Abstract
Background
Dengue (DEN) viruses have become a public health problem that affects approximately 100 million people worldwide each year. Prevention measures rely on vector control programs, which are inefficient. Therefore, a vaccine is urgently needed.
Methods
The main goal of our laboratory is to develop an efficient tetravalent DEN DNA vaccine. In this study, we constructed four DEN-2 DNA vaccines expressing prM/env genes, using the homologous leader sequence (VecD2, VRD2E) or the tissue plasminogen activator (tPA) secretory signal (VecD2tpa, VRD2tpa). In vitro expression was tested by transient transfections and Western blot. The immunogenicity and protective efficacy of the vaccine candidates was evaluated in BALB/c mice, using intramuscular (IM) and intradermal (ID) vaccination routes.
Results
Envelope (E) protein expression was detected in transfected COS-7 or 293T cells. We found statistical differences in the antibody responses induced by these vaccine candidates. In addition, the strongest antibody responses and protection were observed when the vaccines were delivered intramuscularly. Moreover, the tPA leader sequence did not significantly improve the vaccine immunogenicity since VecD2 and VecD2tpa induced similar antibody responses.
Conclusions
We demonstrated that most of our DNA vaccine candidates could induce antibody responses and partial protection against DEN-2 virus in mice. These results provide valuable information for the design and construction of a tetravalent DEN DNA vaccine.
Keywords: Dengue-2 virus, DNA vaccines, BALB/c mice
Dengue (DEN) viruses belong to the Flavivirus genus, and consist of four antigenically different serotypes (DEN-1, DEN-2, DEN-3, DEN-4). These viruses are transmitted mainly by the mosquito Aedes aegypti and are a major cause of epidemics in the tropical and subtropical regions of the world (1). Infection with these viruses induces diseases ranging from dengue fever (DF) to dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) (1–2). There is no specific treatment available for these diseases and the prevention of epidemics is based on vector population control programs and community education. Thus, the development of a vaccine will be essential in controlling the 100 million new cases of DF distributed worldwide and the up to 500,000 cases of DHF that arise every year (2).
Prototype DEN vaccines have been developed and evaluated in different laboratories for more than 30 years. Diverse vaccine strategies have been employed including: live attenuated virus (3–4), inactivated virus particles (5–6), recombinant subunit proteins (6–7), and chimeric viruses (8–9). From these, live attenuated and chimeric virus vaccines are the most advanced in clinical studies (10–12). Although these vaccines are capable of inducing antibody and cellular immune responses against DEN viruses, some problems have arisen in adjusting the virus dose of each serotype in the tetravalent formulas in order to obtain a balanced response (4, 13). In addition, there is a potential for reversion to pathogenic stage in the attenuated vaccines (14) or to cause adverse reactions in immunocompromised subjects due to their replication ability (15). Thus, a strategy that can stimulate cellular and humoral immune responses without imposing the risks associated with virus replication may be an excellent alternative for the development of an effective DEN vaccine.
DNA vaccines have been applied as an alternative approach for the development of DEN vaccines (16–20). DNA vaccines have several potential advantages over traditional immunization strategies. First, since the antigen is expressed intracellularly, it can be presented by MHC class I molecules to stimulate cytotoxic T lymphocytes (CTL). Second, vaccination with plasmid DNA may lead to long-lasting immunity due to the continual priming or re-stimulation of the immune system. Studies using reporter genes and PCR have shown that the injected plasmid DNA remains in the muscle tissue several months after injection (21). Finally, DNA vaccines are easy to prepare and are not reactogenic (22–23).
Despite the potential of DNA vaccines in inducing anti-viral immunity, occasionally the immune responses they elicit are too weak to provide protection against viral pathogens. Thus, several approaches have been used to increase the immunogenicity induced by the expression vectors, such as using heterologous leader sequences (24) or different immunization routes (18, 25). Likewise, DNA-based vaccines have been immunologically assessed in the context of a prime-boost regimen, using pathogen-specific proteins to improve the humoral response elicited by DNA alone (26). These strategies for DNA vaccine improvement were employed in this study. Hence, the aim of this study was to compare the humoral immune responses and protection elicited in mice by four different DEN-2 envelope (E) protein expression vectors.
Materials and Methods
Cells
C6/36 and Vero cells, provided by Dr. Vance Vorndam (CDC Dengue Branch, San Juan, PR), were maintained in MEM and M199 media, respectively, supplemented with 10% FBS and 1% gentamycin (Invitrogen, Carlsbad, CA). COS-7 cells were obtained from the American Tissue Culture Collection (ATCC, Manassas, VA) and were maintained in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). 293T cells were obtained from Dr. Edmundo Kraiselburd’s laboratory and were maintained in DMEM supplemented with 10% FBS (Invitrogen, Carlsbad, CA).
Plasmids
A molecular clone of DEN-2 virus (New Guinea c [NGC] strain) that was provided by Dr. Radma Padmanabhan (University of Kansas) (27), was used to amplify the prM/env genes. The DEN-2 viral genome was divided into two clones; the 5′ clone containing nucleotides (nt) 1--2203 and the 3′ clone, which contained the rest of the DEN-2 genomic sequences (nt 2203--10723). The eukaryotic expression vectors pJW4304 (provided by Dr. Jim Mullins from University of Washington) (28) and pVR1020 (provided by Vical, Inc., San Diego, CA) (29) were used for the construction of DEN-2 DNA vaccines. Both vectors contain the cytomegalovirus (CMV) immediate early promoter, including the intron A, the bovine growth hormone polyadenylation signal, and the tPA leader sequence. The advantage of pVR1020 over pJW4304 is that, according to FDA regulations (30), the former does not contain the SV40 origin of replication or the ampicillin resistance gene, making it suitable for further use in clinical trials.
Construction of DEN-2 E protein expression vectors
Four expression vectors containing the DEN-2 prM/env genes were constructed using the parental plasmids pJW4304 and pVR1020 (Table 1). The differences among these vectors are mainly in the leader sequence that precedes the viral genes. Vectors VecD2 and VRD2E use the homologous leader sequence, which consists of the last 39 nucleotides of the capsid gene. This leader sequence was replaced for the secretory signal of the tissue plasminogen activator (tPA) protein to construct vectors VecD2tpa and VRD2tpa. The resistance gene is also different, where the pJW4304-derived vectors contain ampicillin and those derived from pVR1020 have kanamycin.
Table 1.
Description of the E protein expression vectors.
| Vector Name | Parental Vector | Resistance Gene | Leader Sequence |
|---|---|---|---|
| VecD2 | pJW4304 | Ampicillin | Homologous |
| VecD2tpa | pJW4304 | Ampicillin | Heterologous (tPA) |
| VRD2tpa | pVR1020 | Kanamycin | Heterologous (tPA) |
| VRD2-E | pVR1020 | Kanamycin | Homologous |
A molecular clone of DEN-2 was used to construct VecD2. To obtain the full-length sequence, clone 5′ was digested with ClaI and BamHI and was ligated to clone 3′ digested with BamHI and XbaI. The full-length construct was used to PCR-amplify the prM/env region. Primers D2E.F (cccaagcttatggcaggcatgatcattatgctgattcc) and D2E.R (cgtctagatcacttcagttctttgtttttccagc) were used to construct VecD2, which uses the last 39 nucleotides of capsid as leader sequence for the prM/env genes. The PCR reaction was performed using Platinum® Taq DNA polymerase High Fidelity (Invitrogen, Carlsbad, CA) and the product obtained (2059 bp long) was cloned into pJW4304 using the HindIII and NheI sites. To construct VRD2E, the DEN-2 prM/env sequences were amplified from VecD2 and cloned into the SalI and BglII sites of pVR1020. Two modified versions of these vectors were constructed containing the tPA signal already present in either pJW4304 or pVR1020. For this, the prM/env genes were PCR-amplified from VecD2 using primers D2E5′ (ttccatttaaccacaagtaacg) and D2E3′ (tcacttcagttctttgtttttccagc). The PCR product (2022 bp long) was cloned into the unique NheI and MluI sites of pJW4304, or the unique BamHI site of pVR1020, in-frame with the tPA leader to create VecD2tpa or VRD2tpa, respectively. All vectors were sequenced at Lark Technologies Inc. (Dallas, TX) and the results were compared to the GenBank-published sequences of DEN-2 NGC strain (accession number M29095) using the DNAsis version 2.1 program (Chicago, IL).
