Abstract
Nicotinamide phosphoribosyltransferase (Nampt) catalyzes the rate-limiting step of nicotinamide adenine dinucleotide (NAD+) synthesis and is required for cell growth, survival, DNA replication and repair, and angiogenesis. Nampt expression increases gene expression which promotes cell survival and increases SirT1 activity, promoting angiogenesis, and it is increased in several human malignancies. Recently, others have shown that ovarian serous adenocarcinomas (OSAs) express high levels of activated Stat3. Since Nampt expression is increased by Stat3, we hypothesized that Nampt protein might be highly expressed in OSAs. Using tissue microarray (TMA) and the avidin-biotin complex immunohistochemical technique we examined Nampt expression in 47 samples of benign ovarian tissue and 49 samples of ovarian serous adenoacarcinomas. Our data show that Nampt protein expression is significantly increased in OSAs as compared to benign ovarian tissue (0.49+/-0.12 benign vs. 4.78+/-0.46 malignant; +/-standard error of the mean). This is the first report demonstrating Nampt overexpression in OSA, which may shed light on the pathogenesis of OSA. Further studies of the role of Nampt overexpresion in OSA may shed light on the prognosis and clinical course of OSA. Last, since an effective pharmacologic Nampt inhibitor is currently in clinical use, further studies of Nampt overexpression in OSA may be used in selecting patients for Nampt inhibitor therapy.
Keywords: Nicotinamide phosphoribosyltransferase, serous adenocarcinoma, ovarian cancer, nicotinamide adenine dinucleotide, Stat3, Interleukin-6
Introduction
Ovarian cancer is the fifth leading cause of female cancer deaths and confers the highest malignancy-related mortality within gynecological malignancies. Roughly 80% of these ovarian malignancies are serous adenocarcinomas (OSAs), the majority of which are high-grade [1]. Unlike many malignancies, OSAs typically emerge in the absence of a recognizable preexisting lesion, and often carry a poor prognosis, with less than 25% of OSAs detected at stages I and II. Commonly OSAs present with diffuse peritoneal involvement, limiting surgical options to debulkingonly. Additionally, despite the introduction of new chemotherapeutic agents, little progress has been made in improving cure rates, a parameter still largely dependent upon stage at presentation. For these reasons the long-term survival of patients with OSAs is poor, with more than 50% of patients eventually succumbing to the disease [1,2].
Nicotinamide phosphoribosyltransferase (Nampt) catalyses the rate limiting step of nicotinamide adenine dinucleotide (NAD+) synthesis [3]. Nampt expression promotes cell growth and survival, angiogenesis, and is highly expressed in gastric and colorectal carcinomas, and malignant astrocytomas/glioblastomas [3-8]. It has been shown that Nampt may be induced (∼1.5 to 2.0-fold above basal levels) by factors such as hypoxia, serum deprivation, and methylmethane sulfonate in cell culture, and fasting in animals, via a Stat3-dependent mechanism [9-10]. cDNAand tissue microarray studies of ovarian malignancies have shown overexpression of activated signal transducer and activator of transcription 3 (Stat3), particularly in high-grade serous carcinomas [11,12]. Stat3 is a transcription factor that acts as an oncogene in several human malignancies including OSA [13,14].
We hypothesized that the increased Stat3 expression may result in increased Nampt expression in OSA. Using tissue microarray technology and immunohistochemistry we studied Nampt protein expression pattern in OSA as compared to benign ovarian tissue (BOT).
Materials and methods
Upon Institutional Review Board approval, Tissue Microarray (TMA) Human ovarian cancer tissue microarrays were purchased from Protein Biotechnologies, San Diego, CA, and AccuMax Array, Seoul, South Korea, catalog numbers TMA-006 and A213II, respectively. The Protein Biotechnologies microarray consisted of 80 ovarian samples, 1.5 mm in diameter each, including 39 benign ovarian tissue samples (BOT), 39 OSAs, 1 clear cell carcinoma, and 1 mucinous carcinoma. The AccuMax Array consisted of 52 ovarian samples in duplicate (including benign cases), 1.0 mm in diameter each. This included 8 BOT, 10 OSAs, 1 serous borderline tumor, 2 fibromas, 3 dysgerminomas, 3 granulosa cell tumors, 5 clear cell carcinomas, 2 mucinous cystadenomas, 1 mucinous borderline tumor, 3 mucinous adenocarcinomas, 5 endometrioid carcinomas, 4 yolk sac tumors, 3 transitional cell tumors, 1 Brenner tumor, and 1 serous cystadenocarcinoma.
