Abstract
Background
Sustained hyperglycemia induces increased renal oxygen consumption resulting in reduced oxygen availability in the diabetic kidney. We investigated the roles of the nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase and the neuronal nitric oxide synthase (nNOS) for the increased oxygen consumption in streptozotocin-diabetic rats.
Methods
Oxygen consumption was measured in isolated proximal tubular cells (PTC) from streptozotocin-induced diabetic rats (n=7–9 per group) with and without chronic treatment with apocynin, a NADPH-oxidase inhibitor, or S-methyl-L-thiocitrulline (SMTC), a selective nNOS inhibitor, or a combination of the two and the results were compared to normoglycemic controls (n=10). Oxidative stress was estimated from thiobarbituric acid reactive substances (TBARS) and protein expressions measured by Western blot.
Results
PTC from untreated diabetics had increased oxygen consumption compared to controls (40.6±7.9 vs. 10.9±2.0 nmol/mg protein/min). All treatments reduced the diabetes-induced increase in oxygen consumption (apocynin 10.5±1.7, SMTC 19.7±3.0 and apocynin+SMTC 21.6±3.6 nmol/mg protein/min). Neither apocynin nor SMTC had any effect on the oxygen consumption in cells pre-incubated with ouabain, an inhibitor of active electrolyte transport. Oxidative stress was elevated in the diabetic kidney and inhibited by all treatments. The increased oxygen consumption by diabetic PTC correlated with increased protein expressions of p47phox and nNOS, and the treatments prevented these increases.
Conclusions
Diabetes induces oxidative stress, which increases oxygen consumption in PTC. Inhibition of either NADPH-oxidase or nNOS prevented the increased oxygen consumption. The effect of blocking both these enzymes was not additive suggesting a common pathway which warrants further studies.
Keywords: NADPH-oxidase, neuronal nitric oxide synthase, diabetes mellitus, oxygen consumption, oxidative stress
Introduction
Sustained hyperglycemia is commonly associated with increased formation of reactive oxygen species (ROS) resulting in increased oxygen consumption and limited oxygen availability in the diabetic kidney [1, 2]. Although we have previously shown that antioxidant treatment prevents these changes in the intrarenal oxygen metabolism [2], the mechanism for the excessive ROS formation in the diabetic kidney is presently unknown. There are several potential sources of superoxide formation in the kidney, including the nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase, uncoupled nitric oxide synthase (NOS), mitochondria and xanthine oxidase [3–7]. Indeed, increased activity of the NADPH-oxidase during diabetes has been reported [4]. Furthermore, increased NOS uncoupling in the diabetic has been shown [5] and Nishikawa and co-workers showed that normalized superoxide levels at the level of the mitochondria prevented all of the major pathways proposed to result in the development of diabetes-induced complications [8].
Recently, the involvement of tissue hypoxia for the development of kidney dysfunction has gained growing support [9–12]. Körner et al. [1] showed that proximal tubular cells (PTC) isolated from diabetic rats had increased oxygen consumption, which we later linked to significantly reduced oxygen tensions in the diabetic kidneys [2, 13–15]. We and others have proposed that the chronically reduced kidney oxygenation is a crucial mechanism for the development of diabetes nephropathy [10, 12, 16]. The pioneering study by Deng et al. [17] showed that the kidney as well as isolated PTC oxygen consumptions are highly regulated by nitric oxide (NO) originating from the neuronal (n) NOS. The diabetic kidney has reduced NO levels, which directly influences oxygen availability independently of blood flow [15].
Taken together, a large number of studies have highlighted the involvement of ROS and hypoxia for the development of diabetes-induced kidney dysfunction. Therefore, we investigated the specific role of the NADPH-oxidase and the nNOS for the increased oxygen consumption by PTC isolated from diabetic kidneys.
Materials and methods
All chemicals were from Sigma-Aldrich (St. Louis, MO, USA) and of highest grade available if not otherwise stated.
