Abstract
Background
Lowering LDL-cholesterol and blood pressure in patients with diabetes can significantly reduce the risk of cardiovascular disease. However, previous studies have not assessed variability in the benefit and harm from pursuing LDL and blood pressure targets.
Methods
Our sample was comprised of subjects aged 30-75 with diabetes participating in the National Health and Nutrition Examination Survey-III. We used Monte Carlo methods to simulate a treat-to-target strategy, in which patients underwent treatment intensification with the goal of achieving LDL cholesterol and blood pressure targets of 100 mg/dl and 130/80, respectively. Patients received up to 5 titrations of statin therapy and 8 titrations of antihypertensive therapy. Treatment side effects and polypharmacy risks and burdens were incorporated using disutilities. Health outcomes were simulated using a Markov model.
Results
Treating to targets resulted in gains of 1.50 (LDL) and 1.35 (BP) quality-adjusted life years (QALYs) of lifetime treatment-related benefit, which declined to 1.42 and 1.16 QALYs after accounting for treatment-related harms. The majority of the total benefit was limited to the first few steps of medication intensification or to tight control for a limited group of very high risk patients. However, because of treatment-related disutility, intensifying beyond the 1st step (LDL) or 3rd step (BP) resulted in either limited benefit or net harm for patients with below-average risk.
Conclusion
The benefits and harms from aggressive risk factor modification vary widely across the US diabetes population depending on a patient's underlying CVD risk, suggesting a personalized approach could maximize a patient's net benefit from treatment.
Introduction
Nearly all diabetes practice guidelines recommend aggressive treatment of LDL cholesterol and blood pressure to lower a patient's risk of developing cardiovascular disease (CVD) or preventing its sequelae.1-2 These recommendations, which are based on the average results of trials evaluating the relative benefits of intensive risk factor control,3-6 are not tailored to an individual's underlying CVD risk. While this approach is often advocated in patients without diabetes,2 there is an implicit assumption that all patients with diabetes are at equally high risk, requiring all patients to be treated aggressively. However, the benefit of intensifying treatment in order to attain low risk factor targets, or “treating to targets,” could vary greatly across the diabetic population depending on the distribution of CVD risk in the population.
Older clinical trials have demonstrated that intensive risk factor control can provide significant benefits on average for persons with diabetes, but many of these trials enrolled patients with higher than average CVD risk. Two recent sub-studies of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial have confirmed that intensive BP control and intensive treatment with lipid-lowering therapies offer no survival advantage overall but may be beneficial to higher risk groups. But for at least three reasons, even results from these trials provide limited guidance for a typical clinical decision making context. First, since many of the studies of tight risk factor control enrolled patients with a range of CVD risk but did not stratify the results accordingly, the relative benefit of tight control for patients with specific risk levels cannot be determined. Second, clinical trials on primary prevention in patients with diabetes have rarely examined whether the benefits derive from the first few steps of medication intensification (moderate dose statins and low to moderate doses of 2-3 antihypertensive medications) or from later intensifications (high doses of statins or high doses of 3-4 antihypertensive medications). This is important because later intensifications tend to reduce the risk factor less effectively than the initial intensifications. Adding a second antihypertensive therapy, for example, produces a 16% (35%) lower systolic (diastolic) blood pressure reduction than would be expected if the treatment effects were additive,7 implying that combination therapy provides a smaller marginal health benefit.
Treatment harm is a third, and often overlooked factor that determines the relative benefit of tight risk factor control. All treatments used to lower CVD risk factors are associated with adverse events and burdens, and their combined effects could be substantial when polypharmacy is used to reach tight control targets. When the benefits of treatment are small or accrue mainly to a subset of patients, incorporating a small treatment-related disutility can significantly lessen or negate the benefit of treatment.8 Most trials report adverse event rates that far exceed discontinuation rates,9 indicating that patients will persist with burdensome regimens despite experiencing adverse events. No studies have assessed the impact of treatment-related disutility on the benefit of treating to aggressive LDL and blood pressure targets in patients with diabetes.
We therefore examined heterogeneity in the benefits, harms, and net benefit of aggressively treating CVD risk factors in the US diabetes population. Using risk factor reductions obtained in clinical trials of specific treatments and estimates of the associated CVD risk reduction, we developed a simulation model of a treat-to-target strategy. We used the model to assess: 1) the expected health benefit of treating to aggressive risk factor targets, 2) the expected net health benefit after accounting for treatment-related disutility, and 3) the net benefit of individual treatment steps. To assess variation in the net benefit across the population we stratified all analyses according to a measure of CVD risk—a patient's expected loss in quality-adjusted life expectancy if risk factors were to remain uncontrolled.
