Abstract
In this report, we examined the antitumor activity of photodynamic therapy (PDT) in combination with 5,6-dimethylxanthenone- 4-acetic acid (DMXAA), a vascular disrupting agent currently undergoing clinical evaluation. BALB/c mice bearing subcutaneous CT-26 colon carcinomas were treated with PDT using the second-generation chlorin-based sensitizer, 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (Photochlor) with or without DMXAA. Long-term (60-days) treatment outcome, induction of tumor necrosis factor-alpha (TNF-α) and interleukin- 6 (IL-6), vascular damage (microvessel density, MVD) were evaluated as endpoints. In addition, treatment selectivity was evaluated using magnetic resonance imaging (MRI) and the foot response assay. A highly synergistic interaction was observed with the combination of low-dose DMXAA and PDT (48 J cm−2 at 112 mW cm−2) resulting in ~60% long-term cures. The duration of the PDT session for this combination therapy protocol was only 7 min, while the duration of a monotherapy PDT session, selected to yield the equivalent cure rate, was 152 min. MRI showed markedly less peritumoral edema after DMXAA + short-duration PDT compared with long-duration PDT monotherapy. Similarly, DMXAA + PDT caused significantly less phototoxicity to normal mouse foot tissue than PDT alone. Increased induction of cytokines TNF-α and IL-6 (P < 0.001) was observed at 4 h followed by extensive vascular damage, demonstrated by a significant reduction in MVD at 24 h after combination treatment. In conclusion, Photochlorsensitized PDT in combination with DMXAA exhibits superior efficacy and improved selectivity with clinically feasible illumination schemes. Clinical evaluation of this novel combination strategy is currently being planned.
INTRODUCTION
Over the last decade, photodynamic therapy (PDT) has become an accepted treatment modality for a variety of solid tumors. PDT involves the selective deposition of cytotoxic singlet oxygen in situ through photoactivation of a tissue-localized drug, the sensitizer (1,2). The effectiveness of PDT is dependent on the optimization of multiple factors such as sensitizer dose, the interval between sensitizer injection and photoactivation, the incident light dose (fluence) and light dose rate (fluence rate) (1–3). In current clinical practice, PDT is carried out using prescribed drug doses and fluences as well as fixed drug-light intervals and irradiances. Initial treatment responses after clinical PDT are usually positive; however, in some cases recurrences can occur and the outcome for the patients is poor. Therefore, methods to improve the efficacy of this treatment modality are required.
There is growing evidence that the relatively high irradiances used in a typical PDT session may cause the depletion of ground-state oxygen (3O2) almost immediately following the start of the illumination of the target tissue (4–6). This reaction can be treatment-limiting as a rich supply of 3O2, converted to cytotoxic singlet oxygen (1O2) during the photodynamic process, is required all through the course of tissue illumination. The extent of photochemical consumption of 3O2 is directly related to sensitizer concentration and irradiance in addition to other factors that are outside the clinicians’ control (e.g. capillary density) (7,8). In a dose-ranging study of Photofrin®-based PDT in patients with basal cell carcinomas the step-wise reduction in the photosensitizer dose resulted in proportionally less initial tumor response and a concomitant decrease in response durability (9). In preclinical models, the rational selection of very low irradiances, based on theoretical models, has been an effective and dramatic means of minimizing photodynamic oxygen depletion and maximizing treatment efficacy (4–6). However, these irradiances require long treatment times that may not be clinically feasible; additionally, preclinical and clinical studies of PDT have shown that low fluence rate treatments can result in more damage to normal tissue (10,11). It is therefore essential to identify approaches that result in improved PDT efficacy without concomitant increases in normal tissue toxicity, ideally with the use of short, clinically feasible illumination schemes.
As clinical application of PDT is not precluded by prior treatment, we hypothesized that a combination therapy approach will compensate for the shortfalls associated with attempts to improve PDT by manipulating only PDT treatment parameters. Indeed, a number of previous studies have demonstrated improved outcomes using PDT in combination with surgery, radiation and chemotherapy (12–14). Recently, the therapeutic potential of PDT in combination with anti-angiogenic therapy has also been investigated (15,16). In a previous report, using the Food and Drug Administration-approved sensitizer Photofrin®, we have shown improved efficacy of PDT in combination with 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a vascular disrupting agent (VDA) that is currently undergoing Phase II clinical evaluation (17). While Photofrin® is an effective sensitizer that is widely used in clinical PDT, it is also associated with prolonged and sometimes severe cutaneous phototoxicity in patients (18). This limitation has been the major impetus behind the synthesis of newer sensitizers. One such sensitizer that has shown favorable photophysical and pharmacokinetic properties in preclinical studies is the second-generation, chlorin-based compound, 2-[1-hexyloxyethyl]-2-devinylpyropheophorbide-a (Photochlor; HPPH) (19,20). Clinical Phase I–II studies of HPPH conducted in patients with early/late stage lung and esophageal cancers have also demonstrated excellent response rates (21). In a recent clinical study we have demonstrated that, in addition to its impressive photodynamic efficacy, HPPH is associated with minimal, rapidly diminishing cutaneous sensitivity in patients, a significant clinical advantage over Photofrin® (22).