Animals and induction of diabetes
Age-matched male Wistar Furth rats weighing approximately 330 g were purchased from M&B (Ry, Denmark). Animals had free access to water and standard rat chow (Na 0.3%, K 0.8%, protein 21%; R3, Ewos, Södertälje, Sweden) throughout the study if not otherwise stated. All experiments were performed in accordance with the NIH guidelines for use and care of laboratory animals and approved by the Animal Care and Use Committee for Uppsala University. Diabetes was induced by an injection of streptozotocin (STZ; 45 mg/kg) in the tail vein. Animals were considered diabetic if blood glucose concentrations increased to above 18 mmol/L within 24 hours after STZ injection and remained elevated. Blood glucose concentrations were determined with test reagent strips (MediSense, Bedford, MA, USA) from blood samples obtained from the cut tip of the tail.
Experimental protocols
The experimental groups consisted of control and diabetic rats with and without the combined chronic treatment of apocynin (16 mg/kg bw/24h) [4] and SMTC (0.5 mg/kg bw/24h) [18] for a total of 16 days (throughout the course of diabetes). Additional diabetic rats were chronically treated with either apocynin (16 mg/kg bw/24h) or SMTC (0.5 mg/kg bw/24h).
Metabolic cages
Nine days after induction of diabetes, the rats were fed nitrate/nitrite-free diet (Harlan Teklad, Madison, WI, USA) for five days and thereafter placed in the metabolic cages. Before the start of urine collection, the animals were allowed a 24-hour equilibration period. Urine was collected for the subsequent 24 hours and stored at −80°C until analyzed for oxidative stress markers, electrolytes and nitrite/nitrate.
Measurements of oxygen consumption in vitro
PTC were isolated from normoglycemic controls and STZ-diabetic rats as previously described [1, 2]. In brief, the renal cortical tissue was minced through a metallic mesh strainer and immediately placed in an ice-cooled buffer solution and incubated in collagenase (0.05% wt/vol) at 37°C for 60 minutes while the buffer was equilibrated with 95% O2/5% CO2. Thereafter, the cell suspension was cooled to 4°C and filtrated through graded filters with pore sizes of 180, 75, 53 and 38 μm, respectively. The cells were pelleted by a slow centrifugation (100 g, 4 minutes) and re-suspended in a collagenase-free buffer, a procedure that was repeated three times. The suspension was kept on ice until O2 consumption was measured according to a previously described procedure [2]. In brief, a custom-made thermostatically controlled (37°C) gas-tight plexiglass chamber with a total volume of 1.10 mL was used. The chamber was filled with buffer solution (in mmol/L: NaCl 113.0; KCl 4.0; NaHCO3 27.2; KH2PO4 1.0; MgCl2 1.2; CaCl2 1.0; HEPES 10.0; Ca lactate 0.5; glutamine 2.0, and streptomycin 50 U/mL; osmolality 298±2 mOsm/kg H2O; pH 7.40) and continuously stirred with an air driven magnetic stirrer. The glucose concentration in the medium was 5.8 mmol/L for cells from normoglycemic controls and 23.2 mmol/L for cells from diabetic animals. The O2 content was measured by a modified Unisense-500 O2 sensor (Unisense A/S, Aarhus, Denmark), calibrated with the air-equilibrated buffer solution as 228 μmol/L O2 and Na2S2O5-saturated buffer as zero. 100 μl of cell suspension was then injected into the chamber, and the rate of O2 disappearance was measured. At the end of each experiment, a 100 μL sample was taken to determine the protein concentration using DC Protein Assay (Bio-Rad Laboratories, Hercules, Calif., USA). O2 consumption was calculated as the rate of O2 disappearance adjusted for protein concentration. Measurements were performed during baseline and after incubation with ouabain (1 mmol/L), apocynin (1 mmol/L), S-methyl-L-thiocitrulline (SMTC, 50 μmol/L; Cayman Chemicals, Ann Arbor, MI, USA), or a combination of apocynin and SMTC.
Urine volume and electrolytes
The urine volumes were measured gravimetrically and urinary sodium and potassium concentrations by flame spectrophotometry (IL543, Instrumentation Lab, Milan, Italy).
