Abstract
Osteoporosis-related fractures occur more frequently in women compared with men, but mortality is greater in men compared with women. Peak bone mass is a significant predictor of osteoporosis and fracture risk; therefore, it is important to optimize peak bone mass during young adulthood. Several recent longitudinal studies, which are summarized in this article, have investigated bone changes among young men. Cortical bone loss does not appear to be significant until individuals reach their mid-30s and is associated with decreased sex hormone concentrations. Significant trabecular bone loss in young men aged in their 20s has been reported and is associated with reduced lean mass and activity levels, especially among former athletes. Whether changes in activity levels among nonathletes lead to bone loss among young men requires further investigation.
Keywords: activity, androgen, cortical, estrogen, exercise, male, osteoporosis, trabecular
Epidemiology
Osteoporosis is an important disease affecting approximately 75 million people in Europe, the USA and Japan [1]. In 2000, there were an estimated 9 million new osteoporotic fractures worldwide, with approximately 1.7 million forearm fractures, 1.6 million hip fractures and 1.4 million vertebral fractures [2]. Although osteoporosis and osteoporosis-related fractures occur more frequently in women, with a female-to-male ratio of 1.6 [2], the estimated lifetime risk of an osteoporotic fracture in men over the age of 50 years is substantial at 30%. Increased mortality risk 5–10 years following a low trauma fracture has also been observed in both older Australian men and women [3]. Although the prevalence of osteoporotic fractures is lower in Australian men than in Australian women, the overall fracture-related mortality is greater in men aged 60 years and older, with a 20% mortality in the first 12 months following a hip fracture [4]. These data emphasize the importance and significance of understanding factors associated with bone gain and later bone loss in men.
Peak bone mass is thought to be a significant predictor of future osteoporosis and fracture risk. This is based on studies that have demonstrated that dual energy x-ray absorptiometry (DXA) measures of bone mineral density (BMD) are associated with future fracture risk [5], and that BMD tracks within an individual both in childhood [6] and adulthood [7]. Low BMD is also an important determinant of current fracture risk. In a longitudinal study of 2179 Canadian men aged 50–90 years, over 50% of those who experienced low-trauma fractures had osteopenia, and the incidence of repeat fractures was approximately doubled in the men with osteopenia [8].
Bone as a tissue
Bone is generally classified into two types: trabecular and cortical bone. Cortical bone (also known as compact bone) is less porous than trabecular bone and is found primarily in the shaft of long bones, in the outer shell surrounding trabecular bone at the end of long bones and at the vertebrae. Trabecular bone (also known as spongy bone) is more porous and is found at the end of long bones, in vertebrae and in flat bones such as the pelvis.
Most longitudinal bone growth ceases by the end of puberty and is thought to be a result, in both males and females, of the actions of increased pubertal estrogen concentrations to induce epiphyseal maturation and closure [9–11]. By the late teens, longitudinal bone growth is negligible in most individuals, although growth in bone width continues through bone modeling and periosteal expansion. Bone modeling involves osteoblasts depositing bone matrix and later mineralizing it on the periosteal surface, while osteoclasts resorb bone on the endosteal surface [12]. Modeling leads to expansion of the periosteal surface and an increase in the size of the marrow cavity. Bone remodeling also occurs through successive cycles of bone resorption and bone formation, but this process occurs along the same bone surface with osteoclast-mediated resorption of bone and subsequent osteoblast-mediated laying down of new bone. Usually, bone removal and addition are closely balanced so that there is little to no net effect on the total amount of bone. However, the remodeling process is important for repairing microfractures and allowing for dynamic adaptation to variable external stresses.
Methods for assessing bone
The strength of bone is a function of both bone shape or architecture and bone density. Density is the mass of an object divided by its volume. In standard bone nomenclature, the term ‘bone density’ usually refers to the degree to which a radiation beam is attenuated by a bone. DXA is the most widely used densitometric method for diagnosing osteoporosis; advantages of DXA are its wide availability, relatively low radiation exposure and short scanning times. However, bone measures by DXA are only in 2D and they provide estimates of the amount of bone mineral content (BMC) and bone area within a specific region or in the total body. BMD is then calculated as BMC/bone area (g/cm2). Because this is a 2D measurement, and not an actual volumetric measurement, DXA results are often referred to as areal BMD (aBMD). aBMD measurements are influenced by bone size; larger bones will by definition have greater aBMD even though the actual volumetric (3D) density is the same [13]. This presents problems when interpreting aBMD differences within and across different ages and sexes.
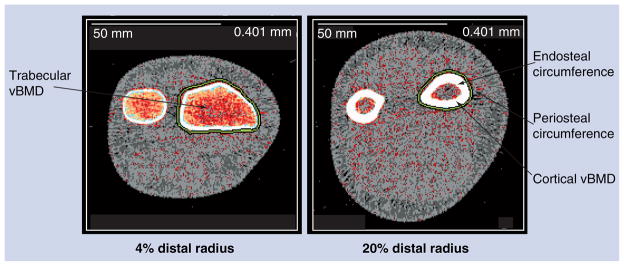
Quantitative computed tomography (QCT) provides a 3D assessment of bone size and geometry and permits analysis of cortical and trabecular BMD (volumetric BMD [vBMD]). In addition, specific geometric parameters of cortical bone can be derived from cross-sectional images (e.g., periosteal and endosteal circumferences, cortical thickness and cortical area) (Figure 1). Cortical vBMD measured by QCT methods is an integrated measure of both the material density of the cortical bone itself, as well as the cortical porosity. Increased cortical porosity is seen during periods of high bone turnover. Although studies show that aBMD measured by DXA is greater in males than females, studies using QCT or peripheral QCT (pQCT) indicate that cortical vBMD is actually higher in females than males following puberty and up until the time of menopause [14,15]. Most studies suggest that the overall actions of estrogen are to decrease bone turnover [15], thereby explaining the higher cortical vBMD in women than men during the reproductive years.
Figure 1. Peripheral quantitative computed tomography image of the 4 and 20% distal radius showing periosteal and endosteal circumferences and cortical and trabecular volumetric bone mineral density.
vBMD: Volumetric bone mineral density.
Longitudinal adult studies have shown aBMD by DXA to predict future fracture risk [16,17], although the sensitivity for assessing vertebral fracture risk is relatively low (65% using WHO criteria) [18]. Criteria for diagnosing osteoporosis are based on the aBMD T-score, which is defined as the observed aBMD expressed in standard deviation (SD) units based on the mean and SD of a normal young adult. A T score of less than −1 SD defines osteopenia and a T score of less than or equal to −2.5 SD defines osteoporosis [19]. Although aBMD results from regional spine and hip DXA scans predict future fracture risk and are usually the primary outcomes reported in studies, they are not as informative for assessing bone structure or assessing cortical and trabecular vBMD. Bone size and geometry, both of which are known to significantly influence bone strength [20], can be measured using QCT.