In vitro expression
The E protein expression vectors (VecD2, VRD2E, VecD2tpa and VRD2tpa) and their respective parental vectors (pJW4304 and pVR1020) were individually transfected (5 μg each) into COS-7 or 293T cells using lipofectamine (Invitrogen, Carlsbad, CA). Transfected cells were cultured for 48 hrs. and cells were either fixed on poly-lysine-treated slides for immunofluorescence assays or lysed (1.8 mM NaH2PO4, 8.4 mM Na2HPO4, 5% Triton-X, 0.1% SDS, 0.1 M Nacl, 0.5% sodium deoxycholate, and 1 mM PMSF) for Western blot analysis. Cell supernatants were ultracentrifuged at 20,000 rpm for 2 hrs. in a sucrose cushion to test for the presence of virus-like particles (VLPs).
Western blot
Proteins were solubilized at room temperature in 10 μL of sample buffer (3% SDS, 33% Glycerol, 0.2 M Tris, 2.5 mM PMSF, and 0.02% bromophenol blue) for 30 minutes. Known protein concentrations were electrophorased on 15% polyacrylamide gels. Resolved proteins were transferred electrophoretically to nitrocellulose membrane sheets (Bio Rad, Hercules, CA). E protein was detected using monoclonal antibody (MAb) 3H5 and the anti-mouse IgG alkaline phosphatase Western blot kit from Vector laboratories (Burlingame, CA).
Mice
BALB/c mice, age 6–8 weeks, were purchased from Harlan (Indianapolis, IN). Mice were housed in a temperature controlled, light cycled room. Bleedings were performed by retro-orbital puncture using sterile, glass capillary tubes. Mice were euthanized by cervical dislocation of the spinal cord. All procedures were performed under ketamine (Ketaset, Fort Dogde, Iowa) and xylazine anesthesia. Animal care complied with the National Institutes of Health guidelines for the humane use of laboratory animals. Animal protocols were approved by the Institutional Animal care and Use committee (IACUC).
Immunization and bleeding schedule
DNA immunizations were performed at 4–6 or 3-weeks intervals by needle injection using 100 μg of plasmid DNAdiluted in 50 μl of endotoxin-free H2O. IM immunizations were performed at the quadriceps while ID inoculations were performed at the upper back portion. Boost was performed subcutaneously with formalin-inactivated DEN-2 virus adjuvanted in alum. For challenge, DNA injections were performed intramuscularly on weeks 0 and 2, followed by the virus inoculation on week 3. Bleedings were performed every 2 or 3 weeks after each immunization. Individual serum samples were used for antibody detection.
Indirect immunofluorescence assay (IFA) and reduction neutralization test (PRNT)
Serum samples from mice were assayed for DEN-2 specific antibodies by IFA and for virus neutralizing antibodies by PRNT. For IFA, HTC slides coated with DEN-2-infected C6/36 cells were used. Serum samples from vaccinated mice were serially diluted (five-fold, starting at a 1:5 dilution) with PBS containing 10% goat serum. Ten μL of each dilution was added to the wells in duplicate and incubated for 30 minutes at 37°C. Slides were washed and incubated with a goat anti-mouse FITC-conjugated IgG. Slides were covered with glycerol and observed under a fluorescence microscope. PRNT were performed as previously described (5). Briefly, two fold dilutions (starting at a 1:20 dilution) were prepared in 0.3 ml of PBS supplemented with 30% FBS. An equal volume of the DEN-2 virus sample (3000 pfu/mL) was added to the serially diluted sera preparations. Individual samples were mixed and incubated at 25°C for 2 hours. Duplicate Vero cell monolayers were infected with 0.2 ml of each sample in 6-wells plate. Viral plaque counting was performed after 5 days of inoculation at 37°C, 10% CO2. The number of plaques observed in the sample incubated with serum obtained from unvaccinated mice were taken to represent 100%. PRNT50 (serum dilution that caused 50% reduction in plaque formation) was calculated using Probit regression from the SPSS program (version 11.5, Chicago, IL).
Intracranial challenge
Challenge was performed as previously described (5). Briefly, six week old vaccinated mice were inoculated intracranially with 100 pfu of live, mouse adapted DEN-2 virus (NGC strain, provided by Dr. Robert Putnak, WRAIR) in 25 μL Hanks balanced salt solution. After challenge, the animals were monitored for 16--18 days until they regained normal mobility. Morbidity status was scored as follows: 0 = healthy; 1 = ruffled coat, lethargic; 2 = partial hind limb paralysis; 3 = complete hind limb paralysis, wasting.
Statistical analysis
All statistical analyses were performed using the SPSS version 11.5 Program (Chicago, IL). Fisher’s exact test (using Crosstabs) was used to compare the percentage of seroconversion among the vaccinated groups. Geometric mean titers (GMT) were calculated after log transformation of reciprocal titers. Multiple comparisons of GMT were performed by analysis of variance (One-way ANOVA) using Games Howell. Survival times were estimated by the Kaplan-Meier method and compared with Fisher’s exact test (using Crosstabs). All p values were two-tailed.
Results
In vitro analysis of the E protein expression vectors
All four DEN-2 vectors were constructed by PCR and therefore, were sequenced to confirm the fidelity of the amplification (data not shown). Sequencing results were compared to the published sequences of prM/env of DEN-2 NGC strain. We found three point mutations in VecD2 and VRD2E but only two can cause amino acid (aa) substitutions. The changes at nucleotide (nt) 608 (A to G) and at nt 1075 (G to A) can cause a Lys to Arg substitution at aa 57 of prM, and a Glu to Lys mutation at aa 49 of the E protein, respectively. A silent mutation at aa 257 of E protein was identified as a C to A change at nt 1701. A fourth mutation was identified in VecD2tpa and VRD2tpa at nt 2337, which was also a silent mutation at aa 469 of the E protein. VRD2tpa also contained four additional amino acids at the 3′ end of the tPA leader that resulted from cloning into the BamHI site of pVR1020.