Methods
Data
We used the third wave of the National Health and Nutrition Examination Survey (NHANES-III) to create a nationally representative cohort of patients aged 30 through 75 with diabetes. NHANES-III was fielded from 1988-1994, a time when statins had only recently been introduced and antihypertensives were used much less intensively, and thus provides distributions of risk factors in a relatively untreated state. In addition, in the absence of data on an individual's treatment history (e.g. contraindications, intolerances, and previous treatment failures) NHANES-III provides more valid inferences for the benefits of treating to targets than recent waves of the survey. We abstracted patients’ baseline medications; hemoglobin A1c levels, blood pressure, and LDL values; and diabetes-related complications. We used multivariate imputation by chained equations (MICE)10 to impute missing data and used sample weights to account for the survey's complex sampling design.11
Treatments
We specified intensification regimens for patients having risk factors above targets at baseline (LDL>100 mg/dL [2.59 mmol/L] and blood pressure >130/80) that differed according to each subject's baseline medications (Table 1). Patients who were not receiving lipid-lowering medications at baseline were started on simvastatin 20 mg and medications were titrated up to 5 times as needed, ending with simvastatin/ezetimibe combination therapy. Patients receiving no antihypertensives at baseline were started on standard doses of a thiazide, and then given an ACE inhibitor, beta blocker, and calcium channel blocker as necessary. Those taking medications at baseline were intensified beyond their initial medications as needed in the same sequence. Patients who failed to reach the target on standard antihypertensive doses were intensified by doubling each dose. We chose this approach because the treatment literature shows greater blood pressure reductions from initial medication doses than from dose titration.12
Table 1.
Treatment intensification regimens
| Intensification treatment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline treatment | Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | Step 6 | Step 7 | Step 8 | |
| LDL | None |
Add SMV20 |
Intensify to SMV40 |
Switch to ATV40 |
Intensify to ATV80 |
Switch to SMV/EZE |
- |
- |
- |
| Non-statin |
Add SMV20 |
Intensify to SMV40 |
Switch to ATV40 |
Intensify to ATV80 |
Switch to SMV/EZE |
- |
- |
- |
|
| Low-dose statin | Intensify to SMV40 | Switch to ATV40 | Intensify to ATV80 | Switch to SMV/EZE | - | - | - | - | |
| | |||||||||
| BP | None |
Add THI |
Add ACE |
Add BBL |
Add CCB |
Intensify THI |
Intensify ACE |
Intensify BBL |
Intensify CCB |
| Thiazide (THI) |
Add ACE |
Add BBL |
Add CCB |
Intensify THI |
Intensify ACE |
Intensify BBL |
Intensify CCB |
- |
|
| ACE Inhibitor (ACE) |
Add THI |
Add BBL |
Add CCB |
Intensify THI |
Intensify ACE |
Intensify BBL |
Intensify CCB |
- |
|
| Beta blocker (BBL) |
Add THI |
Add ACE |
Add CCB |
Intensify THI |
Intensify ACE |
Intensify BBL |
Intensify CCB |
- |
|
| Calcium channel blocker (CCB) | Add THI | Add ACE | Add BBL | Intensify THI | Intensify ACE | Intensify BBL | Intensify CCB | - | |
Note: SMV20=Simvastatin 20 mg, ATV=Atorvastatin, SMV/EZE=Simvastatin 80mg/Ezetimibe 10mg. Patients on low dose statins at baseline were assumed to be on the equivalent of simvastatin 20 mg. For patients treated with multiple antihypertensive classes at baseline, medications were added in the following order as needed: THI, ACE, BBL, CCB.
Treatment efficacy model
Our simulation was comprised of two sets of models, a “treatment efficacy” model, that simulated the efficacy of medication intensification in reducing risk factor levels, and an “outcomes” model, that translated changes in risk factor levels into risks of diabetes complications and quality-adjusted life expectancy. The treatment efficacy model used Monte Carlo simulation to 1) estimate the LDL and blood pressure reductions produced by each treatment for each subject, and 2) to simulate adverse events and discontinuation from each treatment. Patients not reaching targets after the first set of treatments were intensified with step 2 treatments, and the simulation continued until all patients were below targets or until all treatments had been exhausted. We ran patients through the Monte Carlo simulation 500 times to estimate each patient's average risk factor reduction and overall treatment disutility.
Model parameters
Treatment efficacy
Our treatment efficacy parameters (i.e., risk factor reductions from each treatment) came from two meta-analyses of randomized placebo-controlled trials, both by Law et al.,12-13 and a head-to-head trial comparing ezetimibe combination therapy with statin monotherapy.14 For each treatment we abstracted difference-in-difference estimates (reduction in the treatment arm minus reduction in the placebo arm) and their standard errors. We incorporated a diminishing marginal efficacy in blood pressure reduction for combination antihypertensive therapy, a 16%/35% lower systolic/diastolic reduction for any second therapy added to monotherapy, as demonstrated by Wu.7 Because no studies have assessed the relative efficacy of combinations of three or more classes, we assumed each subsequent class had an additional 16% (35%) lower systolic (diastolic) efficacy (e.g. 29% (58%) lower efficacy for a 3rd class, and 41% (73%) lower efficacy for a 4th class). We modeled treatment effects on risk factors as a percentage change from baseline to allow larger reductions for patients having higher baseline LDL and blood pressure levels (Appendix Tables A1 and A2).
