Abstract
Approximately 11% of U.S. women undergo surgery for pelvic floor dysfunction, including genital organ prolapse and urinary and fecal incontinence. The major risk factor for developing these conditions is giving vaginal birth. Vaginal birth is a remarkable event about which little is known from a biomechanical perspective. We first review the functional anatomy of the female pelvic floor, the normal loads acting on the pelvic floor in activities of daily living, and the functional capacity of the pelvic floor muscles. Computer models show that the stretch ratio in the pelvic floor muscles can reach an extraordinary 3.26 by the end of the second stage of labor. Magnetic resonance images provide evidence that show that the pelvic floor regions experiencing the most stretch are at the greatest risk for injury, especially in forceps deliveries. A conceptual model suggests how these injuries may lead to the most common form of pelvic organ prolapse, a cystocele.
Keywords: vaginal birth, biomechanics, stretch ratio, pelvic floor, injury, prolapse
INTRODUCTION: EPIDEMIOLOGY OF PELVIC FLOOR DYSFUNCTION IN WOMEN
Women have been expected to pay a lifelong price for a baby’s birth. Pelvic floor dysfunction, in the form of a type of hernia termed pelvic organ prolapse (including cystocele, rectocele, and/or uterine prolapse) and urinary and fecal incontinence are considered inevitable sequelae for some women who experience injuries during birth. Of the risk factors that have been examined, parity has the strongest association with risk of requiring surgery for pelvic organ prolapse. Compared with nulliparous women, women with one child were 4 times more likely and those with two children were 8.4 times more likely to develop pelvic organ prolapse requiring hospital admission (1). Vaginal delivery is also the single most important risk factor for developing stress urinary incontinence, and the need for treatment increases inexorably with advancing age.
With the burgeoning elderly population, the latent injuries caused in childbirth will affect more and more women. Although surgery for pelvic organ prolapse is effective in restoring anatomy, functional outcomes have not been as satisfactory. Despite the obvious fact that these problems are structural failures, it is only recently that researchers have begun biomechanical analyses to evaluate the mechanisms of normal support and failure.
THE FUNCTIONAL ANATOMY OF THE PELVIC FLOOR MUSCLES
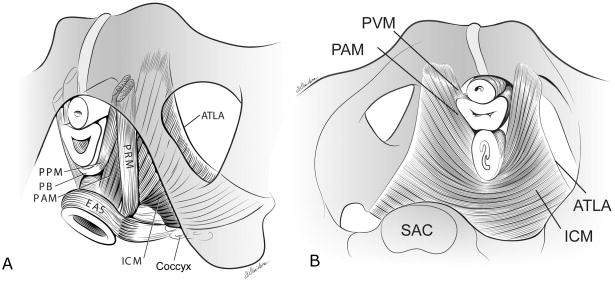
The term pelvic floor refers to all of the muscles, the connective tissue, and the organs that fill the cavity of the pelvic canal. The pelvic floor muscles form a diaphragm that spans the pelvic cavity. Its fibers have a U shape surrounding the levator hiatus, an arrangement that allows their constant activity to close the hiatus, providing support for the superincumbent pelvic and abdominal organs (Figure 1). The levator ani muscle consists of five parts: the pubovaginal, puboperineal, puboanal portions, which form the pubovisceral complex, and the puborectalis and iliococcygeus muscles. These parts of the levator ani muscle form three different regions of the pelvic floor. Region 1 is the iliococcygeal portion, which forms a relatively flat, horizontal shelf spanning the potential gap from one pelvic sidewall to the other near the sacrum. Region 2 includes the pubovisceral muscle, consisting of fatigue-resistant (type 1) muscle fibers that arise from the pubic bone on either side of the symphysis and attach to the walls of the pelvic organs and the perineal body; these help to close the urogenital hiatus (the opening in the levator ani through which the urethra and vagina pass). Region 3, the puborectal muscle, forms a sling around and behind the rectum, just cephalad to the external anal sphincter.
Figure 1.
(a) Schematic view of the levator ani muscles from below, after the vulvar structures and perineal membrane have been removed, that shows the arcus tendineus levator ani (ATLA); the external anal sphincter (EAS); the puboanal muscle (PAM); the perineal body (PB) uniting the two ends of the puboperineal muscle (PPM); the iliococcygeal muscle (ICM); and the puborectal muscle (PRM). Note that the urethra and vagina have been transected just above the hymenal ring. (b) The levator ani muscle seen from above, looking over the sacral promontory (SAC), showing the pubovaginal muscle (PVM), sometimes called the pubococcygeal muscle. The urethra, the vagina, and the rectum have been transected just above the pelvic floor. PAM denotes the puboanal muscle. (The internal obturator muscles have been removed to clarify levator muscle origins.) From Kearney R, Sawhney R, and DeLancey JO. Levator ani muscle anatomy evaluated by origin-insertion pairs. Obstet Gynecol. 2004;104:168–73. © DeLancey 2003
The normal baseline activity of the levator ani muscle keeps the urogenital hiatus closed against the opening action of intrabdominal pressure. The muscle exerts a resultant force in a ventro-cephalic direction, thereby helping to compress the rectum, vagina and urethra, from back to front, as it equilibrates intraabdominal pressure. To counteract increased hydrostatic pressure and the effect of greater abdominal wall muscle tone, this postural activity exerts a 92% larger vaginal closure force in the upright posture than in the supine posture, in an analogous manner to the erector spinae muscle activity required to maintain upright trunk posture. Indeed, in the upright posture, the pelvic floor muscles are preactivated before rapid arm movements, just as the erector spinae muscles are (2).
