Abstract
Gastrointestinal stromal tumors (GIST) usually form a well-circumscribed mass. However, patients with germline mutations in c-KIT, PDGFRA and NF1 may present with diffuse interstitial cell of Cajal (ICC) hyperplasia along the Auer-bach plexus without forming a discrete mass. To our knowledge, sporadic diffuse ICC hyperplasia replacing the gut wall has not been described previously. We describe herein two such cases. Case 1 was a 59-yr-old woman who presented with signs of ileus and a large mass submitted as Meckel diverticulum. The resection specimen showed a large GIST with diverticulum-like and solid areas. The diverticular component showed a diffuse proliferation of spindle cells extending for several centimetres from the solid tumor replacing the full thickness of the gut wall and lined by intact mucosa. Mutation analysis revealed a combined deletion/insertion in c-KIT exon 11 (V560delEins) in both the solid and the diffuse tumor component. Case 2 was a 66-yr-old man who underwent segmental sigmoid colon resection for adenocarcinoma in a villous adenoma. Random sections from grossly unremarkable colonic wall showed a diffuse proliferation of CD117+/CD34+ spindle cells completely replacing the muscularis propria for a length of 6 mm. Molecular analysis revealed a somatic point mutation/ deletion in exon 11 of c-KIT (Q575L; L576_W582del). Absence of multiple lesions and demonstration of a wild-type sequence for c-KIT in surrounding normal tissue ruled out the possibility of a germline mutation in both cases. This peculiar diffuse form of sporadic ICC hyperplasia results from somatic c-KIT mutations and must be distinguished from syndromic ICC hyperplasia associated with hereditary GIST syndromes.
Keywords: GIST, Meckel Diverticulum, ICC hyperplasia, KIT mutation, hereditary GIST
Introduction
Gastrointestinal stromal tumors (GIST) are the most common primary mesenchymal neoplasms of the GI tract [1]. GISTs are KIT-positive and KIT-signalling driven neoplasms that most commonly originate in the stomach and small bowel but may occur at any site along the GI tract [2]. GISTs are uncommon in the colon and rectum (5–10%) [2]. Somatic mutation in the c-KIT(∼80%) [3,4] and PDGFRA (∼10%) [5,6] are considered initiating early molecular events in a majority of cases. GISTs usually present as a discrete well-circumscribed but non-encapsulated tumor mass with strikingly varied gross appearances including luminal-polypoid, intramural, extramural, pedunculated forms or a variable combination thereof [2,7]. A few GISTs were reported to arise within a “Meckel diverticulum” [7,8], but the diverticular aspect in most of them is obviously the consequence of deep cavitating tumor ulceration with central necrosis and occasional cyst formation [7,9].
GISTs are currently thought to derive from or differentiate similar to the gastrointestinal pacemaker cells, the interstitial cells of Cajal (ICCs) [10]. ICCs are CD117-positive and variably CD34-expressing slender bipolar mesenchymal cells that form a network surrounding the auto-nomic nerves of the Auerbach plexus and are also distributed within the inner and outer layer of the muscularis propria [11]. The term ICC hyperplasia has been applied to a variety of microscopic CD117-expressing spindle cell lesions detected incidentally in surgical or autopsy specimens harbouring larger GISTs or other non-GIST lesions [10,12,13]. ICC hyperplasia may occur either as a sporadic (incidental) lesion or present in a syndromic setting known to predispose to multiple GIST tumors at different sites. The latter situation has been observed in patients with hereditary GIST syndromes caused by germline mutations in c-KIT [14] or PDGFRA [15] and in patients with neurofibromatosis type 1 (NF-1) [16]. The incidence of sporadic incidental ICC hyperplasia (also called microscopic GIST) in esophagogas-tric resections in previous studies ranged from 10%–35%, based on the method used for detection [12,13,17,18]. To our knowledge, sporadic ICC hyperplasia showing diffuse longitudinal growth pattern completely replacing the muscularis propria has not been reported to date. We herein describe two such cases.
