Abstract
Autoimmune pancreatitis (AIP) is a rare form of chronic pancreatitis that is characterized by lymphoplasmacytic infiltrate, storiform fibrosis, obliterative phlebitis, and increased IgG4+ plasma cells. Serum IgG4 levels usually are elevated. Patients with AIP frequently have disease affecting other organs or sites; these tissues show similar histologic changes, including increased IgG4+ plasma cell infiltrate and response to corticosteroid therapy. A new clinicopathologic concept of IgG4-related systemic disease (ISD) has been proposed. These diseases often are not limited to the pancreas, and the pancreas may not be involved at all. In this article, we review the literature and our own experience to detail the clinicopathologic features of AIP and extrapancreatic lesions in ISD.
Keywords: Autoimmune pancreatitis, IgG4, IgG4-related systemic disease
Introduction
Autoimmune pancreatitis (AIP) is a rare form of chronic pancreatitis, first described in 1961 as “primary inflammatory sclerosis of the pancreas” [1]. Subsequent reports have described the disease as lymphoplasmacytic sclerosing pancreatitis, chronic sclerosing pancreatitis, nonalcoholic duct-destructive chronic pancreatitis, and inflammatory pseudotumor [2-5]. The concept of AIP first was proposed by Yoshida et al [6] in 1995. In that report, a patient with chronic pancreatitis also had hyperglobulinemia, was autoantibody-positive, and responded to corticosteroid therapy. The authors suspected that the disease was caused by autoimmune factors. Since then, many studies of this unique type of chronic pancreatitis have shown that autoimmune mechanisms are involved in its pathogenesis.
AIP has become a widely accepted term because clinical, serologic, histologic, and immunohistochemical findings suggest an autoimmune mechanism. AIP is occasionally associated with other autoimmune disorders such as Sjogren syndrome, idiopathic retroperitoneal fibrosis, and inflammatory bowel disease (IBD) [6-9]. Most affected patients have hypergam-maglobulinemia and increased serum levels of IgG, particularly IgG4 [10], [11]. Patients also may have autoantibodies directed against lacto-ferrin, carbonic anhydrase II and IV, rheumatoid factor, smooth muscle antigens, and nuclear antigens [8]. AIP is characterized histologically by a diffuse lymphoplasmacytic infiltration, accompanied by obliterative phlebitis and interstitial fibrosis [12, 13]. Immunohistochemical typing reveals a predominance of CD8+ and CD4+ T lymphocytes, with few B lymphocytes [14]. Importantly, increased IgG4+ plasma cell infiltrate in the pancreas is a very useful marker for the histologic diagnosis of AIP[15-18]. Finally, AIP responds well to corticosteroid therapy [19-21].
Patients with AIP often have diseases affecting other organs or sites. The association of chronic pancreatitis with sclerosing cholangitis and Sjogren syndrome was recognized as early as 1984 [22]. Nearly 20 years later, the concept of a systemic IgG4 disease was introduced by Kamisawa et al [23], who showed that patients with AIP had extensive IgG4+ plasma cell infiltrate in other organs, including peripancreatic tissue, bile duct, gallbladder, portal area of the liver, gastric mucosa, colonic mucosa, salivary glands, lymph nodes, and bone marrow. They proposed the term “IgG4-related systemic disease” (ISD) to describe this condition. Their observations were confirmed by several subsequent studies [16, 18, 24-26]. ISD is defined as a syndrome characterized by elevated serum IgG4 levels, prominent lymphoplasmacytic infiltrates with increased IgG4+ plasma cells, and dense sclerosis. The fibrosis associated with ISD may damage and even partially destroy an affected organ, but the inflammatory process typically responds to corticosteroid therapy [27]. Although the pancreas is the most commonly affected organ, the presence of AIP is not essential in this systemic disease. In the series by Kamisawa et al [28], 2 patients had AIP develop only during follow-up of sclerosingsialadenitis.
Extra pancreatic presentations can include scle-rosing cholangitis, retroperitoneal fibrosis, scle-rosing sialadenitis (Küttner tumor), lymphadenopathy, nephritis, and interstitial pneumonia. Increased IgG4+ plasma cell infiltrate has been reported in sclerosing lesions from other organ sites, including inflammatory pseudotumors of liver, breast, mediastinum, orbit, and aorta, and it has been observed with hypophysitis and IgG4 -associated prostatitis [29-36]. Furthermore, we have observed abundant IgG4+ plasma cells in Riedel thyroiditis, sclerosing mesenteritis, and inflammatory pseudotumor of the orbit and stomach. In this review, we describe the clinical and histologic presentations of AIP, its associated extra pancreatic manifestations, and other related entities.
Autoimmune Pancreatitis
AIP is a rare disorder with characteristic clinical, histologic, and morphologic findings [21, 27]. Most of the literature about AIP comes from Japan, where the incidence appears to be increasing, perhaps because of increased recognition of the disease [37]. However, AIP has been described in several countries in Europe, as well as in the United States and Korea, which suggests that it is a worldwide entity [38]. Clinically, patients can present with abdominal pain, weight loss, and jaundice, and liver function tests will show an obstructive pattern. Imaging usually shows diffuse enlargement of the pancreas, but tumor-like local swelling can occur. The pancreatic duct is diffusely or segmentally narrowed. Such presentations of AIP mimic pancreatic cancer. Until recently, almost all AIP was diagnosed in patients undergoing pancreati-coduodenectomy for presumed pancreatic cancer[39, 40]. Despite growing awareness of the condition, differentiating between AIP and pancreatic cancer remains challenging, particularly for patients with radiologic evidence of a tume-factive lesion. Because the condition responds so well to corticosteroid treatment, the correct preoperative diagnosis is highly desirable. Recently, Mayo Clinic introduced criteria for diagnosing AIP; summarized by the mnemonic HI-SORt, these criteria include 5 cardinal features of AIP in histology, imaging, serology, other organ involvement, and response to corticosteroid therapy [41].
A possible marker for AIP is elevated serum levels of IgG4. A hallmark study by Hamano et al [10] reported that serum IgG4 levels were highly sensitive (95%) and highly specific (97%) for AIP. However, elevated IgG4 levels have been observed in patients with atopic dermatitis, asthma, some parasitic diseases, pemphigus vulgaris, and pemphigus foliaceus [42-45], which suggests that it is not entirely AIP-specific. In a recent large cohort study, Ghazale et al [46] showed that elevated serum IgG4 levels were a characteristic but not diagnostic feature of AIP. Elevated IgG4 levels were observed in 3% to 10% of patients without AIP, including those with primary sclerosing cholangitis (PSC), pancreatic cancer, and acute and chronic pancreatitis, as well as patients without any pancreatic disease. Thus, elevated serum IgG4 levels alone are not sufficient to make the diagnosis of AIP.
Grossly, AIP may diffusely involve the entire pancreas, or it may focally affect the pancreatic head and mimic pancreatic cancer both clinically and radiologically [6, 47, 48]. Because of dense fibrosis, the pancreas is firm upon gross inspection. A distinct mass or nodule usually is not present, even though a tumescent mass may be suggested by imaging studies. The pancreatic parenchyma is fibrotic, and the lobulated architecture can be partially destroyed by fibrosis [2]. Calcification is an uncommon finding. The pancreatic duct may be diffusely or segmentally narrowed. The common bile duct often is involved and can have a thickened wall, narrowing, and dilation at the proximal part.