A maximum voluntary contraction of the levator ani muscles causes the pubovisceral muscles and the puborectalis muscles to additionally increase the vaginal closure force by 46%, further compressing the rectum, distal vagina and urethra behind the pubic bone distally and against intraabdominal pressure more proximally. It is this compressive force and pressure that one feels when palpating a pelvic floor muscle contraction vaginally. Finally, maximal contraction of the mid and dorsal iliococcygeus muscles (Region 1) elevates the central region of the posterior pelvic floor but likely contributes little to a vaginal measurement of levator strength because these muscles do not act about the vagina.
PELVIC FLOOR MUSCLE ISOMETRIC CONTRACTILE PROPERTIES
A popular method of testing pelvic floor muscle strength has involved the use of vaginally placed balloon-type sensors. However, their radial compliance precludes the isometric measurements favored by muscle physiologists. We therefore invented a two-billed instrumented speculum to make the isometric measurements of vaginal closure force outlined in the two preceding paragraphs. Knowing that the physiological cross-sectional area of the Region 2 muscles is approximately 2.8 cm2 (3) and that striated muscle can maximally develop 2.8 Newtons (N) cm−2 parallel with its fibers, we can predict that the right and the left sides of this muscle each develop approximately 5.6 N, or a total force acting to close the vagina of 11 N. This is consistent with the mean (± SD) maximum value of 10 (± 2) N of vaginal closure force we have measured in upright stance (4).
PELVIC FLOOR DIMENSIONS AND LOADING
The pelvic floor, viewed cranially, is a hexagonally shaped structure connected to the bony pelvis at the sacrum, ischial spines, and pubic bones. In the upright posture it is loaded by the hydrostatic load due to the height of the fluid abdominal contents beneath the diaphragm, by part of the weight of the genital organs, and by any abdominal pressurization due to abdominal muscle and diaphragm muscle postural tone and/or respiratory activity. In published studies, we have measured hydrostatic pressures of 40 cmH2O (the traditional pressure units in urology and gynecology) in the bladder, just above the pelvic floor, so this is approximately the hydrostatic load carried by the pelvic floor in the upright posture. The area of the average female pelvic floor is approximately 94 cm2, distributed with approximately 53 cm2 located anterior to and 41 cm2 located posterior to the ischial spines (5).
Knowing pelvic floor area and the pressure acting on it, one can estimate the load acting normal to the pelvic floor (calculated as the product of the hydrostatic pressure times the floor area) as approximately 37 N in quiet standing. This load decreases to approximately 19 N in the supine posture. However, during a maximum cough, the strong diaphragmatic contraction causes intravesical pressure to reach approximately 140 cmH2O, thereby placing a peak load of 129 N on the pelvic floor. A small percentage of this load is inertial as downward momentum of the abdominal contents is arrested by the pelvic floor. During another common activity of daily living, straining at stool, co-contractions of the diaphragm and the abdominal wall cause the peak intravesical pressure to typically reach 100 cmH2O over several seconds, thereby exerting a peak load on the pelvic floor of 92 N. Inhibition of the levator ani muscle allows the urogenital hiatus to open for bowel and/or bladder evacuation.
THE SECOND STAGE OF LABOR
During labor, the pelvic floor undergoes remarkable changes to allow a 3.5 kg baby to be passed from inside to outside of the body. The first stage of labor consists of progressively stronger uterine contractions that help to drive the fetal head through a ripened cervix causing it to dilate and thin. The second stage of labor starts after the cervix is completely dilated and the fetal head begins to touch the posterior pelvic floor. During this approximately 90 min stage, spontaneous uterine contractions occur every 3 min for a duration of 1 min. These contractions increase the uterine pressure near the fetal head from 2.6 to 8.5 kPa (6), engaging the head in the pelvis and helping to push it through the birth canal along what is known as the Curve of Carus. When, during the second stage of labor, the woman is instructed to push during uterine contractions by bearing down via a maximal contraction of her diaphragm, this increases the intrauterine pressure to approximately 19 kPa.
If the average diameter of the molded fetal head is 9 cm, then its cross-sectional area is approximately 63 cm2. The above intrauterine pressures would then apply expulsive forces on the fetal head: 16 N at rest, 54 N during a uterine contraction, and 120 N during a volitional push. In a recently published computer simulation, which takes into account the viscoelastic nature of the pelvic floor muscles, we show that a single volitional push timed to coincide with the height of each uterine contraction is a more efficient pushing style than pushing at other times. If the woman becomes exhausted and can no longer make progress, then an instrumented delivery is indicated. A vacuum device is applied to the fetal head to apply an additional traction force of up to 113 N, typically with up to four pulls, each coinciding with a uterine contraction and push (7). In the cases when obstetric forceps have to be used, the additional traction force can reach 200 N (8).