Treatment-related disutility
We considered two main sources of disutility: treatment-specific adverse events and the burdens and safety risks from polypharmacy. We limited statin-related adverse events to myalgia; liver failure and rhabdomyolysis were considered too rare to significantly impact our results. Using data from an observational study,15 we assumed that 5.25% of patients beginning a statin developed myalgia and were not intensified further. For patients proceeding to high dose statins, we used a back-titration rate from atorvastatin 80 mg to 40 mg from the IDEAL trial of 13%16 to reflect intolerance of myalgia symptoms. Because a 40 mg dose of atorvastatin was not assessed in IDEAL, we assigned back-titration rates of 6.5% to both 40 mg and 80 mg doses, under the assumption that patients beginning each dose were equally likely to back-titrate to avoid symptoms.
For the antihypertensives, we calculated adverse event rates attributable to each treatment from one of the largest trials to publish these data for all four drug classes.17 Adverse event rates on high dose antihypertensives were estimated to be 46% higher than standard doses for each class.12 Patients who experienced two or more side effects on standard doses of any antihypertensive class were not titrated to the higher dose, while those having two or more side effects after titrating to high doses back-titrated to the standard dose.
Disutility values for side effects found in the literature varied widely depending on the population and estimation methods used. Estimates for diarrhea, for example, ranged from 0.00518 through 0.01419. We therefore decided on a common, conservative yearly disutility of 0.005 for each side effect, with the exception of myalgia (0.10), which we varied in sensitivity analyses (Appendix Table A4).
We assumed all patients incurred a disutility from the burden of polypharmacy, including the inconvenience of daily use of each medication as well as safety risks. Polypharmacy is associated with a greater incidence of drug-drug interactions that reduce the efficacy of beneficial diabetes treatments20-21 and increase the incidence of adverse events22 and depression23. In our base case, we assumed a very small incremental disutility of 0.001 for each drug class added (i.e. 0.001, 0.002, 0.003, and 0.004 for the first four drug classes). For comparison, a disutility of 0.001 is a commonly cited value for the inconvenience of taking aspirin daily. We equated the disutility of a fifth medication (0.005) with that of a typical drug side effect. In sensitivity analyses we assumed a constant 0.001 disutility per class. Given their small magnitude, we assumed all disutilities were additive.
Treatment discontinuation
We simulated discontinuation separately for patients who did and did not experience adverse events on each treatment to accurately reflect the frequency with which patients continued treatment despite having side effects. We estimated average, all-cause discontinuation rates across several large statin trials;3, 24-27 Bruckert15 documented a 20% discontinuation rate for patients experiencing myalgia. A meta-analysis by Ross et al.9 provided both all-cause and adverse event-related discontinuation rates for all four categories of antihypertensives.
Outcomes model
We used a previously published Markov model28-29 to estimate the health benefits associated with the simulated LDL and blood pressure reductions. The model simulates the progression of diabetes as a function of an individual's age, time since diagnosis, prior complications, and baseline measures of LDL, A1c, and blood pressure. Patients proceed through the model, incurring complications (blindness, amputation, nephropathy, renal failure, stroke, and coronary artery disease), in accordance with probabilities derived from randomized controlled trials30-32 and prospective cohort studies.5, 33 The fundamental underlying relationship between risk factors levels and outcomes is a log-linear relationship that has been firmly established in cohort studies and analyses of randomized trials.34-36 Details of this model can be found in Appendix B. We used the Markov model to estimate the gain in quality-adjusted life expectancy associated with each subject's average risk factor reduction, and we used a 3% annual discount rate for estimating the value of future health benefits. Additional sensitivity analyses are described in Appendix A.
Results
The weighted NHANES-III data provide representative estimates for the nearly 8 million individuals with diabetes between the ages of 30 and 75 in the early 1990s, a period when aggressive LDL and BP treatment was uncommon. After excluding the 15% of patients with diabetes that had an LDL < 100 at baseline, the mean LDL in the remainder of the population was approximately 151 mg/dl [3.90 mmol/L]. The majority of patients were not receiving lipid-lowering treatment at baseline, and only four percent were taking statins (Table 2). Nearly 38% of patients had a baseline BP < 130/80. The average BP among those lacking tight BP control at baseline was 144/79; 53% of these patients were taking one or two medications to control their blood pressure, while 43% were on no BP medications.
Table 2.
Base case simulation results
| Baseline treatment | Prevalence (%) | Baseline level | Absolute reduction | Target attainment | QALY gain |
||
|---|---|---|---|---|---|---|---|
| unadjusted* | adjusted | ||||||
| LDL | No treatment | 89.9 | 150.4 mg/dl | 56.0 | 78.6 | 1.49 | 1.40 |
| Non-statin | 5.9 | 153.3 | 57.9 | 76.5 | 1.75 | 1.67 | |
| Low dose statin | 4.2 | 157.7 | 44.2 | 48.4 | 1.57 | 1.55 | |
| Overall | 150.8 | 55.7 | 77.2 | 1.50 | 1.42 | ||
| | |||||||
| BP | No treatment | 42.5 | 141.5, 78.8 mmHg | -16.4, -6.6 | 80.0 | 1.59 | 1.43 |
| 1 class | 31.6 | 145.4, 77.8 | -15.2, -4.9 | 58.0 | 1.28 | 1.08 | |
| 2 classes | 21.7 | 145.9, 80.9 | -12.4, -3.6 | 27.5 | 1.14 | 0.90 | |
| 3 classes | 3.5 | 154.8, 80.1 | -8.2, -2.0 | 18.5 | 0.58 | 0.44 | |
| 4 classes | 0.6 | 141.6, 87.9 | -3.7, -1.0 | 0.1 | 0.62 | 0.42 | |
| Overall | 144.1, 79.0 | -14.8, -5.2 | 59.0 | 1.35 | 1.16 | ||
Unadjusted for treatment-related harms. QALY denotes quality-adjusted life years
Note: Targets were 100 mg/dl [2.59 mmol/L] (LDL) and 130/80 mmHg (blood pressure). Measurement scale for absolute reductions is the same as that used for baseline levels. Results for blood pressure are systolic, diastolic.