Case histories
Case 1
A 59-year-old woman presented with abdominal symptoms and signs of ileus. Clinical examination showed signs of a mechanical ileus. In addition, imaging procedures revealed signs of small bowel adhesions and a large small bowel diver-ticulum with evidence of chronic inflammation. At surgery, multiple small bowel adhesions have been resolved together with segmental resection of the small bowel containing the diverticu-lar structure and appendectomy. No further tumors or metastasis were seen intra-operatively. The patient is alive and well 12 months after surgery. She has no features of NF-1 or other relevant familial history.
Case 2
A 66-year-old man was diagnosed with adeno-carcinoma arising within a villous adenoma in the sigmoid colon. Because of worry about residual cancer after polypectomy, a segmental resection of the sigmoid colon was performed. The patient had no history of GIST, evidence of other GISTs at surgery or signs of NF-1. He is alive and well without evidence of new tumors or recurrence 15 months after surgery.
Material and methods
Tumor tissue was fixed in formalin and embedded routinely for histological examination. Im-munohistochemical stains were performed on 5-μm sections cut from paraffin blocks using the primary antibodies CD117 (polyclonal, 1:200, DakoCytomation, Glostrup, Denmark), CD34 (1:200, DakoCytomation), protein S100 (polyclonal, 1:2500, DakoCytomation), desmin (1:250, DakoCytomation) and alpha-smooth muscle actin (1:200, DakoCytomation) according to manufacturer's recommendation. For mutational analysis, DNA was extracted from formalin-fixed paraffin embedded tissue and amplified using primers specific for exons 9, 11, 13 and 17 of the c-KIT gene and exons 12 and 18 of the PDGFRA gene followed by purification of the PCR products and direct bi-directional sequencing using the methods described previously [4,5,19].
Results
Pathological findings
Case 1: The resection specimen was composed of a 7-cm small bowel segment and a separate 4 cm large saccular specimen (submitted as Meckel diverticulum) containing a firm nodular mass measuring 1.5 cm in diameter with a grey-whitish cut-surface (Figure 1A-B). The adjacent diverticular structure was lined by intact mu-cosa with focal haemorrhages, but no ulcera-tion. Histologically, the diverticular component represented peripheral extension (continuity) of the solid nodule without intervening normal tissue between them (Figure 1C). The proliferation occupied and replaced the full thickness of the gut wall in a manner similar to case 2, extending for a distance of 2.5 cm. A remarkable finding within the saccular component was the presence of thick-walled dysplastic blood vessels within the thickened muscularis mucosae and also within adjacent submucosa, focally replac-ingthe entire thickness of the bowel wall (Figure 1D). The cellular density of the longitudinal tumor infiltrate diminished gradually as one moves away from the solid nodule (Figure 1D). Using desmin immunostaining, the muscularis propria was almost completely deficient in most of the saccular lesion with only occasional attenuated smooth muscle fibres seen in the sub-serosa (Figure 1E). Histological examination of the solid mass showed a cellular neoplasm composed of admixed spindled and rounded/ epithelioid tumor cells displayingfinely vesicular chromatin and palely staining cytoplasm arranged in short intersecting fascicles and diffuse sheets (Figure 1F). Numerous Periodic acid Schiff-positive globular deposits consistent with skeinoid fibers were seen between tumor cells. There was no significant atypia and the mitotic rate was 1/50 high power field. Immunohisto-chemistry revealed strong and diffuse expression of CD117 with focal alpha-smooth muscle actin reactivity. Desmin, CD34 and protein S100 were not expressed in the tumor cells. The tumor histology was similar in both the solid and the diffuse parts. Additional discrete spindle cell proliferations consistent with multifocal ICC hyperplasia or multifocal microscopic GISTs were not seen in the remaining bowel segment.