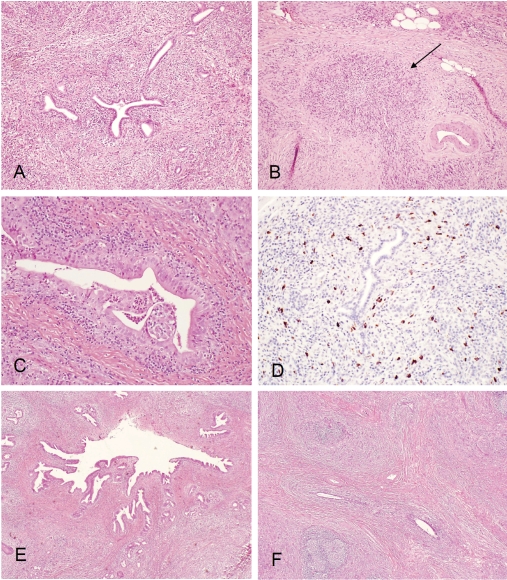
The typical histologic features of AIP are as follows: dense fibrosis with a focal storiform-like pattern that is intermixed with inflammatory cells and diffuse, lymphoplasmacytic infiltration centered on pancreatic ducts, accompanied by obliterative phlebitis and acinar atrophy (Figure 1A) . The obliterative phlebitis (Figure 1B) is a very helpful feature when making a diagnosis of AIP, but it is not pathognomonic. Lymphoid aggregates can be identified in most cases in intra pancreatic and extra pancreatic tissue.
Figure 1.
(A), Type 1 autoimmune pancreatitis (lymphoplasmacytic sclerosing pancreatitis): low power view showing periductal lymphoplasmacytic infiltrate and storiform fibrosis with inflammatory cellular stroma. (B), Obliterative phlebitis (arrow): dense peri- and intra-venular inflammatory infiltrate with fibrosis destroying the endothelium and obliterating the lumen. (C), Granulocyte epithelial lesion (GEL) in type 2 autoimmune pancreatitis (idiopathic duct centric pancreatitis): periductal lymphoplasmacytic and neutrophilic infiltrate with intra-epithelium and intra-lumen neutro-philic infiltrate; destruction of small ducts and ductal epithelium, lobular lymphoplasmacytic and neutrophilic infiltrate. (D), IgG4 immunostain: markedly increased (>30/high power filed) periductal IgG4+ plasma cell infiltrate. (E), IgG4 associated cholangitis: low power view showing periductal lymphoplasmacytic infiltrate and storiform fibrosis. (F), Chronic sclerosingsialadenitis (Küttner tumor) showing dense lymphoplasmacytic infiltrate and storiform fibrosis destroying glandular structures.
Two histologic subgroups of AIP have been recognized recently; they are designated as lymphoplasmacytic sclerosing pancreatitis (type 1) and idiopathic duct-destructive pancreatitis (type 2) [12, 49]. Type 1 AIP usually has the histologic features described above. Although the 2 groups have some histologic overlap, type 2 AIP is characterized by granulocytic epithelial lesions, which show neutrophils in the duct epithelium or duct epithelial damage in the lumen (or both) (Figure 1C) [13]. It is still unclear whether the 2 subgroups represent different diseases or different stages of the same disease. A recent, large, cohort study [50] showed that type 1 and type 2 AIP have distinct clinical profiles. Patients with type 1 AIP were older than those with type 2 AIP (mean [SD] age, 62 [14] vs 48 [19] years) and had a greater prevalence of increased serum levels of IgG4 (47/59 [80%] vs 1/6 [17%] patients). Patients with type 1 AIP were more likely to have proximal biliary, retroperitoneal, renal, or salivary disease (60% vs 0%) and also were more likely to have relapse (47% vs 0%). After a median follow-up of 58 months (for type 1 patients) and 89 months (for type 2 patients), the 5-year survival rates for both groups were similar to those of an age- and sex-matched US population.
The histologic diagnosis of AIP can be difficult, especially if the tissue sample is small (eg, from a core needle biopsy), and also because of patchy distribution of the disease [51]. After the report of elevated serum IgG4 levels in AIP by Hamano et al [10], numerous studies have evaluated the contribution of IgG4 immunohistochemical staining when diagnosing AIP [16-18, 23, 25]. Although all studies indicated that increased IgG4+ plasma cell infiltrate in the pancreas was a helpful marker for AIP, like elevated serum IgG4 levels, it is not entirely specific for AIP. In our study [17], moderately increased IgG4+ plasma cell infiltrate in the pancreas (>10 cells per high-powered field) could be seen in 72% of AIP (Figure 1D), but it also was present in 11% of patients with alcoholic chronic pancreatitis and 12% of patients with pancreatic adenocarcinoma. Findings of IgG4 immunostaining should be interpreted cautiously when the tissue sample is small and IgG4+ plasma cell infiltrates are limited (ie, 5-10 cells per high-powered field). Because AIP is considered a systemic disease and high levels of IgG4 staining have been found in other organs of patients with AIP, some authors suggested that positive IgG4 staining in extra pancreatic tissue may confirm a definitive diagnosis of AIP for patients those with clinical evidence of pancreatic disease, thereby eliminating the need for pancreatic biopsy or surgical exploration [16, 18, 23, 52]. However, in our experience, IgG4 immunostaining on duodenal mucosa biopsy specimens from patients with known AIP did not show more IgG4+ cells than control patients with normal mucosa or with giardiasis, celiac disease, peptic duodenitis, or adenoma. The diagnostic value of IgG4 immunostaining in extrapancreatic tissue for AIP is still undetermined.
Extra pancreatic Organ Involvement in ISD
Bile Duct
The biliary tract is the most commonly involved extra pancreatic site in ISD with AIP. Several case series have described the association between AIP and biliary strictures. The overall rate of extrahepatic bile duct involvement in AIP is 71% to 100% [23, 25, 28, 46, 53] [54]. In a recent review, we introduced the term “IgG4-associated cholangitis” (IAC) to describe biliary manifestations of ISD [55]. The involvement of the biliary tree in AIP usually is determined radiographically by bile duct wall thickening and biliary stricture. Although serum IgG4 elevation is characteristic of IAC, it is not a pathognomonic finding. The sensitivity of serum IgG4 for IAC in our study was 74%, similar to that seen in AIP [54]. The specificity and positive predictive value of elevated serum IgG4 levels for IAC is unknown.
Histologically, the bile duct wall is characterized by diffuse lymphoplasmacytic infiltration, marked interstitial fibrosis with focal storiform-like pattern, and occasional obliterative phlebitis (Figure 1E). The biliary epithelium is usually spared of injury. The features are similar to changes in the pancreas. Immunohistochemically, moderate infiltration of IgG4+ plasma cells was observed in 88% of patients in our series [54].
Like AIP, other organ involvement is an important clue in the diagnosis of IAC. The presence of unexplained pancreatic disease in patients with biliary strictures should raise clinical suspicion for IAC. Although AIP is present in most patients with IAC, we recently identified 7 patients with IAC who had no obvious pancreatic disease (according to clinical and imaging criteria) and did not have elevated serum IgG4 levels. All underwent surgical exploration because of a high suspicion of extra hepatic cholangiocarcinoma. The resected specimen had typical histologic features of IAC, as described above, and all showed marked periductal IgG4+ plasma cell infiltrate. Therefore the absence of AIP and normal serum IgG4 levels should not exclude IAC from the differential diagnosis. Like AIP, biliary strictures in IAC respond to corticosteroids. In our study [54], complete resolution of strictures or normalization of liver tests (or both) were observed in approximately two-thirds of patients, and improvement was noted for the remaining one-third.