Role of androgen & estrogen on bone in young adult men
The notion that osteoporosis would be expected to be a disease affecting a significant percentage of men is increasingly accepted. This is due not only to the obvious impact of disease-related androgen deficiency on the male skeleton, but also due to recent studies demonstrating the effect of waning androgen sufficiency on aging male bone [21]. Estrogen, which has been known to influence female bone health for decades, has also recently been shown to be important in growing and aging males for bone growth and metabolism [9,22,23]. In males, unlike females where the ovaries are the primary source of estrogen, the majority of the estrogen in the circulation is derived from the peripheral aromatization of testicular-derived androgens by the cytochrome P450 enzyme, aromatase [24]. Studies of individuals with androgen insensitivity support the role of androgens on maintaining trabecular bone [25], and androgen receptors have been reported in human osteoblasts [26]. The importance of estrogen for normal bone mineralization in the male skeleton is particularly supported by reports of markedly decreased spine aBMD in aromatase-deficient men and their dramatic improvement within 6 months of estrogen treatment [23,27–32]. Similarly, histomorphometric analysis of a man with an estrogen receptor-α point mutation (hERKO) revealed decreased trabecular thickness and volume, but a preservation of trabecular number [33]. DXA measures of the spine aBMD were profoundly low with a Z score of −3.9. pQCT measures of the radius showed normal periosteal circumference coupled with greater endosteal circumference resulting in a smaller cortical thickness compared with controls. Both decreased trabecular and cortical vBMD were also observed. Interestingly, Rochira and coworkers found a significant increase in spine aBMD following androgen administration of an aromatase-deficient man who had been receiving estrogen replacement for approximately 2 years [34]. A clear consensus is emerging from these studies that androgen and estrogen individually, and in a complex collaborative manner, act to promote healthy bone growth and mineralization.
Role of body composition on bone in adolescent & young adult men
Weight and height are significantly associated with bone measures, part of which is a reflection of differences in bone size [13,35]. However, it is becoming increasingly clear that the amount of both lean and fat mass, as well as total body mass, has an important influence on bone. The data on the relationship between bone and lean mass is quite consistent and indicates that bone outcomes are positively associated with lean mass [36–42]. By contrast, the association between bone outcomes and fat mass, if any, is not clear. Whether the relationship between bone and lean mass is due to genetic factors influencing body size or an indirect measure of the influence of bone-loading activities on bone is not known.
A popular analytical approach to investigate the role of fat mass has been to directly relate bone outcomes to fat mass and other covariates using multivariate regression models. Use of this type of analysis in cross-sectional studies of children and adolescents has resulted in both positive and negative associations between fat mass and some bone measures [36–39]. Some of the studies that included males and found a negative association of fat mass and bone measures used regression models containing both body weight and fat mass [43,44], which has been criticized because inclusion of body weight and fat mass in the same model complicates the interpretation of the regression coefficient for fat mass [45]. However, other studies did not have this problem and still found negative associations between DXA-measured bone outcomes and fat mass [40,41]. It has been suggested that detrimental effects of fat mass may be partly due to displacement of lean mass [36]. In this study, both lean mass and fat mass were positively associated with bone mass, but the magnitude of the fat mass effect was much less than the lean mass effect. Thus, an increase in fat mass at a fixed body weight (increase in percentage fat mass) would attenuate the influence of lean mass and effectively be detrimental to bone.
A cross-sectional study conducted in twins found that femoral neck BMD was associated with lean mass independent of fat mass and height. Upon further analyses, these authors concluded that 60–80% of the individual variances in both femoral neck BMD and lean mass, and greater than 50% of their covariance, were accounted for by genetic factors. They also speculated that the relationship between BMD and lean mass was a result of genes that regulated body size, since the cross-twin correlations between BMD and lean mass were no longer different between monozygotic and dizygotic twins when height and fat mass were included in the analysis.
There is one cross-sectional study of healthy male siblings (aged 25–45 years) that investigated the associations of fat mass and lean mass with pQCT-measured bone outcomes for the radius and tibia [42]. At both the radius and tibia, lean mass was found to be positively associated with cortical area and periosteal circumference, but negatively associated with cortical vBMD. By contrast, fat mass was negatively associated with cortical area and periosteal circumference for radius and tibia, and no significant association with cortical vBMD was observed. Leptin, which is produced by adipocytes, has been shown in animal models to influence bone mass both locally (negative association with bone mass) and centrally (positive association with bone mass) through the hypothalamus [46]. Taes and coworkers found that in multivariate models with age and height, leptin was negatively associated with cortical bone area and periosteal circumference at the radius and tibia. When fat mass was added to the statistical model, the effect of leptin was no longer significant, whereas a negative association with fat mass remained. These results suggest that leptin is not responsible for the negative association between bone and fat mass.
Some cross-sectional studies have specifically addressed gender-dependent effects of fat mass on bone outcomes, and although some differences between males and females are noted, there are no consistent trends. A problem with comparing results among these studies is the use of different bone measures. One finding consistent in two studies was a significant negative association between spine aBMD and fat mass in males, but in females the association was positive [41] or not significant [40].
As previously noted, the longitudinal study design allows investigation of associations that reflect relationships within individuals. Changes in a bone measure over time inferred from a cross-sectional association across individuals of different ages may not accurately reflect what occurs over time within an individual. Indeed, Clark et al. found positive cross-sectional associations, as well as negative longitudinal associations, between fat mass and total body BMC and bone area for young girls that depended on pubertal stage [37]. A small number of other longitudinal studies of DXA-measured bone accrual during growth suggest that weight gain in the form of fat mass has a negative effect on bone geometry [47] and aBMD adjusted for height [6].
Peak bone mass & subsequent bone loss in young adult men
Approximately 90% of adult bone mass in both sexes is gained in the first two decades of life and peak bone mass is typically achieved by the early-to-mid 20s. As peak bone mass is a significant predictor of later osteoporosis and fracture risk, optimizing peak bone mass during young adulthood is hypothesized to be important in reducing osteoporosis and fractures later in life.
The National Health and Nutrition Examination Survey (NHANES) III study provides the most widely used adult male total hip aBMD cross-sectional reference dataset [48]. Based on these data and other cross-sectional studies, aBMD for the hip and spine peaks during or before 20–30 years of age, while the whole body and forearm aBMD appears to peak after 30 years of age [49,50]. The age at which peak vBMD occurs is much younger. Gilsanz and coworkers, in a cross-sectional study of 150 females aged 2–20 years, found that trabecular vBMD increased around the age of puberty and remained constant thereafter [51]. Race differences were not apparent prior to puberty, but following puberty African–American girls had greater vBMD compared with Caucasian girls.