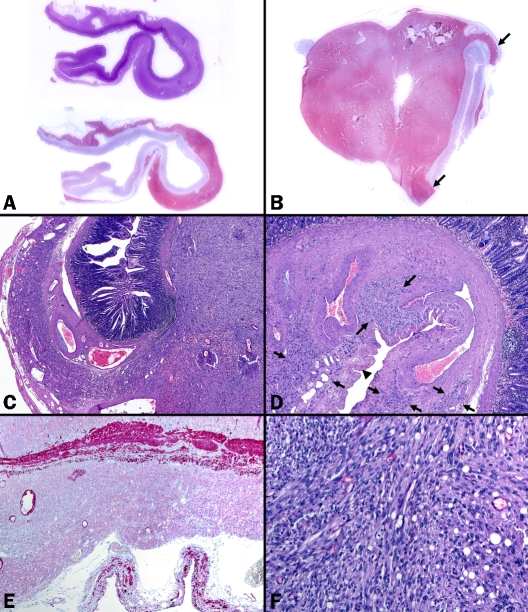
Figure 1.
Histological features of gastrointestinal stromal tumor in case 1. (A) Whole mount section from the diverticu-lar component (actual section length 2.5 cm) showed diffuse submucosal growth highlighted by CD117 (upper H&E, lower CD117). (B) Solid tumor component at the tip of a diffuse saccular structure (arrows) highlighted in the CD117 immunostaining. (C) Interphase between the two components, note dysplastic vessels. (D) Dysplastic vessels and intervening attenuated muscle fibers and connective tissue replaced the normal muscularis propria, note hypocellu-lar intervening tumorous tissue (between arrows). Prominent serosal undulations (arrow head) indicated thinning of the gut wall. (E) The muscularis propria was almost completely absent in the diffuse component (desmin stain). (F) Higher magnification of the solid tumor showed fascicular growth of spindled and rounded cells with occasional vacuoles.
Case 2: The sigmoid resection specimen measured 8.0 cm in length and was 7.4 in circumference. The area of previous polypectomy was identified and embedded completely. The muscularis propria looked normal grossly. Multiple random sections were taken away from the previous polypectomy. Histologically, no residual cancer was seen except for ulceration and granulation tissue. Sections of grossly normal sigmoid colon showed a diffuse spindle cell proliferation completely replacing the muscularis propria in its full thickness and extending longitudinally for a length of 6 mm (Figure 2A). Residual smooth muscle fibers of the muscularis propria and occasional ganglion cells as well as scattered inflammatory cells and eosinophils were seen amongst the spindle cell proliferation (Figure 2B). The lesional cells were spindled with elongated to plump nuclei and eosinophilic fibrillar cytoplasm (Figure 2C). There was mild pleomorphism but no mitotic activity or necrosis was seen. Based on the orientation of submitted sections, the proliferation appeared seg-mental with a relatively sharp demarcation at the interface with normal appearing muscularis propria proximally and distally. The spindle cell proliferation was positive for CD117 (Figure 2D) and CD34 and was negative for desmin, alpha-smooth muscle actin and protein S100. The overlying mucosa was intact with mild chronic inflammation within the lamina propria. The remaining colonic wall including resection margins and the previous polypectomy area showed no evidence of similar mesenchymal lesions in the muscularis propria. There was no evidence of diverticular disease in the segment of bowel removed or in the area containing the spindle cell proliferation.
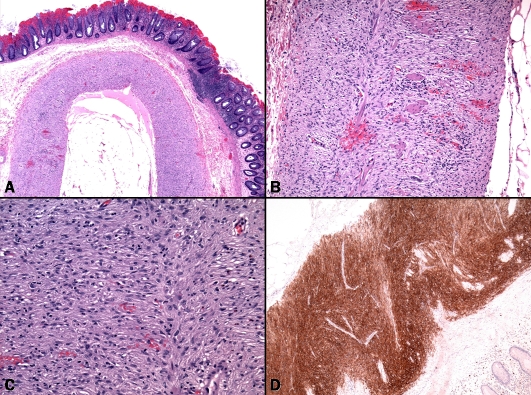
Figure 2.
Pathological findings in case 2. (A) Diffuse spindled proliferation replacing both layers of the muscularis propria in otherwise architecturally unremarkable colonic wall. (B) Residual muscle fibers of the muscularis propria were seen between tumor cells, note circumscribed growth towards both submucosa (left) and subserosa (right). (C) Tumor cells at higher magnification. (D) Diffuse staining with CD117 highlightingthe longitudinal pattern of tumor.