Nampt Immunohistocehmistry (IHC)
The concentration of primary Nampt antibody was optimized on normal kidney as control tissue. The staining of the TMA was performed in the Tissue Core Histology Lab Facility at the Moffitt Cancer Center. The microarray slides were stained using a Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ) as per manufacturer's protocol with proprietary reagents. Briefly, slides were depar-affinized on the automated system with EZ Prep solution (#950-100, Ventana Medical Systems, Tucson, AZ). Heat-induced antigen retrieval method was used in Cell Conditioning 1 (#950-124, Ventana Medical Systems, Tucson, AZ). The mouse monoclonal anti-Nampt antibody (#ALX-804-717, Plymouth Meeting, PA) was used at a 1:1000 concentration in Dako antibody diluent (#S0809, Dako, Carpenteria, CA) and incubated for 60 min. The Ventana Anti-mouse secondary Antibody was used for 16 min. The detection system used was the Ventana OmniMap kit. Slides were then dehydrated and coverslipped as per normal laboratory protocol.
Evaluation of Nampt Stain
Relative Nampt protein immunohistochemical expression was determined as the product of immunostain intensity and percent of cells stained. Both were scored on a 0-3 scale, with 3 being maximal. Immunostain intensity was scored with no staining being 0, light staining as 1, moderate staining as 2, and heavy staining as 3. The percent of cell stained was measured with no detectable staining as 0, 1-33% as 1, 34-66% as 2, and 67-100% as 3. The final IHC score was the product of the percent of cells stained score multiplied by the intensity score, allowing for a maximal score of 9 and a minimal score of 0. Nuclear staining was seen in all tissue samples examined, while cytoplasmic staining was seen in some, but not all tumor samples. We therefore measured and quantified Nampt staining within the nuclear compartment.
Statistical Analysis
The standard error of the mean (SEM) was calculated by using the standard deviation in the staining of each tumor type and dividing this number by the square root of the sample size. For the TMA with duplicate tumors samples (AccuMax), the same tumor sample was scored twice and the average of the two scores was used as a data point. SEM calculations could not be performed for those samples consisting of only 1-2 tumors.
Results
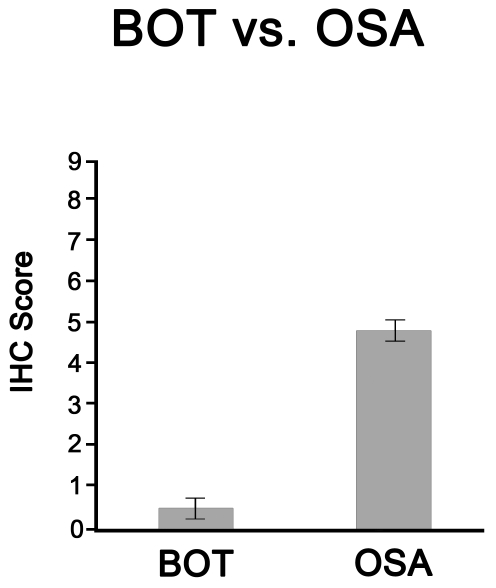
After eliminating those samples which lost tissue during the IHC processes, we examined Nampt expression in 47 BOT samples and 49 OSAs. Nampt protein expression was low in BOT (IHC score 0.49+/-0.12), and it was significantly increased in OSA (IHC score 4.87+/-0.46, Table 1 and Figure 1). The other ovarian tumor types, represented on the TMA, also revealed higher Nampt protein expression as compared to BOT. In particular, the highest Nampt expression was noted in the surface epithelial tumors, and in the endometroid and clear cell types (Table 1 and Figure 2).
Table 1.
Nampt IHC scoring in different ovarian neoplasms
| Tissue Type | Sample Number | Staining | SEM |
|---|---|---|---|
| Benign Ovarian Tissue | 47 | 0.49 | 0.12 |
| Serous Adenocarcinoma | 49 | 4.87 | 0.46 |
| Clear Cell Carcinoma | 6 | 5.44 | 0.94 |
| Endometrioid Carcinoma | 5 | 4.89 | 0.68 |
| Yolk sac tumor | 4 | 0.75 | 0.25 |
| Mucinous Adenocarcinoma | 4 | 6.60 | 0.64 |
| Dysgerminoma | 3 | 1.00 | 0.25 |
| Granulosa cell tumor | 3 | 3.00 | 0.79 |
| Transitional Cell Carcinoma | 3 | 3.17 | 0.74 |
| Fibroma | 2 | 0.75 | - |
| Mucinous cystadenoma | 2 | 1.00 | - |
| Mucinous borderline tumor | 1 | 1.00 | - |
| Brenner Tumor | 1 | 3.00 | - |
| Serous borderline tumor | 1 | 1.00 | - |
Figure 1.
The relative IHC score of BOT compared to OSA. 47 BOT and 49 OSA samples were examined, scored, and the standard error of the mean was calculated.