Among patients above goal at baseline, our treat-to-target simulation resulted in 77.2% reaching the LDL target (100mg/dl) and 59.0% reaching the blood pressure target (130/80) (Table 2). Those already on medication at baseline were much less likely to attain tight control. For example, patients taking low dose statins at baseline were 30 percentage points less likely to reach an LDL <100, reflecting the diminishing return of dose titration for statins. Results for blood pressure were even more extreme. Whereas 80% of those untreated at baseline reached the BP target, patients on three or more treatments at baseline achieved targets less than 20% of the time. The population undergoing LDL intensification had a mean quality-adjusted life expectancy at baseline of 11.5 years while those undergoing the BP intensification had a mean quality-adjusted life expectancy of 10.1 years. Aggressively treating LDL and blood pressure led to an average gain of 1.50 and 1.35 QALYs, respectively, but after accounting for treatment-related harms, the net benefit was reduced to 1.42 and 1.16 QALYs, respectively.
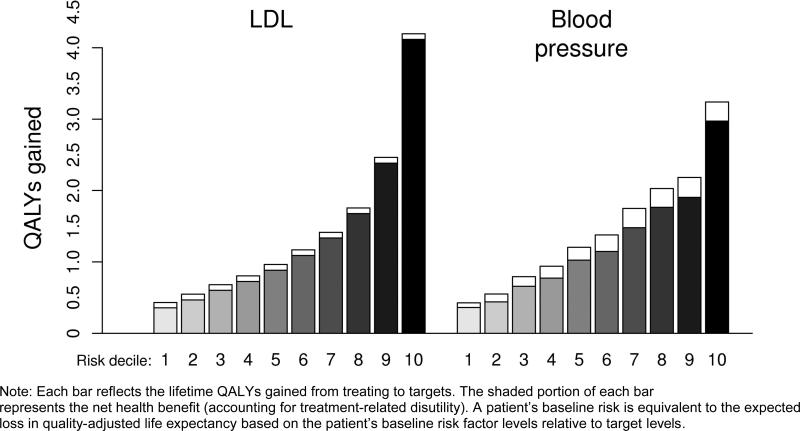
The magnitude of the benefit depended highly on a subject's baseline risk of developing diabetes complications, a measure of risk that incorporates expected losses in both the quality and quantity of life. To identify the baseline risk, we used our outcomes model to estimate the QALYs at risk due to a patient's baseline risk factor level relative to the target level. We then stratified our analyses of treatment intensification by this measure of risk. Figure 1 indicates that patients who were below the median (“below-average”) risk had an average net gain of less than 1 QALY from treating to LDL targets, while patients in the highest risk decile had a net gain of 4.1 QALYs. A similar pattern was found for blood pressure—the net health gains from treating to targets ranged from 0.4 to 3.0 QALYs across risk groups. Disutilities from treatment had a much larger impact on the net benefit of treating to blood pressure targets compared to LDL targets. Because of the skewed distribution of baseline risk in the population, average-risk patients had similar benefits to those with below-average risk, while the top two risk deciles account for nearly 50% (40%) of the population benefit attained by treating to LDL (blood pressure) targets.
Figure 1.
Health benefit and net health benefit of treating to targets, by baseline risk level.
Note: Each bar reflects the lifetime QALYs gained from treating to targets. The shaded portion of each bar represents the net health benefit (accounting for treatment-related disutility). A patient's baseline risk is equivalent to the expected loss in quality-adjusted life expectancy based on the patient's baseline risk factor levels relative to target levels.