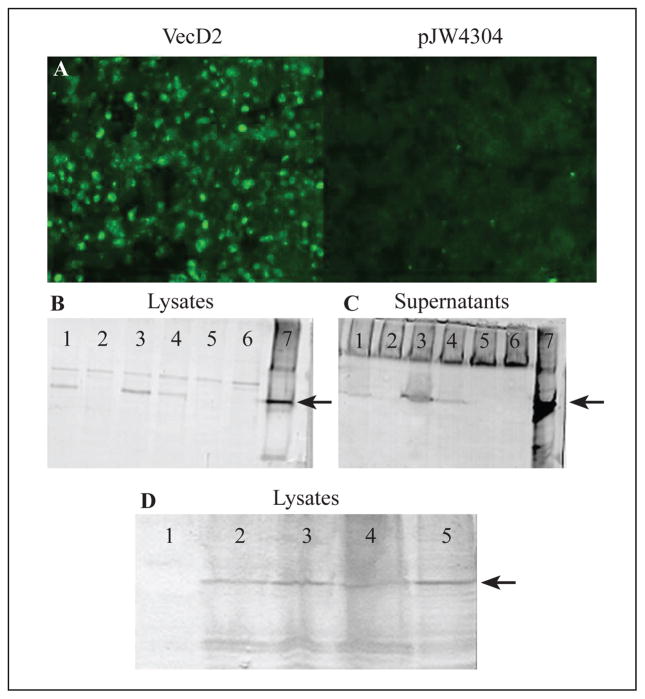
In vitro expression of the DEN-2 E vectors was determined by direct immunofluorescence assays and Western blot analysis of transfected cells. We found that FITC-conjugated anti-DEN immune serum reacted to VecD2-transfected cells (Figure 1A). Similar results were obtained with the other three vectors (data not shown). No fluorescence was detected in COS-7 cells transfected with either of the parental vectors, pJW4304 (Figure 1A) and pVR1020 (data not shown). Western blot analysis was performed under non-denaturing conditions using the conformation-dependent MAb 3H5. The E protein (54 kDa) was detected in lysates of COS-7 cells transfected with VRD2tpa, VecD2tpa or VecD2 (Figure 1B, lanes 1, 3 and 4, respectively), but not from lysates of pVR1020- or pJW4304-transfected cells (Figure 1B, lanes 2 and 5, respectively). In addition, the molecular weight of the recombinant E protein is similar to that of the native E protein obtained from DEN-2-infected C6/36 cells (Figure 1B, lane 7). These results suggest that the DEN-2 vectors expressed the E protein in its native conformation. This protein was also detected in ultracentrifuged cell-free supernatants from VRD2tpa-, VecD2tpa-, and VecD2-transfected COS-7 cells (Figure 1C, lanes 1, 3 and 4, respectively), suggesting that the E protein may be associated with VLPs. A densitometry analysis showed that expression of the E protein in lysates and supernatants of VecD2tpa-transfected cells was 5-fold higher than that of cells transfected with VecD2 (Figure 1B and C, lanes 3 and 4). However, only a 2-fold increase in envelope protein expression was detected in the lysates but not in the supernatants of cells transfected with VRD2tpa when compared to VecD2-transfected cells (Figure 1B and C, lanes 1 and 4). This data suggests that the tPA leader was capable of enhancing the intracellular expression of the DEN-2 E protein.
Figure 1.
Detection of the DEN-2 E protein in transient transfected cells. FITC-conjugated anti-DEN-2 hyperimmune serum and the MAb 3H5 were used for detection of the DEN-2 E protein by direct immunofluorescence (A), and Western blot (B--D), respectively. Cell lysates (B) and ultracentrifuged cell-free supernatants (C) of non-transfected COS-7 cells or DEN-2 infected C6/36 cells were used as negative and positive controls, respectively for Western blot. Lane 1: VRD2tpa, Lane 2: pVR1020, Lane 3: VecD2tpa, Lane 4: VecD2, Lane 5: pJW4304, Lane 6: Control COS-7, Lane 7: D2 virus. Arrows indicates the position of DEN-2 E protein. (D) Lane 1: pVR1020, Lane 2: VecD2 48hrs, Lane 3: VecD2 24 hrs, Lane 4: VRD2E 48 hrs, Lane 5: VRD2E 24 hrs.
Expression of the E protein by VRD2E was also tested by transient transfection in 293T cells and compared to that of VecD2. Both vectors expressed similar levels of the E protein in both, cell lysates (Figure 1D) and supernatants (data not shown), 24 and 48 after transfection.
Immunogenicity of DEN-2 vaccine candidates
The first vector that was constructed and tested for its immunogenicity in mice was VecD2. Groups of seven 6–8 week-old BALB/c mice were intramuscularly inoculated with either VecD2 or pJW4304 on weeks 0, 4, 10, and 16. Serially diluted serum samples, obtained from inoculated mice, were tested for DEN-2 specific antibodies by IFA. Antibody responses were first detected on week 10 (6 weeks after the second DNA dose) but only in one animal inoculated with VecD2 (data not shown). Seroconversion was observed in 2/7 and 5/7 VecD2-vaccinated animals two weeks after the third and fourth DNA inoculations, respectively (data not shown). Although, 100% seroconversion was not reached after administration of four DNA doses, the difference in seroconversion between VecD2-vaccinated mice and the control animals was statistically significant on week 18 (5/7 vs. 0/7, p < 0.05). Neutralizing antibodies (NAb) were also measured by PRNT but were not detected in animals inoculated with VecD2, even after four DNA inoculations (data not shown). As expected, seroconversion was not found in control mice that were inoculated with pJW4304.
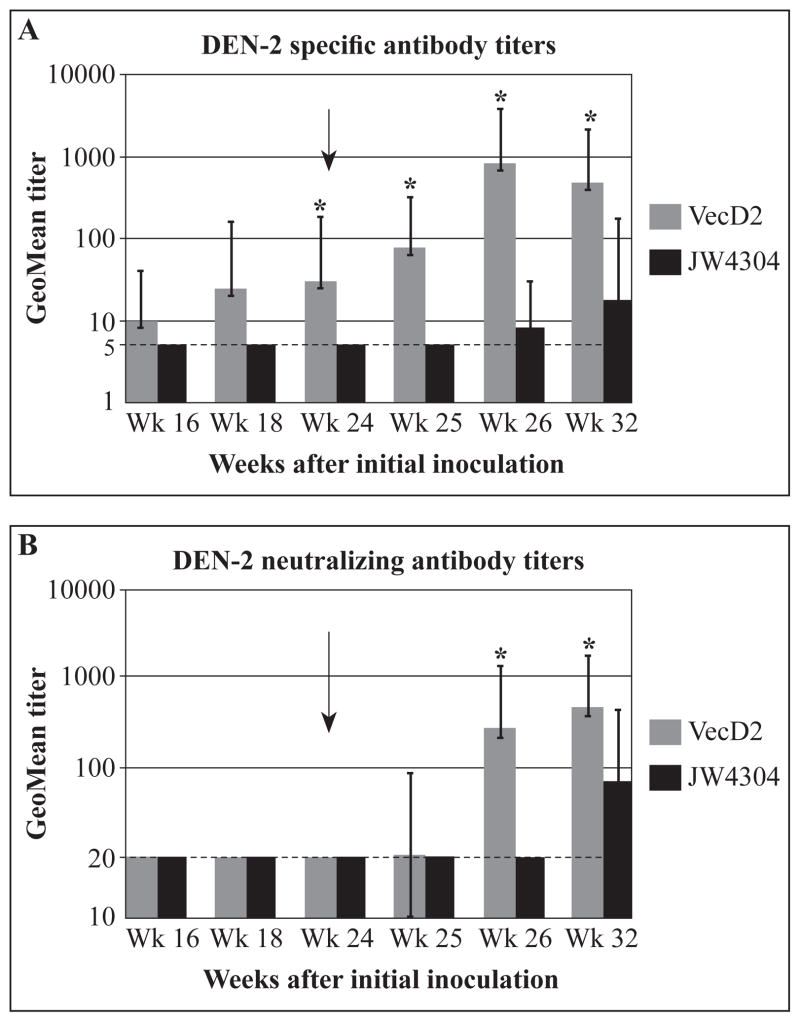
Geometric mean titers (GMT) of DEN-2 specific antibodies (Ab, measured by IFA) and NAb are shown in Figure 2. Up to week 24, VecD2-vaccinated animals developed low Ab titers (GMT < 32) and non-detectable NAb responses (Figure 2A and B). Although IFA titers of mice inoculated with VecD2 were higher than those observed in pJW4304-inoculated animals, the difference in titers between these groups were statistically significant only on week 24 (p < 0.05).
Figure 2.
Figure 2A & 2B. Specific and neutralizing antibody titers induced by VecD2. All animals (experimental and control) received four DNA doses (on weeks 0, 4, 10, 16) and a boost with inactivated DEN-2 virus (on week 24). Geometric mean antibodies titers with their respective 95% confidence limits are shown. Comparison among groups were performed by analysis of variance (ANOVA) using Games Howell test (SPSS), and * indicates when the difference in antibody titers between Vec D2 and pJW4304 was statistically significant (p < 0.05). (A) Titers of DEN-2 specific antibodies were measured by IFA and negative results were given titer of 5 (limit of detection) (B) PRNT50 titers were obtained by probit analysis (SPSS), and negative results were given a titer of 20 (limit of detection).