Molecular findings
Using PCR and bi-directional sequencing analysis a heterozygous somatic combined deletion/ insertion involving codon 560 in c-KIT exon 11 (V560delEins) was detected in both the solid and the diffuse component of case 1 (Figure 3A-B). In case 2, a point mutation involving codon 575 combined with a 21 bp-deletion (Q575L; L576_W582del) were detected in the juxtam-embrane domain of c-KIT (exon 11). In both patients, analysis of surrounding normal tissue revealed wild-type sequence for c-KIT thus ruling out the possibility of an inherited germline mutation (Figure 3C). There was no evidence for the presence of a PDGFRA mutation in any of the cases.
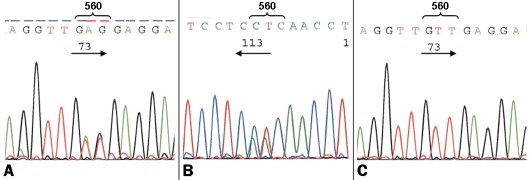
Figure 3.
Molecular findings in case 1. (A) A heterozygous V560delEins mutation involving exon 11 of c-KIT was detected in both the solid and the diffuse component in case 1. (B) Reverse sequencing confirmed the mutation. (C) Surrounding normal tissue revealed a wild-type sequence.
Discussion
The two cases presented herein displayed a unique and, to our knowledge, to date unre-ported diffuse microscopic growth pattern in sporadic ICC hyperplasia (microscopic GIST). The growth pattern in our cases differs from both the sporadic (incidental) and the diffuse (hereditary) ICC hyperplasia reported previously. Based on the presence of interspersed ganglions cells and residual muscle fibers together with the absence of a grossly discernible tumor-ous mass, the term “sporadic diffuse segmental ICC hyperplasia” would thus be most appropriate for our case 2 and for the diffuse microscopic component in case 1.
Although the ICC hyperplasia pattern we described herein may superficially mimic the diffuse hereditary form in patients with germline mutations in c-KIT, PDGFRA and NF-1, the findings in our cases are different in terms of extent (diffuse but segmental) and involvement of the entire thickness of the muscularis propria. ICC hyperplasia in syndromic settings usually presents as a thin layer of spindle cell proliferation along the Auerbach plexus occupying the inter-muscular space between the inner circular and outer longitudinal muscle layer [14,20]. Some of these hereditary ICC hyperplasia lesions may grow further to form discrete GIST nodules (tumorlets) that usually are similar to small incidental and microscopic GISTs in their circumscription and their gradual mergence with the surrounding muscle layer [20]. The detection of a somatic c-KIT mutation in exon 11 in our cases, absence of clinical features of NF-1, the unifocality of the lesions and localization of one lesion in the distal colon are features that together practically ruled out the possibility of NF-1 as an underlying aetiology. GISTs and their precursors in NF-1 patients commonly present as multifocal gross or microscopic lesions with a striking predilection for the small bowel [16]. Most importantly, they lack activating c-KIT and PDGFRA mutations [16,21]. In both our cases, demonstration of a wild-type sequence in normal tissue excluded a germline c-KIT mutation. Of note, sporadic ICC hyperplasia and minute incidental GISTs in the stomach may present as multiple lesions [22,23]. A careful evaluation of the growth pattern and demonstration of distinct heterozygous somatic mutations in the individual lesions as opposed to a wild-type sequence in adjacent normal tissue should facilitate recognition of their sporadic non-hereditary nature [23,24]. On the other hand, rare familial GISTs may present with isolated localized disease in the large bowel suggesting a forme fruste or attenuated inherited disorder thus underscoring the value for analyzing normal tissue to exclude a germline mutation in such cases [25].