Longitudinal studies are used over cross-sectional studies when determining age at peak bone mass. However, these studies are more difficult, and to better define peak bone mass the age range for such a cohort study would need to span adolescence and early adulthood. A longitudinal study of bone acquisition in healthy youth of diverse races (Asian, Hispanic, black and Caucasian) found that increases in aBMD in boys leveled off at approximately 16 years of age for the total hip and at 18 years for spine and whole body [52]. However, this study included 193 males aged 8.8–25.9 years at enrollment, but only 12 boys were aged 19 years or older. The use of a longitudinal study design to define peak bone mass is preferred since peak bone mass inferred from cross-sectional data may be biased owing to a cohort effect if there is a secular trend in bone mass.
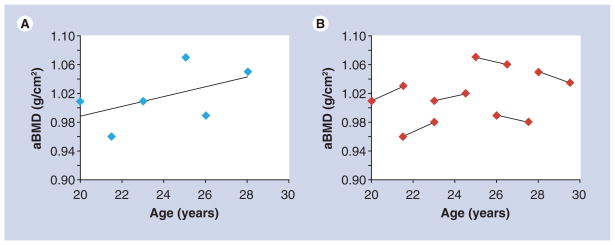
As individuals age following attainment of peak bone mass there is loss of bone, and attenuating this age-related reduction in bone is an important strategy for prevention of osteoporosis. The majority of investigations to estimate rates of bone loss in the period after peak bone mass have focused on the transition from pre- to post-menopause in women. The majority of studies that have investigated factors influencing bone loss in adult men use a cross-sectional design. Data from cross-sectional studies have been shown to both overestimate or underestimate rates of change observed in longitudinal studies [53,54]. The reason for these discrepancies are illustrated in Figure 2, which shows the difference in the estimated rate of change based on cross-sectional (panel A) and longitudinal data (panel B). Regression lines are fitted to cross-sectional data and the rates of loss estimated based on the regression equation, whereas with longitudinal data, the actual rates of change are calculated for an individual and the mean rates of change are then calculated. In this example, the reader would conclude, based on cross-sectional data, that there is a constant rate of change in aBMD with increasing age, but in actuality individual rates of change reveal an age-dependent influence on loss of aBMD.
Figure 2. Difference in rate of change estimation based on cross-sectional (A) and longitudinal (B) data.
Regression lines can be fitted to cross-sectional data and the rates of loss estimated based on the regression equation (A), whereas longitudinal data allow for the calculation of actual rates of change within an individual and mean rates of change can then be calculated (B). aBMD: Areal bone mineral density.
Longitudinal bone changes in young adult men
Another strategy for increasing bone mass in aging men is to increase the peak bone attained as an adolescent or young adult. Several recent longitudinal studies have investigated bone change in young men (Table 1). These investigations can be grouped according to their purpose. Two studies were performed to describe the rates of bone gain or loss [7,54]; two studies focused on the relationships between the rate of bone gain or loss and sex hormone concentrations [55,56]; and several defined the impact of lean mass or physical activity on rates of bone gain or loss [57–59]. These studies are summarized in Table 1 and described in detail in the following sections.
Table 1.
Summary of longitudinal bone studies of young men.
| Study | Study population | Length of follow-up (measurement times) | Number | Bone sites measured | Comments | Significant findings | Ref. |
|---|---|---|---|---|---|---|---|
| Descriptive studies | |||||||
| Emaus et al. (2005) | Men enrolled in the Tromso (Norway) Osteoporosis Study (population-based study) | 6.4 years (0 and 6.4 years) | 147 men stratified into 25–29 (n = 25), 30–34 (n = 31), 35–39 (n = 45) and 40–44 (n = 46) year age groups. | Single x-ray absorptiometry: at baseline and average of 6.4 years later; Distal and ultradistal radius: aBMD, BMC, BA and BMAD | Tromso study started 20 years prior to initial bone measurements | Peak aBMD occurred in 30–34 year age group with losses occurring after this age. Rates of change in the younger age group (25–34 years) was not different from 0 | [7] |
| Lauretani et al. (2008) | Population-based sample (Tuscany, Italy: InCHIANTI study) of men and women aged 21–102 years age-stratified up to 65 years and then random sample | 6 years (0, 3 and 6 years) | 1173 (540 men) enrolled; 345 men had baseline bone measures | pQCT 4% tibia: total and trabecular vBMD; pQCT 38% tibia: total and cortical bone area, medullary area and CSMI | Numbers of men with 3- and 6-year data were not given. Totals (men and women) were 926 at 3 years, and 809 at 6 years | Periosteal expansion increases in younger men. This, combined with a similar medullary expansion across all ages, leads to greater increase in cortical bone area among younger men compared with older men. Decrease in trabecular vBMD is greater in younger versus older men | [54] |
| Hormone-related studies | |||||||
| Khosla et al. (2001) | Age-stratified random, population-based sample of men in Rochester, MN, USA | 4 years (0, 2 and 4 years) | 22–39 (31) years (n = 88); 40–59 (50) years (n = 97); 60–90 (74) years (n = 130); total n = 315 (> one visit) | DXA: spine, hip and mid-distal radius aBMD | Interested in relationship between sex steroids and longitudinal bone changes | Decrease in spine aBMD occurs at all ages and is not related to estrogen or testosterone levels. Increase in hip and radius aBMD were observed in younger men. Greater increase in radius aBMD, but not hip, were associated with increased serum estrogen levels in young men | [55] |
| Riggs et al. (2008) | Age-stratified random, population-based sample of men aged 21–97 years in Rochester, MN, USA | 3 years (annually for radius; baseline and 3 years for spine) | 309 men with at least 1 and up to 3 years of radial pQCT data; 260 men with baseline and 3 years spine QCT data | Spiral QCT and pQCT: spine and radius trabecular and cortical vBMD | – | Decrease in trabecular vBMD observed in young men (30–39 years) and not associated with hormone levels. Rate of change in cortical vBMD was associated with bioactive E2, testosterone and IGF-I (higher hormones, lower rates of loss). Rate of change in cortical vBMD in men younger than 40 years was not different from 0 | [56] |
| Activity-related studies | |||||||
| Bakker et al. (2003) | Participants of the Amsterdam Growth and Health Longitudinal Study (AGAHLS; The Netherlands) | 10 years | 225 men enrolled at age 13 years; bone measured at 27 (n = 84), 32 (n = 195) and 36 (n = 170) years of age; n = 48 with 27- and 32-year visits; n = 41 with 32- and 36-years visits |
DXA: spine aBMD and BMC | Interested in relationship between lean mass and longitudinal bone changes | Decrease in spine aBMD between 27 and 32 years of age. Spine BMC did not change: aBMD decrease between 27 and 32 years may be due to increase in bone size. Lean mass was the most significant predictor of spine aBMD and BMC (increase in lean mass associated with increased aBMD and BMC) | [57] |
| Nordstrom et al. (2007) | Males recruited from two northern Swedish high schools and ice hockey and badminton clubs (volunteers, not population based) | 7.7 years (ages 17, 19, 23 and 25 years) | 116 at 17 years; 107 at 19 years; 102 at 25 years | DXA: spine, femoral neck and hip aBMD, BMC and bone area; spine and FN BMAD | Mixed effect model including age, physical activity, height, weight and pubertal status | Decrease in hip and femoral neck aBMD after 19 years, which paralleled BMC loss. Physical activity decrease from 7.1 to 4.2 h vigorous/week between 17 and 25 years of age. Decrease in activity associated with greater decrease in aBMD loss at all sites | [58] |
| Tervo et al. (2008 and 2009) | Additional follow-up reports based on the population in the initial report by Nordstrom et al. | 12 years (ages 17, 19, 23, 25 and 29 years) | 116 aged 17 years; 99 aged 29 years | DXA: spine, FN and total body aBMD | – | Categorized men into former athletes, active athletes and controls. Decreases in aBMD were increased among former athletes compared with current athletes and controls, and paralleled changes in activity levels, especially at trabecular bone sites | [59,68] |
aBMD: Areal bone mineral density; BA: Bone area; BMAD: Bone mineral apparent density; BMC: Bone mineral content; CSMI: Cross-sectional moment of inertia; DXA: Dual energy x-ray absorptiometry; E2: Estradiol; FFM: Fat free mass; FN: Femoral neck; n: Number of individuals; pQCT: Peripheral quantitative computed tomography; QCT: Quantitative computed tomography; TB: Total body; vBMD: Volumetric bone mineral density.