IAC can be confused with PSC, especially because of overlapping radiologic findings, but only the former responds well to corticosteroid therapy. Clinically, IAC usually occurs abruptly, with obstructive jaundice, compared with PSC; the PSC diagnosis often is made in asymptomatic patients after liver test abnormalities are identified [56]. Radiologic findings of IAC usually include segmental strictures, dilation after confluent stricture, and strictures of the lower common bile duct. IgG4 immunostaining shows that the degree of infiltration of IgG4+ plasma cells around the bile duct in the portal areas and the extrahepatic bile duct is markedly lower with PSC than with AIP-associated cholangitis [57]. We recently found that nearly one quarter of explanted livers that carry a clinical diagnosis of PSC contain increased IgG4+ periductal plasma cell infiltrates and positive serum IgG4 levels. However, none of the explants show histologic features diagnostic of IAC. PSC with tissue IgG4 positivity has a more aggressive clinical course manifested by shorter time to transplant and a higher likelihood of recurrence than IgG4 negative PSC [58]. However, most PSC patients with elevated IgG4 had a good biochemical response to steroids [59].
Liver
Liver dysfunction frequently is observed in patients with AIP. It can be due either to extrahepatic obstructive lesions or to inflammatory liver injury. Hirano et al [60] reported lymphoplasmacytic infiltration in the portal area for 7 of 7 liver biopsy samples in their study. Another recent study [61] proposed the term “IgG4 -hepatopathy” and described 5 histologic liver patterns in AIP: 1) evident portal inflammation with or without interface hepatitis, 2) large bile-duct obstructive features, 3) portal sclerosis, 4) lobular hepatitis, and 5) canalicular cholestasis. Some of these histologic features coexisted in the same liver specimen. The number of IgG4+ plasma cells was significantly higher in patients with AIP than in control patients with autoimmune hepatitis, primary biliary cirrhosis, PSC, or chronic viral hepatitis [62] and was significantly correlated with serum IgG4 concentration. Corticosteroid therapy reduces IgG4+ plasma cell infiltration in the liver and ameliorates other histologic findings. Interestingly, a recent case report [63] described a patient with severe hepatitis, elevated serum IgG4 levels, and IgG4+ plasma cell infiltrates in the liver, but the patient had no evidence of pancreatic disease.
Another aspect of liver involvement in ISD is hepatic inflammatory pseudotumor 32, 62. The recent study by Zen et al [64] defined 2 types of hepatic inflammatory pseudotumors, fibrohistiocytic and lymphoplasmacytic. In their description, “histiocytic inflammatory pseudotumors were characterized by xanthogranulomatous inflammation, multinucleated giant cells, and neutrophilic infiltration, [which] mostly occurred in the peripheral hepatic parenchyma as mass-forming lesions. In contrast, lymphoplasmacytic inflammatory pseudotumors showed diffuse lymphoplasmacytic infiltration and prominent eosinophilic infiltration, and were all found around the hepatic hilum. In addition, venous occlusion with little inflammation and cholangitis without periductal fibrosis were frequently observed in the fibrohistiocytic type, whereas obliterative phlebitis and cholangitis with periductal fibrosis were common features of the lymphoplasmacytic type. IgG4-positive plasma cells were significantly more numerous in the lymphoplasmacytic than fibrohistiocytic type” [64]. The authors concluded that the lymphoplasmacytic type has histologic features similar to AIP and could belongto ISD.
Gallbladder
Gallbladders frequently are affected in AIP; they are characterized by a diffuse, acalculous, lymphoplasmacytic cholecystitis [65-67]. Although a pattern of diffuse, lymphoplasmacytic, chronic cholecystitis is highly specific for extrahepatic biliary tract disease, it does not distinguish between primary and secondary cholangiopathies such as PSC, malignancy-associated obstructive jaundice, or cholelithiasis [68]. Lymphoplasmacytic cholecystitis associated with AIP usually shows deep mural and extramural inflammation. Phlebitis and inflammatory nodules were more frequently noted in patients with AIP. AIP gallbladders show increased IgG4+ plasma cell and higher IgG4+/IgG+ plasma cell ratios than gallbladders from patients with pancreatic carcinoma or PSC, and IgG4 immunostaining may be a useful marker for AIP-associated cholecystitis [65,67].
Gastrointestinal Tract
Scattered IgG4+ plasma cells usually are present in normal gastrointestinal mucosa. In the initial report describing ISD[23], increased IgG4+ plasma cells were observed in the stomach and colon. Deheragoda etal [18] reported a marked increase in IgG4+ plasma cells in gastrointestinal mucosa in AIP and suggested that immunostaining of involved tissue for IgG4 may be useful when AIP is suspected clinically. Another study suggested that IgG4-immunostaining of biopsy specimens from the major duodenal papilla may support the diagnosis of AIP [52]. However, the small number of patients in these studies limits the genera l izability of these findings. In our experience, IgG4 immunostaining of duodenal mucosa biopsy specimens did not show more IgG4+ cells in patients with AIP compared with controls.
IBD occasionally is associated with AIP [9, 13]. Zamboni et al [13] showed a high prevalence of IBD with AIP and convincingly showed that idiopathic duct-destructive pancreatitis and granulocytic epithelial lesions were more frequently associated with IBD. The clinical significance of this finding is unknown.
Salivary and Lacrimal Glands
Salivary and lacrimal glands are frequently involved in ISD [13] [23, 25, 69, 70]. Küttner tumor is a chronic, sclerosing sialadenitis that presents with asymmetric, firm swelling of the submandibular glands. Kitagawa et al [69] reported a series of 12 patients with Küttner tumors and reported that 5 had sclerosing lesions in extrasalivary glandular tissues. Geyer et al [71] reported 13 cases recently, of which 3 presented with ISD. Histologically, the salivary glands showed marked lymphoplasmacytic infiltration with fibrosis and the destruction of glandular lobules (Figure 1F). Obliterative phlebitis often was observed. Immunohistochemically, the proportion of IgG4+/IgG+ plasma cells was more than 45% in patients with Küttner tumor, whereas it was less than 5% for control patients with sialolithiasis or Sjögren syndrome. The similarity in clinicopathologic features between Küttner tumor and AIP suggests the same IgG4-related disease origin. Thus, IgG4immunostaining may be useful for distinguishing between chronic, sclerosing sialadenitis and other forms of sialadenitis.
Mikulicz disease is an idiopathic, bilateral, painless, and symmetric swelling of the lacrimal, parotid, and submandibular glands. Microscopically, tissues show marked lymphoplasmacytic infiltration, with lymphoid follicles surrounding solid epithelial nests (epimyoepithelial islands), stromal fibrosis, acinar atrophy and destruction, and lymphoepithelial lesions. Because Mikulicz disease and Sjögren syndrome are histologically similar, Mikulicz disease has been considered a subtype of Sjögren syndrome, even though the 2 diseases have some clinical differences. Patients with Mikulicz disease have elevated serum IgG4 concentrations and infiltration of IgG4+ plasma cells into the lacrimal and salivary glands [70, 72-74]. Thus, Mikulicz disease is now considered as an ISD.
Sjögren syndrome occasionally is observed in patients with AIP [7, 22, 75, 76]. Although Sjögren syndrome has some histologic overlap with AIP, a few studies showed that the submandibular gland of patients with AIP differ from those with typical Sjögren syndrome. Serum IgG4 levels in Sjögren syndrome are significantly lower than those in patients with AIP [10, 74]. Rare IgG4+ plasma cells are seen in Sjögren syndrome [69, 77], which suggests that IgG4 may not have an important role in the cause of Sjögren syndrome.
Kidney
Renal lesions in ISD usually present as tubulointerstitial nephritis. Clinically, patients can present with renal insufficiency, vasculitis, or a “renal mass"; these symptoms often are associated with AIP [78-84]. Histologically, renal lesions are characterized by a densely patchy or diffuse tubulointerstitial lymphoplasmacytic infiltrate. Numerous eosinophils are often seen. Tubulitis and tubular injury are present, along with tubular atrophy and focally thickened tubular basement membranes. Glomeruli usually are uninvolved [82, 83]. Immunohistochemistry shows a marked increased IgG4+ plasma cell infiltrate [80-83]. One study also showed IgG4 immune-complex deposits in the tubular basement membranes, as evidenced by immunofluo-rescence or immunohistochemistry and by electron microscopy [83]. Like AIP, tubulointerstitial nephritis associated with AIP usually responds favorably with corticosteroid therapy [84].
