Abstract
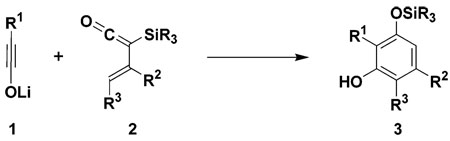
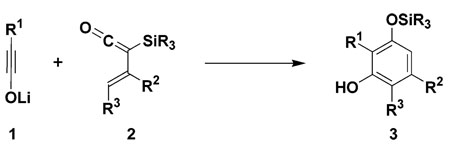
(Trialkylsilyl)vinylketenes react with lithium ynolates to produce highly substituted phenols in a new benzannulation strategy that proceeds via the 6π electrocyclization of an intermediate 3-(oxido)dienylketene.
Vinylketenes1 function as versatile four-carbon building blocks in a variety of useful methods for the synthesis of carbocyclic and heterocyclic compounds. For example, research in our laboratory has shown that [2 + 2] cyclo-additions of vinylketenes can serve as triggering steps in several “pericyclic cascade” strategies for the synthesis of six- and eight-membered carbocyclic compounds.2 Like most ketenes, however, vinylketenes are rarely isolable species and generally must be generated as transient intermediates for in situ trapping with ketenophilic π bonds. In previous studies, we have demonstrated that (trialkylsilyl)vinylketenes (“TAS-vinylketenes”) are remarkably stable ketenes and exhibit reactivity complementary to other vinylketenes in many useful synthetic reactions. In these transformations, the silyl substituent3 suppresses the tendency of vinylketenes to undergo dimerization and [2 + 2] cycloaddition, allowing them to express their underlying reactivity as electron-rich dienes in Diels-Alder cycloadditions4 and as reactive carbonyl compounds in [4 + 1] annulation reactions.5,6 In this Letter we now report a new transformation of these versatile synthons: the reaction of TAS-vinylketenes with lithium ynolates in a new benzannulation strategy for the synthesis of substituted phenols (eq 1).
 |
(1) |
The TAS-vinylketenes 6a–6d required for this investigation were prepared via the photochemical Wolff rearrangement of α′-silyl-α′-diazo-α,β -unsaturated ketones4b (Scheme 1). The requisite photo-Wolff substrates (5a–5d) were synthesized by silylation7 of the corresponding diazo ketones (4a–4d), which were obtained employing our detrifluoro-acetylative diazo transfer procedure.8 As noted previously, TAS-vinylketenes are remarkably robust ketenes, stable at 25 °C and at mildly elevated temperatures, and amenable to purification using conventional silica gel chromatography.
Scheme 1.
a For the prior preparation of 4a,b, 5a,b, and 6a,b, see ref 4b.
As shown in eq 1, lithium ynolates (1) serve as the second reaction partner in the proposed benzannulation. Recent studies have demonstrated that “ynolate anions” function as valuable synthetic intermediates in a number of useful transformations, and several reliable methods are now available for their preparation.9 For our initial studies, we focused our attention on the generation of lithium ynolates via the cleavage of siloxy alkynes (“silyl ynol ethers”) with methyllithium. This method, first described by Kowalski,10 is a variant of the well-known strategy for the regiospecific generation of enolates introduced by Stork and Hudrlik.11 For our purposes, this process offered the attraction that it takes place under mild conditions and produces only inert tetraalkylsilanes as byproducts. In addition, the siloxy alkynes that serve as ynolate precursors can be conveniently prepared in one step from readily available acetylenes or esters. As shown in Scheme 2, siloxy alkynes 9d-9g were thus prepared in 69–90% yield employing the method of Julia,12 and alkynes 9a–9c were obtained via the Kowalski homologation of esters 7a–7c.10,13 As noted previously, these TIPS ynol ethers can be purified by distillation or careful chromatography and are stable to extended storage in solution at 0 °C.