Longitudinal bone changes in men
The Tromso Study was a population-based study in Norway that followed men (n = 147, aged 25–44 years at enrollment) for 6.4 years [7]. The subjects were stratified into 25–29, 30–34, 35–39 and 40–44 year age groups and single x-ray absorptiometry was used to measure aBMD at the distal and ultradistal radius: single x-ray absorptiometry is similar to DXA, but is only used at peripheral sites, where the soft tissue surrounding the bone is more homogenous. aBMD of the distal and ultradistal radius were obtained at baseline and at 6.4 years. A significant age effect on rates of bone change was observed among men with peak aBMD occurring in the 30–34 year age group. Significant aBMD losses at the distal (primarily cortical bone) radius were observed in both the 35–39 and 40–44 year age groups, while loss at the ultradistal radius was only significant in the 35–39 year age group.
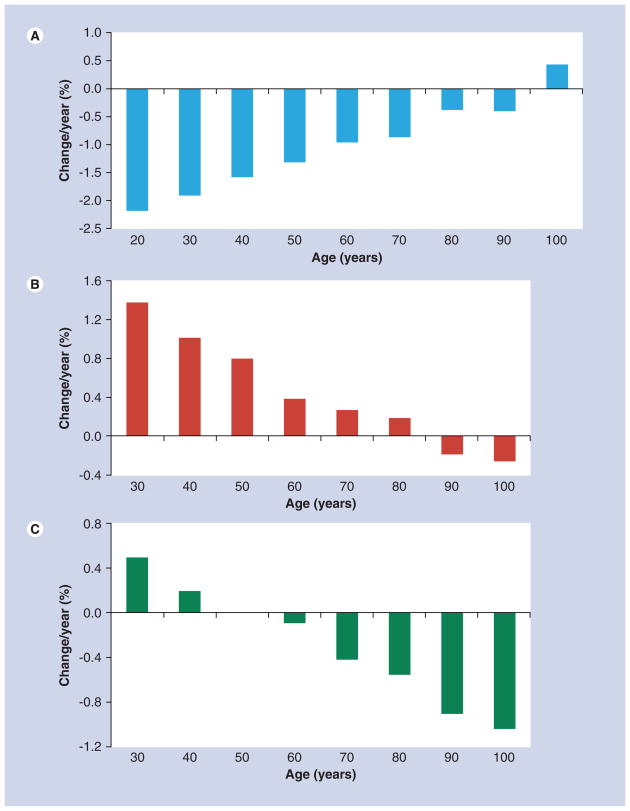
The InCHIANTI study (Invecchiare in Chianti, aging in the Chianti area) was a population-based study of Italian men (n = 345) and women (n = 464) aged 21–102 years who were followed over a 6-year period with tibial pQCT measurements at baseline and at 3 and 6 years [54]. As shown in Figure 3, younger men had greater rates of loss of trabecular vBMD, but greater gains in total and cortical bone area than older men. An estimate of the rate of loss of vBMD in the men aged 20 years was calculated from the regression equation and was 0.6%/year, similar to the findings of Riggs et al. [56]. The increase in periosteal apposition that was observed among the young men in this study, as seen by the increase in total bone area, may explain why others have found decreases in aBMD among young men without an apparent change in BMC [57]. The increase in bone size, with no change in bone mass, would lead to a decrease in aBMD. Although a decrease in aBMD may be viewed as an increase in fracture risk, the larger periosteal circumference would actually lead to a greater cross-sectional moment of inertia and increased bone strength [60].
Figure 3. Percent change per year according to baseline age decade in men aged 20–100 years for (A) trabecular volumetric bone mineral density, (B) total bone area and (C) cortical bone area.
Data taken from [54].
Relationship of sex hormones to bone changes in young adult men
Two studies have investigated the relationship between longitudinal bone changes and sex hormone concentrations among men [55,56]. One of the first studies to report a bone loss in young men was a population-based study by Khosla and coworkers [55] who studied 346 men aged 22 years and older (88 were 22–39 years at baseline). Spine, hip and radius aBMD were measured at baseline and at 2 and 4 years to determine the relationship between longitudinal bone changes and sex steroid concentrations. They categorized men into 22–39, 40–59 and over 60 year age groups and found that significant loss in spine aBMD occurred at all ages and was not associated with estrogen or testosterone concentrations. However, increases in hip and radius aBMD were observed among the younger men in the 22–39 and 40–59 year age groups, and greater increases in radius aBMD were observed among men with higher serum estrogen levels. These results showed the importance of estrogen in increasing radial aBMD in males, and that losses in spine aBMD are seen in men as young as 22–39 years of age and are not related to estrogen or testosterone concentrations.
An age-stratified, random, population-based sample of men aged 21–97 years were enrolled in a prospective study in Rochester, MN, USA [56]. There were 309 men with at least 1, and up to 3 years of follow-up including pQCT of the radius and tibia, and spiral QCT spine measurements. Sex steroid concentrations and bone turnover markers, as well as serum IGF-1 and IGFBP-3 concentrations, were measured in fasting blood. Men were categorized into decade of life and significant decreases in trabecular vBMD at both the ultradistal radius and spine were observed in all age categories beginning at 30–39 years up to greater than 80 years; however, while most age groups had at least 45 men, the 20–29 year age group only had eight men. Changes in trabecular vBMD were not associated with any of the sex steroid concentrations. Rates of cortical vBMD loss at the distal radius occurred in men older than 40 years and were associated with bioactive estradiol, testosterone and IGF-I concentrations: lower rates of bone loss were associated with higher hormone concentrations.