Retroperitoneum and Mesentery
Retroperitoneal fibrosis is a disease characterized by the proliferation of fibrous tissue in the retroperitoneum (specifically, marked lymphoplasmacytic infiltrations encompassed by a dense fibrosis). The lesions show active and chronic inflammatory infiltration and sclerosis, and lymphoid follicles with germinal centers are also present. Most infiltrating IgG+ plasma cells are IgG4+ [85].No causative factor is identified for most patients, although autoimmune mechanisms have been suggested. Some cases of retroperitoneal fibrosis associated with AIP have been reported [9, 85-88] and were resolved by corticosteroid therapy. Serum IgG4 levels were elevated for most patients.
Sclerosing mesenteritis is a rare fibroinflammatory disorder of unknown cause; it primarily affects the small bowel mesentery. Histologically, the most frequent finding is prominent fibrosis with scant inflammation and some fat necrosis. Involvement of the pancreas in patients with sclerosing mesenteritis has been reported previously [89, 90]. One study showed that 33% of patients with sclerosing mesenteritis have abundant IgG4+ plasma cell infiltrates in the tissue [91]. The authors speculated that IgG4-related immunopathologic processes also might be involved in the pathogenesis of some cases of sclerosing mesenteritis.
Thyroid
About a quarter of patients with AIP have clinically significant hypothyroidism [25, 92]. Patients with hypothyroidism more commonly have antithyroglobulin antibodies than those without hypothyroidism, but other laboratory test findings are similar, including serum IgG4 concentration. No histologic findings or tissue samples showing IgG4+ plasma cell infiltrate were available. The authors suggested evaluating thyroid function in patients with AIP. [92]
Riedel thyroiditis is an uncommon form of chronic thyroiditis, in which the thyroid gland is replaced by fibrous tissue. Although the cause of Riedel thyroiditis is unclear, it is considered part of a systemic fibroinflammatory process also involving other organs; it may be associated with retroperitoneal fibrosis, sclerosing mediastinitis, sclerosing cholangitis, or orbital pseudotumors [93, 94]. We have observed numerous IgG4+ plasma cells in Riedel thyroiditis. Like inflammatory pseudotumors in other organs, Riedel thyroiditis likely also is an IgG4-related systemic fibroinflammatory process.
Breast
Inflammatory pseudotumor of the breast is an extremely rare condition. A case of IgG4-related inflammatory pseudotumor of the breast has been reported [36]. This patient presented with an induration in the left breast, and the lesion had histologic features similar to those of AIP. Furthermore, the patient also had elevated serum IgG4 levels and many IgG4+ plasma cells within the lesion. This case may be a manifestation of ISD in breast.
Lung
Pulmonary involvement in ISD can present as interstitial pneumonia or as an inflammatory pseudotumor. Hamed et al [95] described a patient with a lung nodule that mimicked lung cancer clinically. The patient also had elevated serum IgG4 levels and inflammatory lesions in his prostate, submandibular glands, and bile ducts. The biopsy of the lung nodule showed many IgG4+ plasma cells. A final diagnosis of ISD was made and the patient was treated with corticosteroids.
Two patients with AIP-associated interstitial pneumonia have been reported. Taniguchi etal [96] described a patient with bilateral, interstitial pneumonia of the lower lung fields during follow-up for AIP. A transbronchial lung biopsy showed marked thickening of the alveolar septum, with considerable lymphoplasmacytic infiltrate and increased IgG4+ cells. Nieminen et al [97] also reported a patient with idiopathic pancreatitis, sclerosing cholangitis, sialadenitis, and nodular interstitial pneumonia. In both cases, patients responded to corticosteroid therapy.
IgG4-related immunopathologic processes might be involved in the pathogenesis of the pulmonary lesions. Zen et al [98] reported 9 patients with pulmonary inflammatory pseudotumors; the pseudotumors had histologic features similar to those of AIP, with dense lymphoplasmacytic infiltrates intermixed with fibrosis and, in some cases, prominent eosinophilic infiltration, irregular narrowing of bronchioles entrapped in nodules, and an interstitial pneumonia pattern at the boundaries of nodules. Obliterative phlebitis was present in all cases, and 5 lesions also had obliterative arteritis. Shrestha et al [99] described lung biopsies of 6 patients with AIP; specimens showed endothelialitis of pulmonary vessels, active fibrosis, lymphangitic inflammatory infiltrates rich in plasma cells and histiocytes (with or without nodule formation), and fibrinous pleuritis. Immunostaining showed many IgG4+ plasma cells diffusely distributed within nodules.
Lymph Node
Concomitant lymphadenopathy is common in ISD [100-102]. A recent study [103] detailed morphologic features of the lymph nodes in ISD. The authors categorized these features into 3 patterns: 1) Castleman disease-like features, 2) follicular hyperplasia, and 3) interfollicular expansion by immunoblasts and plasma cells. However, lymph nodes often lacked typical storiform fibrosis or phlebitis. When compared with 54 control lymph nodes from patients with various reactive conditions, the percentage of IgG4+/IgG+ plasma cells in patients with AIP and lymphadenopathy was markedly elevated (mean, 62% vs. 9.9%). Most patients responded to corticosteroid therapy. The clinical significance of lymph node involvement in ISD remains uncertain.
Summary
Since the term “autoimmune pancreatitis” was first introduced by Yoshida et al [6] in 1995, tremendous clinical, serologic, radiologic, and pathologic studies to characterize this relatively new entity. To facilitate clinical management of AIP, diagnostic criteria have been established, and serologic markers and pathologic features have been identified. Cumulatively, the evidence suggests that AIP is a part of a new clinicopathologic entity of IgG4-related autoimmune diseases. The extra pancreatic lesions typically have pathologic features similar to those of AIP; they are characterized by lymphoplasmacytic infiltrate with dense fibrosis, obliterative phlebitis, and increased IgG4+ plasma cells. Serum IgG4 levels are often elevated, but this is not a disease-specific finding. Not all ISD patients have AIP, although the pancreas is the most commonly involved organ.
Importantly, AIP is frequently associated with various extra pancreatic lesions. The involvement of other organs has been widely reported (Table 1), and the prevalence and distribution of extra pancreatic lesions has been proposed in a recent study [25]. ISD can affect only 1 organ (usually presenting as an inflammatory pseudotumor), or it can affect 2 to 4 organs. It is particularly important for surgical pathologists to be aware of this disease and to make the correct diagnosis; treatment with corticosteroids will result in rapid and sustained resolution without unnecessary surgical procedures.
Table 1.