To determine if VecD2-immunized mice were primed for the development of NAb responses, an inactivated virus boost was administered 8 weeks after the last DNA injection (week 24). All serum samples obtained after this boost were analyzed by IFA and PRNT. We found that Ab titers significantly increased after the inactivated virus boost in VecD2-inoculated mice, reaching their maximum on week 26 (GMT = 817) (Figure 2A). Ab titers induced by the virus boost were statistically higher in the DNA-primed animals than in the controls. NAb responses were also observed on week 26 (two weeks after the virus boost) in 5/6 animals inoculated with VecD2 but not in the control group (data not shown). In addition, six weeks after the virus boost (week 32), all VecD2-vaccinated animals and only one control developed NAb responses. These differences in seroconversion were statistically significant (p < 0.05). NAb titers also increased dramatically in the VecD2 group, reaching statistical significance, (GMT = 230 and 403 on weeks 26 and 32, respectively, Figure 2B) after the virus boost.
In an attempt to improve the immunogenicity of VecD2, we constructed two new vectors containing the tPA leader (VecD2tpa and VRD2tpa). In addition, we used two different routes of inoculation (IM and ID) and shorter intervals between inoculations (3 weeks instead of 4–6 weeks intervals). Groups of 8–10 BALB/c mice were inoculated with VecD2, VecD2tpa, VRD2tpa or the parental vectors (pJW4304 + pVR1020, control group) on weeks 0, 3, 6, and 9. As above, serum samples were tested by IFA and PRNT. Ab responses were first detected 3 weeks after the second DNA dose in mice vaccinated intramuscularly with VecD2 (3/9), VecD2tpa (5/8) or VRD2tpa (1/8) (data not shown). A statistically different seroconversion was observed on week 9 (3 weeks after the third DNA injection) in mice vaccinated with either VecD2 (7/9) or VecD2tpa (7/8), in comparison to the control group (0/8) or the VRD2tpa group (2/8). These two groups (VecD2tpa and VecD2) reached 100% seroconversion on week 15 (six weeks after the fourth DNA dose) when the vaccine candidates were administered intramuscularly. Seroconversion was slower in the VRD2tpa group than in the other experimental groups and 100% seroconversion was never reached. Surprisingly, none of the vaccine candidates elicited Ab responses when delivered intradermally (data not shown).
NAb were first detected on week 6 (3 weeks after the second DNA dose) in the VecD2- and VecD2tpa-vaccinated groups (1/9 and 1/8 respectively, data not shown) but not in the VRD2tpa group. Interestingly, on week 9 (3 weeks after the third DNA dose) the proportion of VecD2tpa-inoculated mice that showed NAb responses was significantly higher than in the naked vector group (6/8 vs. 0/8, respectively, data not shown). However, it was not until week 15 (6 weeks after the fourth DNA inoculation) that the same was true for the VecD2 group (6/9 vs. 0/8, data not shown). Both groups, VecD2tpa and VecD2, reached 100% seroconversion for NAb on weeks 15 and 18, respectively. In VRD2tpa-vaccinated mice, NAb was first detected on week 12 but only in 2/8 animals and this response was transient (data not shown). Of importance, this vaccine group never reached 100% seroconversion and the NAb induction was statistically lower than in the other two experimental groups (VecD2 and VecD2tpa) towards the end of the experiment (on weeks 15 and 18). As with Ab responses, NAb were not detected in intradermally inoculated mice with any of the DEN-2 expression vectors.
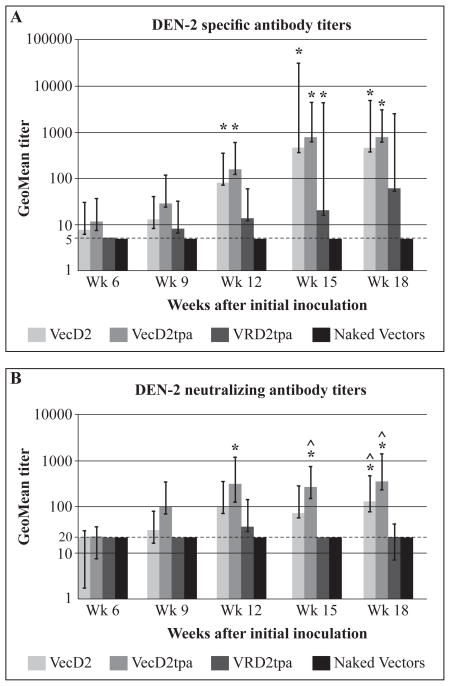
Geometric mean titers (GMT) of specific antibody (measured by IFA) and NAb are shown in Figure 3. IFA titers were weak after three DNA doses (week 9) in all vaccinated mice (GMT ≤ 25, Figure 3A). However, those titers increased in VecD2- (GMT = 79) and VecD2tpa- (GMT = 152) vaccinated mice after the fourth DNA inoculation, reaching statistical significance (p ≤ 0.05) when compared to titers from mice inoculated with naked vectors (GMT ≤ 5). These two groups produced GMT above 100 on weeks 12 (VecD2tpa) and 15 (VecD2). IFA titers were consistently lower in VRD2tpa-vaccinated mice than in the other experimental groups, but not statistically different (Figure 3A). VecD2-vaccinated mice developed weak NAb titers on weeks 6 and 9 (GMT~30, Figure 3B). This was also true in VecD2tpa group, except on week 9 when these mice developed a GMT of a 100. VecD2 and VecD2tpa elicited strong NAb responses (GMT > 100) by week 12 (3 weeks after the fourth DNA dose) and these titers were maintained during the entire experiment. NAb responses in VecD2tpa group were significantly higher than in the negative control (GMT > 20) from week 12 to the end of the experiment. VecD2 group reached a significant level of NAb only on week 18. Although VecD2tpa developed stronger NAb titers than VecD2, the difference in titers was not statistically significant. On the other hand, VRD2tpa group only induced weak NAb (GMT ≤ 50) during the entire experiment and those responses never reached statistical significance when compared to the control group. Moreover, NAb titers of mice inoculated with VecD2 and/or VecD2tpa were statistically higher (p < 0.05) than those of the VRD2tpa-vaccinated animals on week 15 (VecD2tpa only) and on week 18 (both).
Figure 3.
Specific and neutralizing antibody titers induced by the DEN-2 E protein expression vectors. Mice were vaccinated intramuscularly on week 0, 3, 6 and 9. Geometric mean antibodies titers with their respective 95% confidence limits are shown. Comparison among groups were performed by analysis of variance (ANOVA) using Games Howell test (SPSS), and * indicates when the difference in antibody titers between experimental and control (naked vectors) group was statistically significant (p < 0.05). ^ indicates when the difference in antibody titers between VecD2tpa or VecD2 and VRD2tpa group was statistically significant (p < 0.05). (A) Titers of DEN-2 specific antibodies were measured by IFA and negative results were given titer of 5 (limit of detection) (B) PRNT50 titers were obtained by probit analysis (SPSS), and negative results were given a titer of 20 (limit of detection).