Scheme 2.
a 9a: ref 10. b 9b: Danheiser, R. L.; Helgason, A. L. J. Am. Chem. Soc. 1994, 116, 9471. c 9d: Zang, L.; Kozmin, S. A. J. Am. Chem. Soc. 2004, 126, 10204. d 9e: Sweis, R. F.; Schramm, M. P.; Kozmin, S. A. J. Am. Chem. Soc. 2004, 126, 7442.
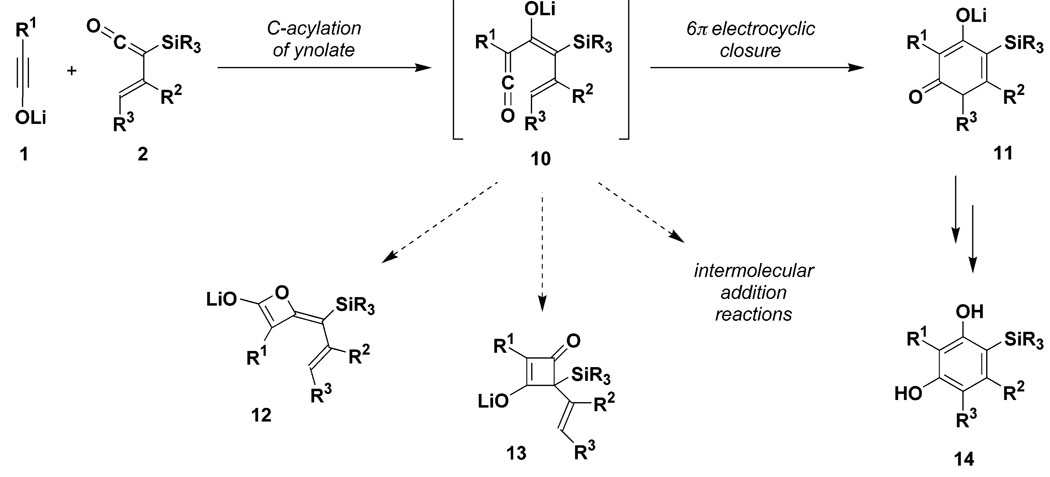
Scheme 3 outlines the mechanistic pathway envisaged for the proposed ynolate benzannulation as well as several of the possible side reactions that we anticipated might compete with the desired reaction. C-Acylation of the ynolate by the TAS-vinylketene was expected to produce intermediate 10, with addition to the ketene occurring anti to the bulky trialkylsilyl group to afford the indicated (Z)-enolate. Several modes of cyclization for this densely functionalized intermediate are then conceivable. The addition of ynolates to aldehydes and ketones leads to the formation of β-lactone enolates,9 and an analogous reaction in this case would give rise to products of type 12. An alternative mode of ring closure would generate the four-membered carbocycle 13, an enolate derivative of a substituted 1,3-cyclobutanedione. Our expectation, however, was that intermediate 10 would most likely undergo facile 6 π electrocyclic ring closure to afford the desired cyclohexadienone 11.14–16 Favoring this mode of cyclization is the (Z)-enolate geometry of 10, which enforces close proximity between the C-1 and C-6 carbon atoms at which bond formation is desired to occur. Also important is the significant increase in charge stabilization that should develop in the transition state leading to the 1,3-dicarbonyl enolate system in 11. It was our expectation that these factors would suffice to favor the desired mode of ring closure over alternative cyclization pathways and intermolecular condensation reactions.
Scheme 3.
The feasibility of the benzannulation was initially investigated using TAS-vinylketene 6a and the ynolate derived from the siloxy hexyne 9e. Exposure of 9e to 1 equiv of methyllithium in THF at room temperature led to complete consumption of the siloxy alkyne within 3 h as monitored by TLC analysis. Upon addition of TAS-vinylketene 6a, a new aromatic product rapidly appeared that was isolated in 62% yield after purification by silica gel chromatography. Interestingly, this benzannulation product was identified as the silyl ether 15 (Table 1) rather than the originally expected resorcinol of type 14. We speculate that the initially formed electrocyclization product 11 isomerizes under the conditions of the benzannulation to produce a 6-silyl-2,4-cyclohexadienone intermediate that aromatizes via a 1,3 carbon → oxygen silyl shift. 1,3-Silyl shifts in α-silyl ketones to form silyl enol ethers are well-known processes,17 and related rearrangements involving silylcyclohexadienones have been observed in our laboratory4b and others.18
Table 1.