Organs affected by IgG4-related systemic disease
| Organ or Site | Clinicopathologic Features | References |
|---|---|---|
| Pancreas | Lymphoplasmacytic sclerosing pancreatitis (type 1 autoimmune pancreatitis) Idiopathic, duct-centric, chronic pancreatitis or granular epithelial lesion (type 2 autoimmune pancreatitis) | 10, 12, 13,16, 17, 21,27, 38, 41, 49, 50 |
| Bile duct | Sclerosing cholangitis or IgG4-associated cholangitis Inflammatory pseudotumor | 46, 53, 54, 55, 56 |
| Liver | Sclerosing cholangitis involving intrahepatic ducts Portal inflammation, with or without interface hepatitis Large bile-duct obstruction Portal sclerosis Lobular hepatitis Canalicular cholestasis Inflammatory pseudotumor | 35, 60-64 |
| Gallbladder | Diffuse, acalculous, lymphoplasmacytic cholecystitis | 65-68 |
| Gastrointestinal tract | Increased IgG4-positive cells in mucosa Inflammatory bowel disease | 13, 18, 23, 52 |
| Salivary and lacrimal glands | Küttner tumor (chronic sclerosing sialadenitis) Mikulicz disease Chronic, sclerosing dacryoadenitis | 69-74 |
| Kidney | Tubulointerstitial nephritis Membranous glomerulopathy, with IgG4 immune complex deposits in tubular basement membrane | 78-84 |
| Retroperitoneum and mesentery | Retroperitoneal fibrosis Sclerosing mesenteritis | 85-91 |
| Thyroid | HypothyroidismRiedel thyroiditis | 92-94 |
| Breast | Inflammatory pseudotumor | 36 |
| Lung | Interstitial pneumonia Inflammatory pseudotumor | 97-101 |
| Aorta | Inflammatory abdominal aortic aneurysm | 31, 32 |
| Orbit | Inflammatory pseudotumor | 30 |
| Mediastinum | Sclerosing mediastinitis | 29 |
| Pituitary gland | Hypophysitis Inflammatory pseudotumor | 33 |
| Prostate | IgG4-associated prostatitis | 34 |
| Lymph nodes | Castleman disease-like lymphadenopathy Lymphadenopathy with follicular hyperplasia Lymphadenopathy with interfollicular expansion by immunoblasts and plasma cells | 100-103 |
References
- 1.Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreasan autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–698. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- 2.Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387–395. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- 3.Sood S, Fossard DP, Shorrock K. Chronic sclerosing pancreatitis in Sjogren's syndrome: a case report. Pancreas. 1995;10:419–421. doi: 10.1097/00006676-199505000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, Lankisch P, Stolte M, Luttges J, Kremer B, Kloppel G. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41:263–268. doi: 10.1136/gut.41.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wreesmann V, van Eijck CH, Naus DC, van Velthuysen ML, Jeekel J, Mooi WJ. Inflammatory pseudotumour (inflammatory myofibroblastic tumour) of the pancreas: a report of six cases associated with obliterative phlebitis. Histopathology. 2001;38:105–110. doi: 10.1046/j.1365-2559.2001.01056.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 7.Kino-Ohsaki J, Nishimori I, Morita M, Okazaki K, Yamamoto Y, Onishi S, Hollingsworth MA. Serum antibodies to carbonic anhydrase I and II in patients with idiopathic chronic pancreatitis and Sjogren's syndrome. Gastroenterology. 1996;110:1579–1586. doi: 10.1053/gast.1996.v110.pm8613065. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki K, Uchida K, Ohana M, Nakase H, Uose S, Inai M, Matsushima Y, Katamura K, Ohmori K, Chiba T. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573–581. doi: 10.1016/s0016-5085(00)70264-2. [DOI] [PubMed] [Google Scholar]
- 9.Fukukura Y, Fujiyoshi F, Nakamura F, Hamada H, Nakajo M. Autoimmune pancreatitis associated with idiopathic retroperitoneal fibrosis. AJR Am J Roentgenol. 2003;181:993–995. doi: 10.2214/ajr.181.4.1810993. [DOI] [PubMed] [Google Scholar]
- 10.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 11.Hirano K, Kawabe T, Yamamoto N, Nakai Y, Sasahira N, Tsujino T, Toda N, Isayama H, Tada M, Omata M. Serum IgG4 concentrations in pancreatic and biliary diseases. Clin Chim Acta. 2006;367:181–184. doi: 10.1016/j.cca.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinico-pathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119–1127. doi: 10.1097/00000478-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Zamboni G, Luttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Kloppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–563. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 14.Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, Egawa N, Nakajima H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683–687. doi: 10.1136/gut.52.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamisawa T, Okamoto A, Funata N. Clinicopathological features of autoimmune pancreatitis in relation to elevation of serum IgG4. Pancreas. 2005;31:28–31. doi: 10.1097/01.mpa.0000167000.11889.3a. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande V, Chicano S, Finkelberg D, Selig MK, Mino-Kenudson M, Brugge WR, Colvin RB, Lauwers GY. Autoimmune pancreatitis: a systemic immune complex mediated disease. Am J Surg Pathol. 2006;30:1537–1545. doi: 10.1097/01.pas.0000213331.09864.2c. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Notohara K, Levy MJ, Chari ST, Smyrk TC. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2007;20:23–28. doi: 10.1038/modpathol.3800689. [DOI] [PubMed] [Google Scholar]
- 18.Deheragoda MG, Church NI, Rodriguez-Justo M, Munson P, Sandanayake N, Seward EW, Miller K, Novelli M, Hatfield AR, Pereira SP, Webster GJ. The use of immunoglobulin g4 immunostaining in diagnosing pancreatic and extrapancreatic involvement in autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1229–1234. doi: 10.1016/j.cgh.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Nakano I, Koyanagi S, Miyahara T, Migita Y, Ogoshi K, Sakai H, Matsunaga S, Yasuda O, Sumii T, Nawata H. Autoimmune pancreatitis as a new clinical entity. Three cases of autoimmune pancreatitis with effective steroid therapy. Dig Dis Sci. 1997;42:1458–1468. doi: 10.1023/a:1018862626221. [DOI] [PubMed] [Google Scholar]
- 20.Kamisawa T, Yoshiike M, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Treating patients with autoimmune pancreatitis: results from a long-term follow-up study. Pancreatology. 2005;5:234–238. doi: 10.1159/000085277. discussion 238-240. [DOI] [PubMed] [Google Scholar]
- 21.Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670–2676. doi: 10.1056/NEJMra061200. [DOI] [PubMed] [Google Scholar]
- 22.Montefusco PP, Geiss AC, Bronzo RL, Randall S, Kahn E, McKinley MJ. Sclerosing cholangitis, chronic pancreatitis, and Sjogren's syndrome: a syndrome complex. Am J Surg. 1984;147:822–826. doi: 10.1016/0002-9610(84)90212-5. [DOI] [PubMed] [Google Scholar]