VAGINAL BIRTH IS A MAJOR CAUSATIVE EVENT IN PELVIC FLOOR DYSFUNCTION
All women sustain stretching of their pelvic floor during birth, but only some experience injury (Figure 2). Specific features of injury during vaginal birth influence whether a woman develops prolapse later in life. Several factors that can be grouped together as descriptors of difficult vaginal delivery are associated with increased occurrence of prolapse: forceps delivery, a prolonged second stage of labor, and large infant birth weight have all been associated with pelvic organ prolapse. Unfortunately, because of the overlapping nature of these different factors, it is difficult to determine which of them is causal and which of them is associated. Forceps delivery is used when there has been a prolonged second stage of labor, and both of these factors are increased in infants of large size. The role of childbirth in causing damage to the levator ani muscle, which is associated with both vaginal delivery and with pelvic organ prolapse, is probably the mediating mechanism in these injuries.
Figure 2.
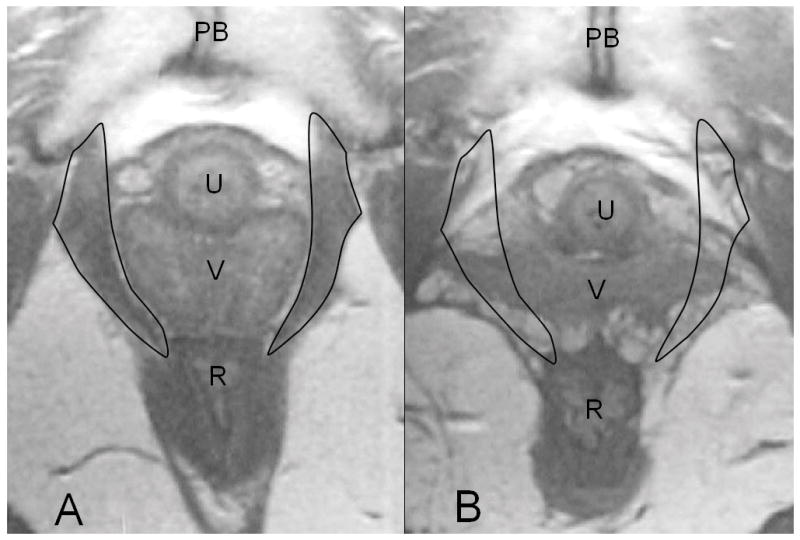
(a) The axial proton density magnetic resonance scan shows a normal pubovisceral muscle with the muscle outlined at the level of the mid-urethra. The scan shows the pubic sympysis (PS), urethra (U), vagina (V) and rectum ( R). (b) A similar image from a woman with complete loss of the pubovisceral muscle (expected location of pubovisceral muscle shown by outline). From DeLancey JO. The hidden epidemic of pelvic floor dysfunction: achievable goals for improved prevention and treatment. Am J Obstet Gynecol. 2005;192:1488–95. © DeLancey 2005
INJURY FROM VAGINAL BIRTH
In one study of 160 primiparous women we found that 32 (20%) exhibited damage to the levator ani muscles on magnetic resonance (MR) scans. None of the 80 nulliparous women serving as controls had injuries, thereby identifying birth as a cause of the type of levator ani muscle injury seen in women with pelvic floor dysfunction. Twenty-nine of these injuries occurred in the pubovisceral muscle; only three injuries occurred in the iliococcygeal portion of the muscle. This was a study originally designed to study stress urinary incontinence: equal numbers of women that developed de novo stress incontinence and women that remained continent after their first birth were recruited. Because the stress-incontinent group were twice as likely to have defects as the continent group, the occurrence of these defects in a group of primiparous women not oversampled for stress incontinence would be somewhat lower than this estimate. However, even if this estimate were halved, it would still indicate that 1 in 10 women delivering their first infant will sustain levator damage. An association has been demonstrated between MR-visible injury and de novo stress urinary incontinence as well as pelvic organ prolapse. In the case of the de novo stress urinary incontinence, use of forceps, anal sphincter laceration, and episiotomy increased the odds ratio for levator muscle injury by 14.7-, 8.1-, and 3.1-fold, respectively (9). Women with levator injuries were 3.5 years older and had a 78-min-longer second stage. These morphological studies identify the role of vaginal birth in the injury and indicate where some of the damage is occurring but do not yield information about the mechanism(s) of injury. In a more recent study, three-dimensional (3D) ultrasound scans of women before and after vaginal birth have confirmed that these types of injury occur during vaginal delivery. There is great variation in the amount of injury, from a few fibers on one side of the levator to the entire pubovisceral muscle, both with and without distortion of surrounding tissues.