Intracranial challenge
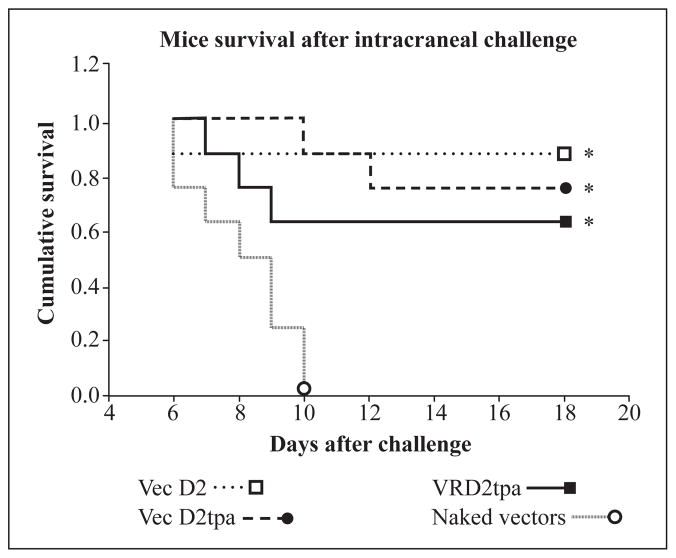
A challenge experiment was performed to evaluate if the DEN-2 vaccine candidates would induce immune responses that protect mice against intracranial inoculation of live virus. Groups of 8 animals were inoculated intramuscularly with 100 μg of each expression vector on weeks 0 and 2. All animals were challenged with 100 pfu of live, mouse adapted DEN-2 virus (NGC strain). Partial protection was observed in VecD2- and VecD2tpa-inoculated mice, where 6/8 and 7/8 animals, respectively survived challenge after 18 days of observation (Figure 4). These results were statistically significant when compared to the survival of the naked vectors group (0/8, p < 0.05). Survival of VRD2tpa-inoculated animals was also statistically higher (5/8, p < 0.05) than in the control group. IFA titers were detected pre-challenge especially in some mice immunized with VecD2tpa (4/8, GMT = 20), VecD2 (3/8, GMT = 11) and VRD2tpa (1/8, GMT < 10) (data not shown). Pre-challenge NAb responses were not observed in the vaccinated mice. However, anamnestic Ab (including NAb) responses were observed in all survivors (GMT > 100) after challenge, but the levels of these Ab did not reach a significant difference between the experimental and control groups (data not shown).
Figure 4.
Mice survival after intracranial challenge. Animals were inoculated by needle on weeks 0 and 2, followed by the intracranial challenge on week 3 and were monitored for signs of paralysis for 18 days until they regained normal mobility. Survival analysis was performed using the Kaplan-Meier method (SPSS) and the proportion of animals that survived challenge was compared by Fisher exact test. * indicates when the difference in survival between experimental and control (naked vectors) group was statistically significant (p < 0.05).
Antibody responses induced by VRD2E
Despite the fact that VecD2 and VecD2tpa were more immunogenic and induced stronger protection against intracranial challenge in mice than VRD2tpa, these vectors cannot be used in human trials. Therefore, we constructed a modified version of VRD2tpa, called VRD2E, by using the natural DEN-2 leader sequence instead of the tPA signal. This new vector was also tested in vivo. Two groups of 13 BALB/c mice received four intramuscular inoculations (100 μg, on weeks 0, 3, 6, 9) of VRD2E or pVR1020 as control group. Mice were bled and serum was tested as described previously. Seroconversion for specific Ab was first observed 3 weeks after the initial DNA inoculation (week 3) in 9/13 inoculated with VRD2E (data not shown). This proportion was significantly higher than the seroconversion rate observed in the VecD2- (from previous experiment) or pVR1020- vaccinated animals (0/9 and 0/13; respectively). The VRD2E group reached 100% seroconversion on week 6 (3 weeks after the second DNA dose) while the VecD2 group required 4 DNA doses to reached similar levels of seroconversion. NAb responses were also observed in the majority of the inoculated animals (10/13) after the first DNA dose. However, those responses were only maintained in 2/13 and 7/13 of the VRD2E-vaccinated mice after the second and third DNA inoculation, respectively (data not shown). Nevertheless, seroconversion for NAb was faster in the VRD2E group than in those animals inoculated with VecD2. Indeed, three DNA doses of VRD2E were required to maintain a significantly higher seroconversion rate than the control group while four DNA doses of VecD2 were necessary to observe the same results.
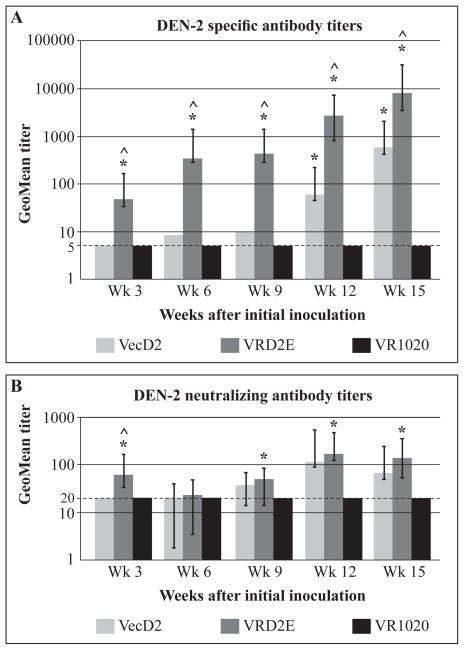
For comparison purposes Ab responses (including NAb) elicited by VecD2 in the previous experiment are shown in Figure 5 together with the data obtained from VRD2E- and pVR1020-vaccinated mice. We observed that the VRD2E induced stronger IFA titers (GMT= 46) than VecD2 or pVR1020 (GMT≤5) after the first DNA inoculation. This response was statistically significant over the other two groups and the difference was maintained during the entire experiment. Specific Ab titers above 100 were observed in the VRD2E group on week 6 (3 weeks after the second DNA dose) and at the end of the experiment (week 15, Figure 5A) those titers increased to a GMT = 8,414. In contrast, VecD2 did not induce specific GMT near 100 until week 12 (three weeks after the fourth DNA dose), when those titers were significantly higher than in the control group. VRD2E also induced strong NAb responses (GMT=58), which were significantly higher than those elicited by VecD2 or pVR1020 after the initial DNA inoculation (week 3, Figure 5B). However, those responses were not sustained and after the second DNA dose, NAb titers were similar in both experimental groups; VRD2E and VecD2. Four DNA doses of VRD2E and VecD2 were required to induce NAb titers ≥ 100 (week 12, Figure 5B). However, those responses were significantly higher than those of the control group in the VRD2E group, but not in the VecD2 group (up to week 15).
Figure 5.
Specific and neutralizing antibody titers induced by VRD2E. Mice were vaccinated intramuscularly on week 0, 3, 6 and 9. Geometric mean antibodies titers with their respective 95% confidence limits are shown. Comparison among groups were performed by analysis of variance (ANOVA) using Games Howell test (SPSS), and * indicates when the difference in antibody titers between experimental and control (pVR1020) group was statistically significant (p < 0.05). ^ indicates when the difference in antibody titers between VecD2 and VRD2E group was statistically significant (p < 0.05). (A) Titers of DEN-2 specific antibodies were measured by IFA and negative results were given titer of 5 (limit of detection) (B) PRNT50 titers were obtained by probit analysis (SPSS), and negative results were given a titer of 20 (limit of detection).
A challenge experiment was also performed as previously described to evaluate if VRD2E could induce immune responses that protect mice against intracranial inoculation of mouse adapted DEN-2 virus. The survival rate for the VRD2E group was 9/10 but in the control group (pVR1020) high survival was also observed (6/10, data not shown). This result was unexpected and, as a consequence, significant differences in the survival rates between the experimental and the control groups could not be established. Therefore, we could not determine the level of protection against DEN-2 induced by VRD2E.