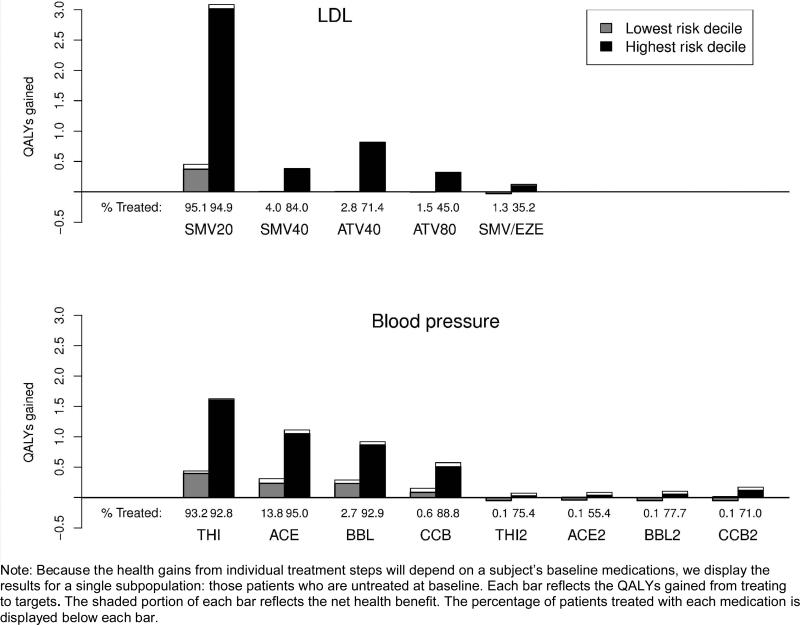
In Figure 2 we display the health gains attributable to individual treatment steps for the population of patients that was untreated at baseline. First-line treatments were associated with the largest gains in health outcomes. There was no incremental benefit of intensifying beyond simvastatin 20 mg for below-average risk patients, and intensification produced net harm at step 5 due to the incremental disutility of combination lipid-lowering therapy. For the blood pressure simulation, below-average risk patients began experiencing significantly diminished benefits with the fourth treatment step (addition of calcium channel blocker) and net harm at all subsequent steps. The dramatic diminishing of health benefits from later treatment intensifications depicted in Figure 2 has two sources. First, each additional treatment used provides diminishing efficacy in decreasing the risk factor (Appendix Tables A1 and A2(note)). Second, a subject's pre-treatment risk of CHD decreases at each successive step because of the risk factor reductions (and health benefits) achieved from prior steps.
Figure 2.
Health benefit and net health benefit of treating to targets, by baseline risk level and treatment.
Note: Because the health gains from individual treatment steps will depend on a subject's baseline medications, we display the results for a single subpopulation: those patients who are untreated at baseline. Each bar reflects the QALYs gained from treating to targets. The shaded portion of each bar reflects the net health benefit. The percentage of patients treated with each medication is displayed below each bar.
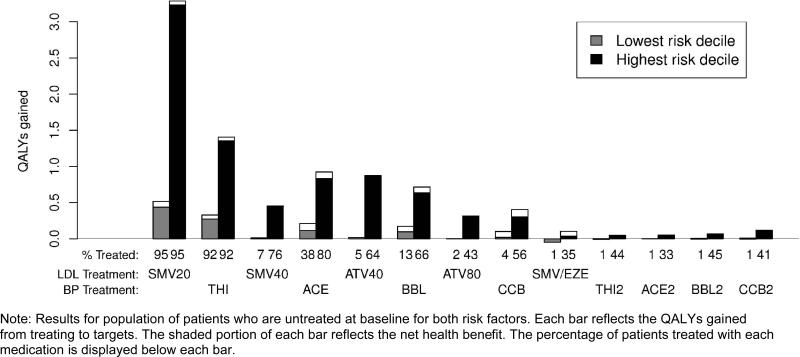
The magnitude of the treatment benefit also depends on whether patients undergo intensification for one or both risk factors. Each treatment is associated with a smaller marginal health gain for patients treated for both risk factors, again due to the fact that treating each risk factor lowers a patient's overall cardiovascular risk. Figure 3 illustrates the benefits for those who had both elevated LDL and blood pressure values and who were untreated at baseline, and is a more accurate representation of the expected health benefits than that depicted in Figure 2, which assumed that the other risk factor was not treated at all. If we alternate intensifying lipid and BP treatments, the marginal gain in QALYs at each treatment step is much smaller than that displayed in Figure 2 (although the overall risk reduction is greater). For example, thiazide therapy produced an incremental gain of 1.6 QALYs for high risk patients when considered independently, but only 1.3 QALYs after a subject began treatment with simvastatin 20 mg. Among patients with below-average risk undergoing treatment intensification, nearly half the benefit of blood pressure treatments was lost to medication harms after step 2 (adding standard dose ACE inhibitors), whereas in the isolated analysis it occurred at step 4 (adding standard dose calcium channel blockers).
Figure 3.
Health benefit and net health benefit of treating to targets, by baseline risk level and treatment, combined analysis.
Note: Results for population of patients who are untreated at baseline for both risk factors. Each bar reflects the QALYs gained from treating to targets. The shaded portion of each bar reflects the net health benefit. The percentage of patients treated with each medication is displayed below each bar.
The results of our sensitivity analyses are provided in Appendix Table A4. Lower adherence, higher discontinuation rates, and the use of discounting, had the greatest impact on the benefit of treating to targets, but the relative QALY benefit for the highest risk group was not significantly diminished in any sensitivity analysis. We did not explore the implications of alternative parameter values on the risks and benefits of individual treatment steps, but we would expect much higher rates of net harm for patients with below-average risk when any individual parameter was changed to be less favorable to a treat-to-target strategy.