Western blot analysis
Samples were homogenized in 700 μL buffer (1.0% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mmol/L NaF, 80 mmol/L Tris, pH 7.5) containing enzyme inhibitors (Phosphatase inhibitor cocktail-2; 10 μL/mL, and Complete Mini; 1 tablet/1.5 mL; Roche Diagnostics, Mannheim, Germany). Samples were run on 12.5% Tris-HCl gels with Tris/glycine/SDS buffer and the proteins detected, after transfer to nitrocellulose membranes, using goat-anti p47phox antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and HRP-conjugated secondary antibody (donky anti-goat, 1:10,000; Kirkegaard and Perry Laboratories, Gaithersburg, MD), and mouse anti-nNOS (1 μg/ml; Zymed Laboratories; Invitrogen, Carlsbad, CA, USA) and HRP-conjugated goat anti-mouse antibody (1:5,000; Santa Cruz) by an ECL-camera (Kodak image station 2000; New Haven, CT). β-actin was detected using mouse anti-rat β-actin antibody (1:10,000) and secondary HRP-conjugated goat-anti mouse antibody (1:60,000; Kirkegaard and Perry Laboratories).
TBARS and nitite/nitrate
Thiobarbituric acids reactive substances (TBARS) were determined fluorometrically after boiling the samples with thiobarbituric acid. 200 μL of tissue homogenate was heated to 97°C for 60 minutes together with 250 μL 42 mmol/L thiobarbituric acid (Merk, Darmstadt, Germany). Standard samples were prepared from malondialdehyd-bis-(diethylacetate) (Merk-Schuchart, Schuchart, Germany). The samples were subsequently cooled on ice and precipitated with a mixture of methanol and 1 mol/L NaOH (91:9) and centrifuged at 3000 rpm for five minutes. Fluorescence was measured in the supernatant (ex. 532 nm, em. 553 nm).
Nitrite/nitrate was analyzed using commercially available kit according to manufacturers’ instructions (Cayman Chemicals; catalog no. 780001).
Statistical evaluation
All statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA). Multiple comparisons between different groups were performed using analysis of variance (ANOVA) followed by Dunnett’s Multiple Comparisons Test. Multiple comparisons within the same group were performed using repeated measures ANOVA followed by Dunnett’s post-hoc test for paired comparisons. When comparing before and after a treatment within the same animals, paired Student’s t-test was used. Descriptive statistics are presented as mean values ± SEM. For all comparisons, P <0.05 was considered statistically significant.
Results
Diabetic rats displayed reduced body weight gain, increased blood glucose levels and increased urine production and urinary excretion of Na+ and K+ compared to the controls (Table 1). These parameters were unaffected by the chronic treatments to the diabetic rats, and only body weight was affected by the chronic treatment to the normoglycemic control rats.
Table 1.
Body weight (BW), blood glucose, 24h urine production and urinary excretion of Na+, K+ in normoglycemic control and diabetic rats with and without the different treatments.
| Treatment | N | BW (g) | Blood glucose (mmol/L) | Urine production (mL/24h) | Na+ excretion (mmol/24h) | K+ excretion (mmol/24h) | |
|---|---|---|---|---|---|---|---|
| Normoglycemic | - | 10 | 372±5 | 6.0±0.3 | 20±1 | 1.72±0.20 | 3.29±0.23 |
| Apocynin + SMTC | 10 | 337±4*# | 5.4±0.2 | 15±2# | 1.51±0.16# | 3.34±0.15# | |
| Diabetic | - | 10 | 256±10* | 24.4±0.8* | 215±26* | 2.92±0.29* | 7.12±0.64* |
| Apocynin | 8 | 283±4* | 24.7±0.9* | 210±29* | 3.75±0.40* | 6.80±0.72* | |
| SMTC | 10 | 273±15* | 25.6±0.7* | 282±18* | 3.90±0.40* | 9.15±0.71* | |
| Apocynin + SMTC | 12 | 273±4* | 23.8±1.7* | 171±11* | 2.95±0.38* | 6.58±0.92* |
All values are ±SEM.
denotes P <0.05 when compared to untreated normoglycemic control, and
denotes P <0.05 when compared to the untreated diabetic group.
