Abstract
Carney complex is a syndrome that may include cardiac and mucocutaneous myxomas, spotting skin pigmentation, and endocrine lesions. Many patients with Carney complex have been shown to have a stop codon mutation in the PRKAR1A gene in the 17q22-24 region. Here we present the case of a 57 year-old man with multiple skin lesions and cardiac myxomas. Histology of the skin lesions showed lentigenous melanocytic hyperplasia and cutaneous myxomas, confirming the diagnosis of Carney complex. Lesional and control normal tissue from the patient were identified and sequenced for the PRKAR1A gene. A germline missense mutation was identified at exon 1A. This is the first report of this mutation, and one of the few reported missense mutation associated with Carney complex. This finding strengthens the argument that there are alternative ways in which the protein kinase A 1-alpha subunit plays a role in tumorigenesis
Keywords: Carney complex, PRKAR1, mutation, myxomas
Introduction
In 1985, J.A. Carney first described a constellation of lesions in a group of 40 patients, which included cardiac myxomas, skin pigmentation, primary pigmented nodular adrenocortical hyperplasia, myxoid mammary fibroadenomas, testicular tumors and pituitary adenomas. The mean age at the time of diagnosis was 18 years [1]. A total of 353 patients with Carney complex (CNC) have been identified, with 43% male to 57% female representation. Most patients (70%) were part of an affected family, while 88 individuals were considered to be sporadic [2]. A majority of cases were shown to have an affected parent, suggesting an autosomal dominant inheritance. Since this complex is often correlated with large-cell calcifying Sertoli cell tumor (LCCSCT), this may decrease the fertility of affected males [3], thus affecting the observed inheritance rate.
In 2000, two independent groups identified mutations in the PRKAR1A gene in the 17q22-24. The PRKAR1A is a tumor suppressor gene that encodes for the protein kinase A regulatory 1-alpha subunit [4, 5]. In a study of 51 patients with Carney complex, 65% had mutations of PRKAR1A that resulted in a truncated protein that is not translated [6]. The majority of mutations are nonsense, frameshift and splicesite mutations that undergo nonsense-mediated mRNA decay(NMD) with resultant haploinsufficency. They are primarily seen in two hotspots, delTG576-577 and C769T [7]. Recently, Bertherat and Stratakis reviewed 353 patients with CNC and/or PPNAD or those with a pathogenic PRKAR1A mutation. They found 258 (73%) patients had a defect in PRKAR1A. Of the 80 types of defects, only 6 coded for missense mutations. In their correlation with phenotype, they found that patients with a defect in PRKAR1A were more likely to have myxomas in all locations, thyroid tumors, psammomatous melanotic schwannomas (PMS), and LCCSCT's. Mutations found in exons were associated with acromegaly, cardiac myxomas, lentigines and PMS. Finally, patients with mutations that escaped NMD had an increased number of CNC tumors [8]. In vitro studies of missense mutations that do not undergo NMD found that the mutated R1α protein was associated with increased PKA activity, similar to what is seen in complete loss of R1α protein [9].
Case Presentation
Clinical history
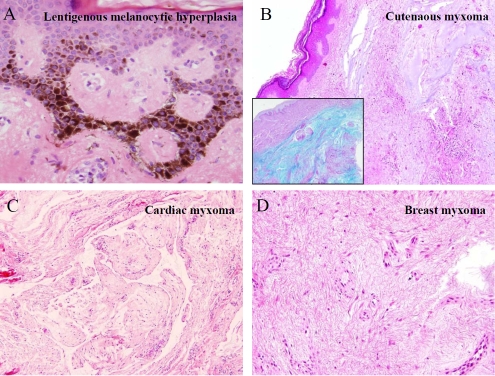
A 57 year-old man presented with multiple skin lesions of the eyebrow, flank and mid-back in January 2004. At this time, the clinical differential diagnosis included neurofibromata, dysplastic nevi, and malignant melanoma. His previous surgical history was significant for prior removal of two atria l polyps in 1996 and 2003. The lesion from skin showed lentigenous melanocytic hyperplasia (Figure 1A). The histology of the atrial lesions showed a myxomatous polyp (Figure 1C). The patient was diagnosed at that time with Carney complex. The patient denied any family history of cutaneous, cardiac or endocrine lesions. In March of 2004, a 2.3-cm nodule was identified on the right buttock. Histology revealed a cutaneous myxoma (Figure 1B). In May of 2004, a 0.5-cm, right areolar lesion was identified as another myxoma (Figure 1D).
Figure 1.
Features of Carney complex: Lentigenous melanocytic hyperplasia (A), multiple myxomas in the skin (B), heart (C) and breast (D). B inset: Alcian blue stain.