MECHANISMS OF LEVATOR MUSCLE INJURY
There have been several suggestions for why the levator ani muscles might be injured. Information from electrodiagnostic studies has demonstrated that birth causes changes in mean motor unit duration after vaginal birth as well as changes in pudendal terminal motor latency. Abnormal tests have also been found in women with prolapse or stress incontinence. Although the pudendal nerve innervates the voluntary urethral and anal sphincters, it does not innervate the levator ani muscles, which receive their own nerve supply from the sacral plexus. Currently, it is not clear whether the visible levator defects are from neurological or stretch injury.
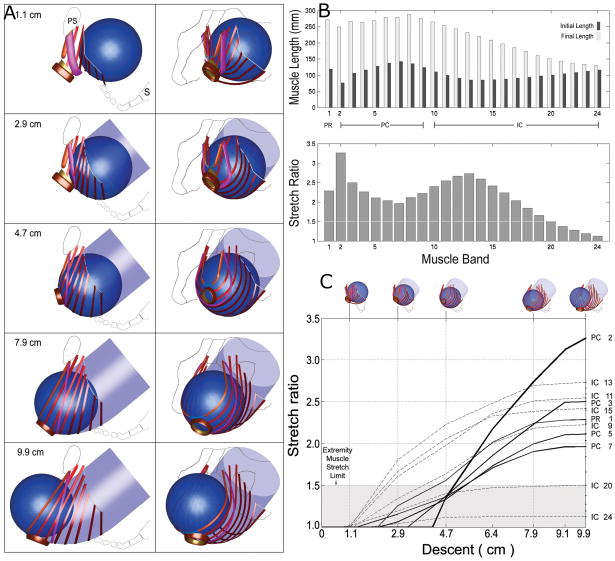
A geometric model (Figure 3) has suggested that some muscle damage during the second stage of labor may come from overstretching because those parts of the muscle that are stretched the most are those parts that are seen to be injured (10). The pubovisceral (pubococcygeal) muscle, the shortest and most medial levator ani muscle, had the largest tissue strain with a stretch ratio of 3.26. Regions of the iliococcygeus, pubococcygeus, and puborectalis muscles reached maximal stretch ratios of 2.73, 2.50, and 2.28, respectively. These values considerably exceed the maximum stretch ratio of 1.5 tolerated by striated muscle in nonpregnant animal preparations. Tissue stretch ratios were found to be proportional to fetal head size. For example, increasing fetal head diameter by 9% increased medial pubovisceral stretch by the same amount. This analysis revealed that the region of muscle injured most often, the pubovisceral (pubococcygeal) portion, was the portion of the muscle that underwent the greatest degree of stretch, and the second area of observed injury, the iliococcygeal muscle, was the muscle stretched second most. Furthermore, when the portion of the muscle at risk was identified in cross sections cut in the same orientation as axial MR scans, the pattern of predicted injury matched the injury seen in MR scans (Figure 4). Finally, the maximum stretch ratio was found at the end of the second stage of labor when the fetal head crowned (Figure 3). These theoretical findings suggesting stretch-induced injury are supported by studies showing increased insulin-like growth factor-1 splice variants that indicate stretch and overload in women after first vaginal birth.
Figure 3.
(a) Simulated effect of fetal head descent on the levator ani muscles in the second stage of labor. At top left, a left lateral view shows the fetal head (as a sphere) located posteriorly and inferiorly to the pubic symphysis (PS) in front of the sacrum (S). The sequence of five images at left shows the fetal head as it descends 1.1, 2.9, 4.7, 7.9, and 9.9 cm below the ischial spines while the head passes along the curve of Carus (indicated by the transparent, light blue, curved tube). The sequence of five images at right are front-left, three-quarter views corresponding to those shown at left. (b) The upper bar graph compares, by muscle, initial and final muscle lengths corresponding to 1.1 and 9.9 cm model fetal head descent, respectively. The lower bar graph shows the maximum corresponding stretch ratio found in each levator ani muscle band. Note that the value of the stretch ratio is not simply proportional to initial or final length. For both graphs, muscles are arranged left to right, in ventral to dorsal order of origin location. (c) The relationship between fetal head descent (abscissa, icons at top) and the resulting muscle stretch ratios (ordinate) in selected levator ani muscles. The labels at right identify the pubovisceral (PC), iliococcygeus (IC), and puborectalis (PR) muscle bands. The largest stretch is induced in the medial-most pubovisceral (PC2) muscle, the last muscle to be engaged by the fetal head. The shaded region denotes the values of stretch tolerated by nongravid appendicular striated muscle without injury. From Reference 10.
© Biomechanics Research Lab, University of Michigan, Ann Arbor 2003.
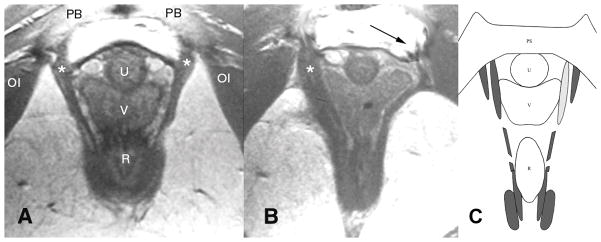
Figure 4.