- 23.Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 24.Ohara H, Nakazawa T, Sano H, Ando T, Okamoto T, Takada H, Hayashi K, Kitajima Y, Nakao H, Joh T. Systemic extrapancreatic lesions associated with autoimmune pancreatitis. Pancreas. 2005;31:232–237. doi: 10.1097/01.mpa.0000175178.85786.1d. [DOI] [PubMed] [Google Scholar]
- 25.Hamano H, Arakura N, Muraki T, Ozaki Y, Kiyosawa K, Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006;41:1197–1205. doi: 10.1007/s00535-006-1908-9. [DOI] [PubMed] [Google Scholar]
- 26.Neild GH, Rodriguez-Justo M, Wall C, Connolly JO. Hyper-IgG4 disease: report and characterisation of a new disease. BMC Med. 2006;4:23. doi: 10.1186/1741-7015-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS, Farnell MB. Diagnosis of Autoimmune Pancreatitis: The Mayo Clinic Experience. Clin Gastroenterol Hepatol. 2006;4:1010–1016. doi: 10.1016/j.cgh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, Okamoto A. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology. 2006;6:132–137. doi: 10.1159/000090033. [DOI] [PubMed] [Google Scholar]
- 29.Inoue M, Nose N, Nishikawa H, Takahashi M, Zen Y, Kawaguchi M. Successful treatment of sclerosing mediastinitis with a high serum IgG4 level. Gen Thorac Cardiovasc Surg. 2007;55:431–433. doi: 10.1007/s11748-007-0154-2. [DOI] [PubMed] [Google Scholar]
- 30.Mehta M, Jakobiec F, Fay A. Idiopathic fibro-inflammatory disease of the face, eyelids, and periorbital membrane with immunoglobulin G4-positive plasma cells. Arch Pathol Lab Med. 2009;133:1251–1255. doi: 10.5858/133.8.1251. [DOI] [PubMed] [Google Scholar]
- 31.Sakata N, Tashiro T, Uesugi N, Kawara T, Furuya K, Hirata Y, Iwasaki H, Kojima M. IgG4-positive plasma cells in inflammatory abdominal aortic aneurysm: the possibility of an aortic manifestation of IgG4-related sclerosing disease. Am J Surg Pathol. 2008;32:553–559. doi: 10.1097/PAS.0b013e31815a04db. [DOI] [PubMed] [Google Scholar]
- 32.Stone JH, Khosroshahi A, Deshpande V, Stone JR. IgG4-related systemic disease accounts for a significant proportion of thoracic lymphoplasmacytic aortitis cases. Arthritis Care Res (Hoboken) 2010;62:316–322. doi: 10.1002/acr.20095. [DOI] [PubMed] [Google Scholar]
- 33.Wong S, Lam WY, Wong WK, Lee KC. Hypophysitis presented as inflammatory pseudotumor in immunoglobulin G4-related systemic disease. Hum Pathol. 2007;38:1720–1723. doi: 10.1016/j.humpath.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimura Y, Takeda S, Ieki Y, Takazakura E, Koizumi H, Takagawa K. IgG4-associated prostatitis complicating autoimmune pancreatitis. Intern Med. 2006;45:897–901. doi: 10.2169/internalmedicine.45.1752. [DOI] [PubMed] [Google Scholar]
- 35.Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193–1203. doi: 10.1097/01.pas.0000136449.37936.6c. [DOI] [PubMed] [Google Scholar]
- 36.Zen Y, Kasahara Y, Horita K, Miyayama S, Miura S, Kitagawa S, Nakanuma Y. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 level: histologic similarity to sclerosing pancreatitis. Am J Surg Pathol. 2005;29:275–278. doi: 10.1097/01.pas.0000147399.10639.f5. [DOI] [PubMed] [Google Scholar]
- 37.Okazaki K. Autoimmune pancreatitis is increasing in Japan. Gastroenterology. 2003;125:1557–1558. doi: 10.1016/j.gastro.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Kim KP, Kim MH, Lee SS, Seo DW, Lee SK. Autoimmune pancreatitis: it may be a worldwide entity. Gastroenterology. 2004;126:1214. doi: 10.1053/j.gastro.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 39.Hardacre JM, Iacobuzio-Donahue CA, Sohn TA, Abraham SC, Yeo CJ, Lillemoe KD, Choti MA, Campbell KA, Schulick RD, Hruban RH, Cameron JL, Leach SD. Results of pancreati-coduodenectomy for lymphoplasmacytic sclerosing pancreatitis. Ann Surg. 2003;237:853–858. doi: 10.1097/01.SLA.0000071516.54864.C1. discussion 858-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber SM, Cubukcu-Dimopulo O, Palesty JA, Suriawinata A, Klimstra D, Brennan MF, Conlon K. Lymphoplasmacytic sclerosing pancreatitis: inflammatory mimic of pancreatic carcinoma. J Gastrointest Surg. 2003;7:129–137. doi: 10.1016/s1091-255x(02)00148-8. discussion 137-129. [DOI] [PubMed] [Google Scholar]
- 41.Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic's HISORt criteria. J Gastroenterol. 2007;42(Suppl 18):39–41. doi: 10.1007/s00535-007-2046-8. [DOI] [PubMed] [Google Scholar]
- 42.Aalberse RC, Van Milligen F, Tan KY, Stapel SO. Allergen-specific IgG4 in atopic disease. Allergy. 1993;48:559–569. doi: 10.1111/j.1398-9995.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 43.Rock B, Martins CR, Theofilopoulos AN, Balderas RS, Anhalt GJ, Labib RS, Futamura S, Rivitti EA, Diaz LA. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–1469. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 44.Bhol K, Mohimen A, Ahmed AR. Correlation of subclasses of IgG with disease activity in pemphigus vulgaris. Dermatology. 1994;189(Suppl 1):85–89. doi: 10.1159/000246938. [DOI] [PubMed] [Google Scholar]
- 45.Ding X, Diaz LA, Fairley JA, Giudice GJ, Liu Z. The anti-desmoglein 1 a utoanti bodies in pemphigus vulgaris sera are pathogenic. J Invest Dermatol. 1999;112:739–743. doi: 10.1046/j.1523-1747.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 46.Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT, Vege SS, Farnell MB. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646–1653. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 47.Kamisawa T, Egawa N, Nakajima H. Autoimmune pancreatitis is a systemic autoimmune disease. Am J Gastroenterol. 2003;98:2811–2812. doi: 10.1111/j.1572-0241.2003.08758.x. [DOI] [PubMed] [Google Scholar]
- 48.Saito T, Tanaka S, Yoshida H, Imamura T, Ukegawa J, Seki T, Ikegami A, Yamamura F, Mikami T, Aoyagi Y, Niikawa J, Mitamura K. A case of autoimmune pancreatitis responding to steroid therapy. Evidence of histologic recovery. Pancreatology. 2002;2:550–556. doi: 10.1159/000066092. [DOI] [PubMed] [Google Scholar]
- 49.Park DH, Kim MH, Chari ST. Recent advances in autoimmune pancreatitis. Gut. 2009;58:1680–1689. doi: 10.1136/gut.2008.155853. [DOI] [PubMed] [Google Scholar]
- 50.Sah RP CS, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD, Takahashi N, Farnell MB, Vege SS. Differences in Clinical Profile and Relapse Rate of Type 1 vs Type 2 Autoimmune Pancreatitis. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.03.054. in press. [DOI] [PubMed] [Google Scholar]
- 51.Chandan VS, Iacobuzio-Donahue C, Abraham SC. Patchy distribution of pathologic abnormalities in autoimmune pancreatitis: implications for preoperative diagnosis. Am J Surg Pathol. 2008;32:1762–1769. doi: 10.1097/PAS.0b013e318181f9ca. [DOI] [PubMed] [Google Scholar]
- 52.Kamisawa T, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A. Usefulness of biopsying the major duodenal papilla to diagnose autoimmune pancreatitis: a prospective study using IgG4-immunostaining. World J Gastroenterol. 2006;12:2031–2033. doi: 10.3748/wjg.v12.i13.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishino T, Toki F, Oyama H, Oi I, Kobayashi M, Takasaki K, Shiratori K. Biliary tract involvement in autoimmune pancreatitis. Pancreas. 2005;30:76–82. [PubMed] [Google Scholar]