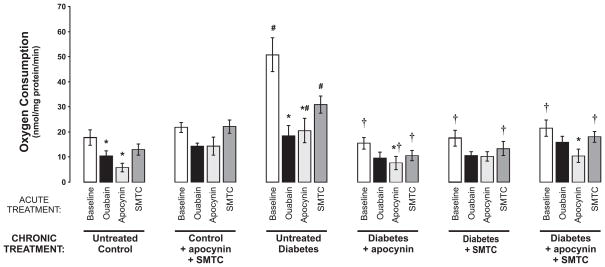
PTC from untreated diabetic rats displayed increased oxygen consumption compared to controls (Fig. 1). Chronic treatments with either apocynin or SMTC prevented the diabetes-induced increase in oxygen consumption. Similar reductions in oxygen consumption were also achieved by acute administration of either apocynin or SMTC to untreated diabetic PTC. Furthermore, acute administration of apocynin reduced the oxygen consumption in all groups, although not reaching statistical significance in the chronically treated controls and the chronically apocynin-treated diabetics. Interestingly, acute inhibition of active electrolyte transport by ouabain only reduced oxygen consumption by PTC from untreated control and diabetic animals. Chronic treatment with the combination of apocynin and SMTC had no effect on PTC from normoglycemic control rats.
Figure 1.
Oxygen consumption by isolated proximal tubular cells from normoglycemic controls and diabetic rats with and without the different chronic and acute treatments. * denotes P<0.05 vs. baseline within the same group, # denotes P<0.05 vs. corresponding group of untreated control, and † denotes P<0.05 vs. corresponding group of untreated diabetics. All values are the means ± SEM.
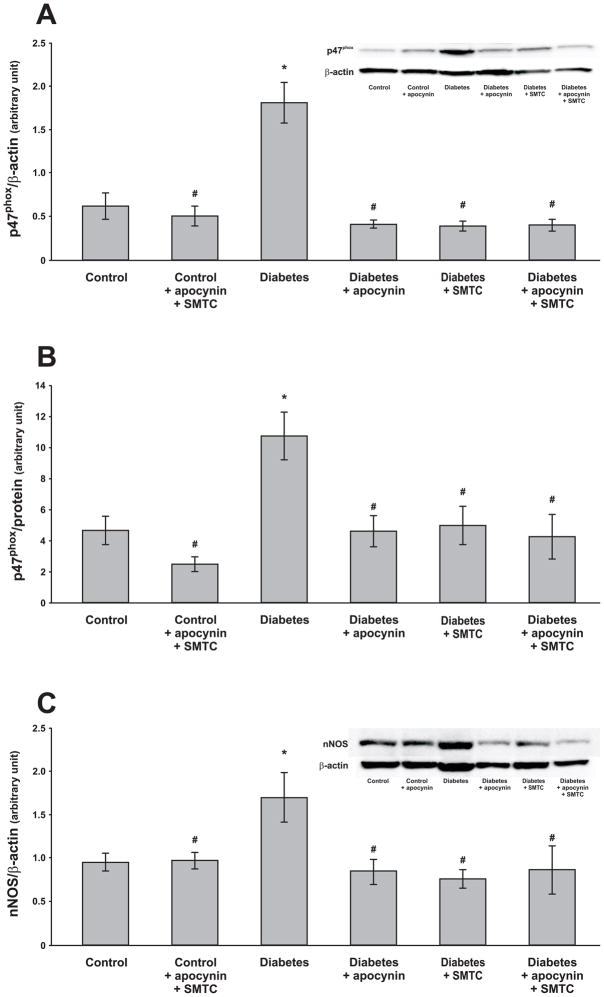
Protein expressions of p47phox were increased in both total cell lysate and membrane fraction in the diabetic kidney (Fig. 2A–B). All the applied chronic treatments prevented these increases in the diabetic rats whereas the combined chronic treatment with apocynin and SMTC had no effect on controls. Protein expression of nNOS was increased in diabetic kidneys and the chronic treatments prevented this increase (Fig. 2C).