(a) Normal anatomy in an axial mid-urethra proton density magnetic resonance image that shows the normal pubovisceral muscle(shown by the asterisks). (b) Woman who has lost a part of the left pubovisceral muscle (displayed on the right side of the image, according to standard medical imaging convention), with lateral displacement of the vagina into the area normally occupied by the muscle. The arrow points to the expected location of the missing muscle. (c) Axial, mid-urethral section through the arch of the pubic bone (see pubic symphysis (PS), top) and the model levator ani muscles corresponding to those from the patient shown in (b). Intact muscles are shown in dark gray. The location of simulated muscle atrophy is illustrated by the light gray shading of the left-hand pubovisceral muscle. This location is shown to correspond with the location of muscle atrophy demonstrated in (b). OI: obturator internus; PB: pubic bone; U: urethra; V: vagina; R: rectum. Modified from Reference 10.
We do not yet know whether, during a birth, injury occurs to a pubovisceral muscle in its active or its passive state or both. The best predictors of stretch injury in active muscle are the strain or the product of force and strain (work), with strain and work essentially equivalent in their predictive value (11). The best predictor of injury in passive muscle, as measured by the force deficit after one min, is the negative work done on the muscle. When a passive muscle is stretched, its force depends on two factors: the strain rate and the product of the strain times the strain rate. An order-of-magnitude increase in the strain rate can increase the peak force by 25%. Hence, a physician performing an instrumented delivery (vacuum or forceps instrumentation) is probably wise to keep the rate of pelvic muscle stretch as low as possible by delivering the fetal head as slowly as is reasonable. We recognize that injury can also occur when a shoulder is delivered or when the head is delivered occiput-posterior, but the mechanics of these injury mechanisms remain to be investigated.
THE FUSIBLE LINK HYPOTHESIS
One might speculate how 85–90% of first time mothers can undergo vaginal birth without the fetal head overstretching and rupturing the U-shaped loop of pubovisceral muscle tissue. One possibility is that the structure that lies in series with this muscle could protect it by stretching more than the muscle itself (12), just as a fusible link protects the wiring harness of an automobile against an electrical short. That structure, the perineal body, located between the vaginal and rectum and comprised of relatively soft connective tissue, has material properties that do appear to change in late pregnancy, but these remain to be quantified.
EFFECT OF PREGNANCY ON PELVIC FLOOR TISSUE PROPERTIES
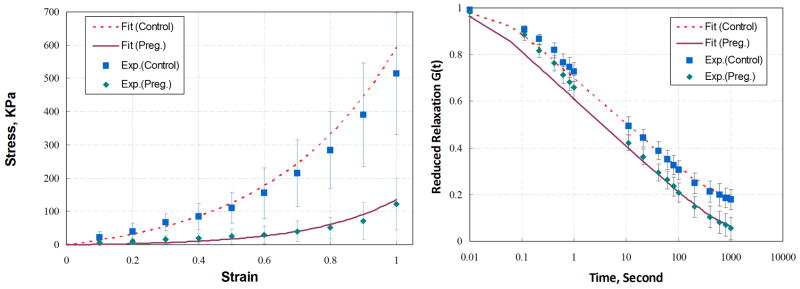
During pregnancy, hormones affect the biochemical composition of the solid matrix and hydration phases constituting each pelvic floor tissue. Remodeling mechanisms lead to changes in the organization, orientation, and diameter of the collagen fibers as well as the crimp structure of the collagen fibrils, reinforcing each tissue. Such effects can significantly affect the short- and longer-term viscoelastic properties of the vaginal wall, the pubovisceral muscles, and the perineal body, for example. They will largely determine (a) the extent and rate at which these structures can be stretched by an expulsive force acting cyclically on the fetal head, and (b) the resistance to stretch provided by those structures. The more a tissue exhibits creep behavior, the further it will stretch under a constant load. And the more it exhibits relaxation behavior, the more the stress in a tissue will decrease over time when held at a constant length, thereby helping to lower the risk of rupture in the next loading cycle. Were a tissue to exhibit viscoplasticity, it would behave like a solid below a critical level of stress, but above that level, it would flow like a viscous liquid. There is evidence that tensile failure in some soft tissues can be predicted by the product of the stress times the strain extant in the tissue, so mechanisms that lower one or both these variables will reduce the risk of rupture. Pregnancy is known to significantly affect the instantaneous stiffness and relaxation behavior of vaginal tissues in rat (14; Figure 5). However, accurate constitutive laws are lacking for pregnant human pelvic floor tissues, and the effects of pregnancy on injury at any tissue level, and on structural failure, are as yet largely unknown.
Figure 5.