Discussion
Previous trials have provided limited information on the variability of benefits and harms of treating to targets across the spectrum of the US diabetes patient population, making it difficult for clinicians to tailor their care to individual patients. We developed a simulation model using the best available evidence from clinical trials and found that diabetes patients with the highest CVD risk accounted for nearly all of the benefits of treating to targets while average-risk patients—nearly three-quarters of the population—received very little benefit. By accounting for treatment-related harms, we identified numerous examples in which intensifying treatment would be contraindicated on the basis of risk/benefit considerations, and many more instances in which the expected benefits are so small, that shared patient-clinician decision-making would appear to be the appropriate medical intervention. Of note, even for very high-risk patients with diabetes, increasing from standard to high doses of BP medications in pursuit of tight control goals was mainly effective for calcium channel blockers, calling into question the wisdom of titrating thiazides, ACE/ARBs or beta-blockers to high doses.
Results similar to our findings have been found frequently when heterogeneity of treatment effects are fully examined. Ioannidis noted over a decade ago that a small minority of high risk patients often account for the vast majority of outcomes in clinical trials.37 Many others have demonstrated how, under such circumstances, using average results from clinical trials can extend treatments to large numbers of patients who get little or no benefit, and sometimes net harm.8 The recent ACCORD trials highlight these risks. Greater use of polypharmacy in the intensive arm of the ACCORD-BP trial (SBP goal of less than 120 mm Hg) was associated with significantly higher rates of at least three types of serious adverse events, but provided no additional reduction in cardiovascular events compared to the non-intensive arm (SBP goal of less than 140 mm Hg). Although there are a number of possible explanations for the lack of benefit of intensive control, one likely explanation is that the majority of the benefit experienced by patients in both arms came from lowering patients’ BP from high levels to moderate levels, or in other words, from lowering blood pressure among the highest risk patients. While there was no overall benefit of combination therapy in the ACCORD-Lipid trial, a subgroup analysis suggested that there might be a benefit for subjects with the lowest levels of HDL and highest levels of triglycerides. Taken together, these results provide additional support for a more nuanced view of selecting risk factor target levels for patients with diabetes.
Our results highlight the implication of heterogeneity of treatment effects for current diabetes treatment guidelines, and that simply because the average CVD risk across all patients with diabetes is high, that does not mean that most people with diabetes have high risk. Most primary prevention guidelines are moving even more strongly to base recommendations on an individual patient's calculated CVD risk, and our results suggest that having diabetes should perhaps no longer be an exception to this general rule. Further, because LDL and blood pressure values are individually poor predictors of a person's overall CVD risk,38 many patients will receive little or no benefit when intensification is based solely on their current LDL and blood pressure levels, while at the same time, some high-risk patients might be undertreated. Disutility from side effects and high levels of polypharmacy have the potential to cause net harm in patients receiving little or no benefit from intensification, especially those who are already taking several medications— which is the majority of patients with diabetes today.39-41 For these patients, the next treatment is likely to have limited efficacy (due to the diminishing benefit of combination therapy), more side effects (because 3rd, 4th, and 5th-line agents and high doses tend to be less well tolerated), and a high polypharmacy burden. While the magnitude of the treatment harm might seem trivial, there are compelling reasons not to discount it. Greater use of polypharmacy is associated with a higher risk of drug interactions; more uncertain long-term safety risks (particularly when newer treatments are used); and significant cost, inconvenience, and side effect burdens that might engender higher rates of non-adherence. Given the large number of comorbidities often associated with diabetes, pursuing small marginal health gains through polypharmacy could have significant opportunity costs. We accounted for these factors using a small disutility that increased with the level of polypharmacy, but formally quantifying these effects is a challenge.
By attempting to simulate a complex clinical process, our model required a number of simplifications. There is no one standard treatment protocol for controlling LDL and blood pressure, and using a different set of treatments, additional treatments, or allowing therapeutic substitutions instead of simply additions or titrations, could impact our results. We restricted our focus to treatments likely to be considered standard in most practice settings and considered only therapies with known efficacy in lowering each risk factor. We did not consider fibrate therapy, for example, which is often prescribed to lower triglycerides and increase HDL, because it has limited efficacy in lowering LDL42 and has no clear effects on reducing CVD mortality.43 While we might have considered substituting therapies for patients having adverse events to lower the contribution of treatment harms, doing so would have resulted in lower rates of successful control and smaller health improvements.
