α-Synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis
The fatty acid-binding protein alpha-synuclein has been proposed to regulate SNARE proteins. This report shows that alpha-synuclein sequesters fatty acids to down-regulate SNARE assembly thereby inhibiting exocytosis.
Keywords: α-synuclein, arachidonic acid, exocytosis, SNARE, syntaxin
Abstract
α-Synuclein is a synaptic modulatory protein implicated in the pathogenesis of Parkinson disease. The precise functions of this small cytosolic protein are still under investigation. α-Synuclein has been proposed to regulate soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins involved in vesicle fusion. Interestingly, α-synuclein fails to interact with SNARE proteins in conventional protein-binding assays, thus suggesting an indirect mode of action. As the structural and functional properties of both α-synuclein and the SNARE proteins can be modified by arachidonic acid, a common lipid regulator, we analysed this possible tripartite link in detail. Here, we show that the ability of arachidonic acid to stimulate SNARE complex formation and exocytosis can be controlled by α-synuclein, both in vitro and in vivo. α-Synuclein sequesters arachidonic acid and thereby blocks the activation of SNAREs. Our data provide mechanistic insights into the action of α-synuclein in the modulation of neurotransmission.
Introduction
α-Synuclein is a cytosolic protein that is enriched in mature nerve terminals and has recently been implicated in the aetiology of Parkinson disease (Spillantini et al, 1997; Cookson, 2009). The physiological function of this abundant brain protein is not yet fully defined. Several studies have indicated a role for α-synuclein in the regulation of intracellular transport (Cooper et al, 2006; Ben Gedalya et al, 2009; Sousa et al, 2009), including synaptic vesicle fusion underlying neurotransmitter release (Abeliovich et al, 2000; Murphy et al, 2000; Cabin et al, 2002; Yavich et al, 2004; Nemani et al, 2010). Recently, α-synuclein was shown to interfere with a late step in exocytosis (Larsen et al, 2006). The discovery of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins forming a ternary complex that drives exocytosis (Sollner et al, 1993) allows a detailed characterization of the mechanistic aspects of the regulatory mechanisms that underlie vesicle fusion. We now know that in the brain, at the neuromuscular junction and in the endocrine organs, a set of three SNARE proteins has a main role in accomplishing fusion of vesicular and plasma membranes. These are syntaxin 1, synaptosomal-associated protein of 25 kDa (SNAP25) and synaptobrevin 2. It is generally accepted that these three proteins, which normally reside on opposing membranes, engage each other and form a tight SNARE complex. The formation of this complex drives membrane fusion, leading to the release of the vesicular cargo into the extracellular space.
Recent studies have highlighted the lipid-based mechanisms underlying various aspects of vesicle fusion and retrieval (Wenk & De Camilli, 2004; Rigoni et al, 2005; Lang et al, 2008; Bader & Vitale, 2009). It is widely recognized that arachidonic acid, released from phospholipid membranes after the action of phospholipases, and its metabolites are intimately involved in the regulation of synaptic transmission (Kreitzer & Regehr, 2002; Bazan, 2005). We showed recently that arachidonic acid upregulates syntaxin and enhances its engagement with the fusogenic SNARE complex (Rickman & Davletov, 2005; Connell et al, 2007). Remarkably, the function of α-synuclein is also linked to fatty acid metabolism (Perrin et al, 2001; Sharon et al, 2001; Kubo et al, 2005; Broersen et al, 2006; Golovko et al, 2006). These observations raise the possibility that the proposed effect of α-synuclein on SNARE-mediated exocytosis might be explained by an arachidonic acid link. Here, we investigated the mechanism of the function of α-synuclein in SNARE assembly in the presence and absence of arachidonic acid. Our results show that α-synuclein efficiently blocks arachidonic-acid-induced SNARE interactions, both in vitro and in vivo, suggesting a physiological role for α-synuclein.
