63 | 84.9 | 79.6 | 0.58 |
| Urinary tract infection | 12.0 | 10.4 | 11.1 | 0.88 | 13.8 | 8.9 | 0.52 | 14.3 | 17.4 | 0.73 |
| Chronic lung disease | 24.6 | 19.5 | 26.3 | 0.30 | 10.6 | 33.6 | 0.04 | 13.9 | 28.2 | 0.17 |
| Body mass index (kg/m2) | ||||||||||
| ≤25 | 22.8 | 13.2 | 29.1 | 0.02 | 17.1 | 21.9 | 0.62 | 6.5 | 31.1 | 0.03 |
| 26–35 | 47.7 | 55.1 | 53.4 | 43.1 | 49.7 | 38.5 | 34.4 | |||
| ≥36 | 29.5 | 31.7 | 17.4 | 39.8 | 28.5 | 55.0 | 34.5 | |||
| Depressive symptoms3 | 45.3 | 45.4 | 36.0 | 0.21 | 46.8 | 41.4 | 0.67 | 58.1 | 60.7 | 0.83 |
| Lifestyle factors | ||||||||||
| Exercise involving bouncing at least once a week | 34.1 | 35.8 | 46.8 | 0.15 | 20.5 | 25.7 | 0.63 | 24.3 | 27.5 | 0.76 |
| Lift or carry ≥30 pounds more than once a week | 69.9 | 66.2 | 78.1 | 0.08 | 57.3 | 79.1 | 0.15 | 50.4 | 62.6 | 0.31 |
| Current cigarette smoking | 26.7 | 23.8 | 15.0 | 0.14 | 24.1 | 41.7 | 0.15 | 26.8 | 35.9 | 0.43 |
| Drink >8 glasses fluid per day | 37.5 | 37.4 | 28.2 | 0.20 | 26.4 | 52.0 | 0.04 | 38.2 | 42.0 | 0.75 |
| Obstetric/Gynecologic history | ||||||||||
| Vaginal parity (number of vaginal births) | ||||||||||
| 0 | 31.3 | 20.0 | 54.5 | <0.0001 | 11.5 | 22.8 | 0.10 | 16.7 | 27.8 | 0.32 |
| 1–2 | 37.1 | 44.2 | 30.1 | 30.9 | 45.5 | 46.1 | 29.6 | |||
| ≥3 | 31.6 | 36.7 | 15.4 | 57.6 | 31.7 | 37.2 | 42.6 | |||
| Current estrogen use | 13.1 | 8.5 | 6.9 | 0.68 | 13.6 | 18.0 | 0.64 | 20.0 | 21.5 | 0.88 |
| Prior surgery for prolapse or urinary incontinence | 7.7 | 1.5 | 6.4 | 0.12 | 7.6 | 8.9 | 0.86 | 7.3 | 17.3 | 0.24 |
| Menopause | 42.7 | 41.6 | 28.7 | 0.08 | 37.3 | 50.6 | 0.29 | 47.3 | 61.0 | 0.26 |
| Prior hysterectomy | 21.6 | 23.6 | 8.5 | 0.006 | 23.5 | 27.0 | 0.75 | 30.3 | 32.6 | 0.84 |
Data are weighted hence no sample sizes are indicated.
Chi-square p-value for comparison between Black and White women within continence category.
Self-reported feelings of sadness, depression, and/or loneliness in the prior week.
Table 2.
Least Square Means and 95% confidence intervals for clinical measures by race adjusted so that they represent the population from which the sample was drawn unaffected by over sampling for urinary incontinence and Black race. EPI Study clinic population (N=335). Analyses are adjusted for age (continuous), body mass index (continuous), vaginal parity (0, 1–2, ≥3), and diabetes.