Discussion
The aim of this study was to evaluate the humoral immune responses elicited in mice by four different DEN-2 DNA vaccine candidates. Our strategy was focused on the DEN E protein because it mediates the receptor binding and the entry steps during virus infection. In addition, NAb against this protein were shown to be protective against pathogenic virus infection and disease (5). Two DEN-2 E protein expression vectors (VecD2 and VRD2E) were constructed using the last 39 nucleotides of capsid as the leader sequence plus prM and the full-length env sequences from DEN-2 NGC strain. The other two vectors constructed, VecD2tpa and VRD2tpa, also contained the prM/env genes but the homologous leader sequence was replaced with the tPA signal. The prM region was necessary to prevent conformational changes in the E protein that may occur when it is exposed to a mildly acidic pH in the Golgi apparatus and the secretory vesicles (31). The full-length env gene was selected to stimulate E protein secretion in the form of VLPs in order to expose conformational antigens that may be critical in eliciting protective immune responses. Studies with recombinant plasmids containing the prM and the full-length env of tick-borne encephalitis (TBE) showed that co-expression of these genes induce the secretion of VLPs from cells (32) and that these VLPs elicited protective NAb responses (33). In addition, Konishi and collaborators (34) developed a Japanese encephalitis (JE) DNA vaccine containing prM/env (100%), which also produced VLPs that were able to protect mice against lethal virus challenge. Moreover, comparative studies of truncated and full-length E protein of DEN-1 have demonstrated that plasmids expressing the entire E protein are better immunogens than those that express truncated forms of this protein (18). Thus, the published data supports the hypothesis that vectors expressing the full-length E protein are able to induce better protective immune responses than those that express truncated forms of this protein.
Three antigenic domains have been identified on the E protein (I, II and III) by MAbs (35). Domain I includes aa 130 to 185 and its role has not yet been defined. Domain II spans from aa 50 to 130 and from aa 185 to 300 and contains the putative membrane fusion sequence (aa 98 to 110). Domain III extends over aa 300 to 400 and contains most of the conformation-dependent virus neutralizing epitopes. Thus, domain III may be the most important in terms of generating NAb responses. Four point mutations were introduced in the DEN-2 E protein expression vectors during PCR amplification of the prM/env genes. Only two of these mutations can cause substitutions at aa 57 of prM and at aa 49 of E protein. The last mutation is at the border of domain II and it is very likely that this mutation did not significantly affect the E protein conformation. Indeed, Western blot analysis performed with MAb 3H5, which recognize a conformation-dependent epitope (aa 383–397) (36) revealed that all vectors expressed a recombinant E protein of similar size as the native protein expressed in DEN-2-infected cells (Figure 1). Thus, the results obtained demonstrated that the epitopes expressed by the recombinant E protein were similar to those expressed by the native E protein of DEN-2 and that these vectors had the ability to produce VLPs.
The homologous prM leader sequence was replaced with the tPA signal in VecD2tpa and VRD2tpa to enhance the expression and secretion of the DEN-2 E protein. Our hypothesis was that enhanced expression and secretion of E protein could lead to an enhanced immunogenicity in mice. This secretory signal peptide was chosen because it could enhance expression and exportation of several viral proteins such as the Rotavirus VP4 protein (37) and the SIV envelope protein (38). In addition, tPA was more efficient for secretion of HIV-1 gp120 than other leader sequences (39). In accordance to the published information, intracellular expression of the DEN-2 E protein was 5-fold and 2-fold higher in cells transfected with VecD2tpa and VRD2tpa, respectively than in VecD2-transfected cells (Figure 1B). However, increased secretion of E protein was only detected in VecD2tpa-transfected cells and not in those transfected with VRD2tpa. Since the tPA leader in VRD2tpa contains four additional amino acids that resulted from cloning into the unique BamHI site of pVR1020, it is possible that this insertion had a negative impact in the secretion of the E protein from this vector.
There is no ideal animal model for the evaluation of DEN vaccine candidates. However, mice are routinely used for initial assessment of vaccine immunogenicity (40). In this study, we used BALB/c mice to test the potential immunogenicity of the DEN-2 E protein expression vectors. Three in vivo experiments were performed using four DNA doses that were administered either intradermally or intramuscularly by needle injection. Only in the first experiment was an inactivated virus boost included in the vaccination regimen. The rationale for this inclusion was to corroborate if VecD2 was capable of priming mice for the development of protective Ab responses (i.e. NAb). VecD2 induced weak specific Ab titers and non-detectable NAb responses after four DNA doses during the first experiment (Figure 2). However, vigorous anamnestic responses were observed in VecD2-vaccinated animals after administration of the inactivated DEN-2 virus boost. Furthermore, antibody titers (both, specific Ab and NAb) were significantly higher in animals inoculated with VecD2 than in the controls. Thus, the data obtained indicates that, despite the weak antibody responses elicited by DNA vaccination alone, the VecD2-inoculated animals were primed for the induction of NAb against DEN-2 virus. It is possible that the long resting periods (4–6 weeks) between DNA inoculations may have been partially responsible for the low immunogenicity of VecD2 in this experiment.
The second experiment described in this report was performed to enhance the immunogenicity of VecD2. Two strategies were followed. First, the replacement of the prM leader sequence with the tPA signal. Two of our vectors, VecD2tpa and VRD2tpa, contained this substitution because, as discussed above, this signal peptide has been shown to increase the expression of several viral proteins. Studies performed using the mouse intracranial virus challenge model revealed that a JE prM/env DNA vaccine containing tPA leader confers higher levels of protection in challenged mice than those with the JE prM leader sequence (41). Putnak, et al. (6) evaluated a DEN-2 DNA vaccine containing prM/env genes and the tPA leader sequences in monkeys and they observed an induction of anti-E NAb responses that partially protected these animals against challenge with live DEN-2 virus. Although VecD2tpa induced faster and stronger antibody responses (including NAb) than VecD2, these responses were not statistically different. Thus, the contribution of the tPA to the immunogenicity of the DEN-2 DNA vaccine candidates could not be clearly established.
The second approach we used to enhance the immunogenicity of VecD2 included some modifications in the vaccination regimen such as shorter resting periods between immunizations (3 weeks instead of 4–6 weeks intervals) and using an alternative inoculation route, ID. Changing the vaccination route from IM to ID can influence the efficacy of DNA vaccines because the skin has more antigen presenting cells (APC) than the muscle. APC can be transfected with the plasmid DNA and move to the lymph node where they can be potent activators of naive T cells (42). Thus, we hypothesized that better antigen presentation could result in better induction of protective immune responses. However, in our second experiment, Ab responses were weak or non-detectable when the vaccine candidates were delivered intradermally. This result was unexpected since previous reports describing the evaluation of DEN DNA vaccines demonstrated induction of both, specific Ab and NAb responses after ID immunization (16, 18). A possible explanation for this result is that we used a different inoculation site (upper back portion) than in the published studies (base of the tail) and it is possible that injections in some anatomical areas are more immunogenic than others.
The immunogenicity of VecD2 and VecD2tpa was improved when delivered intramuscularly at 3-weeks intervals. A statistically significant seroconversion was observed in mice vaccinated with these two vectors after the third DNA inoculation. The proportion of mice that elicited NAb responses was also significant after the third and fourth inoculation with VecD2tpa and VecD2, respectively. Antibody titers (both, specific and NAb) were weak after three DNA doses, except in the VecD2tpa group, which produced strong NAb (Figure 3B). However, after four DNA inoculations, specific Ab and NAb responses were strong in the VecD2 and VecD2tpa groups (GMT > 100 in most cases, Figure 3). Indeed, these titers were higher than those elicited by VecD2 before the virus boost in the first experiment that shorter intervals between inoculations might have improved the boosting effect of subsequent DNA doses, leading to a better immune response.
The Ab titers induced by VRD2tpa were consistently lower than those induced by the other DEN-2 E protein expression vectors. Although specific Ab titers were not statistically different among the three DEN-2 vaccine candidates, NAb responses were statistically higher in the VecD2tpa and VecD2 groups in weeks 15 and 18, respectively, than VRD2tpa group (Figure 3B). Moreover, NAb responses in VRD2tpa group never reached statistical difference when compared to the control group (naked vectors). Therefore, VRD2tpa only induced short-lived, weak protective Ab responses against DEN-2 virus. This may be due to the modified tPA leader which contained 4 additional aa that might have caused a negative impact in the immunogenicity of this vector.