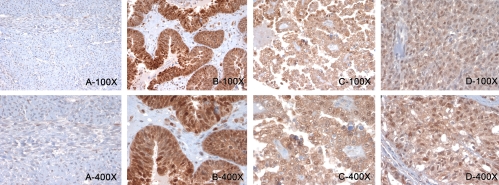
Figure 2.
Representative IHC results for Nampt staining on various ovarian tumors. A. low and high power views of benign ovary. B, C, and D, low and high power fields of an ovarian high grade serous adenocarcinoma, clear cell carcinoma, and a granulosa cell tumor.
Discussion
Based on previous analyses of the molecular alterations underlying ovarian cancers, we hypothesized there would be overexpression of Nampt. This study demonstrated increased Nampt expression in these tumors as compared to BOT Nampt catalyzes the rate-limiting step of the NAD+ synthesis salvage pathway. NAD+ is required for DNA synthesis and repair, cell growth and division, and also promotes angio-genesis, cell migration, and apoptosis inhibition via regulating SirT1 activity [3,15-20]. Interestingly, microarray studies have demonstrated that SirT1 is over-expressed in ovarian serous adenoacarcinomas [21]. Thus Nampt overexpression could have a role in promoting increased cell growth and viability.
Examination of other epithelial neoplasms revealed a general pattern of increasing Nampt expression accompanying malignancy and in particular the higher grade malignant neoplasms. In our study we similarly observed high Nampt staining in clear cell carcinoma, mucinous adenocarcinoma, endometrioid carcinoma, and in the OSAs. Mucinous borderline tumor, serous borderline tumor, ovarian fibroma, and mucinous cystadenoma revealed significantly less Nampt staining. Interestingly, the yolk sac tumor and dysgerminoma had Nampt staining only slightly greater than benign ovarian tissue, while the granulosa cell tumor, transitional cell, and Brenner tumors showed intermediate staining. However, we acknowledge that for these subtypes the number of tumors examined is too low to make any definite conclusion (Table 1). Further studies to clarify the significance of Nampt protein expression in these tumor types are warranted. Other investigators using microarray studies have demonstrated that different ovarian tumors show very different gene expression profiles, specific for different tumor types [1,11,14,22-24]. Additionally, Nampt expression produces NAD+ necessary for cell growth and division and also promotes the expression of genes promoting cell survival [25]. It is therefore not surprising that Nampt expression varies in different ovarian tumor types and is highly expressed in high grade tumors.
Interleukin-6 (IL-6) has been implicated in several malignancies arising in a background of chronic inflammation. IL-6 activates Stat3, resulting in up-regulation of anti-apoptotic gene expression and the promotion of uncontrolled cell proliferation [26]. Stat3 activation also induces Nampt, increasing the NAD+ required for cell proliferation [1,10]. Thus Nampt may be added to the list of genes induced via the IL-6/ Stat3 axis which promotes malignancy. Support for this view comes from the observation that IL-6 levels are elevated in ovarian malignancies, with highest levels seen in mucinous and OSAs, two ovarian malignancies in which we found had very high Nampt expression [27].
Lastly, a specific Nampt inhibitor, FK866 has shown promising results in the treatment of human malignancies [28,29]. Since Nampt is over expressed in high grade OSAs, and the prognosis for these malignancies is often very poor at higher stages [1,11,12], this inhibitor may provide an alternative adjuvant therapeutic modality for these malignancies. Further studies of Nampt in high grade OSAs may be helpful in selecting participants for Nampt inhibitor therapy.
Acknowledgments
We would like to thank Jennifer Burton for help in manuscript preparation, and the Histology Core at Moffitt Cancer Center for IHC stain.