Therefore, in this study, we examined the preclinical activity of HPPH-sensitized PDT in combination with DMXAA using a murine colon adenocarcinoma model, CT-26, implanted subcutaneously in syngeneic BALB/c mice. The objectives of the study were to determine (1) whether DMXAA potentiated the antitumor activity of HPPH-sensitized PDT in vivo, and (2) the potential mechanism(s) of interaction between the two treatments. We compared the efficacy and selectivity of combination therapy with a low irradiance, long-duration monotherapy PDT regimen that was predicted to preserve tissue oxygenation and has been shown to give the maximum long-term tumor control achievable for this model (23). Here we report the interaction between HPPH-sensitized PDT and DMXAA in vivo, the significance of PDT treatment conditions and advantages of this novel combination strategy that could potentially lead to significant clinical benefit.
MATERIALS AND METHODS
Tumor model
Pathogen-free BALB/c-AnNCr mice obtained from the Jackson Laboratory (Bar Harbor, ME) were housed in microisolator cages within a laminar flow unit and fed food and water ad libitum. Murine CT-26 colon carcinoma cells were maintained in RPMI-1640 medium containing 10% FBS and 1% streptomycin-penicillin. Eight-to-ten-week-old animals were inoculated subcutaneously under the right shoulder with 1 × 106 CT-26 cells in 50 μL of culture medium. Studies were carried out approximately 7–8 days after inoculation when the tumors reached ~5–7 mm in diameter. All experimental procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Drugs
Solid DMXAA (courtesy of Gordon Rewcastle, University of Auckland, New Zealand) was stored at room temperature in the dark prior to use. For combination studies, DMXAA was freshly prepared in 5% sodium bicarbonate and injected intraperitoneally (i.p.) 2 h prior to start of light treatment. Clinical-grade HPPH was diluted in sterile PBS and injected at a dose of 0.4 μmol kg−1 via tail vein injection in a volume of 0.01 mL g−1 body weight.
PDT
Tumor-bearing mice were restrained in Plexiglas® holders and tumor illumination was carried out using a 20-W argon laser (Model 2080; Spectra Physics, Mountain View, CA) pumping a dye laser (Coherent, Santa Clara, CA) circulating 4-dicyanomethylene-2-methyl-6-p-dimethylaminostyryl-4H-pyran (DCM) dye (Cooper Lasersonics, Palo Alto, CA) and tuned to 665 nm. A custom-designed beam splitter device allowed simultaneous illumination of up to eight animals through 200-μm diameter quartz fiber optic cables; fibers were terminated in microlenses to provide a uniform 1 cm-diameter illumination over the tumor. Power densities were measured using a radiometer (Coherent Lasermate). Tumor illumination was carried out using a high irradiance regimen (incident fluence of 48 J cm−2 delivered at a fluence rate of 112 mW cm−2, ~7 min) and a highly effective, low irradiance PDT regimen (128 J cm−2 at 14 mW cm−2, ~152 min).
Tumor response and analysis
Tumor dimensions were measured with vernier calipers every 1–3 days after treatment and volumes calculated. The end points included time (days) to reach a tumor volume of 400 mm3 and number of tumor-free animals at the end of 60 days following treatment. Time to reach a tumor volume of 400 mm3 was estimated using a custom-designed Microsoft Excel spreadsheet as described previously (17). Animals were considered cured if they remained tumor free for 60 days after treatment. Mice were humanely killed when tumors exceeded a volume of 400 mm3.
Cytokine measurements
Intratumoral protein levels of the cytokines, tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were measured in CT-26 tumors 4 h after treatment with HPPH-PDT alone, DMXAA alone or the combination, using the enzyme-linked immunosorbent assay (ELISA) similar to methods described by us previously (24). Levels of TNF-α and IL-6 in tumor tissue extracts containing 40 μg of protein were determined using ELISA kits specific for each protein (Quantikine®; R&D Systems, Minneapolis, MN). The assays were performed on samples isolated from three to five mice for each group.
Microvessel density analysis
Vascular damage following treatment was assessed using microvessel density (MVD) based on CD31-immunostaining of tumor sections as described previously (17). Briefly, 24 h after treatment, tumors were excised and fixed overnight in Tris-buffered zinc fixative. The samples were than transferred to 70% ethanol and subsequently embedded in paraffin. Mouse CD31 was detected with a rat MAb (IgG2a; Pharmingen, San Diego, CA) at 1:50 dilution in PBS for 60 min at 37°C followed by biotinylated rabbit anti-rat IgG (12112D; Pharmingen) at 1:100 dilution for 30 min, streptavidin peroxidase (50–242; Zymed, San Francisco, CA) for 30 min and diaminobenzidine for 5 min. CD31 + endothelial cell clusters on immunostained tumor sections were counted under a microscope (20× magnification, at least 10 fields per tumor).