Although we assessed all assumptions in sensitivity analyses, we were unable to find values for several model parameters in the clinical literature. We found no estimates for the relative blood pressure reduction observed with combination therapy involving three or more antihypertensives, so we extrapolated estimates from 2-drug combinations. The absence of these data is alarming given the high level of combination therapy used today. Lacking an estimate of the disutility for statin-induced myalgia, we assigned a value of 0.10; others have reported a higher disutility of 0.18 for myalgia following chemotherapy.44 Our myalgia incidence rates came from the only study we were able to find—a study of patients reporting muscular symptoms on high dose statins in an outpatient setting15—and we assumed patients starting low dose statins had a rate half as large. We did not use myalgia rates reported in statin trials since these estimates are likely to significantly underestimate the true rates because of the use of runin phases for detecting intolerance45 or from the use of inclusion criteria requiring prior tolerance in these trials.16
Our results illustrate the complexities of decision making regarding control of CVD risk factors. The relative benefit of treatment depends on a patient's baseline risk factor level, underlying risk of adverse health outcomes, competing mortality risks, and current medications. It is possible that clinicians already incorporate the issues and principles addressed in our study to help make treatment decisions that are more in line with an individual patient's risks and benefits. Therefore, our model and assumptions may or may not represent how clinicians currently practice but rather models what would happen if current guidelines were followed rigidly by clinicians. Further, we are not proposing that clinicians should solely consider quantitative estimates of models such as ours when making individual patient decisions. However, having such estimates could greatly assist clinicians in helping their patients make personalized decisions, and given the complexity of the factors involved in estimating risks and benefits of lipid and blood pressure treatments, it seems likely that information systems that can assimilate this information and support evidence-based treatment recommendations will be needed for models like ours to be used in clinical practice. Decision support is even more important due to the speed with which the clinical literature evolves in this area.
Conclusion
Aggressively treating cardiovascular risk factors to achieve low targets can produce large health benefits on average, but these benefits accrue disproportionately to a small subset of very high risk patients; below-average to average-risk patients appear to receive virtually no net benefit from titrating beyond standard doses of a statin and two to three blood pressure medications, even if commonly recommended LDL and BP goals have not been met. Given the large set of factors that moderate the benefit of treatment intensification, including patients’ underlying CVD risk, the diminishing efficacy of combination therapy, and increasing polypharmacy and side effects, we recommend a strategy of tailoring treatments to individual patients on the basis of their expected benefit of intensifying treatment. Current treatment approaches that encourage uniformly lowering risk factors to common targets can be both inefficient and cause inappropriate harm.
Supplementary Material
Acknowledgements
This work was supported in part by the VA Health Services Research & Development Service (IIR 06-253) and by the Measurement Core of the Michigan Diabetes Research & Training Center (NIDDK of The National Institutes of Health [P60 DK-20572]). The funding agencies played no role in the design, analysis, or reporting of these findings. All authors had full access to all the data in the study and all take responsibility for the integrity o the data and the accuracy of the data analyses. All authors contributed to the design and analysis of the study, and agree to the manuscript as written.
This work was supported in part by the VA Health Services Research & Development Service (IIR 06-253) and by the Measurement Core of the Michigan Diabetes Research & Training Center (NIDDK of The National Institutes of Health [P60 DK-20572]).
These funders played no direct role in the design, analysis or reporting of these findings.
Footnotes
Dr. Vijan serves on an advisory board for Sanofi-Aventis. The authors received no compensation for conducting this analysis.
A portion of these results were presented at the AcademyHealth Annual Research Meeting in Chicago, Illinois on June 29, 2009
References
- 1.American Diabetes Association Standards of medical care in diabetes--2008. Diabetes Care. 2008 Jan;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004 Jul 13;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 3.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002 Jul 6;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. New England Journal of Medicine. 2004 Apr 8;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 5.UKPDS Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998 Sep 12;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998 Jun 13;351(9118):1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Kraja AT, Oberman A, et al. A summary of the effects of antihypertensive medications on measured blood pressure. American Journal of Hypertension. 2005 Jul;18(7):935–942. doi: 10.1016/j.amjhyper.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Zulman DM, Vijan S, Omenn GS, Hayward RA. The relative merits of population-based and targeted prevention strategies. Milbank Quarterly. 2008 Dec;86(4):557–580. doi: 10.1111/j.1468-0009.2008.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross SD, Akhras KS, Zhang S, Rozinsky M, Nalysnyk L. Discontinuation of antihypertensive drugs due to adverse events: a systematic review and meta-analysis. Pharmacotherapy. 2001 Aug;21(8):940–953. doi: 10.1592/phco.21.11.940.34520. [DOI] [PubMed] [Google Scholar]
- 10.Multivariate Imputation by Chained Equations [computer program]. Version 1.162007.
- 11.Analysis of complex survey samples [computer program]. Version 3.102008.