Figure 2.
Protein expression and a representative blot of p47phox in kidney cortex lysate (A) and in the membrane fraction (B) as well as nNOS in kidney cortex and a representative blot (C) of control and diabetic rats with and without the different chronic treatments. The bands at the molecular weights of 47 kDa for p47phox and 160 kDa for nNOS were quantified by densitometry from 8 rats in each group. * denotes P<0.05 vs. untreated control group and # denotes P<0.05 vs. untreated diabetic group. All values are means ± SEM.
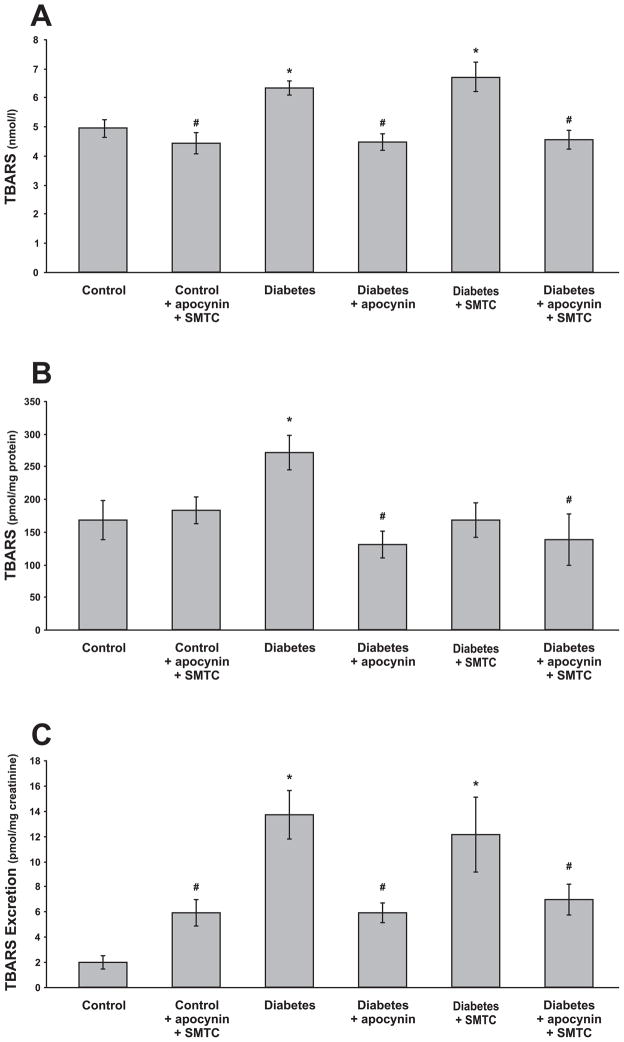
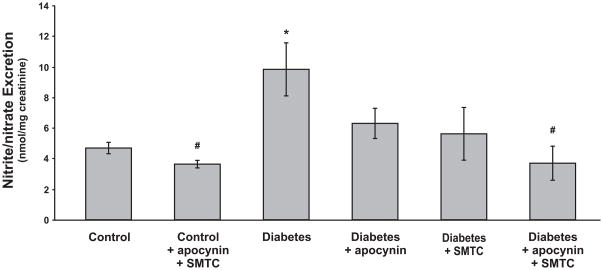
TBARS in the plasma (Fig. 3A), kidney tissue (Fig. 3B) and urinary excretion (Fig. 3C) were increased in diabetics. Chronic apocynin or combined apocynin and SMTC treatments prevented all of these changes, whereas only kidney TBARS was prevented by the chronic SMTC treatment. Urinary excretion of nitrate/nitrite was increased in the diabetic rats and all of the applied chronic treatments prevented this increase (Fig. 4). Nitrite/nitrate excretion was unaffected by the combined chronic treatment with apocynin and SMTC to control rats.