Challenge studies are performed to test the efficacy of DEN vaccines to protect mice against live virus inoculation. These studies require the mice to be inoculated intracranially with DEN viruses on or before 6-weeks of age (43). This gives enough time for only two DNA inoculations at 2 week intervals. Since the challenge is one week after the second DNA inoculation, it is possible that 21 days is not enough time to produce a mature immune response in these young animals. This was evident in our studies, since DEN-2-specific antibody responses were absent in the pre-challenge serum of most vaccinated mice. Thus, this might explain why the DEN-2 vaccine candidates only provided partial protection against intracranial challenge (Figure 4).
Although two of the DEN-2 vaccine candidates (VecD2 and VecD2tpa) induced Ab responses, including NAb, both vectors were derived from a plasmid (pJW4304) that cannot be used in human trials (30). Therefore, we constructed and evaluated a new DEN-2 E protein expression vector called VRD2E. The results obtained with VRD2E in the immunogenicity studies were compared with those obtained with VecD2 because this vector contains the same viral sequences present in VRD2E. This comparison was not extended to the other two DEN-2 expression vectors (VRD2tpa and VecD2tpa) because in these vectors the homologous leader sequence was replaced by the tPA secretory signal (Table 1).
Our data demonstrated that mice immunized with the VRD2E vaccine candidate seroconverted earlier for both specific and NAb responses, than those immunized with VecD2. In addition, VRD2E elicited statistically higher levels of DEN-2 specific Ab than those generated by VecD2, during the entire experiment (Figure 5A). However, NAb titers were similar in the two experimental groups and were not statistically different except on week. Thus, it is possible 3 (Figure 5B). On the other hand, NAb titers in the VRD2E group were statistically higher than the control at most time points tested (except week 6) but the same was not true for the VecD2 group. These results demonstrated that VRD2E induced faster and stronger DEN-2 specific Ab responses than VecD2, although NAb titers did not increase accordingly.
To determine if the immune enhancement of VRD2E was due to a better expression and secretion of the DEN-2 E protein, transient transfection studies were performed to compare the E protein expression between VRD2E and VecD2. Results obtained by Western blot analysis demonstrated that both expression vectors produced similar levels of the E protein both intracellularly (Figure 1D) and ofVRD2tpa, extracellularly (data not shown). Therefore, the level of expression is not responsible for the enhancement in the immune response observed in VRD2E-vaccinated mice.
Our DEN-2 DNA vaccines elicited responses that are comparable to those elicited by DNA vaccines developed by other groups, despite the differences in the experimental protocols (6, 16, 18–20). The DNA vaccine candidate developed by Konishi and collaborators (17) closely resembles our vaccine candidates since it includes the prM leader, prM and the full-length env sequences. In their experiments, they performed three intramuscular inoculations (at intervals of two weeks) with 100 μg of the vaccine candidate and only detected NAb (1:10, at 90% plaque reduction) in one out five vaccinated mice. However, strong anamnestic responses (titers > 1:40, at 90% plaque reduction) were observed after a virus boost. These results are similar to those obtained in our first experiment where NAb were not elicited by VecD2 alone, but we demonstrated that the immunized animals were primed for protective immune responses.
In summary, data collected in this study demonstrates the ability of four DNA vaccine candidates, VecD2, VecD2tpa, VRD2tpa and VRD2E, to express DEN-2 E protein in vitro. In addition, we found that two of our DNA vaccine candidates (VecD2 and VecD2tpa) induced strong protective humoral responses when delivered intramuscularly. Since these two vectors cannot be used in human trials (30), we constructed and evaluated a new vector, VRD2E, which elicited stronger Ab responses than VecD2. Thus, VRD2E merits further evaluation as a DEN-2 vaccine candidate. The findings described in this report are relevant to the design and development of an effective, tetravalent DNA vaccine against DEN virus.
Acknowledgments
We thank Dr. Edmundo Kraiselburd from the Microbiology Department and Dr. Vance Vorndam, Dr. Jorge Muñoz, Manuela Beltran, Mark Verduin, and Edgardo Vergne from the San Juan CDC laboratories for their advice, assistance, and reagents. Expression vectors pJW4304 and pVR1020 were a gift from Dr. Jim Mullins from the University of Washington and Vical, Inc., respectively. We are also grateful to Dr. Robert Putnak from WRAIR for providing the mouse-adapted DEN-2 virus for the intracranial challenge. Additionally, we thank Dr. Brad Biggerstaff (CDC, Ft. Collins, CO.) and Cynthia Rivera (RCMI Clinical Research Center, Medical Sciences Campus) for their help in the statistical analysis of the data.
These studies were supported by NIGMS/MBRS-SCORE award, S06 GM008224, NIGMS/MBRS-RISE award R25-GM61838, and NCRR/NIH award, 1P20-RR11126. Infrastructure was supported by NCRR/NIH award, G12-RR03051.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 2.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhamarapravati N, Yoksan S. Live attenuated tetravalent dengue vaccine. Vaccine. 2000;18:44–47. doi: 10.1016/s0264-410x(00)00040-2. [DOI] [PubMed] [Google Scholar]
- 4.Kanesa-thasan N, Sun W, Kim-Ahn G, et al. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine. 2001;19:3179–3188. doi: 10.1016/s0264-410x(01)00020-2. [DOI] [PubMed] [Google Scholar]
- 5.Putnak R, Barvir DA, Burrous JM, et al. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys. J Infect Dis. 1996;174:1176–1184. doi: 10.1093/infdis/174.6.1176. [DOI] [PubMed] [Google Scholar]
- 6.Putnak RJ, Coller BA, Voss G, et al. An evaluation of dengue type-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine. 2005;23:4442–4452. doi: 10.1016/j.vaccine.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Staropoli I, Frenkiel MP, Megret F, Deubel V. Affinity-purified dengue-2 virus envelope glycoprotein induces neutralizing antibodies and protective immunity in mice. Vaccine. 1995;15:1946–1954. doi: 10.1016/s0264-410x(97)00128-x. [DOI] [PubMed] [Google Scholar]
- 8.Lai CJ, Monath TP. Chimeric flaviviruses: novel vaccines against dengue fever, tick-borne encephalitis, and Japanese encephalitis. Adv Virus Res. 2003;61:469–509. doi: 10.1016/s0065-3527(03)61013-4. [DOI] [PubMed] [Google Scholar]
- 9.Guirakhoo F, Pugachev K, Zhang Z, et al. Safety and efficacy of chimeric yellow Fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J Virol. 2004;78:4761–4775. doi: 10.1128/JVI.78.9.4761-4775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabchareon A, Lang J, Chanthavanich P, et al. Safety and immunogenicity of a three dose regimen of two tetravalent live-attenuated dengue vaccines in five- to twelve-year-old Thai children. Pediatr Infect Dis J. 2004;23:99–109. doi: 10.1097/01.inf.0000109289.55856.27. [DOI] [PubMed] [Google Scholar]
- 11.Sun W, Cunningham D, Wasserman SS, et al. Phase 2 clinical trial of three formulations of tetravalent live-attenuated dengue vaccine in flavivirus-naiuml;ve adults. Hum Vaccin. 2008;5:33–40. doi: 10.4161/hv.5.1.6348. [DOI] [PubMed] [Google Scholar]