MRI
Studies were performed using a 4.7T/33-cm horizontal bore MR scanner (GE NMR Instruments, Fremont, CA) incorporating AVANCE digital electronics (Bruker Medical, Billerica, MA), a removable gradient coil insert (G060; Bruker Medical) generating a maximum field strength of 950 mT m−1, and a custom-designed RF transreceiver coil. Tumor-bearing mice (n = 6 total) were anesthetized using 4% isoflurane (Abbott Laboratories, Chicago, IL), secured in a mouse coil chamber and positioned in the scanner. Anesthesia was maintained at 1–2% during imaging and a circulating water bath maintained at 37°C was used to keep the animals warm inside the magnet. T2-weighted axial fast spin-echo images were acquired 4 h after treatment with PDT alone (low irradiance regimen, 128 J cm−2 at 14 mW cm−2) or PDT + DMXAA (high irradiance regimen, 48 J cm−2 at 112 mW cm−2 + 25 mg kg−1) using the following acquisition parameters: matrix size 128 × 128, TR/TE = 2744/41 ms, slice thickness = 1.0 mm, field of view 3.2 × 3.2 cm, RARE factor = 8, number of averages = 4). Image processing and analysis was carried out using commercially available software (Analyze PC, Version 7.0; AnalyzeDirect, Overland Park, KS).
Foot response studies
Nontumor-bearing BALB/c mice (five mice per time point) were restrained in Plexiglas® holders designed to expose only the right hind foot to laser light. Mouse foot response was assessed following treatment with the combination of PDT (48 J cm−2 at 112 mW cm−2) + DMXAA (25 mg kg−1, i.p.) and compared to treatment with PDT alone (128 J cm−2 at 14 mW cm−2). Each treated foot was always compared with the contralateral hind foot and graded on a subjective scale of 0–1.3 (Table 1) for a period of 3 days following treatment as described previously (17).
Table 1.
Foot response assay – grading scale.
| 0 | No reaction |
| 0.1 | Very slight edema |
| 0.2 | Slight erythema |
| 0.3 | Slight edema |
| 0.4 | Slight edema + slight erythema |
| 0.5 | Moderate edema |
| 0.6 | Moderate edema + slight erythema |
| 0.7 | Large edema |
| 0.8 | Moderate erythema |
| 1.0 | Erythema + edema and/or slight epilation |
| 1.1 | Large edema + erythema/slight epilation |
| 1.2 | Large erythema + slight epilation and/or edema |
| 1.3 | Moderate epilation and/or moderate edema |
Statistical analyses
All measured values have been reported as mean ± SEM. Kaplan–Meier survival curves based on hours-to-end point (400 mm3) and median time to regrowth (MTR) were analyzed for statistical significance using the log rank test (17). One-way analysis of variance (ANOVA) with Neuman–Keuls multiple comparisons test was used to compare TNF-α and IL-6 levels between control and treatment groups. The two-tailed Student’s t-test was used to compare differences in MVD between control and treatment groups. Normal tissue response was compared between groups using the Kruskal– Wallis test. P < 0.05 was considered statistically significant. All statistical calculations and analyses were performed using Graph Pad (Version 5.00; GraphPad Software, Inc., San Diego, CA).
RESULTS AND DISCUSSION
Enhanced antitumor activity of HPPH-sensitized PDT in combination with DMXAA
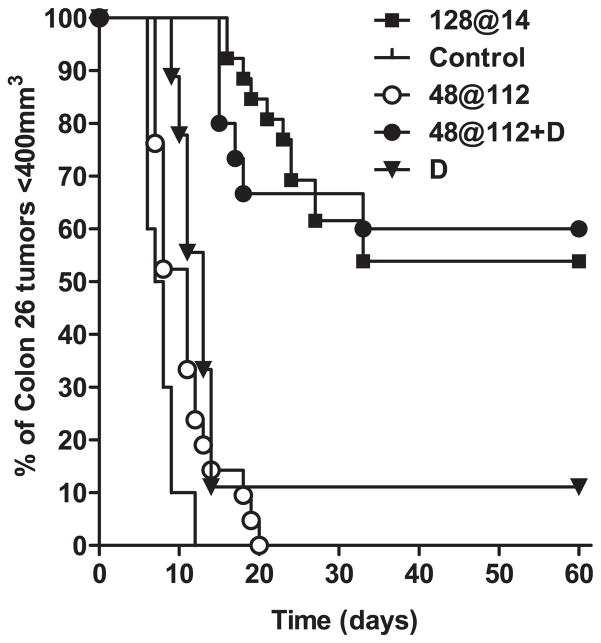
Prior to evaluating the antitumor activity of PDT–DMXAA combination therapy in vivo, dose–response studies were carried out using graded doses of DMXAA (25, 27.5 and 30 mg kg−1). Based on the results of these studies, a low, nontoxic, minimally effective dose of DMXAA (25 mg kg−1, i.p.) was chosen (25). DMXAA monotherapy at this dose resulted in a marginal increase in tumor growth delay (MTR 13 vs 7.5 days for untreated controls, P < 0.001, Fig. 1). We explored the antitumor activity of DMXAA in combination with PDT using a high-irradiance, short-duration, PDT regimen (48 J cm−2 delivered at a fluence rate of 112 mW cm−2) (Table 2). In a previous study, mathematical modeling predicted that this PDT regimen would rapidly deplete tissue 3O2 (23). Consistent with previous findings, treatment with this high irradiance PDT regimen was ineffective against CT-26 tumors as a monotherapy, with only a mild growth delay observed compared to untreated controls (MTR 11 vs 7.5 days, Fig. 1). Remarkably, administration of DMXAA (25 mg kg−1) 2 h prior to start of light treatment using this regimen resulted in a highly synergistic antitumor effect with ~60% of the animals remaining tumor-free for the 60-day period following treatment (P < 0.001, Fig. 1). In agreement with a previous report (23), treatment with PDT alone using the low irradiance regimen, 128 J cm−2 at 14 mW cm−2, also resulted in ~60% long-term cures (Fig. 1, P < 0.001). However, the treatment times between the highly effective monotherapy regimen (~152 min) and the regimen used for combination therapy (7 min) were dramatically different.