Benzannulation via Reaction of TAS-Vinylketenes with Lithium Ynolates
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| entry | alkyne | ketene | product | yield (%)a | entry | alkyne | ketene | product | yield (%)a |
| 1 | 9e | 6a | 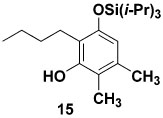 |
62 | 5 | 9d | 6c | 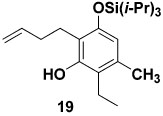 |
68–70 |
| 2 | 9b | 6a | 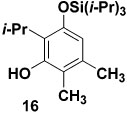 |
65 | 6 | 9c | 6d |  |
44–46 |
| 3 | 9a | 6b |  |
67–71 | 7 | 9g | 6b | 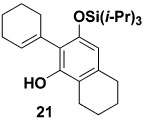 |
43 |
| 4 | 9c | 6c | 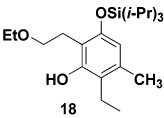 |
46 | 8 | 23 | 6b | 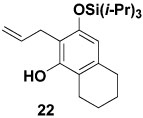 |
44–48 |
Isolated yield of products purified by column chromatography.
Previous studies in our laboratory have demonstrated that TAS-vinylketenes behave as electron-rich dienes in Diels–Alder reactions and react best with electron-deficient dienophiles.4b,c In view of these prior observations, we were not surprised to find that no reaction occurs upon heating TAS-vinylketene 6a and siloxy alkyne 9e in refluxing toluene, and attempted reaction in the presence of Lewis and Bronsted acids (e.g., ZnI2, TiCl4, AgNTf2, HNTf2) resulted only in complex mixtures of products.
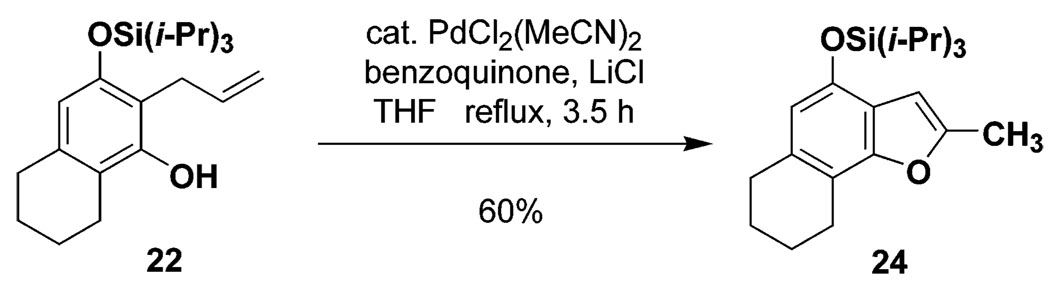
Table 1 delineates the scope of the ynolate benzannulation reaction. Optimization studies revealed methyllithium to be the most effective agent for the generation of ynolates from siloxy alkynes 9a–9g, and low yields of the desired benzannulation products were obtained when TBAF, TBAT, or KOEt19 were substituted for MeLi in the reaction. A variety of siloxy alkynes and vinylketenes participate in the benzannulation, and branching is accommodated on either annulation component. Unfortunately, attempts thus far to extend the reaction to include TAS-arylketenes have not been successful. In addition, low yields of the desired product were obtained upon attempted benzannulation with the allyl-substituted acetylene 9f, apparently as a result of competitive metalation at the methylene carbon by MeLi during the ynolate generation step.20 This problem was easily circumvented, however, by substituting the TBDMS ynol ether 2321 for the TIPS derivative 9f as the ynolate precursor (entry 8). Cleavage of this siloxy alkyne with MeLi is complete within minutes, and upon reaction with TAS-vinylketene 6b the desired benzannulation product 22 is obtained in good yield.