- 54.Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT, Vege SS, Lindor K, Farnell MB. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–715. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Björnsson ECS, Smyrk TC, Lindor K. IgG4 associated cholangitis: Description of an emerging clinical entity based on review of the literature. Hepatology. 2007;45:8. doi: 10.1002/hep.21685. [DOI] [PubMed] [Google Scholar]
- 56.Nakazawa T, Ohara H, Sano H, Ando T, Aoki S, Kobayashi S, Okamoto T, Nomura T, Joh T, Itoh M. Clinical differences between primary sclerosing cholangitis and sclerosing cholangitis with autoimmune pancreatitis. Pancreas. 2005;30:20–25. [PubMed] [Google Scholar]
- 57.Ohara H, Nakazawa T, Ando T, Joh T. Systemic extrapancreatic lesions associated with autoimmune pancreatitis. J Gastroenterol. 2007;42(Suppl 18):15–21. doi: 10.1007/s00535-007-2045-9. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Lewis JT, Abraham SC, Smyrk TC, Leung S, Chari ST, Poterucha JJ, Rosen CB, Lohse CM, Katzmann JA, Wu TT. IgG4+ plasma cell infiltrates in liver explants with primary sclerosing cholangitis. Am J Surg Pathol. 34:88–94. doi: 10.1097/PAS.0b013e3181c6c09a. [DOI] [PubMed] [Google Scholar]
- 59.Björnsson E CS, Silveira M, Gossard A, Takahashi N, Smyrk T, Lindor K. Primary Sclerosing Cholangitis Associated with Elevated ImmunoglobulinG4: Clinical Characteristics and Response to Therapy. Am J Ther. 2010 doi: 10.1097/MJT.0b013e3181c9dac6. (Epub) [DOI] [PubMed] [Google Scholar]
- 60.Hirano K, Shiratori Y, Komatsu Y, Yamamoto N, Sasahira N, Toda N, Isayama H, Tada M, Tsujino T, Nakata R, Kawase T, Katamoto T, Kawabe T, Omata M. Involvement of the biliary system in autoimmune pancreatitis: a follow-up study. Clin Gastroenterol Hepatol. 2003;1:453–464. doi: 10.1016/s1542-3565(03)00221-0. [DOI] [PubMed] [Google Scholar]
- 61.Umemura T, Zen Y, Hamano H, Kawa S, Nakanuma Y, Kiyosawa K. Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology. 2007;46:463–471. doi: 10.1002/hep.21700. [DOI] [PubMed] [Google Scholar]
- 62.Deshpande V, Sainani NI, Chung RT, Pratt DS, Mentha G, Rubbia-Brandt L, Lauwers GY. IgG4-associated cholangitis: a comparative histological and immunophenotypic study with primary sclerosing cholangitis on liver biopsy material. Mod Pathol. 2009;22:1287–1295. doi: 10.1038/modpathol.2009.94. [DOI] [PubMed] [Google Scholar]
- 63.Umemura T, Zen Y, Hamano H, Ichijo T, Kawa S, Nakanuma Y, Kiyosawa K. IgG4 associated autoimmune hepatitis: a differential diagnosis for classical autoimmune hepatitis. Gut. 2007;56:1471–1472. doi: 10.1136/gut.2007.122283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zen Y, Fujii T, Sato Y, Masuda S, Nakanuma Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod Pathol. 2007;20:884–894. doi: 10.1038/modpathol.3800836. [DOI] [PubMed] [Google Scholar]
- 65.Kamisawa T, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A, Horiguchi S. Sclerosing cholecystitis associated with autoimmune pancreatitis. World J Gastroenterol. 2006;12:3736–3739. doi: 10.3748/wjg.v12.i23.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abraham SC, Cruz-Correa M, Argani P, Furth EE, Hruban RH, Boitnott JK. Lymphoplasmacytic chronic cholecystitis and biliary tract disease in patients with lymphoplasmacytic sclerosing pancreatitis. Am J Surg Pathol. 2003;27:441–451. doi: 10.1097/00000478-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Wang WL, Farris AB, Lauwers GY, Deshpande V. Autoimmune pancreatitis-related cholecystitis: a morphologically and immunologically distinctive form of lymphoplasmacytic sclerosing cholecystitis. Histopathology. 2009;54:829–836. doi: 10.1111/j.1365-2559.2009.03315.x. [DOI] [PubMed] [Google Scholar]
- 68.Abraham SC, Cruz-Correa M, Argani P, Furth EE, Hruban RH, Boitnott JK. Diffuse lymphoplasmacytic chronic cholecystitis is highly specific for extrahepatic biliary tract disease but does not distinguish between primary and secondary sclerosing cholangiopathy. Am J Surg Pathol. 2003;27:1313–1320. doi: 10.1097/00000478-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Kitagawa S, Zen Y, Harada K, Sasaki M, Sato Y, Minato H, Watanabe K, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Tsuneyama K, Saito K, Haratake J, Takagawa K, Nakanuma Y. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Kuttner's tumor) Am J Surg Pathol. 2005;29:783–791. doi: 10.1097/01.pas.0000164031.59940.fc. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Shinomura Y, Imai K. A new conceptualization for Mikulicz's disease as an IgG4-related plasmacytic disease. Mod Rheumatol. 2006;16:335–340. doi: 10.1007/s10165-006-0518-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geyer JT, Ferry JA, Harris NL, Stone JH, Zukerberg LR, Lauwers GY, Pilch BZ, Deshpande V. Chronic sclerosing sialadenitis (Kuttner tumor) is an IgG4-associated disease. Am J Surg Pathol. 2010;34:202–210. doi: 10.1097/PAS.0b013e3181c811ad. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto M, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Takahashi H, Imai K. Elevated IgG4 concentrations in serum of patients with Mikulicz's disease. Scand J Rheumatol. 2004;33:432–433. doi: 10.1080/03009740410006439. [DOI] [PubMed] [Google Scholar]
- 73.Takahira M, Kawano M, Zen Y, Minato H, Yamada K, Sugiyama K. IgG4-Related Chronic Sclerosing Dacryoadenitis. Arch Ophthalmol. 2007;125:1575–1578. doi: 10.1001/archopht.125.11.1575. [DOI] [PubMed] [Google Scholar]
- 74.Masaki Y, Dong L, Kurose N, Kitagawa K, Morikawa Y, Yamamoto M, Takahashi H, Shinomura Y, Imai K, Saeki T, Azumi A, Nakada S, Sugiyama E, Matsui S, Origuchi T, Nishiyama S, Nishimori I, Nojima T, Yamada K, Kawano M, Zen Y, Kaneko M, Miyazaki K, Tsubota K, Eguchi K, Tomoda K, Sawaki T, Kawanami T, Tanaka M, Fukushima T, Sugai S, Umehara H. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. 2009;68:1310–1315. doi: 10.1136/ard.2008.089169. [DOI] [PubMed] [Google Scholar]
- 75.Pickartz T, Pickartz H, Lochs H, Ockenga J. Overlap syndrome of autoimmune pancreatitis and cholangitis associated with secondary Sjogren's syndrome. Eur J Gastroenterol Hepatol. 2004;16:1295–1299. doi: 10.1097/00042737-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 76.Kulling D, Tresch S, Renner E. Triad of sclerosing cholangitis, chronic pancreatitis, and Sjogren's syndrome: Case report and review. Gastrointest Endosc. 2003;57:118–120. doi: 10.1067/mge.2003.40. [DOI] [PubMed] [Google Scholar]
- 77.Aoki S, Nakazawa T, Ohara H, Sano H, Nakao H, Joh T, Murase T, Eimoto T, Itoh M. Immunohistochemical study of autoimmune pancreatitis using anti-IgG4 antibody and patients’ sera. Histopathology. 2005;47:147–158. doi: 10.1111/j.1365-2559.2005.02204.x. [DOI] [PubMed] [Google Scholar]