Figure 1.
Long-term response of CT-26 murine colon carcinomas to low-dose DMXAA, HPPH-sensitized PDT and PDT–DMXAA combination therapy. Kaplan–Meier survival curves of BALB/c mice bearing subcutaneous CT-26 tumors treated with low-dose DMXAA (D; 25 mg kg−1, n = 9) alone, HPPH-PDT alone (48 at 112, n = 21 and 128 at 14, n = 21), HPPH-PDT in combination with DMXAA (48 at 112 + D, n = 15) or no treatment (control, n = 10). Log rank test P < 0.001 between 48 at 112 + D vs controls, D and 48 at 112.
Table 2.
Comparative analysis of PDT using a low irradiance regimen and PDT–DMXAA combination therapy using a high irradiance regimen against CT-26 murine tumors.
TNF-α and IL-6 expression following combination therapy
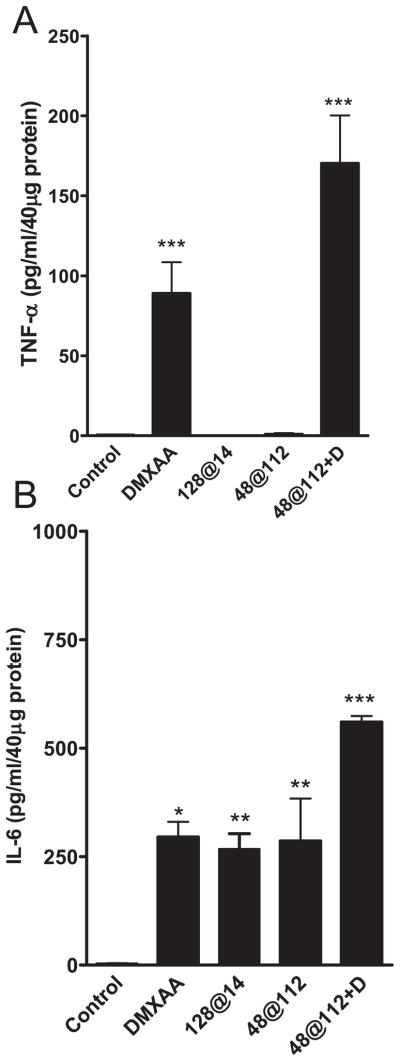
We then investigated the potential mechanisms of interaction between the two treatments. The antivascular activity of DMXAA is, in part, mediated by the induction of cytokines such as TNF-α (26,27). TNF-α is a pleiotropic cytokine that has been shown to cause experimental tumor necrosis through toxic effects on the tumor vasculature (28). The rationale for evaluating the combination of PDT and DMXAA was also based on the observation that exogenous TNF-α potentiated the antitumor activity of PDT in vivo (29). To determine the role of TNF-α in PDT–DMXAA combination therapy, intratumoral levels of the cytokine were measured using the ELISA 4 h after treatment with PDT alone, DMXAA alone or the combination and differences analyzed using ANOVA. Treatment with HPPH-PDT alone did not result in a significant increase in protein levels of TNF-α (Fig. 2A). Administration of low-dose DMXAA resulted in a significant increase in TNF-α protein levels (89 ± 19 pg mL−1/40 μg protein) compared with untreated controls (0.46 ± 0.26 pg mL−1/40 μg protein, P < 0.001). Tumors obtained from mice treated with the high irradiance regimen in combination with DMXAA (25 mg kg−1) showed the greatest increase in TNF-α protein levels (170 ± 30 pg mL−1/40 μg protein) compared with untreated controls (P < 0.001), PDT monotherapy using this regimen (P < 0.001) and low-dose DMXAA alone (P < 0.001, Fig. 2A). These results indicate that induction of TNF-α is an important mechanism behind the observed enhancement of antitumor activity seen with combination treatment.
Figure 2.