| All White (n=145) | All Black (n=190) | p-value | |||
|---|---|---|---|---|---|
| LS Mean | 95% CI | LS Mean | 95% CI | ||
| URETHRAL FUNCTION | |||||
| Maximal Closure Pressure | 55.8 | (52.4, 59.2) | 68.0 | (63.4,72.6) | <0.0001 |
| Pressure increase with maximal contraction | 17.6 | (15.7,19.6) | 20.4 | (17.7,23.1) | 0.11 |
| URETHROVAGINAL SUPPORTS | |||||
| Urethral support; Q-tip angle | |||||
| Rest | −0.95 | (−2.8,0.85) | −2.3 | (−4.8,0.19) | 0.39 |
| Valsalva | 24.7 | (21.9, 27.4) | 23.4 | (19.7, 27.1) | 0.59 |
| Pelvic Muscle Contraction | −14.8 | (−16.7, −12.9) | −14.9 | (−17.5, −12.2) | 0.99 |
| Utero-vaginal Support | |||||
| Anterior Wall (Point Aa) | −1.2 | (−1.5, −0.99) | −1.0 | (−1.3, −0.68) | 0.27 |
| Apex (Point C) | −6.4 | (−6.7, −6.2) | −6.6 | (−6.9, −6.2) | 0.53 |
| Posterior Wall (Point B) | −1.2 | (−1.3, −1.1) | −1.1 | (−1.3, −0.93) | 0.57 |
| Hiatus Measurements | |||||
| Genital Hiatus at rest | 2.9 | (2.7, 3.0) | 2.9 | (2.7, 3.1) | 0.66 |
| Genital Hiatus with Valsalva | 3.4 | (3.2, 3.5) | 3.5 | (3.3, 3.6) | 0.48 |
| Vaginal Closure Force | |||||
| Rest | 3.6 | (3.3, 4.0) | 4.1 | (3.6,4.6) | 0.15 |
| Maximal contraction | 3.0 | (2.7,3.3) | 2.9 | (2.5,3.2) | 0.50 |
| URODYNAMICS | |||||
| Bladder Pressure | |||||
| Rest | 21.2 | (20.0, 22.4) | 23.6 | (22.0, 25.3) | 0.02 |
| Maximal Cough | 155.9 | (149.9, 161.9) | 174.4 | (166.2, 182.6) | 0.0004 |
| PVR | 40.7 | (34.8, 46.6) | 22.9 | (14.9, 31.0) | 0.0005 |
| CMG 1st urge | 198.4 | (185.5, 211.3) | 198.6 | (181.2, 216.1) | 0.98 |
| CMG max | 396.2 | (381.5, 410.9) | 376.1 | (356.0, 396.2) | 0.11 |
| Max flow | 29.9 | (27.9, 31.9) | 30.9 | (28.2, 33.7) | 0. 54 |
| Avg flow | 17.1 | (15.6, 18.6) | 19.2 | (17.2, 21.3) | 0.09 |
LS mean, least squares mean; CI, confidence interval; PVR, post void residual urine volume; CMG, cystometrogram
Table 3.
Logistic regression models predicting stress urinary incontinence and urge urinary incontinence by race containing the factors that best explain incontinence type. EPI Study clinic population (N=335).
| Model | Race | Type | Variable | Coefficient | p-value | Adjusted odds ratio | 95% CI | Max rescaled R-square | ROC area |
|---|---|---|---|---|---|---|---|---|---|
| 1 | White | SUI | MUCP | −0.04 | 0.0006 | 0.96 | 0.94, 0.98 | 0.65 | 0.90 |
| Max cough | 0.02 | <0.0001 | 1.02 | 1.01, 1.04 | |||||
| Urethral Axis MVC | 0.05 | 0.004 | 1.05 | 1.02, 1.09 | |||||
| 2 | Black | SUI | MUCP | −0.04 | 0.03 | 0.96 | 0.93, 0.99 | 0.27 | 0.80 |
| Max cough | 0.02 | 0.05 | 0.96 | 0.93, 0.99 | |||||
| Urethral Axis MVC | 0.06 | 0.02 | 1.06 | 1.01, 1.03 | |||||
| 3 | White | UUI | MUCP | 0.05 | 0.0019 | 0.95 | 0.92, 0.98 | 0.62 | 0.87 |
| Max cough | 0.017 | 0.008 | 1.02 | 1.01, 1.03 | |||||
| Urethral Axis MVC | 0.06 | 0.0006 | 1.06 | 1.03, 1.10 | |||||
| 4 | Black | UUI | MUCP | 0.006 | 0.7 | 1.006 | 0.98, 1.03 | 0.18 | 0.70 |
| Urethral Axis MVC | 0.02 | 0.4 | 1.02 | 0.98, 1.06 | |||||
| Max cough | 0.01 | 0.1 | 1.01 | 0.99, 1.03 |
SUI, stress urinary incontinence; UUI, urge urinary incontinence; CI, confidence interval; MUCP, maximal urethral closure pressure; MVC, maximal voluntary contraction..
Data are weighted using clinic weights.
All analyses are adjusted for age (continuous), body mass index (continuous), and vaginal parity (0, 1–2, ≥3)
Excluded from this table are 7 women with ‘other’ incontinent symptoms, 29 women who demonstrated SUI on valsalva only, and 22 women who reported symptoms of SUI but did not demonstrate SUI in the clinic.
RESULTS
Demographic, health history, lifestyle, and obstetric/gynecologic characteristics of the study sample, by continence status and race, are shown in Table 1. Among continent women, Black and White women were generally similar on these variables with the exception that White women completed more years of education (p=0.003) and were more likely to be married (p=0.02). Additionally, more continent Black women had a history of diabetes (p<0.0001) or hysterectomy (p=0.006), higher body mass index (p=0.02), and higher vaginal parity (p<0.0001) compared to continent White women. Among SUI women, the only significant difference between the racial groups was that more White women reported drinking over eight glasses of fluid per day (p=0.04) compared to Black women. Among UUI women, White women completed more years of education (p=0.006) and had lower body mass index (p=0.03) compared to Black women.
Population-based Continence System Parameters by Race
Table 2 compares continence system parameters for all Black and all White women, weighted to adjust for over-sampling Black and incontinent women to reflect the population from which the sample was drawn. Maximal urethral closure pressure (MUCP) was 22% higher in Black women than White women. Urethral axis and urethro-vaginal support were similar between the racial groups as was levator ani muscle function. Black women had an 11% higher bladder pressure during maximal cough despite similar resting bladder pressures. Although Blacks had statistically lower post void residual urine volume, both of these values were within the normal clinical range. Other measures of continence system function were similar between the two racial groups.