The above studies by Khosla and Riggs focused on whether bone changes in men were associated with hormonal changes; both studies found a relationship between changes in cortical bone and estradiol and testosterone concentrations. In addition, both studies reported early bone loss in trabecular vBMD or bone sites composed of primarily trabecular bone (i.e., spine) that was not associated with hormone concentrations. The same rapid bone loss seen in trabecular bone was not seen in cortical bone or at bone sites composed of primarily cortical bone (i.e., radius); changes at these cortical bone sites were influenced by sex hormone levels. Riggs and coworkers concluded by stating that “the early-onset, substantial trabecular bone loss in both sexes during sex steroid sufficiency is unexplained and indicates that current paradigms on the pathogenesis of osteoporosis are incomplete [56].
Relationship of lean mass or activity to longitudinal bone changes in young adult men
Animal studies, as well as cross-sectional and longitudinal bone studies among athletes, have supported the theory that structural adaptations to bone occur with mechanical loading. Bone marrow fat has been shown to be inversely associated with vertebral vBMD in young adults, supporting the hypothesis that there may be a common progenitor cell capable of a mutually exclusive differentiation into cell lineages responsible for fat and bone formation [61]. In vivo studies found that running rats exhibited a decrease in marrow fat volume and an increase in bone formation rate compared with sedentary rats [62]. These authors also found in tissue and cell culture studies that cyclic loading lowered PPAR-γ, which typically has proadipocytic and antiosteoblastic activity. The results of these studies indicate that mechanical stimuli may modify the balance between adipogenesis and osteoblastogenesis. Unfortunately, there are no studies that have investigated longitudinal changes in activity levels, marrow fat and bone density in young men.
Although some studies suggest that the bone response is greater during growth [63], there is also evidence that bone-loading activities are important postpuberty [64,65]. Some studies have reported long-term benefits of early bone loading [66,67], while other studies do not find persistent skeletal effects [64]. It has become increasingly clear that the response to mechanical loading is site specific and may differ in trabecular and cortical bone [59,68].
Few studies have measured physical activity levels longitudinally, but insight into the role of activity on longitudinal bone changes can also be obtained by investigating the relationship with changes in lean mass, which is considered a surrogate for activity levels. There are several longitudinal studies on bone changes in young men and the relationship to either changes in activity levels or lean mass (Table 1).
Bakker and coworkers reported the results of the Amsterdam Growth and Health Longitudinal Study conducted in The Netherlands [57]. A total of 225 men were enrolled at 13 years of age and spine bone measurements were made at age 27 (n = 84), 32 (n = 195) and 36 (n = 170) years. Variables that were considered in their study included weight, height, BMI, skinfold measurements, body composition variables, and measures of physical activity and calcium intake. They reported a significant loss in spine aBMD between 27 and 32 years of age. Spine BMC did not change, suggesting that the loss in aBMD was due to an increase in spine bone area. Lean mass was the most significant predictor of change in spine aBMD and BMC, with higher lean mass being associated with greater aBMD and BMC. These investigators found that lean mass explained 6% of the variance in spine BMC and 4% of the variance in spine aBMD.
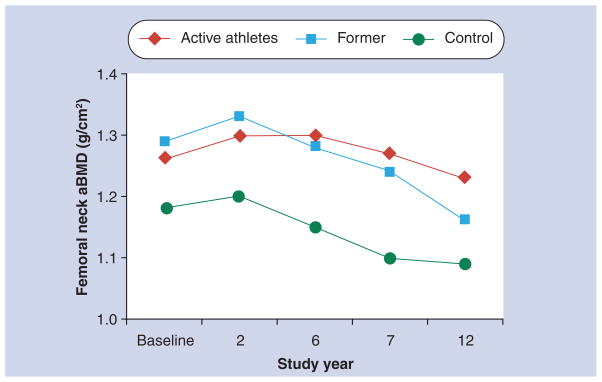
Nordstrom and coworkers recruited 107 male volunteers from two northern Swedish high schools and ice hockey and badminton clubs at a mean age of 17 years [58]. They measured hip, femoral neck and spine aBMD at baseline and at mean ages 19, 23 and 25 years. Although they found no significant change in spine aBMD, they did observe significant losses in hip and femoral neck aBMD after 19 years that paralleled losses in BMC. Significant declines in physical activity levels were also observed, declining from 7.1 to 4.2 h of vigorous activity per week between 17 and 25 years of age. Men with the larger declines in activity had the greatest aBMD loss. In a subsequent paper, changes in aBMD were compared among those athletes who stopped their active careers during follow-up (n = 51), those who continued to be active throughout the follow-up (n = 16) and 25 controls [59]. As shown in Figure 4, significant declines in femoral neck aBMD occurred among the former athletes, while active athletes maintained high aBMD throughout the study compared with controls. Similar results were observed for spine and total body aBMD. A subsequent follow-up paper presented the results at 29 years of age and found that over the 12-year follow-up period reduced physical activity was more strongly associated with changes in trabecular aBMD sites than cortical aBMD sites [68]. The beneficial effect of high activity levels earlier in life was still apparent at predominantly cortical bone sites. These authors concluded that conflicting results in the literature on whether there is a long-term bone benefit of increased activity levels early in life are probably due to which bone site is studied.
Figure 4. Unadjusted mean femoral neck areal bone mineral density in active athletes, former athletes and controls.
aBMD: Areal bone mineral density.
Data taken from [59].
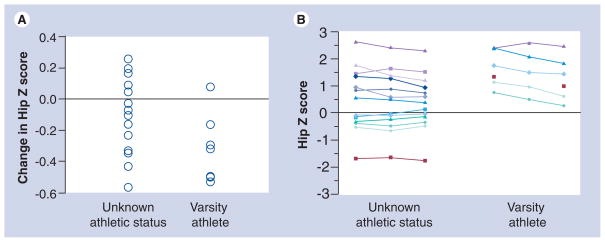
Preliminary results of an ongoing longitudinal 3-year study of bone loss in rural and nonrural men from South Dakota aged 20–66 years at enrollment were consistent with those described above, including significant loss in hip aBMD and trabecular vBMD in young men [69]. A subset of these men were known to play varsity sports (e.g., American football and basketball) while in college and changes in their hip Z score over the 3-year study are shown in Figure 5 compared with age-matched men whose sport status was unknown. These results are consistent with Nordstrom et al. [58], indicating that change in activity levels in young men may have a significant effect on bone changes and may simply reflect a ‘normalization’ of bone to current loads that are placed upon it.
Figure 5.
Change in hip areal bone mineral density Z-scores (A) and areal bone mineral density over 3 years (B) in men known to play varsity sports while in college compared with age-matched men whose sport status during college is unknown.