Induction of TNF-α and IL-6 in CT-26 tumors following HPPH-PDT, low-dose DMXAA and PDT–DMXAA combination therapy. TNF-α and IL-6 levels in tumors were determined 4 h after treatment using the ELISA. Data represent mean ± SE; n = 3–6 mice per group. *P < 0.05, **P < 0.01, P < 0.001 vs controls.
While the cytokine TNF-α is a major biologic mediator responsible for the antitumor activity of DMXAA, tumor necrosis has been observed following DMXAA treatment in TNF knock out mice indicating that other biologic mediators could effectively substitute for the antivascular effects of TNF-α, especially at higher doses of DMXAA (30). A recent study by Jassar et al. had shown that in addition to induction of TNF-α, administration of DMXAA also resulted in an ~13-fold increase in mRNA and ~8-fold increase in protein levels of IL-6 (31). HPPH-sensitized PDT has also been shown to result in increased intratumoral induction of IL-6 in murine tumors (32). We therefore measured IL-6 levels in CT-26 tumors 4 h after treatment with PDT alone, DMXAA alone and combination treatment. As shown in Fig. 2B, significant increase in IL-6 levels was observed following PDT monotherapy compared with control tumors (P < 0.01, ANOVA). Administration of low-dose DMXAA (25 mg kg−1) also resulted in a significant increase in intratumoral IL-6 levels after treatment (P < 0.05 vs controls, Fig. 2B). No significant differences in IL-6 levels were observed between DMXAA and PDT monotherapies. However, the combination of DMXAA and the high irradiance PDT regimen resulted in a marked increase in IL-6 (560 ± 14 pg mL−1/40 μg protein) over levels seen following DMXAA administration alone (295 ± 35 pg mL−1/40 μg protein, P < 0.05) and PDT alone (286 ± 96, P < 0.05, ANOVA) suggesting a potential role for IL-6 in tumor response to combination therapy.
Improved selectivity with PDT–DMXAA combination therapy
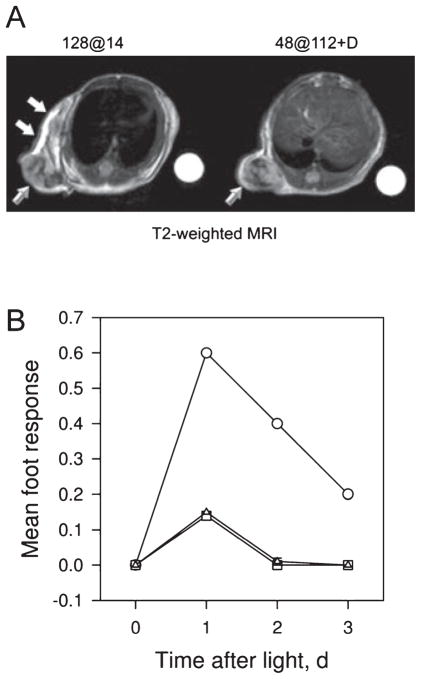
The selectivity of the response to PDT–DMXAA combination therapy was assessed using MRI and the mouse foot response assay. Four hours after treatment with PDT monotherapy using the highly effective low irradiance regimen, T2-weighted MRI showed significant hyperintense areas in the peritumoral region (Fig. 3A, white arrows) suggestive of treatment-induced edema and inflammation along with hypointense regions within the tumor (beveled arrow) indicative of vascular damage. In comparison, images acquired 4 h after DMXAA + PDT treatment (high irradiance PDT + 25 mg kg−1 DMXAA) did not show any evidence of peritumoral tissue damage highlighting the selectivity of combination treatment. Hypointense regions suggestive of vascular damage and hemorrhaging were visible within the tumor following PDT + DMXAA treatment as well (beveled arrow). Treatment with the high irradiance regimen alone or DMXAA alone revealed minimal intratumoral changes in T2-weighted signal with no evidence of peritumoral tissue damage (data not shown).
Figure 3.
MRI and response of normal mouse foot tissue to PDT, DMXAA and combination treatment. (A) T2-weighted axial MR images acquired 4 h following treatment with PDT alone (128 at 14) and PDT + DMXAA (48 at 112 + D). Beveled arrows indicate hypointense regions within the tumor suggestive of treatment-induced vascular damage. Significant peritumoral tissue damage and edema demonstrated by hyperintense regions (white arrows) in the images was seen following treatment with PDT alone (using the low irradiance regimen) compared with PDT + DMXAA (using the high irradiance regimen) highlighting the selectivity of combination therapy. (B) Foot responses were graded according to the scale described in Materials and Methods using five mice/point following treatment with PDT using the high irradiance regimen, 48 at 112 (triangles), low irradiance regimen, 128 at 14 (circles) and combination of 48 at 112 and low-dose DMXAA (squares).