In summary, TAS-vinylketenes react with lithium ynolates in a regiocontrolled benzannulation process that provides efficient access to highly substituted aromatic compounds. We anticipate that these annulation products should serve as useful intermediates in a variety of further synthetic transformations. Phenols such as 21 and 22 are of particular interest, as the unsaturated ortho substitutents can provide the basis for subsequent cyclization reactions to form a variety of oxygen heterocycles including benzofurans and benzopyrans.22 For example, as illustrated in Scheme 4, treatment of 22 with catalytic PdCl2(MeCN)2 in the presence of benzoquinone and LiCl23 furnished the tetrahydronaphthofuran 24 in good yield.
Scheme 4.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health (GM 28273) and Merck Research Laboratories for generous financial support. Y.Z. was supported by a Po Ting Ip Fellowship.
Footnotes
Supporting Information Available: Experimental procedures and characterization data for 4c,d, 5c,d, 6c,d, 9c,f,g, and 15–24. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Reviewed in: Tidwell TT. Ketenes. New York: Wiley; 1995.
- 2.(a) Danheiser RL, Martinez-Davila C, Sard H. Tetrahedron. 1981;37:3943. [Google Scholar]; (b) Danheiser RL, Gee SK, Sard H. J. Am. Chem. Soc. 1982;104:7670. [Google Scholar]; (c) Danheiser RL, Gee SK. J. Org. Chem. 1984;49:1672. [Google Scholar]; (d) Danheiser RL, Brisbois RG, Kowalczyk JJ, Miller RF. J. Am. Chem. Soc. 1990;112:3093. [Google Scholar]
- 3.For reviews of the chemistry of silylketenes, see ref 1 and Pommier A, Kocienski P, Pons J-M. J. Chem. Soc., Perkin Trans. 1998;1:2105. Schaumann E, Scheiblich S. In: Methoden der Organischen Chemie (Houben Weyl) parts 2 and 3. Kropf E, Schaumann E, editors. Vol. E15. Stuttgart, Germany: Thieme; 1993. Pons J-M, Kocienski PJ. In: Science of Synthesis: Houben Weyl Methods of Molecular Transformations. section 4.31. Fleming I, editor. Vol. 4. Stuttgart, Germany: Thieme;
- 4.(a) Danheiser RL, Sard H. J. Org. Chem. 1980;45:4810. [Google Scholar]; (b) Loebach JL, Bennett DM, Danheiser RL. J. Org. Chem. 1998;63:8380. [Google Scholar]; (c) Bennett DM, Okamoto I, Danheiser RL. Org. Lett. 1999;1:641. doi: 10.1021/ol9907217. [DOI] [PubMed] [Google Scholar]
- 5.(a) Loebach JL, Bennett DM, Danheiser RL. J. Am. Chem. Soc. 1998;120:9690. [Google Scholar]; (b) Dalton AM, Zhang Y, Davie CP, Danheiser RL. Org. Lett. 2002;4:2465. doi: 10.1021/ol026014m. [DOI] [PubMed] [Google Scholar]; (c) Rigby JH, Wang Z. Org. Lett. 2003;5:263. doi: 10.1021/ol0272141. [DOI] [PubMed] [Google Scholar]; (d) Davie CP, Danheiser RL. Angew. Chem., Int. Ed. doi: 10.1002/anie.200501579. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For related reactions involving silylated bisketenes, see: Colomvakos JD, Egle I, Ma J, Pole DL, Tidwell TT, Warkentin J. J. Org. Chem. 1996;61:9522. Huang W, Tidwell TT. Synthesis. 2000:457. Allen AD, Huang W-W, Moore PA, Far AR, Tidwell TT. J. Org. Chem. 2000;65:5676. doi: 10.1021/jo000508k.