Effect of term pregnancy on the tissue properties of the rat vaginal wall. (a) Uniaxial stress-strain and (b) reduced relaxation--function relationships for pregnant and nonpregnant animals. Data are aggregated for 5 mm-long by 3.5 mm-diameter proximal, middle, and distal vaginal ring specimens excised from ten E-15 Fisher 344 rats at 20 days pregnancy and 10 age-matched nonpregnant controls. Specimens were stretched at a constant 1 mm s−1 rate to double their length, and then they were held at that length for 15 min. The quasilinear theory-of-viscoelasticity and custom software were used to identify the material constants (for example, A, B, C, τ1, and τ2), based on a nonlinear optimization algorithm. Reproduced from Reference 13.
FINITE-ELEMENT MODELS OF VAGINAL BIRTH
One finite-element model of the second stage of vaginal birth has estimated the maximum levator muscle stretch to be 1.6 (15). This estimate is artificially low because the geometric data for the model’s levator muscles were taken from a cadaver. Because a cadaver naturally lacks pelvic muscle tone and its pelvic floor is often stretched during embalming, it normally exhibits an enlarged levator hiatus and this will reduce the calculated stretch ratio. A more recent finite-element model (16), based on the MR data of the levator muscles from a living woman having levator muscle tone, demonstrated a maximum stretch ratio of 3.5, thereby corroborating the maximum stretch ratio predicted by Lien (10, 12; Figure 6). The maximum stretch ratio is expected to depend on a number of factors, including the size of the fetal head relative to that of the maternal pelvis, the degree of molding of the fetal head, perineal descent, and the subpubic arch angle. Hence, the maximum stretch ratio is expected to range widely among individuals.
Figure 6.
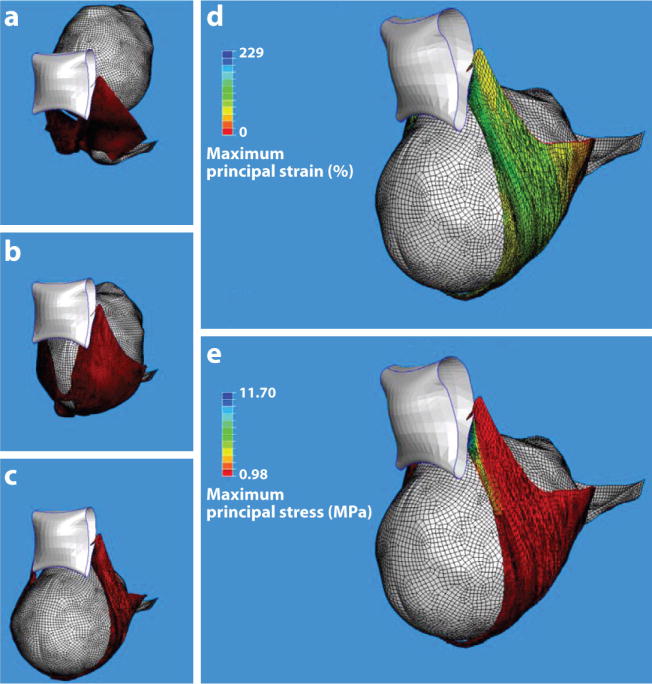
Finite element model results for a simulated single (120 N) push delivery for a 31-year-old mother and fetus at 40 weeks gestation. (a–c) An inferior three-quarter view of the pelvic floor viscohyperelastic soft tissue deformations at three stages during the second stage of labor: (a) Station +2, (b) a middle station, and (c) delivery of the fetal head. The levator ani muscle (red), fetal head (gray), and public bone (white) are shown. (d) The maximum predicted principal strain and (e) stress distributions in the pelvic floor soft tissues at time of delivery. The largest principal strain (d) reached 259% (3.59 stretch ratio). The blue region nearest the pubic bone indicates the local region of highest stress (e), corresponding to the location of muscle defects observed on magnetic resonance scans. Reproduced from Reference 12.
PUDENDAL NERVE STRETCH DURING VAGINAL BIRTH
A geometric model has also been used to predict the stretch ratios in the nerves innervating the levator ani, urethra, and anal sphincter during the second stage of vaginal labor (17). The results showed that the inferior rectal branch exhibited the maximum strain, 35%, and this strain varied by 15% from the scenario with the least perineal descent to that with the most perineal descent. The strain in the perineal nerve branch innervating the anal sphincter reached 33%, whereas the branches innervating the posterior labia and urethral sphincter reached values of 15% and 13%, respectively. It was concluded that during the second stage (a) nerves innervating the anal sphincter are stretched beyond the 15% strain threshold known to cause permanent damage in nonpregnant appendicular nerve, and (b) the degree of perineal descent is shown to influence pudendal nerve strain.
HOW DOES LEVATOR MUSCLE INJURY RELATE TO PELVIC ORGAN PROLAPSE?