Comparisons within Race Groups by Continence Status and Type
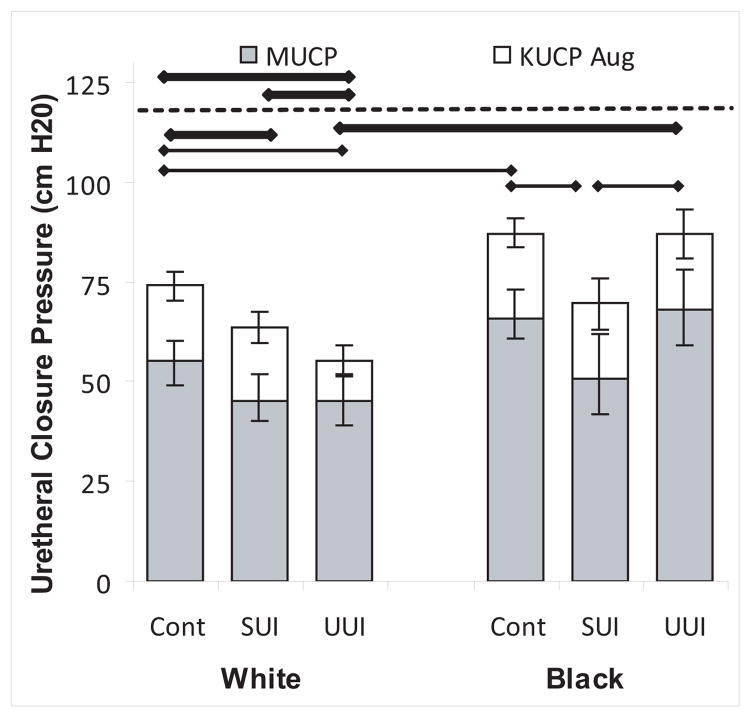
Figure 1 shows that white women with SUI and UUI both had lower urethral pressures compared to white continent women, whereas in black women, lower MUCP occurred only in those with stress urinary incontinence. Therefore, Black women with UUI had higher urethral closure pressures compared to White women with UUI.
Figure 1. Maximal urethral closure pressure.
Maximal urethral closure pressure (grey bars) and the increase during pelvic muscle contraction (open bars) by continence status and race are shown with confidence intervals. Thick horizontal bar indicates statistical significance with Bonferroni correction and thin bars, without. Bars below the dotted line are for maximal urethral closure pressure and those above for increase during muscle contraction. (© DeLancey 2009)
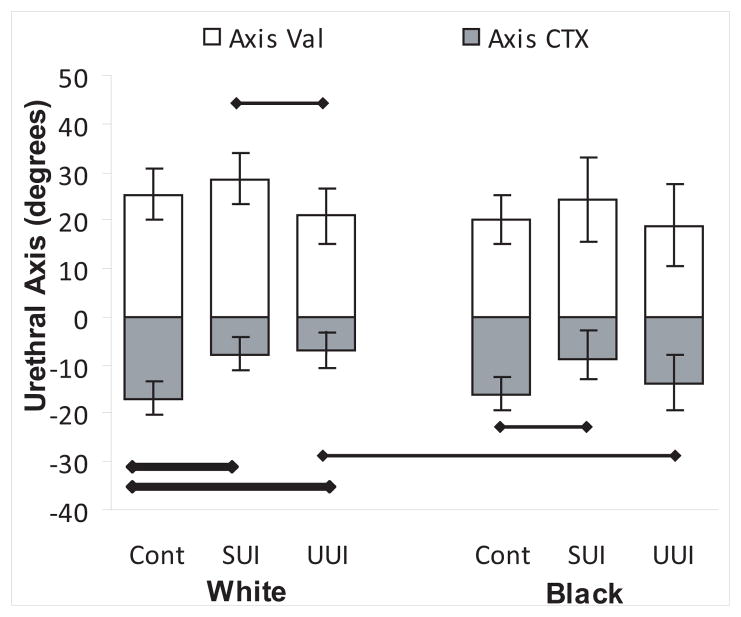
Urethral axis during Valsalva and with maximal contraction is shown in Figure 2. Both White and Black women with SUI showed greater urethral axis change (more mobility) during Valsalva compared to UUI women, although only the difference in Whites was statistically significant. During pelvic muscle contraction, both White and Black SUI women were less able to elevate their urethra than continent women. Women with UUI did not differ from continent women in support during Valsalva in either Black or White race. White UUI, but not Black UUI, women were less able to elevate the urethra during pelvic muscle contraction compared to same race continent women.
Figure 2. Urethral axis.