Novel PRKAR1A gene mutation
Paraffin sections from the atrial myxoma, breast myxoma, skin myxoma, and pigmented skin lesions were selected, also including appropriate normal controls. Genomic DNA was extracted from macro-dissected paraffin sections. The area of interest was identified by pathologist. The tissue of interest was isolated by direct visualization and was excised with a scalpel. The sections were de-paraffinized. DNA was isolated with Qiagen kit, according to the manufacture's instructions. Primers of PRKAR1A exons 1-10 were designed according to the study by Stratakis CA2. PCR amplification was performed in a total volume of 10 μl, containing 20 ng of DNA and a reaction mixture of 1.5 mM MgCl2, 5mM dNTP, 0.1 μl Taq DNA polymerase, and 3 μM corresponding primer. PCR products were then sequenced using ABI Big Dye Terminator Chemistry (Version 3.1) and analyzed on an ABI 3730 Capillary Sequencer (Memorial Sloan-Kettering Cancer Center, New York, NY). The results were analyzed using NCBI alignment algorithm (National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov, Bethesda, M.D.).
Analysis revealed a novel point mutation atexon 1A of the PRKAR1A gene. The mutation of GCG to GAG results in a change from alanine to glutamic acid (Table 1). This mutation was identified in both the lesional tissue and the normal controls, thus consistent with a germline mutation.
Table 1.
Mutation in amino acid 29 of PKA
| Wild type PKA | Mutant PKA | |
|---|---|---|
| Nucleotide | GCG | GAG |
| Amino acid | Alanine | Glutamic Acid |
Discussion
In the literature, one prior case of Carney complex was associated with a missense mutation at C307T. Here we report a novel mutation of PRKAR1A. In the knockout mice study by Veugelers et a l., the missense mutation resulted in the coding of a mutant R1α protein, where the majority of mutations led to a stop codon and nonsense mediated decay of the mRNA. However, neither mutation had any affect on the expressed R1α's inhibition of protein kinase A activity. They hypothesized that the missense mutation may affect the ultrastructure of the R1 α protein, ultimately altering its interaction with partner proteins other than those of the protein kinase tetramer. Although the majority of patients with Carney complex are associated with a noncoding mutation and haploinsufficiency, the fact that a significant fraction, approximately 30%, of patients without identifiable mutations along with the increasing but small number of reported missense mutations, further strengthens the argument that there are additional mechanisms of tumorigenesis in this disease. Additional studies of these missense mutations may help to further characterize these mechanisms.
References
- 1.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine. 1985;64:270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Stratakis CA, Kirschner LS, Carney JA. Carney complex: diagnosis and management of the complex of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas. Am J Med Genet. 1998;80:183–185. doi: 10.1002/(sici)1096-8628(19981102)80:2<183::aid-ajmg19>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Premkumar A, Stratakis CA, Shawker TH, Papanicolaou DA, Chrousos GP. Testicular ultrasound in Carney complex: report of three cases. J Clin Ultrasound. 1997;25:211–214. doi: 10.1002/(sici)1097-0096(199705)25:4<211::aid-jcu10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 5.Casey M, Vaughan CJ, He J, Hatcher CJ, Winter JM, Weremowicz S, Montgomery K, Kucherlapati R, Morton CC, Basson CT. Mutations in the protein kinase A R1alpha regulatory subunit cause familial cardiac myxomas and Carney complex. J Clin Invest. 2000;106:R31–38. doi: 10.1172/JCI10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veugelers M, Wilkes D, Burton K, McDermott DA, Song Y, Goldstein MM, La Perle K, Vaughan CJ, O'Hagan A, Bennett KR, Meyer BJ, Legius E, Karttunen M, Norio R, Kaariainen H, Lavyne M, Neau JP, Richter G, Kirali K, Farnsworth A, Stapleton K, Morelli P, Takanashi Y, Bamforth JS, Eitelberger F, Noszian I, Manfroi W, Powers J, Mochizuki Y, Imai T, Ko GT, Driscoll DA, Goldmuntz E, Edelberg JM, Collins A, Eccles D, Irvine AD, McKnight GS, Basson CT. Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prkar1a haploinsufficient mice. Proc Natl Acad Sci. 2004;101:14222–14227. doi: 10.1073/pnas.0405535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadella KS, Kirschner LS. Disruption of protein kinase a regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res. 2005;65:10307–10315. doi: 10.1158/0008-5472.CAN-05-3183. [DOI] [PubMed] [Google Scholar]
- 8.Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. Mutations in regulatory subunit type 1A of cyclic adenosine 5'-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94:2085–2091. doi: 10.1210/jc.2008-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene EL, Horvath AD, Nesterova M, Giatzakis C, Bossis I, Stratakis CA. In vitro functional studies of naturally occurring pathogenic PRKAR1A mutations that are not subject to nonsense mRNA decay. Hum Mutat. 2008;29:633–639. doi: 10.1002/humu.20688. [DOI] [PubMed] [Google Scholar]