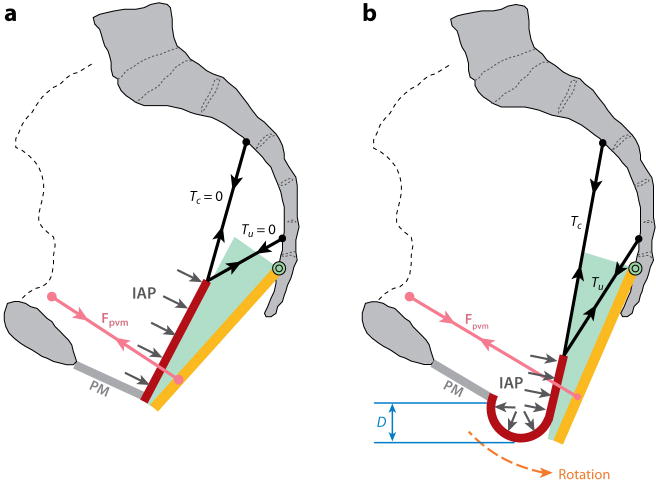
We developed a simplified biomechanical model (Figure 7) of anterior vaginal wall prolapse (cystocele) (18). The anterior vaginal wall is fixed at its lower margin to a structure called the perineal membrane and suspended at its upper end by two mesenteric like structures: the cardinal and uterosacral ligaments. It rests against the rectum and posterior vaginal wall, which are supported by the pubovisceral portion of the levator ani muscles. Intra-abdominal pressure acts perpendicular to the anterior vaginal wall, which pushes against the rectum and levator ani muscles, tending to displace them backward. If muscle force is sufficient to resist this pressure, then there is not net downward displacement of the anterior vaginal wall. If, however, the muscle force is insufficient to resist this pressure, then the hiatus opens and the suspending cardinal and uterosacral ligaments are not stiff enough to prevent downward displacement of the vaginal wall, then the distal vaginal wall will protrude through the genital hiatus. We then examined the effect on model behavior of an impairment of the levator ani muscle, such as that sustained as a birth-related injury. Simulations revealed that the larger the pubovisceral muscle impairment, the larger the anterior wall prolapse became. A 90% impairment of apical support led to an increase in anterior wall prolapse from 0.3 to 1.9 cm at 60% pubovisceral muscle impairment and from 0.7 to 2.4 cm at 80% pubovisceral muscle impairment. These results suggest that a prolapse can develop as a result of impairment of the muscular and apical supports of the anterior vaginal wall. Among women with prolapse, 55% have major injuries to the pubovisceral muscles compared with normal women, who exhibit 16% injury. These findings have functional consequences in that, on average, we found women with prolapse generate 40% less vaginal closure force during maximal voluntary muscle contraction. It is this impairment that may lead to conditions fostering the onset of a cystocele.
Figure 7.
Diagram showing the loading of the anterior vaginal wall and its support system. (a) Loading of the anterior vaginal wall (red) with normal muscular support. (b) Loading of the pelvic floor with defective muscular support and the distal part of the vaginal wall exposed to a pressure differential caused by intra-abdominal pressure (light gray arrows) acting on its superior surface and atmospheric pressure on its inferior surface. IAP: intra-abdominal pressure; Fpvm: reduced tensile force generated by the impaired pubovisceral muscle between the projection of its origin on the pelvic side wall and insertion on the levator plate (orange) which now has been rotated posteriorly under the action of IAP to expose the distal vaginal wall; Tc and Tu: tensile support forces generated by the cardinal and uterosacral ligaments; D: the descent of the most dependent point of the vaginal wall from the end of the perineal membrane (PM), which is used as the measurement of prolapse size in the simulation. Note the descent of the vaginal apex as well as vaginal wall protrusion. Reproduced from Chen LC, Ashton-Miller JA, Hsu Y, DeLancey JOL. Obstet. Gynecol. 108:324–332, 2006, Figure 2.
Summary Points
The pubovisceral portion of the levator ani undergoes a stretch ratio of 3.26 at the end of the second stage of labor.
MR studies show muscle injuries in the region of muscle that undergoes the greatest stretch during the second stage of labor. Use of forceps are associated with the greatest risk of injury to the levator muscles.
Of the main pelvic floor nerves, it is the nerves to the anal sphincter that undergo the greatest strain during the second stage of labor.
Women with prolapse often exhibit MR evidence of an injury to their pelvic floor muscles consistent with those sustained during vaginal birth, and they exhibit a weakness in their maximum isometric vaginal closure force. Biomechanical models suggest that such a weakness can lead to the following sequence of events: the urogenital hiatus being forced open by increases in intraabdominal pressure, exposure of the distal vagina to a pressure differential, apical descent, and the appearance of a cystocele.
Studies are needed on the effects of pregnancy on the constitutive law for and the failure properties of human pelvic floor tissues.
Future Issues
Finite-element models are needed to study the factors that determine whether vaginal birth will progress in a timely manner. Sensitivity analyses are required to examine the effects of hormone- and stretch-related changes on hyperelastic and viscous tissue properties, the degree of fetal head molding, fetal head size and shape, maternal pelvic size and shape, the rate of delivery, the subpubic arch geometry, and so on.
Studies are needed to test the fusible link hypothesis. Does perineal body ripening happen before labor, during labor, or both? If the hypothesis is rejected, does connective tissue in the pubovisceral muscle disaggregate to permit the large stretch ratios?
Measurements of the normal range in the rate of delivery of the fetal head are needed to help validate computer simulations of birth.