References
- 1.Auersperg N, Ota T, Mitchell GW. Early events in ovarian epithelial carcinogenesis: progress and problems in experimental approaches. Int J Gynecol Cancer. 2002;12:691–703. doi: 10.1046/j.1525-1438.2002.01152.x. [DOI] [PubMed] [Google Scholar]
- 2.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garten A, Petzold S, Körner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–8. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima TE, Yamada Y, Hamano T, Furuta K, Gotoda T, Katai H, Kato K, Hamaguchi T, Shimada Y. Adipocytokine levels in gastric cancer patients: resistin and visfatin as biomarkers of gastric cancer. J Gastroenterol. 2009;44:685–90. doi: 10.1007/s00535-009-0063-5. [DOI] [PubMed] [Google Scholar]
- 5.Reddy PS, Umesh S, Thota B, Tandon A, Pandey P, Hegde AS, Balasubramaniam A, Chandramouli BA, Santosh V, Rao MR, Kondaiah P, Somasundaram K. PBEF1/NAmPRTase/ Visfata potential malignant astrocytoma/ glioblastoma serum marker with prognostic value. Cancer Biol Ther. 2008;7:663–8. doi: 10.4161/cbt.7.5.5663. [DOI] [PubMed] [Google Scholar]
- 6.Hufton SE, Moerkerk PT, Brandwijk R, de Bruïne AP, Arends JW, Hoogenboom HR. A profile of differentially expressed genes in primary colorectal cancer using suppression subtractive hybridization. FEBS Lett. 1999;463:77–82. doi: 10.1016/s0014-5793(99)01578-1. [DOI] [PubMed] [Google Scholar]
- 7.Van Beijnum JR, Moerkerk PT, Gerbers AJ, De Bruïne AP, Arends JW, Hoogenboom HR, Hufton SE. Target validation for genomics using peptide-specific phage antibodies: a study of five gene products overexpressed in colorectal cancer. IntJ Cancer. 2002;101:118–127. doi: 10.1002/ijc.10584. [DOI] [PubMed] [Google Scholar]
- 8.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS, Bryant-Greenwood G, Jones SA. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent inter-leukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–95. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- 11.Meinhold-Heerlein I, Bauerschlag D, Hilpert F, Dimitrov P, Sapinoso LM, Orlowska-Volk M, Bauknecht T, Park TW, Jonat W, Jacobsen A, Sehouli J, Luttges J, Krajewski M, Krajewski S, Reed JC, Arnold N, Hampton GM. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2005;24:1053–65. doi: 10.1038/sj.onc.1208298. [DOI] [PubMed] [Google Scholar]
- 12.Rosen DG, Mercado-Uribe I, Yang G, Bast RC Jr, Amin HM, Lai R, Liu J. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer. 2006;107:2730–40. doi: 10.1002/cncr.22293. [DOI] [PubMed] [Google Scholar]
- 13.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis Oncogene. 2000;19:2474–88. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 14.Duan Z, Foster R, Bell DA, Mahoney J, Wolak K, Vaidya A, Hampel C, Lee H, Seiden MV. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin Cancer Res. 2006;12:5055–63. doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]
- 15.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–58. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279 doi: 10.1074/jbc.M408388200. 50754e63. [DOI] [PubMed] [Google Scholar]
- 17.Jeong J, Juhn K, Lee H, Kim SH, Min BH, Lee KM, Cho MH, Park GH, Lee KH. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med. 2007;39 doi: 10.1038/emm.2007.2. 8e13. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Sun D, Li F, Tian L, Li C, Li L, Lin R, Wang S. Downregulation of Sirt1 by antisense oligonucleotides induces apoptosis and enhances radiation sensitization in A549 lung cancer cells. Lung Cancer. 2007;58:21–9. doi: 10.1016/j.lungcan.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–6. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, Nicosia SV, Zhang X. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–60. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 21.Jang KY, Kim KS, Hwang SH, Kwon KS, Kim KR, Park HS, Park BH, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression and prognostic significance of SIRT1 in ovarian epithelial tumours. Pathology. 2009;41:366–71. doi: 10.1080/00313020902884451. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi K, Mandai M, Oura T, Matsumura N, Hamanishi J, Baba T, Matsui S, Murphy SK, Konishi I. Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene. 2010;29:1741–52. doi: 10.1038/onc.2009.470. [DOI] [PubMed] [Google Scholar]
- 23.Hoei-Hansen CE, Kraggerud SM, Abeler VM, Kaern J, Rajpert-De Meyts E, Lothe RA. Ovarian dysgerminomas are characterized by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer. 2007;6(12) doi: 10.1186/1476-4598-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guirguis A, Elishaev E, Oh SH, Tseng GC, Zorn K, DeLoia JA. Use of gene expression profiles to stage concurrent endometrioid tumors of the endometrium and ovary. Gynecol Oncol. 2008;108:370–6. doi: 10.1016/j.ygyno.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, DuMond ME, Krishnakumar R, Yang T, Sauve AA, Kraus WL. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem. 2009;284:20408–17. doi: 10.1074/jbc.M109.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kryczek I, Grybo M, Karabon L, Klimczak A, Lange A. IL-6 production in ovarian carcinoma is associated with histiotype and biological characteristics of the tumour and influences local immunity. British Journal of Cancer. 2000;82:621–628. doi: 10.1054/bjoc.1999.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–42. [PubMed] [Google Scholar]
- 29.Pogrebniak A, Schemainda I, Azzam K, Pelka-Fleischer R, Nüssler V, Hasmann M. Chemopotentiating effects of a novel NAD biosynthesis inhibitor, FK866, in combination with antineoplastic agents. Eur J Med Res. 2006;11:313–21. [PubMed] [Google Scholar]