Urethral axis in degrees during Valsalva (open bars) and with pelvic muscle contraction (grey bars). Confidence intervals are shown. Thick horizontal bar indicates statistical significance with Bonferroni correction and thin bars without correction. Significance levels for Valsalva shown above and for pelvic muscle contraction below. (© DeLancey 2009)
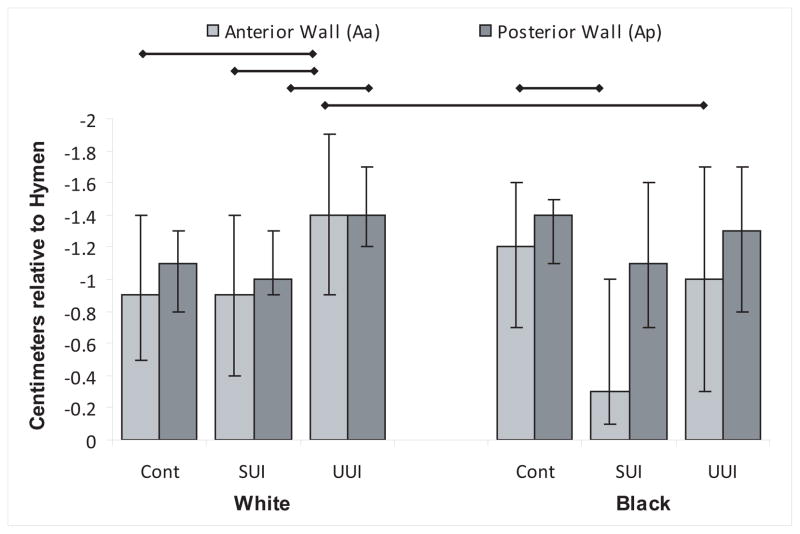
Anterior vaginal wall support is shown in Figure 3. Anterior vaginal wall was lower during Valsalva in Black women with SUI compared with Blacks with urge incontinence. Although White women with SUI also showed lower anterior vaginal wall than UUI women this trend was not statistically significant.
Figure 3. Anterior and Posterior Vaginal Wall Support.
Anterior and posterior vaginal wall support assessed as POP-Q points Aa and Ap. Thin horizontal bar indicates statistical significance without Bonferroni correction. (© DeLancey 2009)
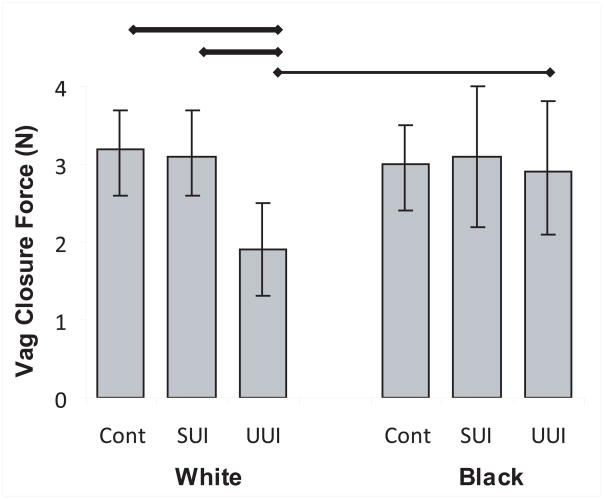
White women with UUI were not able to increase their vaginal closure force compared with continent and SUI White women and Black women with UUI (Figure 4).
Figure 4. Vaginal closure force.
Vaginal closure force during pelvic muscle contraction. Thick horizontal bar indicates statistical significance with Bonferroni correction and thin bars, without. (© DeLancey 2009)
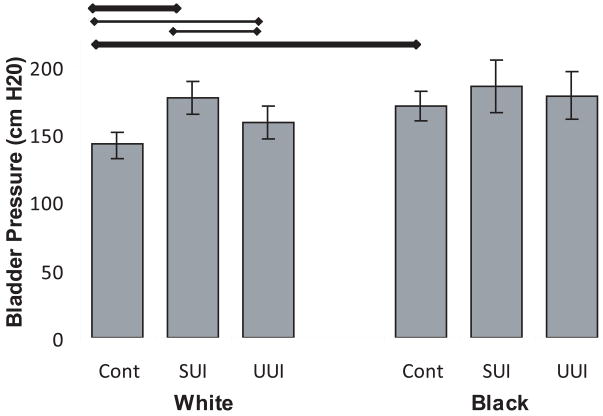
Maximal bladder pressure during a cough was higher among White women with SUI, than continent White women or White women with UUI (Figure 5). The same trend occurred in Blacks but to a lesser extent and did not reach statistical significance. Continent Black women coughed harder than continent White women (143 vs 170 cm H2O respectively).
Figure 5. Maximal bladder pressure.
Maximal bladder pressure during cough. Thick horizontal bar indicates statistical significance with Bonferroni correction and thin bars, without. (© DeLancey 2009)
First urge to void, bladder capacity and flow rates were generally similar between the groups (Appendix A). Although there was a difference in post void residual urine volume between continent Black and White women, all values were in the normal range.