Figure 3.
Lipid peroxidation, estimated as TBARS, in plasma (A), kidney cortex (B) and 24h urinary TBARS excretion (C) in normoglycemic controls and diabetic rats with and without the different chronic treatments. * denotes P<0.05 vs. untreated control group and # denotes P<0.05 vs. untreated diabetic group. All values are means ± SEM.
Figure 4.
Urinary excretion of nitrate/nitrite in normoglycemic controls and diabetic rats with and without the different chronic treatments. * denotes P<0.05 vs. untreated control group and # denotes P<0.05 vs. untreated diabetic group. All values are means ± SEM.
Discussion
The main new finding from the present study is that the increased oxygen consumption in the diabetic kidney is preventable by blocking either the NADPH-oxidase or the nNOS. Furthermore, chronic inhibition of either the NADPH-oxidase by apocynin or the nNOS by SMTC also prevented the diabetes-induced upregulation of the protein expressions of both enzymes and the diabetes-induced oxidative stress in the kidney. These results indicate that oxidative stress originating from NADPH-oxidase and non-functional nNOS triggers a common pathway resulting in increased oxygen utilization by the diabetic kidney.
It is well established that sustained hyperglycemia activates the NADPH-oxidase, manifested as increased phosphorylation of the cytosolic subunit p47phox and subsequent translocation to the membrane [4]. Activation of the NADPH-oxidase results in excessive superoxide production and subsequent oxidative stress in the diabetic kidney [3, 5]. Increased NADPH-oxidase activation was confirmed in the present study as elevated protein expressions of p47phox in both whole tissue lysate and the membrane fraction. Interestingly, inhibition of the phosphorylation of the p47phox subunit by chronic apocynin treatment also prevented the diabetes-induced upregulation of the protein. Similarly, chronic inhibition of the nNOS by SMTC prevented the oxidative stress and the upregulation of the p47phox as well as nNOS protein in the kidney. This finding confirms previous reports of chronic nNOS inhibition resulting in reduced nNOS protein expression in the kidney [19]. However, nNOS inhibition only prevented the oxidative stress in the diabetic kidney tissue, but had no effect on circulating excreted oxidative stress markers. These results indicate that the pro-oxidant effect of nNOS during diabetes is localized only to the kidney. The reason might be that the kidney tissue contains a substantial amount of nNOS [17, 20]. Oxidative stress per se can oxidize tetrahydrobiopterin (BH4), which is required to form the dimer of the NOS subunits and thus a functional NO producing enzyme. However, if the BH4 is converted to oxidized biopterin (BH2) the NOS will instead produce superoxide radicals and thus contribute to the excessive ROS formation commonly associated with the diabetic state. We have previously shown that NO originating from nNOS regulates kidney oxygen consumption in diabetes [21]. However, it was unknown whether the inhibitory effect of NO on mitochondrial oxygen usage was dominating or the increased oxygen consumption induced by oxidative stress originating from uncoupled nNOS. Our results show that the increased oxygen consumption is induced by excessive oxidative stress in the diabetic kidney. However, the pathway requires both NADPH-oxidase and nNOS since inhibition of either of these enzymes completely prevents the excessive ROS formation in the diabetic kidney.
A possible explanation for the decreased plasma concentration of TBARS in the control animals receiving the combined chronic treatment is the increased urinary excretion of TBARS. However, the reason for the increased urinary excretion of TBARS in these animals is unknown, but might include increased glomerular filtration rate, although this was not studied.
Acute administration of apocynin to diabetic PTC significantly reduced the oxygen consumption although not to the same extent as the chronic treatment. This indicates a constant stimulation by the superoxide radicals produced by the NADPH-oxidase on the cellular oxygen consumption. The effect of acute administration of apocynin persisted also in the chronically treated diabetic groups, indicating a sub-maximal effect on cellular oxygen consumption of the chronically administered apocynin. However, the effect of acute SMTC administration was completely abolished in all the chronically treated groups, indicating that the influence of oxidative stress originating from the nNOS involve mechanisms which are substantially slower than the influence of NADPH-oxidase. nNOS blockade has been shown to prevent the diabetes-induced glomerular hyperfiltration [22], which increases the tubular electrolyte load and, therefore, also the demand for active transport and oxygen utilization along the nephron including the proximal tubule.