Microscopic GISTs as incidental findings in patients without syndromic disorders known to predispose to GIST are surprisingly common in the stomach and distal oesophagus (10–35%) [12,17,18], but they are rare in the large bowel (∼0.2% of routinely processed surgical specimens) [13]. None of these previously reported microscopic GISTs (variably referred to as sporadic ICC hyperplasia, microscopic GISTs and seedling mesenchymal tumors) showed a diffuse pattern as in the current study. Interestingly, most of colorectal microscopic GISTs (5 of 7 cases) in our previous study were associated with diverticular disease of the sigmoid colon. Furthermore, diffuse ICC hyperplasia has been encountered in a variety of gastrointestinal mo-tility disorders including one case coexisting with congenital intestinal neuronal dysplasia [26]. One of our current cases showed a large diverticular-like pattern associated with diffuse microscopic ICC hyperplasia. More recently, Schepers and Vanwyck reported a well documented GIST arising within a true diverticulum of the jejunum in a 56-year old woman, but no diffuse ICC hyperplasia-like growth pattern was described in that case [27]. These observations make it likely that the proliferative dynamics of ICCs might be positively influenced by motility disorders, but this remains currently speculative.
The nomenclature of minute gross and microscopic GIST lesions detected incidentally has been a matter of controversy [28]. These lesions have been given different names including sporadic ICC hyperplasia [12], microscopic GISTs [13,17], seedling mesenchymal tumors [18], tumorlets/tumor in situ [29], incidental GISTs [30] and minimal GISTs [2]. It is conceivable that these lesions form a microscopic-macroscopic-continuum with small GISTs reported as sclerosing stromal tumorlets [31] and incidental GISTs [30]. Accordingly, clear-cut discriminating features between lesion on the microscopic and the macroscopic end of the spectrum of these minute lesions are still lacking [28]. Nevertheless, arbitrary criteria using mainly size of the lesion, detection method and whether the lesion forms a discrete tumorous mass may be helpful for their further classification (Table 1). Generally speaking, the term ICC hyperplasia would best fit microscopic lesions that do not form a grossly recognizable tumor mass irrespective of the clinical setting in which they occur. On the other hand, grossly recognizable small nodules found incidentally at autopsy or during surgery and measuring < 1 cm may best be designated stromal tumorlet [28,31].
Table 1.
Suggested classification for benign microscopic proliferative ICC lesions/ minute incidenal GISTs
| Lesion type/reference | Detection method | Size | Circumscription | Focality | Clinical setting | Common location | Molecular features |
|---|---|---|---|---|---|---|---|
| ICC hyperplasia, localized type [12,13,17,18] | Microscopic, rarely through careful palpation/inspection | ≤5 mm, usually <2 mm | Usually irregular borders, blend with M. propria Rarely diffuse segmental | Usually focal, occasionally multifocal | Sporadic Usually incidental histological findings | Esophagogastric junction (∼10%) Proximal stomach (-35%) Colorectum (0.2%) | Somatic heterozygous mutations in c-KIT (∼25%) |
| ICC hyperplasia, diffuse type [14-16,26,29] | Microscopic, rarely thickening of M. propria | Variable, commonly confluent | Diffuse to nodular, Usually both | Usually multifocal or continuous | Hereditary Rarely congenital with neuronal intestinal dysplasia (very rare Carney triad) | Variable, any part of GI tract. In NF-1 mostly in small bowel | Germline c-KIT/PDGFRA mutations NF-1 (wild-type KIT/PDGFRA) |
| GIST tumorlets [30,31] | Grossly visible or palpable | 5-10 mm | Usually irregular borders, blending with M. propria | ∼10% multifocal | Usually sporadic | Proximal stomach, rarely others | Rarely sporadic c-KIT mutations |
| Incidental benign GIST [2,23,24] | Grossly visible or palpable | >10-20 mm | Well circumscribed but nonencapsulated | Rarely multifocal | Usually sporadic | Variable, usually proximal stomach, occasionally small bowel and others | Commonly somatic heterozygous mutations in c-KIT or PDGFRA (∼90%) |
In summary, we described for the first time a diffuse form of sporadic ICC hyperplasia showing a peculiar diffuse longitudinal microscopic growth completely replacing the muscularis pro-pria, thus superficially mimicking diffuse ICC hyperplasia in hereditary GIST syndromes. Detection of heterozygous somatic c-KIT exon 11 mutations ruled out a hereditary disorder in both cases.