In reviewing differences between continence groups for Whites and Blacks it is evident that there are many more differences found in continence function parameters in Whites than Blacks despite similar group size, suggesting these measures may explain continence group differences better in Whites than Blacks (Appendix A, Columns A and B). To further examine the extent to which these different factors explain the occurrence of stress and urge incontinence, we built logistic regression models that included the strongest factors from each of three mechanistic domains: urethral sphincter function (MUCP), load on the system (bladder pressure during maximal cough) and urethral support (Q-tip during maximal contraction) (Table 3). When comparing logistic regression models, the Max-rescaled R2 values indicate that these variables are predictive of stress and/or urge incontinence for White women (Max-rescaled R2=0.65 for SUI and Max-rescaled R2=0.62 for UUI), but provide a relatively poor model for prediction in black women (Max-rescaled R2=0.27 for SUI and Max-rescaled R2=0.18 for UUI).
COMMENT
Black women were less likely than White women to have urinary incontinence and, specifically, stress incontinence on cough. Of the three elements of the stress continence mechanism: urethral function, urethral support, and maximal cough pressures, we found that overall urethral function contributed the most.
Black women had a 22% higher maximal urethral closure pressure compared to White women, which translates into less stress incontinence despite Black women’s higher maximal cough pressure. This finding that Black women had stronger urethras is similar to the findings in White and Black nulliparous women seen in a small non-population based study13 and in a clinical population.18 The present study included women with a broader range of age and parity, providing a fuller representation of the two groups and reducing the effects of selection bias. Additionally, the fact that the women selected for this research could be linked to the population from which they were drawn allows their values to be weighted so as to estimate the values seen in the population, meaning that our findings are not influenced by the number of continent and incontinent women selected.
For many years, the urethral function was not considered to be important to the cause of urinary incontinence in general, and stress incontinence in specific. Recent studies have demonstrated a primary role of urethral function in the etiology of stress incontinence12. The current data lend further support to the importance of urethral function in stress incontinence.
In examining urethral function by race and by continence status it can be seen that there are important differences between the two races. In White women, urethral closure pressure is equally low in SUI and UUI women suggesting that in White women, decreased urethral function contributes to incontinence, regardless of type of symptoms. Similarly, Black women with stress incontinence show lower MUCP when compared to Black continent women. However, Black incontinent women with UUI do not have lower urethral pressures than continent White women and continent Black women. This suggests potentially different mechanisms for UUI in the two groups. One hypothesis explaining these observations is that poor urethral function in Whites, and their inability to augment urethral closure pressure by pelvic muscle contractions, might lead to leakage during the occasional normal episodes of detrusor contraction documented to occur during daily activities.19 Not only was the White women’s’ urethral closure pressure reduced, but the strength of their levator ani muscles in increasing vaginal closure force and their ability to elevate the urethra were also reduced. This may explain many patients’ observations that they “can’t hold on when feeling the urge to urinate.” In Blacks, however, urethral function was better and therefore may require a larger detrusor contraction to cause incontinence. Of course, UUI is multifactorial and involves many potential causes including speculated abnormalities in detrusor muscle, neural, and epithelial factors. If it is true that UUI in Blacks is less likely to be due to a weak urethra, it may be more strongly related to one of these other factors.
If White SUI women and White UUI women have equally low urethral pressures, why do they not have the same symptoms? This difference is likely attributable to differences in urethral support. White women with UUI overall have good urethral support at rest and during Valsalva while those with SUI do not. This observation suggests the following hypothesis: In White women, reduced urethral function puts a woman at risk for incontinence in general and those with both weak urethra and loss of support are at risk for having stress incontinence, while those with urge incontinence may still show normal support. This would be consistent with the wide occurrence of mixed incontinence as there is a continuous spectrum of both urethral support and urethral function.
There is a difference in the ability or our measurements to predict incontinence in White and Black women evident in the many statistically significant differences in continence parameters for White women yet few for Black women. For White women, logistic regression models demonstrated potential use as a predictive model for both stress and urge incontinence. The three significant parameters were urethral sphincteric function, load on the bladder and ability to elevate the bladder position during maximal contraction. For Black women, the same parameters showed relatively poor utility as a predictive model for SUI, and even less so for urge incontinence.
There are several clinical implications to the findings of this study. These results confirm that urethral function is a critical determinant of SUI and suggest that the urethra is an under-appreciated but logical therapeutic target. They also indicate that there is a role for urethral function in the pathogenesis of UUI, at least in White women, and may help to explain why treatments that increase urethral function may improve some women’s symptoms of mixed20 as well as stress incontinence.21 The fact that groups of Black and White women with UUI seem to have different findings regarding urethral function should lead to consideration of whether or not there are different therapeutic responses to treatment. For example, pelvic muscle training for groups of White women with urge or mixed incontinence seems appropriate while it might not be effective in groups of Black women, thus selection criteria for therapeutic value needs close examination. Of course, these issues deserve specific investigation before changes in practice are contemplated. It does, however, emphasize the need for adequate representation of Black participants in such research.