Whereas ouabain reduced oxygen consumption in PTC from untreated control and diabetic rats, it had no significant effect on PTC from any of the chronically treated groups. This indicates that the primary effect of the increased diabetes-induced oxidative stress is on the electrolyte transport-dependent oxygen consumption. Indeed, it has been shown that superoxide radicals can stimulate Na/K-ATPase activity in the proximal tubule [23], which explains the preventable effect of chronic antioxidant treatment for the oxygen consumption by isolated PTC from diabetic rats [2]. Translated to in vivo, these results imply that the chronic treatments with either apocynin and SMTC restores the electrolyte transport efficiency, defined as consumed oxygen per transported Na+, since the data from the metabolic cages show that the urinary excretion of Na+ was unaffected by the different treatments.
The diabetic rats had higher urinary excretion of nitrite/nitrate indicating increased NO production, which is in agreement with previous reports [22, 24]. However, we have previously shown that the bioavailable tissue levels of NO in the diabetic kidney are significantly lower compared to the control kidney and consequently will directly influence oxygen availability in the diabetic kidney [13, 15]. Therefore, the increased NO production during diabetes, indicated by the increased urinary nitrite/nitrate excretion, is likely a feedback response attempting to compensate the increased NO degradation, and therefore reduced bioavailable NO, due to the elevated oxidative stress. Interestingly, reducing the oxidative stress in the kidney, by either inhibition of the NADPH-oxidase or the nNOS, resulted in significantly reduced urinary nitrite/nitrate excretion although the oxidative stress marker was still elevated in the plasma.
In conclusion, these results show that PTC isolated from diabetic rats have increased oxygen consumption due to increased oxidative stress originating from the NADPH-oxidase and the nNOS. Interestingly, whereas inhibition of the NADPH-oxidase prevented the oxidative stress in kidney tissue as well as plasma, selective nNOS inhibition only reduced oxidative lipid damage in the diabetic kidney. These results provide a mechanistic explanation for the increased oxidative stress causing the increased oxygen utilization by the diabetic kidney.
Acknowledgments
This study was supported by the Swedish Medical Research Council (10840), The Swedish Society for Medical Research, The Fredrik and Ingrid Thuring Foundation, The Marcus and Amalia Wallenberg Foundation, The Magnus Bergvall Foundation and NIH/NIDDK R00 grant (DK077858).
References
- 1.Korner A, Eklof AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes. 1994;43:629–633. doi: 10.2337/diab.43.5.629. [DOI] [PubMed] [Google Scholar]
- 2.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46:1153–1160. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 3.Tojo A, Asaba K, Onozato ML. Suppressing renal NADPH oxidase to treat diabetic nephropathy. Expert Opin Ther Targets. 2007;11:1011–1018. doi: 10.1517/14728222.11.8.1011. [DOI] [PubMed] [Google Scholar]
- 4.Asaba K, Tojo A, Onozato ML, et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 5.Satoh M, Fujimoto S, Haruna Y, et al. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F1144–1152. doi: 10.1152/ajprenal.00221.2004. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 7.Desco MC, Asensi M, Marquez R, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51:1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 9.Nangaku M, Inagi R, Miyata T, Fujita T. Hypoxia and Hypoxia-Inducible Factor in Renal Disease. Nephron Exp Nephrol. 2008;110:e1–e7. doi: 10.1159/000148256. [DOI] [PubMed] [Google Scholar]