The results of the foot response assay also showed evidence of pronounced tissue damage and edema (mean foot response 0.6) 24 h following treatment with PDT monotherapy using the highly effective low irradiance regimen (Fig. 3B, circles). Treatment with PDT using the high irradiance, short treatment time regimen (triangles) showed minimal normal tissue toxicity (0.15) at the same time point. Addition of low-dose DMXAA to this regimen resulted in no additional damage to normal mouse foot tissue (0.148, squares). Resolution of normal tissue damage with the low irradiance PDT regimen was observed 5 days after treatment compared to 2 days with combination treatment.
Vascular damage following combination treatment
Finally, as blood vessels are targets for both PDT and DMXAA treatments, we examined the effect of combination therapy on tumor vasculature. Immunohistochemical staining for the pan endothelial cell adhesion molecule (CD31) was performed on tumor sections obtained 24 h after treatment. Using CD31 immunohistochemistry and MVD counts, Henderson et al. have shown that PDT using the low irradiance regimen (128 J cm−2 at 14 mW cm−2) results in marked destruction of tumor vasculature (23). In the same study, it was also shown that the high irradiance regimen (48 J cm−2 at 112 mW cm−2) exhibits no significant effects on MVD (P > 0.05 vs controls) (23). Recently, using contrast-enhanced MRI and fluorescein exclusion, we have also demonstrated that PDT using this regimen exhibits no effect on vascular perfusion (25). At the dose utilized for combination treatment (25 mg kg−1), DMXAA also exhibits minimal antivascular activity (25). Therefore, in this present study, to substantiate the significance of vascular damage following combination treatment, we determined MVD counts following treatment with DMXAA alone and in combination with PDT. The mean MVD of untreated control CT-26 tumors was 8.12 ± 0.44. Twenty-four hours after treatment with DMXAA alone (25 mg kg−1), a significant (P < 0.001, two-tailed Student’s t-test) reduction in MVD (5.10 ± 0.56) was observed. Consistent with our previous observation on tumor vascular damage (25), a dramatic reduction in MVD was seen 24 h following combination treatment (0.85 ± 0.31) compared with untreated controls (P < 0.0001, two-tailed Student’s t-test).
For most sensitizers used in PDT, the treatment regimen, i.e. the amount (fluence) and the rate (fluence rate) at which the light energy is delivered, is a critical factor that determines therapeutic outcome (4–6). Higher fluence rates deplete available tissue oxygen faster than can be replenished by vascular perfusion compromising the efficiency of photodynamic activity (23). In contrast, lower fluence rate treatment regimens are more oxygen conserving and result in greater levels of apoptosis and improved treatment outcomes (4–6,23). While lowering the fluence rate is an effective way of minimizing photodynamic oxygen consumption and maximizing treatment efficacy, several factors need to be considered regarding the use of this approach, especially in the clinical context. First, reducing the fluence rate to achieve maximal antitumor activity results in a substantial increase in illumination time required, typically to a few hours. Such long treatment times may not be clinically feasible. Secondly, preclinical and clinical studies of PDT have shown that low fluence rate treatments often result in pronounced normal tissue damage reducing treatment selectivity (10,11). This is particularly important in the use of PDT for the management of esophageal or endobronchial pathologies as resultant normal tissue toxicity in the form of edema and mucous formation may pose serious complications such as dyspnea and airway stenosis.
The results of the current study show that neoadjuvant administration of a low, minimally effective dose of DMXAA significantly enhances the antitumor activity of HPPH-sensitized PDT in vivo. The combination of DMXAA and PDT allowed the use of a shorter, high irradiance regimen that is clinically feasible (7 min treatment time). Of particular interest is the remarkable potentiation of the noncurative PDT regimen from 0% 60-day cures as a monotherapy to ~60% cures in combination with DMXAA. MRI and mouse foot response assay studies showed that, in addition to durable tumor control, the combination of PDT and DMXAA results in a highly tumor-selective response compared with a low irradiance highly effective PDT monotherapy regimen. DMXAA has successfully completed Phase I evaluation and is undergoing further clinical evaluation in combination with chemotherapy with promising results (33). VDAs such as DMXAA exhibit moderate antitumor activity as monotherapies but their true clinical utility is in combination with other treatments such as chemotherapy or radiation (34). While there are inter-species differences (mouse, rat, man) in pharmacokinetics and pharmacodynamics of DMXAA, our results clearly demonstrate a favorable therapeutic interaction between PDT and DMXAA with definite advantages that warrant clinical investigation. A proposal to conduct a pilot clinical trial to determine the activity of DMXAA and PDT in patients with basal cell carcinomas has been successfully submitted (Bellnier, personal communication). Studies to further investigate the potential mechanisms of interactions between the two treatments are also underway.
Acknowledgments
The authors would like to thank Patricia Maier for excellent technical assistance. This work was supported by the National Cancer Institute Cancer Center Support Grant (CA16056), National Institutes of Health Grant (RO1CA89656) and utilized core facilities at the Roswell Park Cancer Institute.