Several factors must be kept in mind in interpreting the results of this study. There is no perfect way to separate patients into unique racial or incontinence groups 14. The assumptions outlined in the methods were chosen due to our desire to understand the overarching mechanisms of different types of incontinence in self-identified Black and White women rather than the occurrence of incontinence in a population. All women in the SUI group demonstrated the physical finding of cough-induced leakage, but may have also had urge whereas those in the urge incontinent group all had negative stress test and thus less chance of having mixed incontinence in reality. Thus, most women that would be classified clinically as having mixed incontinence are represented in the stress group. It is notable that there is no simple laboratory test that reliably detects the majority of women with incontinence caused by inappropriate detrusor contractions. All forms of pelvic floor testing involve instrumentation and have artifacts due to their performance and we recognize that some degree of artifact is present. For example, our values for urethral axes may seem somewhat lower than are typically seen. This is likely due to our following current recommendations that pelvic floor testing be performed with the individual at a 45° angle, which does tilt the pelvis somewhat. The fact that all individuals were studied in the same manner retains the comparability between subjects in this study but may affect their comparison to other results reported from other units.
We were limited in the extent of testing that was feasible in our study design. We were asking women who received an unsolicited telephone call at home to volunteer to drive to a strange clinic and undergo invasive urodynamic testing by individuals they had never met. For considerations of subject burden, we chose to limit our testing to an examination that could be performed in approximately 30 minutes. This precluded performing extensive provocative maneuvers in an attempt to provoke detrusor contraction and extended pad tests for example. Although these women come from a population based sample it is logical that there is some bias in who decided to come in for urodynamic testing that we cannot completely assess. We were impressed, however, with the altruism demonstrated by these women who did volunteer when they would derive no personal benefit from this information.
In summary, Black women generally have stronger urethras than White women, but both White and Black women with SUI had lower MUCP compared to continent women of the same race. White, but not Black, women with UUI had lower urethral closure pressures. Thus, in White and Black women, SUI may be more likely in those with poor urethral function and hypermobility. In White women, UUI may be more common in those with poor urethral function despite good support. In Black women with UUI, urethral dysfunction did not appear to be as important in the mechanism leading to urge symptoms. Future studies in prevention and therapeutic options, both surgical and pharmacotherapy, should consider these findings in the development of therapeutic options aimed at improving urethral support or urethral function.
Acknowledgments
This research was presented at the American Urogynecologic Society (AUGS) 30th Annual Scientific Meeting, Hollywood, FL, Sept. 24–26, 2009 and given the “Best Oral Presentation Award”
Supported by National Institute of Childhood Diseases and National Institute on Aging R01HD/AG41123. Additional investigator support was provided by Office for Research on Women’s Health’s SCOR on Sex and Gender Factors Affecting Women’s Health and the National Institute of Child Health and Human Development through grants P50 HD044406.
Appendix A: Pelvic Floor Measures for White and Black Women
This table shows the pelvic floor measures for White and Black women, stratified by continence status. Analyses are adjusted for age, body mass index, vaginal parity, and diabetes. Levels of statistical significance for the relevant comparisons are shown on the right. Column A shows which differences between the 3 White continence groups how do women with stress and urge incontinence differ from one another and from continent women. Column B presents similar information for Blacks. Column C shows where there were differences between the two races of the same continence status. (e.g. Black SUI vs. White SUI etc.)