- 78.Takeda S, Haratake J, Kasai T, Takaeda C, Takazakura E. IgG4-associated idiopathic tubulointerstitial nephritis complicating autoimmune pancreatitis. Nephrol Dial Transplant. 2004;19:474–476. doi: 10.1093/ndt/gfg477. [DOI] [PubMed] [Google Scholar]
- 79.Rudmik L, Trpkov K, Nash C, Kinnear S, Falck V, Dushinski J, Dixon E. Autoimmune pancreatitis associated with renal lesions mimicking metastatic tumours. Cmaj. 2006;175:367–369. doi: 10.1503/cmaj.051668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakamura H, Wada H, Origuchi T, Kawakami A, Taura N, Aramaki T, Fujikawa K, Iwanaga N, Izumi Y, Aratake K, Ida H, Taguchi T, Irie J, Akiyama M, Mizokami A, Tsutsumi T, Eguchi K. A case of IgG4-related autoimmune disease with multiple organ involvement. Scand J Rheumatol. 2006;35:69–71. doi: 10.1080/03009740500499484. [DOI] [PubMed] [Google Scholar]
- 81.Watson SJ, Jenkins DA, Bellamy CO. Nephropathy in IgG4-related systemic disease. Am J Surg Pathol. 2006;30:1472–1477. doi: 10.1097/01.pas.0000213308.43929.97. [DOI] [PubMed] [Google Scholar]
- 82.Saeki T, Nishi S, Ito T, Yamazaki H, Miyamura S, Emura I, Imai N, Ueno M, Saito A, Gejyo F. Renal lesions in IgG4-related systemic disease. Intern Med. 2007;46:1365–1371. doi: 10.2169/internalmedicine.46.0183. [DOI] [PubMed] [Google Scholar]
- 83.Cornell LD, Chicano SL, Deshpande V, Collins AB, Selig MK, Lauwers GY, Barisoni L, Colvin RB. Pseudotumors due to IgG4 immune-complex tubulointerstitial nephritis associated with autoimmune pancreatocentric disease. Am J Surg Pathol. 2007;31:1586–1597. doi: 10.1097/PAS.0b013e318059b87c. [DOI] [PubMed] [Google Scholar]
- 84.Yoneda K, Murata K, Katayama K, Ishikawa E, Fuke H, Yamamoto N, Ito K, Shiraki K, Nomura S. Tubulointerstitial nephritis associated with IgG4-related autoimmune disease. Am J Kidney Dis. 2007;50:455–462. doi: 10.1053/j.ajkd.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 85.Miyajima N, Koike H, Kawaguchi M, Zen Y, Takahashi K, Hara N. Idiopathic retroperitoneal fibrosis associated with IgG4-positive-plasmacyte infiltrations and idiopathic chronic pancreatitis. Int J Urol. 2006;13:1442–1444. doi: 10.1111/j.1442-2042.2006.01568.x. [DOI] [PubMed] [Google Scholar]
- 86.Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, Nakazawa K, Shimojo H, Kiyosawa K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–1404. doi: 10.1016/s0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- 87.Uchida K, Okazaki K, Asada M, Yazumi S, Ohana M, Chiba T, Inoue T. Case of chronic pancreatitis involving an autoimmune mechanism that extended to retroperitoneal fibrosis. Pancreas. 2003;26:92–94. doi: 10.1097/00006676-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 88.Ohtsubo K, Watanabe H, Tsuchiyama T, Mouri H, Yamaguchi Y, Motoo Y, Ohnishi I, Gabata T, Sawabu N. A case of autoimmune pancreatitis associated with retroperitoneal fibrosis. Jop. 2007;8:320–325. [PubMed] [Google Scholar]
- 89.Phillips RH, Carr RA, Preston R, Pereira SP, Wilkinson ML, O'Donnell PJ, Thompson RP. Sclerosing mesenteritis involving the pancreas: two cases of a rare cause of abdominal mass mimicking malignancy. Eur J Gastroenterol Hepatol. 1999;11:1323–1329. [PubMed] [Google Scholar]
- 90.Sheikh RA, Prindiville TP, Arenson D, Ruebner BH. Sclerosing mesenteritis seen clinically as pancreatic pseudotumor: two cases and a review. Pancreas. 1999;18:316–321. doi: 10.1097/00006676-199904000-00014. [DOI] [PubMed] [Google Scholar]
- 91.Akram S, Pardi DS, Schaffner JA, Smyrk TC. Sclerosing mesenteritis: clinical features, treatment, and outcome in ninety-two patients. Clin Gastroenterol Hepatol. 2007;5:589–596. doi: 10.1016/j.cgh.2007.02.032. quiz 523-584. [DOI] [PubMed] [Google Scholar]
- 92.Komatsu K, Hamano H, Ochi Y, Takayama M, Muraki T, Yoshizawa K, Sakurai A, Ota M, Kawa S. High prevalence of hypothyroidism in patients with autoimmune pancreatitis. Dig Dis Sci. 2005;50:1052–1057. doi: 10.1007/s10620-005-2703-9. [DOI] [PubMed] [Google Scholar]
- 93.Comings DE, Skubi KB, Van Eyes J, Motulsky AG. Familial multifocal fibrosclerosis. Findings suggesting that retroperitoneal fibrosis, medi-astinal fibrosis, sclerosing cholangitis, Riedel's thyroiditis, and pseudotumor of the orbit may be different manifestations of a single disease. Ann Intern Med. 1967;66:884–892. doi: 10.7326/0003-4819-66-5-884. [DOI] [PubMed] [Google Scholar]
- 94.Dehner LP, Coffin CM. Idiopathic fibrosclerotic disorders and other inflammatory pseudotumors. Semin Diagn Pathol. 1998;15:161–173. [PubMed] [Google Scholar]
- 95.Hamed G, Tsushima K, Yasuo M, Kubo K, Yamazaki S, Kawa S, Hamano H, Yamamoto H. Inflammatory lesions of the lung, submandibular gland, bile duct and prostate in a patient with IgG4-associated multifocal systemic fibrosclerosis. Respirology. 2007;12:455–457. doi: 10.1111/j.1440-1843.2007.01053.x. [DOI] [PubMed] [Google Scholar]
- 96.Taniguchi T, Ko M, Seko S, Nishida O, Inoue F, Kobayashi H, Saiga T, Okamoto M, Fukuse T. Interstitial pneumonia associated with autoimmune pancreatitis. Gut. 2004;53:770. author reply 770-771. [PMC free article] [PubMed] [Google Scholar]
- 97.Nieminen U, Koivisto T, Kahri A, Farkkila M. Sjogren's syndrome with chronic pancreatitis, sclerosing cholangitis, and pulmonary infiltrations. Am J Gastroenterol. 1997;92:139–142. [PubMed] [Google Scholar]
- 98.Zen Y, Kitagawa S, Minato H, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Fujimura M, Nakanuma Y. IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell granu-loma) of the lung. Hum Pathol. 2005;36:710–717. doi: 10.1016/j.humpath.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 99.Shrestha B, Sekiguchi H, Colby TV, Graziano P, Aubry MC, Smyrk TC, Feldman AL, Cornell LD, Ryu JH, Chari ST, Dueck AC, Yi ES. Distinctive pulmonary histopathology with increased IgG4-positive plasma cells in patients with autoimmune pancreatitis: report of 6 and 12 cases with similar histopathology. Am J Surg Pathol. 2009;33:1450–1462. doi: 10.1097/PAS.0b013e3181ac43b6. [DOI] [PubMed] [Google Scholar]
- 100.Ando N, Yasuda I, Saito M, Moriwaki H. Hilar lymphadenopathy associated with autoimmune pancreatitis. Pancreas. 2006;33:101–102. doi: 10.1097/01.mpa.0000226879.58506.ce. [DOI] [PubMed] [Google Scholar]
- 101.Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006;41:613–625. doi: 10.1007/s00535-006-1862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saeki T, Saito A, Hiura T, Yamazaki H, Emura I, Ueno M, Miyamura S, Gejyo F. Lymphoplasmacytic infiltration of multiple organs with immu-noreactivity for IgG4: IgG4-related systemic disease. Intern Med. 2006;45:163–167. doi: 10.2169/internalmedicine.45.1431. [DOI] [PubMed] [Google Scholar]
- 103.Cheuk W, Yuen HK, Chu SY, Chiu EK, Lam LK, Chan JK. Lymphadenopathy of IgG4-related sclerosing disease. Am J Surg Pathol. 2008;32:671–681. doi: 10.1097/PAS.0b013e318157c068. [DOI] [PubMed] [Google Scholar]