References
- 1.Dougherty TJ. A brief history of clinical photodynamic therapy at Roswell Park Cancer Institute. J Clin Laser Med Surg. 1996;14:219–221. doi: 10.1089/clm.1996.14.219. [DOI] [PubMed] [Google Scholar]
- 2.Hopper C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2001;1:212–219. doi: 10.1016/s1470-2045(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 3.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:147–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 4.Foster TH, Hartley DF, Nichols MG, Hilf R. Fluence rate effects in photodynamic therapy of multicell tumor spheroids. Cancer Res. 1993;53:1249–1254. [PubMed] [Google Scholar]
- 5.Coutier S, Bezdetnaya LN, Foster TH, Parache RM, Guillemin F. Effect of irradiation fluence rate on the efficacy of photodynamic therapy and tumor oxygenation in metatetra-( hydroxyphenyl)chlorin (mTHPC)-sensitized HT29 xenografts in nude mice. Radiat Res. 2002;158:339–345. doi: 10.1667/0033-7587(2002)158[0339:eoifro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Henderson BW, Busch TM, Snyder JW. Fluence rate as a modulator of PDT mechanisms. Lasers Surg Med. 2006;38:489–493. doi: 10.1002/lsm.20327. [DOI] [PubMed] [Google Scholar]
- 7.Foster TH, Gao L. Dosimetry in photodynamic therapy: Oxygen and the critical importance of capillary density. Radiat Res. 1992;130:379–383. doi: 10.2307/3578385. [DOI] [PubMed] [Google Scholar]
- 8.Foster TH, Murant RS, Bryant RG, Knox RS, Gibson SL, Hilf R. Oxygen consumption and diffusion effects in photodynamic therapy. Radiat Res. 1991;126:296–303. doi: 10.2307/3577919. [DOI] [PubMed] [Google Scholar]
- 9.Oseroff AR, Blumenson LR, Wilson BD, Mang TS, Bellnier DA, Parsons JC, Frawley N, Cooper M, Zeitouni N, Dougherty TJ. A dose ranging study of photodynamic therapy with porfimer sodium (Photofrin) for treatment of basal cell carcinoma. Lasers Surg Med. 2006;38:417–426. doi: 10.1002/lsm.20363. [DOI] [PubMed] [Google Scholar]
- 10.Blant SA, Woodtli A, Wagnieres G, Fontolliet C, van den Bergh H, Monnier P. In vivo fluence rate effect in photodynamic therapy of early cancers with tetra(mhydroxyphenyl) chlorin. Photochem Photobiol. 1996;64:963–968. doi: 10.1111/j.1751-1097.1996.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 11.Sitnik TM, Henderson BW. The effect of fluence rate on tumor and normal tissue responses to photodynamic therapy. Photochem Photobiol. 1998;67:462–466. [PubMed] [Google Scholar]
- 12.Friedberg JS, Mick R, Stevenson JP, Zhu T, Busch TM, Shin D, Smith D, Culligan M, Dimofte A, Glatstein E, Hahn SM. Phase II trial of pleural photodynamic therapy and surgery for patients with non small-cell lung cancer with pleural spread. J Clin Oncol. 2004;22:2192–2201. doi: 10.1200/JCO.2004.07.097. [DOI] [PubMed] [Google Scholar]
- 13.Pogue BW, O’Hara JA, Demidenko E, Wilmot CM, Goodwin IA, Chen B, Swartz HM, Hassan T. Photodynamic therapy with verteporfin in the radiation-induced fibrosarcoma-1 tumor causes enhanced radiation sensitivity. Cancer Res. 2003;63:1025–1033. [PubMed] [Google Scholar]
- 14.Canti G, Nicolin A, Cubeddu R, Taroni P, Bandieramonte G, Valentini G. Antitumor efficacy of the combination of photodynamic therapy and chemotherapy in murine tumors. Cancer Lett. 1998;125:39–44. doi: 10.1016/s0304-3835(97)00502-8. [DOI] [PubMed] [Google Scholar]
- 15.Ferrario A, Tiehl KV, Rucker N, Schwarz MA, Gill PS, Gomer CJ. Antiangiogenic treatment enhances photodynamic therapy responsiveness in a mouse mammary carcinoma. Cancer Res. 2000;60:4066–4069. [PubMed] [Google Scholar]
- 16.Bhuvaneswari R, Yuen GY, Chee SK, Olivo M. Hypericin-mediated photodynamic therapy in combination with Avastin (bevacizumab) improves tumor response by down regulating angiogenic proteins. Photochem Photobiol Sci. 2007;6:1275–1283. doi: 10.1039/b705763f. [DOI] [PubMed] [Google Scholar]
- 17.Bellnier DA, Gollnick SO, Camacho SH, Greco WR, Cheney RT. Treatment with the tumor necrosis factor-alpha-inducing drug 5,6-dimethylxanthenone-4-acetic acid enhances the antitumor activity of the photodynamic therapy of RIF-1 mouse tumors. Cancer Res. 2003;63:7584–7590. [PubMed] [Google Scholar]