Least Square (LS) Mean weighted to be representative of the population from which the sample was taken for measures of urethral function, vaginal support, genital hiatus, and urethral support by race and continence status. EPI Study clinic population (n=335*). Analyses are adjusted for age (continuous), body mass index (continuous), vaginal parity (0, 1–2, ≥3), and diabetes.
| White | Black | Pair-wise comparisons of LS means p-value** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Continent (Group 1) | SUI (Group 2) | UUI (Group 3) | Continent (Group 4) | SUI (Group 5) | UUI (Group 6) | Column A | Column B | Column C | |
| (n=46) LS Mean | (n=55) LS Mean | (n=44) LS Mean | (n=91) LS Mean | (n=47) LS Mean | (n=52) LS Mean | Differences by Status Among Whites 1v2 2v3 1v3 |
Differences by Status Among Blacks 4v5 5v6 4v6 |
Differences between B&W of same Status 1v4 2v5 3v6 |
|
| URETHRAL FUNCTION CM H2O) | |||||||||
| Maximal urethral closure pressure | 55 | 45 | 45 | 66 | 51 | 68 | 1v2=0.007* 1v3=0.01 |
4v5=0.007 5v6=0.01 |
1v4=0.006 3v6<0.0001* |
| 95% CI | 50, 61 | 38, 50 | 38, 51 | 59, 71 | 40, 60 | 58, 77 | |||
| Pressure increase with maximal contraction | 19.0 | 18.4 | 10.4 | 21.3 | 18.6 | 19.0 | 1v3=0.0006* 2v3=0.002* |
3v6=0.01 | |
| 95% CI | 15.2, 22.7 | 14.5, 22.2 | 6.4, 14.4 | 17.7, 25.0 | 12.1, 25.0 | 13.0, 25.1 | |||
| URETHRAL AXIS “Q-TIP” DEGREES | |||||||||
| Rest | −1 | 7 | 0 | −3 | 1 | −4 | 1v2=0.005* 2v3=0.01 |
||
| 95% CI | −4.1, 2.8 | 2.2, 9.3 | −4.2, 3.2 | −6.8, −0.1 | −4.5, 7.3 | −9.6, 1.4 | |||
| Valsalva | 25.4 | 28.5 | 20.9 | 20.2 | 24.4 | 19.0 | 2v3=0.04 | ||
| 95% CI | 20.2, 30.6 | 23.1, 33.8 | 15.3, 26.6 | 15.1, 25.3 | 15.6, 33.3 | 10.7, 27.3 | |||
| Pelvic Muscle Contraction | −17 | −8 | −7 | −16 | −9 | −14 | 1v2<0.0001* 1v3<0.0001* |
4v5=0.04 | 3v6=0.03 |
| 95% CI | −20.2, −13.3 | −11.3, −4.2 | −10.5, −3.1 | −19.4, −12.5 | −14.9, −3.0 | −19.4, −8.0 | |||
| UTERO-VAGINAL SUPPORT | |||||||||
| Anterior Wall (Point Aa) | −0.9 | −0.9 | −1.4 | −1.2 | −0.3 | −1.0 | 4v5=0.03 | ||
| Apex (Point C) | −1.4, −0.5 −6.4 |
−1.4, −0.4 −6.5 |
−1.9, −0.9 −5.7 |
−1.6, −0.7 −6.5 |
−1.0, −0.5 −6.8 |
−1.7, −0.3 −6.6 |
1v3=0.04 2v3=0.01 |
3v6=0.047 | |
| Posterior Wall (Point B) | −6.9, −5.9 −1.1 |
−7.0, −6.0 −1.0 |
−6.2, −5.2 −1.4 |
−7.0, −6.0 −1.4 |
−7.7, −6.0 −1.1 |
−7.4, −5.8 −1.3 |
1v3=0.04 2v3=0.03 |
||
| −1.3, −0.8 | −1.3, −0.8 | −1.7, −1.2 | −1.6, −1.1 | −1.6, −0.7 | −1.7, −0.8 | ||||
| HIATUS MEASUREMENTS | |||||||||
| Genital Hiatus at rest | 2.9 2.6, 3.1 |
3.2 2.9–3.4 |
3.0 2.8, 3.3 |
2.8 2.5, 3.0 |
3.2 2.8, 3.5 |
2.9 2.5, 3.2 |
1v2=0.04 | ||
| Genital Hiatus with Valsalva | 3.3 3.0, 3.5 |
3.8 3.5, 4.0 |
3.5 3.2, 3.7 |
3.2 2.9, 3.5 |
3.5 3.1, 3.9 |
3.6 3.1, 3.9 |
1v2=0.004* | ||
| VAGINAL CLOSURE FORCE N | |||||||||
| Maximal contraction | 3.2 | 3.1 | 1.9 | 3.0 | 3.1 | 2.9 | 1v3=0.0001* 2v3=0.002* |
3v6=0.04 | |
| 95% CI | 2.7, 3.8 | 2.5, 3.6 | 1.3, 2.5 | 2.5, 3.6 | 2.2, 4.0 | 2.0, 3.7 | |||
| Bladder Pressure Rest | 22 | 22 | 25 | 24 | 25 | 24 | |||
| 19.9, 24.0 | 19.9, 24.3 | 22.4, 26.9 | 22.3, 26.4 | 21.4, 28.6 | 20.6, 27.2 | ||||
| Maximal Cough | 143 | 176 | 158 | 170 | 185 | 178 | 1v3=0.04 1v2<0.0001* 2v3=0.02 |
1v4=0.0001* | |
| PVR | 131, 154 49.2 |
164, 187 42.1 |
146, 170 32.8 |
159, 181 23.1 |
166, 204 26.1 |
160, 195 32.0 |
1v3=0.04 | 1v4=0.0005* | |
| CMG 1st urge | 37.3, 61.1 205.0 |
29.9, 54.3 215.3 |
20.1, 45.6 190.5 |
11.4, 34.8 217.0 |
5.9, 46.3 220.5 |
13.2, 50.8 208.7 |
|||
| CMG max | 179, 231 396.9 |
189, 241 433.7 |
163, 218 389.4 |
192, 242 398.8 |
177, 264 395.0 |
168, 249 374.1 |
2v3=0.03 | ||
| Max flow | 367.8, 426.0 27.8 |
403.7, 463.8 32.8 |
358.3, 420.5 28.9 |
370.2, 427.3 30.0 |
345.2, 444.8 36.4 |
328.0, 420.2 28.3 |
|||
| Avg flow | 23.8, 31.8 17.5 |
28.7, 36.9 18.8 |
24.5, 33.2 15.8 |
26.1, 34.0 19.2 |
29.5, 43.3 22.7 |
22.0, 34.6 18.5 |
|||
| 14.5, 20.6 | 15.7, 21.8 | 12.5, 19.0 | 16.2, 22.1 | 17.6, 27.8 | 13.8, 23.2 | ||||
SUI, stress urinary incontinence; UUI, urge urinary incontinence; LS mean, least squares mean; CI, confidence interval.
Notation:
– remained statistically significant after Bonferroni correction.
only those with p < 0.05 shown
Data are weighted using clinic weights.