The importance of lean mass on longitudinal bone changes in young men was seen in the paper by Bakker et al., which reported lean mass as the most significant predictor of spine aBMD and BMC change in young men [57]. The influence of lean mass, rather than total body weight, on bone is probably a reflection of the effect of loading on bone since lean mass theoretically should be associated with physical activity levels.
There are several cross-sectional and longitudinal studies showing a relationship between bone and bone-loading activities among male athletes and nonathletes [59,70–72]. However, longitudinal data on activity levels in young men who are not athletes are scarce. Sport-related activities have been shown to decrease between 20–29- and 30–39-year-old men [73]. This decrease in bone-loading activities may be responsible for the rapid bone loss observed at the hip and femoral neck in some longitudinal studies [58,59], but not others [55].
Both the studies by Bakker et al. [57] and Nordstrom et al. [58] illustrate how changes in lean mass or activity levels may influence the rate of bone loss in young men. Bakker et al. found that bone changes were associated with changes in lean mass [57], while Nordstrom’s group found significant bone loss in former athletes and no bone loss in controls [58,59]. Bakker et al. found that changes in lean mass influenced changes in spine aBMD, while the studies by Nordstrom and coworkers found that reduced activity levels were associated with significant loss at bone sites consisting predominantly of trabecular bone [58,59,68]. Unfortunately, the hip was not measured in the study by Bakker and coworkers. In our preliminary data, former athletes had higher hip Z scores that decreased more significantly over the 3-year follow-up than the age-matched controls.
Conclusion
Although osteoporosis and osteoporosis-related fractures occur more frequently in women compared with men, the mortality associated with an osteoporotic fracture is greater in men than in women. Estrogen, known to influence bone health in women, has also been shown to be important in males for bone growth and mineralization. Peak bone mass is a significant predictor of future osteoporosis and fracture risk; therefore, it is important to optimize peak bone mass during young adulthood in both males and females.
Several recent longitudinal studies have found significant bone loss in young men and a summary of the findings of these studies is shown in Box 1. Compiling the data from these studies indicates that the early bone loss that has been reported is predominantly in trabecular bone and is independent of sex hormone concentrations. Studies discussed previously indicate that the early loss of trabecular bone is associated with decreases in activity levels and lean mass, especially in former athletes. Whether changes in physical activity levels among nonathletes leads to increased rates of bone loss among young men needs further investigation. Early bone loss in cortical vBMD or aBMD at predominantly cortical bone sites does not appear to be significant, and when it does occur it is associated with decreased sex hormone concentrations.
Box 1. Summary of findings on bone loss in young men.
Cortical volumetric bone mineral density
Trabecular volumetric bone mineral density
Spine areal bone mineral density
Hip areal bone mineral density
Future perspective
It is becoming increasingly clear that the influence of different factors (i.e., lifestyle and hormonal) on bone is site specific and that changes in one bone site do not necessarily translate to changes at all bone sites. Over the next 5–10 years the role of lifestyle and hormonal factors on influencing bone gain and bone loss throughout the life cycle, and the underlying mechanisms for their influence, will be better understood. This will be possible, in part, owing to advances in bone imaging techniques that will lead to a greater understanding of how these factors influence bone geometry and volumetric density, along with the development of relatively inexpensive high-throughput genotyping methods. It is likely that the influence of lifestyle and hormonal factors on bone will vary by the age and genetic make-up of an individual. Large-scale epidemiologic studies are needed to provide the data that will enable investigators to test hypotheses that challenge the current paradigm on the pathogenesis of osteoporosis.
Executive summary.
Epidemiology
Osteoporosis and related fractures occur more frequently in women, but overall fracture-related mortality is greater in men.
Bone as a tissue
Bone modeling leads to increases in bone size, while bone remodeling is important for repairing microfractures and allowing for dynamic adaptation to variable external stresses.
Methods of assessing bone
Dual energy x-ray absorptiometry provides measures of areal bone mineral density (aBMD), not volumetric BMD (vBMD); low aBMD Z scores are associated with increased fracture risk.
Quantitative computed tomography measures cortical and trabecular vBMD and provides a 3D assessment of bone size and geometry.
Role of androgen & estrogen on male bone
Circulating estrogen in men is derived from the aromatization of testicular-derived androgens.
Androgen and estrogen are important for bone growth and mineralization in the male skeleton.
Peak bone mass in males
Peak bone mass is thought to be achieved by the early-to-mid 20s.
Rates of bone change based on cross-sectional and longitudinal studies may differ.
Role of body composition on bone in adolescent & young adult men
Consistent findings of a beneficial effect of lean mass on bone have been reported.
Findings on the effect of fat mass on bone are inconsistent, but tend to indicate an adverse effect of fat mass on bone.
Longitudinal bone changes in young adult men
Significant loss of aBMD at cortical bone sites begins in the mid 30s.
Increases in bone size due to periosteal expansion may explain why decreases in aBMD among young men are observed without an apparent change in bone mineral content.
Relationship of sex hormones to bone changes in young adult men
Changes in cortical vBMD and aBMD at cortical bone sites are associated with circulating concentrations of sex hormones.
The higher rates of loss in trabecular vBMD or aBMD at trabecular bone sites (i.e., spine) among young versus old men are not associated with sex hormone concentrations.
Relationship of lean mass or activity to longitudinal bone changes in young adult men
Structural adaptations in bone occur with mechanical loading; these may vary by bone site and differ in trabecular and cortical bone.