- 10.Palm F, Teerlink T, Hansell P. Nitric oxide and kidney oxygenation. Curr Opin Nephrol Hypertens. 2009;18:68–73. doi: 10.1097/MNH.0b013e32831c4cdf. [DOI] [PubMed] [Google Scholar]
- 11.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 12.Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. 2008;4:216–226. doi: 10.1038/ncpneph0757. [DOI] [PubMed] [Google Scholar]
- 13.Palm F, Buerk DG, Carlsson PO, Hansell P, Liss P. Reduced nitric oxide concentration in the renal cortex of streptozotocin-induced diabetic rats: effects on renal oxygenation and microcirculation. Diabetes. 2005;54:3282–3287. doi: 10.2337/diabetes.54.11.3282. [DOI] [PubMed] [Google Scholar]
- 14.Palm F, Hansell P, Ronquist G, Waldenstrom A, Liss P, Carlsson PO. Polyol-pathway-dependent disturbances in renal medullary metabolism in experimental insulin-deficient diabetes mellitus in rats. Diabetologia. 2004;47:1223–1231. doi: 10.1007/s00125-004-1434-3. [DOI] [PubMed] [Google Scholar]
- 15.Palm F, Friederich M, Carlsson PO, Hansell P, Teerlink T, Liss P. Reduced nitric oxide in diabetic kidneys due to increased hepatic arginine metabolism: implications for renomedullary oxygen availability. Am J Physiol Renal Physiol. 2008;294:F30–37. doi: 10.1152/ajprenal.00166.2007. [DOI] [PubMed] [Google Scholar]
- 16.Palm F. Intrarenal oxygen in diabetes and a possible link to diabetic nephropathy. Clin Exp Pharmacol Physiol. 2006;33:997–1001. doi: 10.1111/j.1440-1681.2006.04473.x. [DOI] [PubMed] [Google Scholar]
- 17.Deng A, Miracle CM, Suarez JM, et al. Oxygen consumption in the kidney: Effects of nitric oxide synthase isoforms and angiotensin II. Kidney Int. 2005;68:723–730. doi: 10.1111/j.1523-1755.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 18.Komers R, Lindsley JN, Oyama TT, Anderson S. Effects of long-term inhibition of neuronal nitric oxide synthase (NOS1) in uninephrectomized diabetic rats. Nitric Oxide. 2004;11:147–155. doi: 10.1016/j.niox.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Komers R, Lindsley JN, Oyama TT, Allison KM, Anderson S. Role of neuronal nitric oxide synthase (NOS1) in the pathogenesis of renal hemodynamic changes in diabetes. Am J Physiol Renal Physiol. 2000;279:F573–583. doi: 10.1152/ajprenal.2000.279.3.F573. [DOI] [PubMed] [Google Scholar]
- 20.Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol. 1995;268:F885–898. doi: 10.1152/ajprenal.1995.268.5.F885. [DOI] [PubMed] [Google Scholar]
- 21.Palm F, Fasching A, Hansell P, Kallskog O. Nitric oxide originating from NOS1 controls oxygen utilization and electrolyte transport efficiency in the diabetic kidney. Am J Physiol Renal Physiol. 2010;298:F416–420. doi: 10.1152/ajprenal.00229.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito A, Uriu K, Inada Y, et al. Inhibition of neuronal nitric oxide synthase ameliorates renal hyperfiltration in streptozotocin-induced diabetic rat. J Lab Clin Med. 2001;138:177–185. doi: 10.1067/mlc.2001.116843. [DOI] [PubMed] [Google Scholar]
- 23.Beltowski J, Borkowska E, Wojcicka G, Marciniak A. Regulation of renal ouabain-resistant Na+-ATPase by leptin, nitric oxide, reactive oxygen species, and cyclic nucleotides: implications for obesity-associated hypertension. Clin Exp Hypertens. 2007;29:189–207. doi: 10.1080/10641960701361585. [DOI] [PubMed] [Google Scholar]
- 24.Shin SJ, Lai FJ, Wen JD, et al. Neuronal and endothelial nitric oxide synthase expression in outer medulla of streptozotocin-induced diabetic rat kidney. Diabetologia. 2000;43:649–659. doi: 10.1007/s001250051354. [DOI] [PubMed] [Google Scholar]