Analyses are adjusted for age (continuous), body mass index (continuous), vaginal parity (0, 1–2, ≥3), and diabetes.
Excluded from this table are 7 women with ‘other’ incontinent symptoms, 29 women who demonstrated SUI on Valsalva only, and 22 women who reported symptoms of SUI but did not demonstrate SUI in the clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson L, Brown JS, Shin GP, Luc KO, Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 2.Bump RC. Racial comparisons and contrasts in urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1993;81:421–5. [PubMed] [Google Scholar]
- 3.Burgio KL, Matthews KA, Engel BT. Prevalence, incidence and correlates of urinary incontinence in healthy, middleaged women. J Urol. 1991;146:1255–9. doi: 10.1016/s0022-5347(17)38063-1. [DOI] [PubMed] [Google Scholar]
- 4.Brown JS, Grady D, Ouslander JG, Herzog AR, Varner RE, Posner SF. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol. 1999;94:66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 5.Fultz NH, Herzog AR, Raghunathan TE, Wallace RB, Diokno AC. Prevalence and severity of urinary incontinence in older African American and Caucasian women. J Gerontol A Biol Sci Med Sci. 1999;54:M299–303. doi: 10.1093/gerona/54.6.m299. [DOI] [PubMed] [Google Scholar]
- 6.Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189:428–34. doi: 10.1067/s0002-9378(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 7.Thom DH, van den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol. 1997;90:983–9. doi: 10.1016/s0029-7844(97)00537-1. [DOI] [PubMed] [Google Scholar]
- 8.Fenner DE, Trowbridge ER, Patel DA, Fultz NH, Miller JM, Howard D, DeLancey JO. Establishing the prevalence of incontinence study: racial differences in women’s patterns of urinary incontinence. J Urol. 2008;179:1455–60. doi: 10.1016/j.juro.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dooley Y, Kenton K, Cao G, Luke A, Durazo-Arvizu R, Kramer H, Brubaker L. Urinary incontinence prevalence: results from the National Health and Nutrition Examination Survey. J Urol. 2008;179:656–61. doi: 10.1016/j.juro.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 10.Sears CL, Wright J, O’Brien J, Jezior JR, Hernandez SL, Albright TS, Siddique S, Fischer JR. The racial distribution of female pelvic floor disorders in an equal access health care system. J Urol. 2009;181:187–92. doi: 10.1016/j.juro.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 11.Kim KJ, Ashton-Miller JA, Strohbehn K, DeLancey JO, Schultz AB. The vesico-urethral pressuregram analysis of urethral function under stress. J Biomech. 1997;30:19–25. doi: 10.1016/s0021-9290(97)81291-2. [DOI] [PubMed] [Google Scholar]
- 12.DeLancey JO, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, Weadock WJ, Ashton-Miller JA. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008;179:2286–90. doi: 10.1016/j.juro.2008.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard D, DeLancey JO, Tunn R, Ashton-Miller JA. Racial differences in the structure and function of the stress urinary continence mechanism. Obstet Gynecol. 2000;95:713–7. doi: 10.1016/s0029-7844(00)00786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas A, Kane Low L, Tumbarello JA, Miller JM, Fenner DE, DeLancey Changes in Self-Assessment of Continence Status Between Telephone Survey and Subsequent Clinical Visit. Neurourology and Urodynamics. 2009;26:1–7. doi: 10.1002/nau.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 16.Tapp K, Connolly A, Visco AG. Evaluation of Aa point and cotton-tipped swab test as predictors of urodynamic stress incontinence. Obstet Gynecol. 2005;105:115–9. doi: 10.1097/01.AOG.0000146642.68543.69. [DOI] [PubMed] [Google Scholar]
- 17.Morgan DM, Kaur G, Hsu Y, Fenner DE, Guire K, Miller JM, et al. Does vaginal closure force differ in the supine and standing positions? Am J Obstet Gynecol. 2005;192:1722–8. doi: 10.1016/j.ajog.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 18.Bump RC. Racial comparisons and contrasts in urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1993;81:421–5. [PubMed] [Google Scholar]
- 19.Schmidt F, Jørgensen TM, Djurhuus JC. Twenty-four-hour ambulatory urodynamics in healthy young men. Scand J Urol Nephrol Suppl. 2004;(215):75–83. doi: 10.1080/03008880410015327. [DOI] [PubMed] [Google Scholar]
- 20.Bent AE, Gousse AE, Hendrix SL, Klutke CG, Monga AK, Yuen CK, Muram D, Yalcin I, Bump RC. Duloxetine compared with placebo for the treatment of women with mixed urinary incontinence. Neurourol Urodyn. 2008;27:212–21. doi: 10.1002/nau.20471. [DOI] [PubMed] [Google Scholar]
- 21.Dmochowski RR, Miklos JR, Norton PA, Zinner NR, Yalcin I, Bump RC. Duloxetine Urinary Incontinence Study Group. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170:1259–63. doi: 10.1097/01.ju.0000080708.87092.cc. [DOI] [PubMed] [Google Scholar]