- 18.Allison RR, Downie GH, Cuenca R, Hu X, Childs CJJ, Sibata CH. Photosensitizers in clinical PDT. Photodiagn Photodyn Ther. 2004;1:27–42. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- 19.Pandey RK, Bellnier DA, Smith KM, Dougherty TJ. Chlorin and porphyrin derivatives as potential photosensitizers in photodynamic therapy. Photochem Photobiol. 1991;53:65–72. doi: 10.1111/j.1751-1097.1991.tb08468.x. [DOI] [PubMed] [Google Scholar]
- 20.Henderson BW, Bellnier DA, Greco WR, Sharma A, Pandey RK, Vaughan LA, Weishaupt KR, Dougherty TJ. An in vivo quantitative structure–activity relationship for a congeneric series of pyropheophorbide derivatives as photosensitizers for photodynamic therapy. Cancer Res. 1997;57:4000–4007. [PubMed] [Google Scholar]
- 21.Bellnier DA, Greco WR, Loewen GM, Nava H, Oseroff AR, Pandey RK, Tsuchida T, Dougherty TJ. Population pharmacokinetics of the photodynamic therapy agent 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a in cancer patients. Cancer Res. 2003;63:1806–1813. [PubMed] [Google Scholar]
- 22.Bellnier DA, Greco WR, Nava H, Loewen GM, Oseroff AR, Dougherty TJ. Mild skin photosensitivity in cancer patients following injection of Photochlor (2-[1- hexyloxyethyl]-2-devinyl pyropheophorbide-a; HPPH) for photodynamic therapy. Cancer Chemother Pharmacol. 2005;57:40–45. doi: 10.1007/s00280-005-0015-6. [DOI] [PubMed] [Google Scholar]
- 23.Henderson BW, Gollnick SO, Snyder JW, Busch TM, Kousis PC, Cheney RT, Morgan J. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64:2120–2126. doi: 10.1158/0008-5472.can-03-3513. [DOI] [PubMed] [Google Scholar]
- 24.Seshadri M, Spernyak JA, Maier PG, Cheney RT, Mazurchuk R, Bellnier DA. Visualizing the acute effects of vascular-targeted therapy in vivo using intravital microscopy and magnetic resonance imaging: Correlation with endothelial apoptosis, cytokine induction and treatment outcome. Neoplasia. 2007;9:28–135. doi: 10.1593/neo.06748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seshadri M, Spernyak JA, Mazurchuk R, Camacho SH, Oseroff AR, Cheney RT, Bellnier DA. Tumor vascular response to photodynamic therapy and the antivascular agent 5,6-dimethylxanthenone-4-acetic acid: Implications for combination therapy. Clin Cancer Res. 2005;11:4241–4250. doi: 10.1158/1078-0432.CCR-04-2703. [DOI] [PubMed] [Google Scholar]
- 26.Joseph WR, Cao Z, Mountjoy KG, Marshall ES, Baguley BC, Ching LM. Stimulation of tumors to synthesize tumor necrosis factor-alpha in situ using 5,6- dimethylxanthenone- 4-acetic acid: A novel approach to cancer therapy. Cancer Res. 1999;59:633–638. [PubMed] [Google Scholar]
- 27.Baguley BC, Ching LM. DMXAA: An antivascular agent with multiple host responses. Int J Radiat Oncol Biol Phys. 2002;54:1503–1511. doi: 10.1016/s0360-3016(02)03920-2. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe N, Niitsu Y, Umeno H, Kuriyama H, Neda H, Yamauchi N, Maeda M, Urushizaki I. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res. 1988;48:2179–2183. [PubMed] [Google Scholar]
- 29.Bellnier DA. Potentiation of photodynamic therapy in mice with recombinant human tumor necrosis factor-alpha. J Photochem Photobiol B Biol. 1991;8:203–210. doi: 10.1016/1011-1344(91)80060-u. [DOI] [PubMed] [Google Scholar]
- 30.Ching LM, Goldsmith D, Joseph WR, Körner H, Sedgwick JD, Baguley BC. Induction of intratumoral tumor necrosis factor (TNF) synthesis and hemorrhagic necrosis by 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF knockout mice. Cancer Res. 1999;59:3304–3307. [PubMed] [Google Scholar]
- 31.Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR, Albelda SM. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone- 4-acetic acid induces an effective CD8+ T-cell mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res. 2005;65:11752–11761. doi: 10.1158/0008-5472.CAN-05-1658. [DOI] [PubMed] [Google Scholar]
- 32.Gollnick SO, Evans SS, Baumann H, Owczarczak B, Maier P, Vaughan L, Wang WC, Unger E, Henderson BW. Role of cytokines in photodynamic-therapy induced local and systemic inflammation. Br J Cancer. 2003;88:1772–1779. doi: 10.1038/sj.bjc.6600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKeage MJ, Fong P, Jeffery M, Baguley BC, Kestell P, Ravic M, Jameson MB. 5,6-Dimethylxanthenone-4-acetic acid in the treatment of refractory tumors: A phase I safety study of a vascular disrupting agent. Clin Cancer Res. 2006;12:1776–1784. doi: 10.1158/1078-0432.CCR-05-1939. [DOI] [PubMed] [Google Scholar]
- 34.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]