- 12.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003 Jun 28;326(7404):1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003 Jun 28;326(7404):1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballantyne CM, Bertolami M, Hernandez Garcia HR, et al. Achieving LDL cholesterol, non-HDL cholesterol, and apolipoprotein B target levels in high-risk patients: Measuring Effective Reductions in Cholesterol Using Rosuvastatin therapY (MERCURY) II. Am Heart J. 2006 May;151(5):975, e971–979. doi: 10.1016/j.ahj.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Bruckert E, Simonetta C, Giral P. Compliance with fluvastatin treatment characterization of the noncompliant population within a population of 3845 patients with hyperlipidemia. CREOLE Study Team. J Clin Epidemiol. 1999 Jun;52(6):589–594. doi: 10.1016/s0895-4356(99)00019-0. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005 Nov 16;294(19):2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 17.The treatment of mild hypertension study. A randomized, placebo-controlled trial of a nutritional-hygienic regimen along with various drug monotherapies. The Treatment of Mild Hypertension Research Group. Arch Intern Med. 1991 Jul;151(7):1413–1423. doi: 10.1001/archinte.151.7.1413. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 18.Ebell MH, Warbasse L, Brenner C. Evaluation of the dyspeptic patient: a cost-utility study. J Fam Pract. 1997 Jun;44(6):545–555. [PubMed] [Google Scholar]
- 19.Anderson JP, Moser RJ. Parasite screening and treatment among Indochinese refugees. Cost-benefit/utility and the General Health Policy Model. JAMA. 1985 Apr 19;253(15):2229–2235. [PubMed] [Google Scholar]
- 20.Elliott WJ. Drug interactions and drugs that affect blood pressure. J Clin Hypertens (Greenwich) 2006 Oct;8(10):731–737. doi: 10.1111/j.1524-6175.2006.05939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhoades KR. Prescribed Medications and OTCs: Interactions and Timing Issues. Diabetes Spectrum. 2002;15(4):256–261. [Google Scholar]
- 22.Kroner BA. Common Drug Pathways and Interactions. Diabetes Spectrum. 2002;15(4):249–255. [Google Scholar]
- 23.Huang ES. Appropriate application of evidence to the care of elderly patients with diabetes. Curr Diabetes Rev. 2007 Nov;3(4):260–263. doi: 10.2174/1573399810703040260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glasziou PP, Irwig L, Heritier S, Simes RJ, Tonkin A. Monitoring cholesterol levels: measurement error or true change? Ann Intern Med. 2008 May 6;148(9):656–661. doi: 10.7326/0003-4819-148-9-200805060-00005. [DOI] [PubMed] [Google Scholar]
- 25.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996 Oct 3;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 26.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998 May 27;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 27.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994 Nov 19;344(8934):1383–1389. No authors listed. [PubMed] [Google Scholar]
- 28.Vijan S, Hofer TP, Hayward RA. Estimated benefits of glycemic control in microvascular complications in type 2 diabetes. Annals of Internal Medicine. 1997 Nov 1;127(9):788–795. doi: 10.7326/0003-4819-127-9-199711010-00003. [DOI] [PubMed] [Google Scholar]
- 29.Schmittdiel J, Vijan S, Fireman B, Lafata JE, Oestreicher N, Selby JV. Predicted quality-adjusted life years as a composite measure of the clinical value of diabetes risk factor control. Med Care. 2007 Apr;45(4):315–321. doi: 10.1097/01.mlr.0000254582.85666.01. [DOI] [PubMed] [Google Scholar]
- 30.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004 Aug 21-27;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 31.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003 Jun 14;361(9374):2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 32.Kjeldsen SE, Hedner T, Jamerson K, et al. Hypertension optimal treatment (HOT) study: home blood pressure in treated hypertensive subjects. Hypertension. 1998 Apr;31(4):1014–1020. doi: 10.1161/01.hyp.31.4.1014. [DOI] [PubMed] [Google Scholar]
- 33.UKPDS Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 34.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000 Aug 12;321(7258):412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000 Aug 12;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994 Feb 5;308(6925):367–372. doi: 10.1136/bmj.308.6925.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ioannidis JP, Lau J. The impact of high-risk patients on the results of clinical trials. J Clin Epidemiol. 1997 Oct;50(10):1089–1098. doi: 10.1016/s0895-4356(97)00149-2. [DOI] [PubMed] [Google Scholar]
- 38.Hayward RA, Kent DM, Vijan S, Hofer TP. Multivariable risk prediction can greatly enhance the statistical power of clinical trial subgroup analysis. BMC Med Res Methodol. 2006;6:18. doi: 10.1186/1471-2288-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grant RW, Devita NG, Singer DE, Meigs JB. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care. 2003 May;26(5):1408–1412. doi: 10.2337/diacare.26.5.1408. [DOI] [PubMed] [Google Scholar]
- 40.Willey CJ, Andrade SE, Cohen J, Fuller JC, Gurwitz JH. Polypharmacy with oral antidiabetic agents: an indicator of poor glycemic control. Am J Manag Care. 2006 Aug;12(8):435–440. [PubMed] [Google Scholar]
- 41.Gu Q, Paulose-Ram R, Dillon C, Burt V. Antihypertensive medication use among US adults with hypertension. Circulation. 2006 Jan 17;113(2):213–221. doi: 10.1161/CIRCULATIONAHA.105.542290. [DOI] [PubMed] [Google Scholar]
- 42.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. New England Journal of Medicine. 1999 Aug 5;341(6):410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 43.Saha SA, Kizhakepunnur LG, Bahekar A, Arora RR. The role of fibrates in the prevention of cardiovascular disease--a pooled meta-analysis of long-term randomized placebo-controlled clinical trials. American Heart Journal. 2007 Nov;154(5):943–953. doi: 10.1016/j.ahj.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Launois R, Reboul-Marty J, Henry B, Bonneterre J. A cost-utility analysis of second-line chemotherapy in metastatic breast cancer. Docetaxel versus paclitaxel versus vinorelbine. Pharmacoeconomics. 1996 Nov;10(5):504–521. doi: 10.2165/00019053-199610050-00008. [DOI] [PubMed] [Google Scholar]
- 45.LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005 Apr 7;352(14):1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.