Studies are needed of the energetics of pushing during the second stage of labor. Different pushing styles have different energetic costs. Which is optimal from an energetic viewpoint? Clearly, the goal is to ensure that the mother does not become exhausted before the baby is delivered.
What are the mechanisms of injury in the pubovisceral muscle? What role does perineal descent play, if any? What is the role of cephalopelvic disproportion ?
Acknowledgments
We apologize in advance to authors whose work could not be cited due to space limitations. We gratefully acknowledge the contributions of our many collaborators and the financial support of PHS grants P50 HD044406, P30 AG024824, and R01 HD 38665.
Footnotes
DISCLOSURE STATEMENT
Dr. De Lancey has consulted for American Medical Systems, Inc., regarding the anatomy of pelvic organ prolapse, and both authors have received grant support from Johnson & Johnson, Inc.
Contributor Information
James A. Ashton-Miller, Email: jaam@umich.edu.
John O.L. DeLancey, Email: delancey@umich.edu.
LITERATURE CITED
- 1.Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Planning Association Study. Brit J Obstet Gynaecol. 1997;104:579–85. doi: 10.1111/j.1471-0528.1997.tb11536.x. [DOI] [PubMed] [Google Scholar]
- 2.Hodges PW, Sapsford R, Pengel LH. Postural and respiratory functions of the pelvic floor muscles. Neurourol Urodyn. 2007;26:362–71. doi: 10.1002/nau.20232. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Hsu Y, Ashton-Miller JA, DeLancey JO. Measurement of the pubic portion of the levator ani muscle in women with unilateral defects in 3-D models from MR images. Int J Gynaecol Obstet. 2006;92:234–41. doi: 10.1016/j.ijgo.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan DM, Kaur G, Hsu Y, Fenner DE, Guire K, et al. Does vaginal closure force differ in the supine and standing positions? Am J Obstet Gynecol. 2005;192:1722–28. doi: 10.1016/j.ajog.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 5.Baragi R, DeLancey JOL, Caspari R, Howard DH, Ashton-Miller JA. Differences in pelvic floor area between African American and European American Women. Am J Obstet Gynecol. 2002;187:111–15. doi: 10.1067/mob.2002.125703. [DOI] [PubMed] [Google Scholar]
- 6.Rempen A, Kraus M. Pressures on the fetal head during normal labor. J Perinat Med. 1991;19:199–206. doi: 10.1515/jpme.1991.19.3.199. [DOI] [PubMed] [Google Scholar]
- 7.Vacca A. Vacuum-assisted delivery: an analysis of traction force and maternal and neonatal outcomes. Austr New Zeal J Obstet Gynaecol. 2006;46:124–27. doi: 10.1111/j.1479-828X.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- 8.Pearse WH. Electronic recording of forceps delivery. Am J Obstet Gynecol. 1963;86:43–51. doi: 10.1016/0002-9378(63)90075-9. [DOI] [PubMed] [Google Scholar]
- 9.Kearney R, Miller JM, Ashton-Miller JA, Delancey JO. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006;107:144–49. doi: 10.1097/01.AOG.0000194063.63206.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lien KC, Mooney B, DeLancey JO, Ashton-Miller JA. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103:31–40. doi: 10.1097/01.AOG.0000109207.22354.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks SV, Zerba E, Faulkner JA. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol. 1995;488(2):459–69. doi: 10.1113/jphysiol.1995.sp020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lien K-C. PhD thesis. Univ. Mich; Ann Arbor: 2006. Biomechanical analyses of the maternal pelvic floor during the second stage of labor; p. 138. [Google Scholar]
- 13.Jing D, Ashton-Miller JA, DeLancey JOL. Effects of pregnancy on the mechanical properties of rat vagina. Presented at the 2005 Am. Soc. Mech. Eng. Summer Bioeng. Conf; Vail. 2005. [Google Scholar]
- 14.Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am J Obstet Gynecol. 2008;198:590.e1–e6. doi: 10.1016/j.ajog.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martens JAC, Pato MPM, Pires EB, Natal Jorge RM, Parente M, et al. Finite element studies of the deformation of the pelvic floor. Ann NY Acad Sci. 2007;1101:316–34. doi: 10.1196/annals.1389.019. [DOI] [PubMed] [Google Scholar]
- 16.Hoyte L, Damaser MS, Warfield SK, Chukkapalli G, Majumdar A, et al. Quantity and distribution of levator ani stretch during simulated vaginal childbirth. Am J Obstet Gynecol. 2008;199:198.e1–e5. doi: 10.1016/j.ajog.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Lien KC, Morgan DM, Delancey JO, Ashton-Miller JA. Pudendal nerve stretch during vaginal birth: a 3D computer simulation. Am J Obstet Gynecol. 2005;192:1669–76. doi: 10.1016/j.ajog.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Hsu Y, Ashton-Miller JA, DeLancey JOL. Interaction between apical support, levator ani impairment, and anterior vaginal wall prolapse. Obstet Gynecol. 2006;108:324–32. doi: 10.1097/01.AOG.0000227786.69257.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]