Changes in lean mass and activity levels are important predictors of change in aBMD at trabecular-rich bone sites in young men.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by funding from the NIH (R01 AR47852). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.European Foundation for Osteoporosis and Bone Disease and National Osteoporosis Foundation. Who are candidates for prevention and treatment for osteoporosis? Osteoporos Int. 1997;7:1. doi: 10.1007/BF01623453. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;4:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 4▪.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. Important and interesting statistics on increased mortality in men following an osteoporosis-related fracture. [DOI] [PubMed] [Google Scholar]
- 5.Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 6.Foley S, Quinn S, Jones G. Tracking of bone mass from childhood to adolescence and factors that predict deviation from tracking. Bone. 2009;44:752–757. doi: 10.1016/j.bone.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Emaus N, Berntsen GKR, Joakimsen RM, Fonnebo V. Longitudinal changes in forearm bone mineral density in women and men aged 25–44 years. Am J Epidemiol. 2005;162:633–643. doi: 10.1093/aje/kwi258. [DOI] [PubMed] [Google Scholar]
- 8.Langsetmo L, Goltzman D, Kovacs CS, et al. Repeat low-trauma fractures occur frequently among men and women who have osteopenic BMD. J Bone Miner Res. 2009;24:1515–1522. doi: 10.1359/jbmr.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 10.Chagin AS, Savendahl L. Genes of importance in the hormonal regulation of growth plate cartilage. Horm Res. 2009;71:41–47. doi: 10.1159/000192435. [DOI] [PubMed] [Google Scholar]
- 11▪.Smith EP, Specker B, Korach KS. Recent experimental and clinical findings in the skeleton associated with loss of estrogen hormone or estrogen receptor activity. J Steroid Biochem Mol Biol. 2009 doi: 10.1016/j.jsbmb.2009.10.016. (Epub ahead of print). Nice review article on the role of estrogen on bone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle–bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–775. doi: 10.1016/j.bone.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 14▪▪.Riggs BL, Melton J, III, Robb RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. One of the few studies that has longitudinal changes in volumetric density in both males and females across a wide age range. [DOI] [PubMed] [Google Scholar]
- 15▪.Jarvinen TL, Kannus P, Sievanen H. Estrogen and bone – a reproductive and locomotive perspective. J Bone Miner Res. 2003;18:1921–1931. doi: 10.1359/jbmr.2003.18.11.1921. A somewhat controversial article that has an interesting discussion on the role of estrogens on bone. [DOI] [PubMed] [Google Scholar]
- 16.Huang C, Ross P, Wasnich RD. Short-term and long-term fracture prediction by bone mass measurements: a prospective study. J Bone Miner Res. 1998;13:107–113. doi: 10.1359/jbmr.1998.13.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, Black DM, Nevitt MC, et al. The Study of Osteoporotic Fractures Research Group: bone density at various sites for prediction of hip fractures. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 18.Greenspan SL, von Stetten E, Emond SK, Jones L, Parker RA. Instant vertebral assessment: a noninvasive dual x-ray absorptiometry technique to avoid misclassification and clinical mismanagement of osteoporosis. J Clin Densitom. 2001;4:373–380. doi: 10.1385/jcd:4:4:373. [DOI] [PubMed] [Google Scholar]
- 19.WHO. WHO Technical Report Series. Geneva: 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group; p. 843. [PubMed] [Google Scholar]
- 20▪.Burr DB, Turner CH. Section I: anatomy and biology of bone matrix and cellular elements. Chapter 8: biomechanics of bone. In: Lian JB, Goldring SR, editors. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. American Society for Bone and Mineral Research; Washington, DC, USA: 2003. pp. 58–64. Excellent review of the biomechanics of bone. [Google Scholar]
- 21.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metabol. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 24.Simpson ER, Clyne C, Rubin G, et al. Aromatase – a brief overview. Ann Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 25.Marcus R, Leary D, Schneider DL, Shane E, Favus M, Quigley CA. The contribution of testosterone to skeletal development and maintenance: lessons from the androgen insensitivity syndrome. J Clin Endocrinol Metabol. 2000;85:1032–1037. doi: 10.1210/jcem.85.3.6428. [DOI] [PubMed] [Google Scholar]
- 26.Colvard DS, Eriksen EF, Keeting PE, et al. Identification of androgen receptors in normal human osteoblast-like cells. Proc Natl Acad Sci. 1989;86:854–857. doi: 10.1073/pnas.86.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carani C, Qin K, Simoni M, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337:91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann BL, Janssen OE, Hahn S, Broecker-Preuss M, Mann K. Effects of estrogen replacement therapy on bone and glucose metabolism in a male with congenital aromatase deficiency. Horm Metab Res. 2005;37:178–183. doi: 10.1055/s-2005-861292. [DOI] [PubMed] [Google Scholar]
- 29.Maffei L, Rochira V, Zirilli L, et al. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol. 2007;67:218–224. doi: 10.1111/j.1365-2265.2007.02864.x. [DOI] [PubMed] [Google Scholar]
- 30.Maffei L, Murata Y, Rochira V, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metabol. 2004;89:61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- 31.Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metabol. 2004;89:6025–6029. doi: 10.1210/jc.2004-0602. [DOI] [PubMed] [Google Scholar]
- 32.Lanfranco F, Zirilli L, Baldi M, et al. A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone. 2008;43:628–635. doi: 10.1016/j.bone.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Smith EP, Specker B, Bachrach BE, et al. Impact on bone of an estrogen receptor-α gene loss of function mutation. J Clin Endocrinol Metabol. 2008;93:3088–3096. doi: 10.1210/jc.2007-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rochira V, Zirilli L, Madeo B, et al. Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: evidence of a priming effect of estrogen for sex steroids action on bone. Bone. 2007;40:1662–1668. doi: 10.1016/j.bone.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Specker B, Binkley T, Fahrenwald N. Rural vs. non-rural differences in BMC, volumetric BMD, and bone size: a population based cross-sectional study. Bone. 2004;35:1389–1398. doi: 10.1016/j.bone.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Ackerman M, Thornton JC, Wang J, Pierson RN, Jr, Horlick M. Sex difference in the effect of puberty on the relationship between fat mass and bone mass in 926 healthy subjects, 6 to 18 years old. Obesity. 2006;14:819–825. doi: 10.1038/oby.2006.95. [DOI] [PubMed] [Google Scholar]
- 37.Clark EM, Ness AR, Tobias JH The Avon Longitudinal Study of Parents and Children Study Team. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metabol. 2006;91:2534–2541. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garnett SP, Hogler W, Blades B, et al. Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr. 2004;80:966–972. doi: 10.1093/ajcn/80.4.966. [DOI] [PubMed] [Google Scholar]
- 39.Reid IR, Plank LC, Evans MC. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab. 1992;75:779–782. doi: 10.1210/jcem.75.3.1517366. [DOI] [PubMed] [Google Scholar]