References
- 1.Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era-a population-based study in western Sweden. Cancer. 2005;103:821–9. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 4.Kim TW, Lee H, Kang YK, Choe MS, Ryu MH, Chang HM, Kim JS, Yook JH, Kim BS, Lee JS. Prognostic significance of c-kit mutation in localized gastrointestinal stromal tumors. Clin Cancer Res. 2004;10:3076–81. doi: 10.1158/1078-0432.ccr-03-0581. [DOI] [PubMed] [Google Scholar]
- 5.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA Activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 6.Wardelmann E, Hrychyk A, Merkelbach-Bruse S, Pauls K, Goldstein J, Hohenberger P, Losen I, Manegold C, Büttner R, Pietsch T. Association of platelet-derived growth factor receptor alpha mutations with gastric primary site and epithelioid or mixed cell morphology in gastrointestinal stromal tumors. J Mol Diagn. 2004;6:197–204. doi: 10.1016/s1525-1578(10)60510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agaimy A, Wünsch PH. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 GISTs to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours. Langenbecks Arch Surg. 2006;391:322–29. doi: 10.1007/s00423-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 8.Kosmidis C, Efthimiadis C, Levva S, Anthimidis G, Baka S, Grigoriou M, Tzeveleki I, Masmanidou M, Zaramboukas T, Basdanis G. Synchronous colorectal adenocarcinoma and gastrointestinal stromal tumor in Meckel's diverticulum; an unusual association. World J Surg Oncol. 2009;7:33. doi: 10.1186/1477-7819-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta N, Schirmer BD, Mishra R, Shami VM. Malignant GIST masquerading as a bleeding duodenal diverticulum. Endoscopy. 2007;39(Suppl 1):E142–3. doi: 10.1055/s-2007-966245. [DOI] [PubMed] [Google Scholar]
- 10.Kindblom L-G, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 11.Streutker CJ, Huizinga JD, Driman DK, Riddell RH. Interstitial cells of Cajal in health and disease. Part II: ICC and gastrointestinal stromal tumours. Histopathology. 2007;50:190–202. doi: 10.1111/j.1365-2559.2006.02497.x. [DOI] [PubMed] [Google Scholar]
- 12.Agaimy A, Wünsch PH. Sporadic Cajal cell hyperplasia is common in resection specimens for distal oesophageal carcinoma. A retrospective review of 77 consecutive surgical resection specimens. Virchows Arch. 2006;448:288–294. doi: 10.1007/s00428-005-0117-x. [DOI] [PubMed] [Google Scholar]
- 13.Agaimy A, Wünsch PH, Dirnhofer S, Bihl MP, Terracciano LM, Tornillo L. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens. A clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am J Surg Pathol. 2008;32:867–73. doi: 10.1097/PAS.0b013e31815c0417. [DOI] [PubMed] [Google Scholar]
- 14.Hirota S, Okazaki T, et al. Cause of familial and multiple gastrointestinal autonomic tumor with hyperplasia of interstitial cells of Cajal is germline mutation of the c-kit gene. Am J Surg Pathol. 2002;24:326–327. doi: 10.1097/00000478-200002000-00045. [DOI] [PubMed] [Google Scholar]
- 15.Chompret A, Kannengiesser C, Barrois M, et al. PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor. Gastroenterology. 2004;126:318–321. doi: 10.1053/j.gastro.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 16.Miettinen M, Fetsch JF, Sobin LH, et al. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90–96. doi: 10.1097/01.pas.0000176433.81079.bd. [DOI] [PubMed] [Google Scholar]
- 17.Kawanowa K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, Hosoya Y, Nakajima T, Funata N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527–1535. doi: 10.1016/j.humpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Abraham SC, Krasinskas AM, Hofstetter WL, Swisher SG, Wu TT. “Seedling” mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol. 2007;31:1629–35. doi: 10.1097/PAS.0b013e31806ab2c3. [DOI] [PubMed] [Google Scholar]