- 12.McArthur JH, Durbin AP, Marron JA, et al. Phase I clinical evaluation of rDEN4Delta30-200,201: a live attenuated dengue 4 vaccine candidate designed for decreased hepatotoxicity. Am J Trop Med Hyg. 2008;79:678–684. [PMC free article] [PubMed] [Google Scholar]
- 13.Guy B, Barban V, Mantel N, et al. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am J Trop Med Hyg. 2009;80:302–311. [PubMed] [Google Scholar]
- 14.Blume S, Geesink I. A brief history of polio vaccines. Science. 2000;288:1593–1594. doi: 10.1126/science.288.5471.1593. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi UC, Shrivastava R, Nagar R. Dengue vaccines: problems and prospects. Indian J Med Res. 2005;121:639–652. [PubMed] [Google Scholar]
- 16.Kochel T, Wu SJ, Raviprakash K, et al. Inoculation of plasmids expressing the dengue-2 envelope gene elicit neutralizing antibodies in mice. Vaccine. 1997;15:547–552. doi: 10.1016/s0264-410x(97)00215-6. [DOI] [PubMed] [Google Scholar]
- 17.Konishi E, Yamaoka M, Khin-Sane W, et al. A DNA vaccine expressing dengue type 2 virus premembrane and envelope genes induces neutralizing antibody and memory B cells in mice. Vaccine. 2000;18:1133–1139. doi: 10.1016/s0264-410x(99)00376-x. [DOI] [PubMed] [Google Scholar]
- 18.Raviprakash K, Kochel TJ, Ewing D, et al. Immunogenicity of dengue virus type 1 DNA vaccines expressing truncated and full length envelope protein. Vaccine. 2000;18:2426–434. doi: 10.1016/s0264-410x(99)00570-8. [DOI] [PubMed] [Google Scholar]
- 19.Konishi E, Kosugi S, Imoto J. Dengue tetravalent DNA vaccine inducing neutralizing antibody and anamnestic responses to four serotypes in mice. Vaccine. 2006;24:2200–2207. doi: 10.1016/j.vaccine.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 20.De Paula So, Lima DM, de Oliveira França RF, et al. A DNA vaccine candidate expressing dengue-3 virus prM and E proteins elicits neutralizing antibodies and protects mice against lethal challenge. Arch Virol. 2008;153:2215–2223. doi: 10.1007/s00705-008-0250-3. [DOI] [PubMed] [Google Scholar]
- 21.Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 22.Rhodes G, Dwark V, Abai A. Injection of expression vectors containing viral genes induces cellular, humoral protective immunity. Vaccine. 1993;93:137–141. [Google Scholar]
- 23.Yankauckas MA, Morrow JE, Parker SE, et al. Long-term anti-nucleoprotein cellular and humoral immunity is induced by intramuscular injection of plasmid DNA containing NP gene. DNA Cell Biol. 1993;12:771–776. doi: 10.1089/dna.1993.12.771. [DOI] [PubMed] [Google Scholar]
- 24.Chang GJ, Hunt AR, Davis B. A single intramuscular injection of recombinant plasmid DNA induces protective immunity and prevents Japanese encephalitis in mice. J Virol. 2000;74:4244–4252. doi: 10.1128/jvi.74.9.4244-4252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gramzinski RA, Millan Cl, Obaldia N, et al. Immune response to a hepatitis B DNA vaccine in Aotus monkeys: a comparison of vaccine formulation, route, and method of administration. Mol Med. 1998;4:109–118. [PMC free article] [PubMed] [Google Scholar]
- 26.Letvin N, Montefiori D, Yasutomi Y, et al. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Nat Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapoor M, Zhang L, Mohan P, Padmanabhan R. Synthesis and characterization of an infectious dengue virus type-2 RNA genome (New Guinea C strain) Gene. 1995;162:175–180. doi: 10.1016/0378-1119(95)00332-z. [DOI] [PubMed] [Google Scholar]
- 28.Harrison RA, Wu Y, Egerton G, Bianco AE. DNA immunisation with Onchocerca volvulus chitinase induces partial protection against challenge infection with L3 larvae in mice. Vaccine. 1999;18:647–655. doi: 10.1016/s0264-410x(99)00274-1. [DOI] [PubMed] [Google Scholar]
- 29.Price BM, Galloway DR, Baker NR, et al. Protection against Pseudomonas aeruginosa chronic lung infection in mice by genetic immunization against outer membrane protein F (OprF) of P. Aeruginosa Infect Immun. 2001;69:3510–3515. doi: 10.1128/IAI.69.5.3510-3515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson HL, Pertmer TM. Current Protocols in Immunology. John Wiley & Sons, Inc; 1998. Nucleic acid immunization; pp. 2.14.1–2.14.19. [DOI] [PubMed] [Google Scholar]
- 31.Heinz FX, Aver G, Stiasny K, et al. The interactions of the flavi-virus envelope protein: implications for virus entry, and release. Arch Virol Suppl. 1994;9:339–348. doi: 10.1007/978-3-7091-9326-6_34. [DOI] [PubMed] [Google Scholar]
- 32.Allison SL, Stadler K, Mandl CW, et al. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and in particulate form. J Virol. 1995;69:5816–5820. doi: 10.1128/jvi.69.9.5816-5820.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinz FX, Allison SL, Stiasny K, et al. Recombinant and virion-derived soluble and particulate immunogens for vaccination against tick-borne encephalitis. Vaccine. 1995;13:1636–1642. doi: 10.1016/0264-410x(95)00133-l. [DOI] [PubMed] [Google Scholar]
- 34.Konishi E, Yamaoka M, Khin-Sane W, et al. Induction of protective immunity against Japanese encephalitis in mice by immunization with a plasmid encoding JE virus premembrane and envelope genes. J Virol. 1998;72:4925–4930. doi: 10.1128/jvi.72.6.4925-4930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandl CW, Guirakhoo FG, Holzmann H, et al. Antigenic structure of the flavivirus envelope protein E at the molecular level, using tick-borne encephalitis virus as a model. J Virol. 1989;63:564–571. doi: 10.1128/jvi.63.2.564-571.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henchal EA, Mc Coun JM, Burk DS, et al. Epitopic analysis of antigenic determinants on the surface of Dengue 2 virions using monoclonal antibodies. Am J Trop Med Hyg. 1985;34:162–169. doi: 10.4269/ajtmh.1985.34.162. [DOI] [PubMed] [Google Scholar]
- 37.Choi AH, Basu M, Rae MN, et al. Particle-bombardment-mediated Dan Vaccination with Rotavirus VP4 or VP7 induces high levels of serum rotavirus IgG but fails to protect mice against challenge. Virology. 1998;250:230–240. doi: 10.1006/viro.1998.9370. [DOI] [PubMed] [Google Scholar]
- 38.Lu S, Arthos J, Montefiori DC, et al. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golden A, Austen DA, Van Schravendijk MR, et al. Effect of promoters and signal sequences on the production of secreted HIV-1 gp120 protein in the baculovirus system. Protein Expr Purif. 1998;14:8–12. doi: 10.1006/prep.1998.0926. [DOI] [PubMed] [Google Scholar]
- 40.Bray M, Zhao B, Markoff L, et al. Mice immunized with recombinant vaccinia virus expressing dengue 4 virus structural proteins with or without nonstructural protein NS1, are protected against fatal dengue virus encephalitis. J Virol. 1989;63:2853–2856. doi: 10.1128/jvi.63.6.2853-2856.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashok MS, Rangarajan PN. Protective efficacy of a plasmid DNA encoding Japanese encephalitis virus envelope protein fused to tissue plasminogen activator signal sequences: studies in a murine intracerebral virus challenge model. Vaccine. 2002;20:1563–1570. doi: 10.1016/s0264-410x(01)00492-3. [DOI] [PubMed] [Google Scholar]
- 42.Tighe H, Corr M, Roman M, Raz E. Gene Vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 43.Cole A, Wisseman CL., Jr Pathogenesis of type 1 dengue virus infection in suckling, weanling and adult mice. 1. The relation of virus replication to interferon and antibody formation. Am J Epidemiol. 1969;89:669–80. doi: 10.1093/oxfordjournals.aje.a120981. [DOI] [PubMed] [Google Scholar]