- 40.Janicka A, Wren TA, Sanchez MM, et al. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metabol. 2007;92:143–147. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 41.Wey CL, Beare T, Biskeborn K, Binkley T, Arneson L, Specker B. High bone density in young Hutterite children. Bone. 2009;44:454–460. doi: 10.1016/j.bone.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 42▪.Taes YEC, Lapauw B, Vanbillemont G, et al. Fat mass in negatively associated with cortical bone size in young healthy male siblings. J Clin Endocrinol Metabol. 2009;94:2325–2331. doi: 10.1210/jc.2008-2501. Well-written study that investigated several bone outcomes (dual energy x-ray absorptiometry and peripheral quantitative computed tomography) along with sex and fat-derived hormones and markers of bone turnover. In addition, siblings were enrolled so that genetic correlations could be obtained. [DOI] [PubMed] [Google Scholar]
- 43.Weiler HA, Janzen L, Green K, Grabowski J, Seshia MM, Yuen KC. Percent body fat and bone mass in healthy Canadian females 10 to 19 years of age. Bone. 2000;27:203–207. doi: 10.1016/s8756-3282(00)00314-8. [DOI] [PubMed] [Google Scholar]
- 44.Lazcano-Ponce E, Tamayo J, Cruz-Valdez A, et al. Peak bone mineral area density and determinants among females aged 9 to 24 years in Mexico. Osteoporos Int. 2003;14:539–547. doi: 10.1007/s00198-002-1363-2. [DOI] [PubMed] [Google Scholar]
- 45.Wang M, Bachrach L, Van Loan M, Hudes M, Flegal K, Crawford P. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone. 2005;37:474–481. doi: 10.1016/j.bone.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 46.Takeda S, Karsenty G. Molecular basis of the sympathetic regulation of bone mass. Bone. 2008;42:837–840. doi: 10.1016/j.bone.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Petit MA, Beck TJ, Hughes JM, Lin HM, Bentley C, Lloyd T. Proximal femur mechanical adaptation to weight gain in late adolescence: a six-year longitudinal study. J Bone Miner Res. 2008;23:180–188. doi: 10.1359/JBMR.071018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 49.Marwaha RK, Tandon N, Shivapasad C, et al. Peak bone mineral density of physically active healthy Indian men with adequate nutrition and no known current constraints to bone mineralization. J Clin Densitom. 2009;12:314–321. doi: 10.1016/j.jocd.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Henry MJ, Pasco JA, Korn S, Gibson JE, Kotowicz MA, Nicholson GC. Bone mineral density reference ranges for Australian men: Geelong Osteoporosis Study. Osteoporos Int. 2009 doi: 10.1007/s00198–009–1042–1047. published on line. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 51.Gilsanz V, Roe TF, Mora S, Costin G, Goodman WG. Changes in vertebral bone density in black girls and white girls during childhood and puberty. N Engl J Med. 1991;325:1597–1600. doi: 10.1056/NEJM199112053252302. [DOI] [PubMed] [Google Scholar]
- 52.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 53.Melton LJ, III, Khosla S, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL. Cross-sectional versus longitudinal evaluation of bone loss in men and women. Osteoporos Int. 2000;11:592–599. doi: 10.1007/s001980070080. [DOI] [PubMed] [Google Scholar]
- 54.Lauretani F, Bandinelli S, Griswold ME, et al. Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res. 2008;23:400–408. doi: 10.1359/JBMR.071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khosla S, Melton J, III, Atkinson EJ, O’Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metabol. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 56.Riggs BL, Melton LJ, Robb RA, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bakker I, Twisk JWR, Van Mechelen W, Kemper HCG. Fat-free body mass is the most important body composition determinant of 10-yr longitudinal development of lumbar bone in adult men and women. J Clin Endocrinol Metab. 2003;88:2607–2613. doi: 10.1210/jc.2002-021538. [DOI] [PubMed] [Google Scholar]
- 58.Nordstrom P, Neovius M, Nordstrom A. Early and rapid bone mineral density loss of the proximal femur in men. J Clin Endocrinol Metab. 2007;92:1902–1908. doi: 10.1210/jc.2006-2613. [DOI] [PubMed] [Google Scholar]
- 59▪▪.Tervo T, Nordstrom P, Neovius M, Nordstrom A. Constant adaptation of bone to current physical activity level in men: a 12-year longitudinal study. J Clin Endocrinol Metabol. 2008;93:4873–4879. doi: 10.1210/jc.2008-1313. Outstanding paper on longitudinal bone changes in former and current male athletes. Results of this paper support the role of decreasing activity levels with age leading to decreasing bone measures in young adult men. [DOI] [PubMed] [Google Scholar]
- 60.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 61.DiIorgi N, Rosol M, Mittleman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metabol. 2008;93:2281–2286. doi: 10.1210/jc.2007-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62▪.David V, Martin A, Lafage-Proust MH, et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor γ in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. Excellent paper on possible molecular mechanisms responsible for the osteogenic effect of bone-loading activities on bone cells. [DOI] [PubMed] [Google Scholar]
- 63.Heinonen A, Sievaenen H, Kannus P, Oja P, Pasanen M, Vuori I. High-impact exercise and bones of growing girls: a 9-month controlled trial. Osteoporos Int. 2000;11:1010–1017. doi: 10.1007/s001980070021. [DOI] [PubMed] [Google Scholar]
- 64.Nordstrom A, Olsson T, Nordstrom P. Bone gained from physical activity and lost through detraining: a longitudinal study in young males. Osteoporos Int. 2005;16:835–841. doi: 10.1007/s00198-004-1749-4. [DOI] [PubMed] [Google Scholar]
- 65.Ma H, Leskinen T, Alen M, et al. Long-term leisure time physical activity and properties of bone: a twin study. J Bone Miner Res. 2009;24:1427–1433. doi: 10.1359/jbmr.090309. [DOI] [PubMed] [Google Scholar]
- 66.Bass S, Pearce G, Bradney M, et al. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. J Bone Miner Res. 1998;13:500–507. doi: 10.1359/jbmr.1998.13.3.500. [DOI] [PubMed] [Google Scholar]
- 67.Kontulainen S, Kannus P, Haapasalo H, et al. Good maintenance of exercise-induced bone gain with decreased training of female tennis and squash players: a prospective 5-year follow-up study of young and old starters and controls. J Bone Miner Res. 2001;16:195–201. doi: 10.1359/jbmr.2001.16.2.195. [DOI] [PubMed] [Google Scholar]
- 68.Tervo T, Nordstrom P, Neovius M, Nordstrom A. Reduced physical activity corresponds with greater bone loss at the trabecular than the cortical bone sites in men. Bone. 2009;45:1073–1078. doi: 10.1016/j.bone.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 69.Specker B. High rates of bone loss in young men. Osteoporosis in men: how far have we come?. Presented at: Impacts of New Paradigms on Skeletal Health Assessment: A Joint ISCD-IOF Meeting; Orlando, FL, USA. 11–14 March 2009. [Google Scholar]
- 70.Fiore CE, Cottini E, Fargetta C, Di Salvo G, Foti R, Raspagliesi M. The effects of muscle-building exercise on forearm bone mineral content and osteoblast activity in drug-free and anabolic steroids self-administering young men. Bone Miner. 1991;13:77–83. doi: 10.1016/0169-6009(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 71.Nikander R, Sievanen H, Heinonen A, Karstila T, Kannus P. Load-specific differences in the structure of femoral neck and tibia between world-class mogul skiers and slalom skiers. Scand J Med Sci Sports. 2008;18:145–153. doi: 10.1111/j.1600-0838.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 72.Rittweger J, Felsenberg D. Recovery of muscle atropy and bone loss from 90 days bed rest: results from a one-year follow-up. Bone. 2009;44:214–224. doi: 10.1016/j.bone.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 73.Verbrugge LM, Gruber-Baldini AL, Fozard JL. Age differences and age changes in activities: Baltimore Longitudinal Study of Aging. J Gerontol B Psychol Sci Soc Sci. 1996;51B:S30–S41. doi: 10.1093/geronb/51b.1.s30. [DOI] [PubMed] [Google Scholar]