- 19.Wardelmann E, Neidt I, Bierhoff E, Speidel N, Manegold C, Fischer HP, Pfeifer U, Pietsch T. c-kit mutations in gastrointestinal stromal tumors occur preferentially in the spindle rather than in the epithelioid cell variant. Mod Pathol. 2002;15:125–36. doi: 10.1038/modpathol.3880504. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Hirota S, Isozaki K, et al. Polyclonal nature of diffuse proliferation of interstitial cells of Cajal in patients with familial and multiple gastrointestinal stromal tumours. Gut. 2002;51:793–6. doi: 10.1136/gut.51.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita K, Hirota S, Isozaki K, Ohashi A, Nishida T, Kitamura Y, Shinomura Y, Matsuzawa Y. Absence of c-kit gene mutations in gastrointestinal stromal tumours from neurofibromatosis type 1 patients. J Pathol. 2004;202:80–5. doi: 10.1002/path.1487. [DOI] [PubMed] [Google Scholar]
- 22.Haller F, Schulten HJ, Armbrust T, Langer C, Gunawan B, Füzesi L. Multicentric sporadic gastrointestinal stromal tumors (GISTs) of the stomach with distinct clonal origdifferential diagnosis to familial and syndromal GIST variants and peritoneal metastasis. Am J Surg Pathol. 2007;31:933–7. doi: 10.1097/01.pas.0000213440.78407.27. [DOI] [PubMed] [Google Scholar]
- 23.Agaimy A, Dirnhofer S, Wünsch PH, Terracciano LM, Tornillo L, Bihl MP. Multiple sporadic gastrointestinal stromal tumors (GISTs) of the proximal stomach are caused by different somatic KIT mutations suggesting a field effect. Am J Surg Pathol. 2008;32:1553–9. doi: 10.1097/PAS.0b013e31817587ea. [DOI] [PubMed] [Google Scholar]
- 24.Agaimy A, Märkl B, Arnholdt H, Wünsch PH, Terracciano LM, Dirnhofer S, Hartmann A, Tornillo L, Bihl MP. Multiple sporadic gastrointestinal stromal tumours arising at different gastrointestinal sites: pattern of involvement of the muscularis propria as a clue to independent primary GISTs. Virchows Arch. 2009;455:101–8. doi: 10.1007/s00428-009-0803-1. [DOI] [PubMed] [Google Scholar]
- 25.Woźniak A, Rutkowski P, Sciot R, Ruka W, Michej W, Debiec-Rychter M. Rectal gastrointestinal stromal tumors associated with a novel germline KIT mutation. Int J Cancer. 2008;122:2160–4. doi: 10.1002/ijc.23338. [DOI] [PubMed] [Google Scholar]
- 26.Jeng YM, Mao TL, Hsu WM, Huang SF, Hsu HC. Congenital interstitial cell of Cajal hyperplasia with neuronal intestinal dysplasia. Am J Surg Pathol. 2000;24:1568–72. doi: 10.1097/00000478-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Schepers S, Vanwyck R. Small bowel gastrointestinal stromal tumor (GIST) arising in a jejunal diverticulum. JBR-BTR. 2009;92:23–4. [PubMed] [Google Scholar]
- 28.Chetty R. Small and microscopically detected gastrointestinal stromal tumours: an overview. Pathology. 2008;40:9–12. doi: 10.1080/00313020701716490. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Atayde AR, Shamberger RC, Kozakewich HW. Neuroectodermal differentiation of the gastrointestinal tumors in the Carney triad. An ultrastructural and immunohistochemical study. Am J Surg Pathol. 1993;17:706–14. doi: 10.1097/00000478-199307000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002;160:1567–72. doi: 10.1016/S0002-9440(10)61103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agaimy A, Wünsch PH, Hofstaedter F, Blaszyk H, Rümmele P, Gaumann A, Dietmaier W, Hartmann A. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113–20. doi: 10.1097/01.pas.0000213307.05811.f0. [DOI] [PubMed] [Google Scholar]