- 7.(a) Maas G, Brückmann R. J. Org. Chem. 1985;50:2801. [Google Scholar]; (b) Brückmann R, Schneider K, Maas G. Tetrahedron. 1989;45:5517. [Google Scholar]
- 8.(a) Danheiser RL, Miller RF, Brisbois RG, Park SZ. J. Org. Chem. 1990;55:1959. [Google Scholar]; (b) Danheiser RL, Miller RF, Brisbois RG. Organic Syntheses. Vol. IX. New York: Wiley; 1998. p. 197. Collect. [Google Scholar]
- 9.For reviews of the chemistry of ynolates, see: Shindo M. Synthesis. 2003:2275. Shindo M. Chem. Soc. Rev. 1998;27:367.
- 10.Kowalski CJ, Lal GS, Haque MS. J. Am. Chem. Soc. 1986;108:7127. [Google Scholar]
- 11.Stork G, Hudrlik PF. J. Am. Chem. Soc. 1968;90:4464. [Google Scholar]
- 12.Julia M, Saint-Jalmes VP, Verpeaux J-N. Synlett. 1993;233 [Google Scholar]
- 13.Siloxy alkyne 9c (see Supporting Information) was prepared using a two-step variant of the Kowalski reaction; see: Kowalski CJ, Fields KW. J. Am. Chem. Soc. 1982;104:321. Smith AB, III, Adams CM, Kozmin SA, Paone DV. J. Am. Chem. Soc. 2001;123:5925. doi: 10.1021/ja0106164.
- 14.The possibility that intermediate 11 might form via a concerted [4 + 2] cycloaddition of 1 and TAS-vinylketene 2 cannot be excluded.
- 15.For a discussion of the 6π electrocyclization of 3-oxido-1,3,5-hexatrienes, see: Magnus P. NouV. J. Chem. 1978;2:555.
- 16.For prior examples of 6π electrocyclization reactions involving enolate derivatives, see: White JD, Skeean RW. J. Am. Chem. Soc. 1978;100:6296. White JD, Skeean RW, Trammell GL. J. Org. Chem. 1985;50:1939. Magomedov NA, Ruggiero PL, Tang Y. J. Am. Chem. Soc. 2004;126:1624. doi: 10.1021/ja0399066. Magomedov NA, Ruggiero PL, Tang Y. Org. Lett. 2004;6:3373. doi: 10.1021/ol048545b. and references therein.
- 17.For a recent theoretical study and leading references, see: Takahashi M, Kira M. J. Am. Chem. Soc. 1999;121:8597.
- 18.(a) Moser WH, Sun L, Huffman JC. Org. Lett. 2001;3:3389. doi: 10.1021/ol016615y. [DOI] [PubMed] [Google Scholar]; (b) Chamberlin S, Wulff WD. J. Org. Chem. 1994;59:3047. [Google Scholar]; (c) Fogel L, Hsung RP, Wulff WD, Sommer RD, Rheingold AL. J. Am. Chem. Soc. 2001;123:5580. doi: 10.1021/ja010091f. [DOI] [PubMed] [Google Scholar]
- 19.Yu W, Jin Z. Tetrahedron Lett. 2001;42:369. [Google Scholar]
- 20.The metalation of 1-phenyl-4-penten-1-yne with MeLi has previously been reported: Klein J, Brenner S, Medlik A. Isr. J. Chem. 1971;9:177.
- 21.The TBDMS siloxy alkyne 23 was prepared from allylacetylene by employing the method of Julia (see Supporting Information). In general, however, the use of TIPS derivatives is preferred because of their increased stability to purification and storage.
- 22.Reviewed in: Zeni G, Larock RC. Chem. Rev. 2004;104:2285. doi: 10.1021/cr020085h.
- 23.These conditions have been employed by Hegedus for the cyclization of 2-allylanilines to indoles. See: Hegedus LS, Allen GF, Bozell JJ, Waterman EL. J. Am. Chem. Soc. 1978;100:5800.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.