Results And Discussion
Effects of α-synuclein on SNARE assembly
First, we analysed the effect of α-synuclein on the formation of the SNARE complex in the presence of arachidonic acid. The defining property of the SNARE complex is its resistance to dissociation by chaotropic agents, which disrupt the three-dimensional structure of macromolecules, and detergents such as sodium dodecyl sulpate (SDS; Hu et al, 2002). When syntaxin 1 is mixed with SNAP25 and synaptobrevin 2 for 20 min, the SNARE complex can be detected directly by SDS–polyacrylamide gel electrophoresis. Fig 1A shows that arachidonic acid enhanced the formation of the SNARE complex, as reported previously (Connell et al, 2007). The addition of α-synuclein in this reaction, however, significantly reduced SNARE assembly, as was confirmed by gel band densitometry (Fig 1A). Further, kinetic measurements showed that arachidonic acid accelerates the rate of SNARE assembly, and α-synuclein blocks this accelerating action (supplementary Fig S1 online). The addition of α-synuclein resulted in a dose-dependent inhibition of SNARE complex formation (Fig 1B). Furthermore, α-synuclein was effective at all the concentrations of arachidonic acid tested, indicating that the micromolar concentrations of this protein are sufficient to reduce free fatty acid levels (Broersen et al, 2006) to the point at which SNARE assembly would not be stimulated (Fig 1B). We also investigated whether α-synuclein can alter SNARE assembly in the absence of arachidonic acid. Interestingly, α-synuclein, even at a high molar excess, did not modify SNARE complex formation in such a scenario. Together, these results show that α-synuclein requires the presence of arachidonic acid to modulate SNARE assembly.
Figure 1.
α-Synuclein blocks arachidonic-acid-mediated SNARE assembly. (A) A Coomassie-stained gel (left) and a bar chart (right) showing an increase in the amount of the ternary SNARE complex in the presence of arachidonic acid (100 μM) in a 20-min reaction. This effect is blocked in the presence of α-Syn (7 μM), Syx1, SNAP25 and Syb (1 μM each). **P<0.01, n=6. (B) Quantification of SNARE complex formation with different concentrations of fatty acid and α-synuclein demonstrates that α-synuclein inhibits arachidonic-acid-induced SNARE complex formation in a dose-dependent manner. (C) α-Synuclein does not affect formation of the ternary SNARE complex in the absence of arachidonic acid. AA, arachidonic acid; SNAP25, synaptosomal-associated protein of 25 kDa; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; Syb, synaptobrevin 2; α-Syn, α-synuclein; Syx1, syntaxin 1.
α-Synuclein inhibits fatty-acid-mediated exocytosis
To probe further the role of α-synuclein in SNARE regulation, we investigated the effect of α-synuclein overexpression on vesicle exocytosis in chromaffin cells. Chromaffin cells were transfected using gold-particle-mediated gene transfer with vectors encoding either α-synuclein or green fluorescent protein (GFP) as a control. Transfected cells were identified by the presence of gold particles and also by immunolocalization of α-synuclein or GFP (data not shown). We monitored exocytosis in individual transfected cells using the membrane dye FM1-43, which becomes fluorescent when vesicles fuse with the plasma membrane after photorelease of caged Ca2+ (Giner et al, 2007). The treatment of the cells with arachidonic acid led to a significant increase in calcium-triggered exocytosis in agreement with previous studies (Latham et al, 2007). When a similar experiment was repeated with α-synuclein-overexpressing cells, the increase in exocytosis was largely blocked, indicating that α-synuclein can efficiently control arachidonic-acid-dependent functions (Fig 2A).
Figure 2.
α-Synuclein inhibits arachidonic-acid-induced exocytosis and SNARE interactions in neuroendocrine cells. (A) Representative curves (left) and a bar chart (right) showing an increase in FM1-43 fluorescence following photorelease of caged Ca2+ in chromaffin cells, with or without a 30-min pretreatment with arachidonic acid (AA; 100 μM). Expression of α-Syn blocks the increase in FM1-43 fluorescence mediated by AA. Normalized responses (right) were calculated at 5 s after the flash photolysis pulse and averaged for the indicated numbers of cells (n). **P<0.01. (B) Western immunoblots (left) and a bar chart (right) showing that AA (100 μM) increases the amount of Syx1 immunoprecipitated with SNAP25 (IP) from PC12 neuroendocrine cells. This effect is blocked after overexpression of α-Syn. Note that the α-Syn is not immunoprecipitated with the SNARE proteins. n=6; **P<0.01. (C) IP of α-Syn from rat brain synaptosomes treated or untreated with AA (100 μM) confirms the lack of interaction between endogenous α-Syn and the SNARE proteins Syx1 and Syb. AA, arachidonic acid; IP, immunoprecipitation; SNAP25, synaptosomal-associated protein of 25 kDa; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; Syb, synaptobrevin 2; α-Syn, α-synuclein; Syx1, syntaxin 1.
An earlier study showed that arachidonic acid specifically activates the first step in the SNARE assembly pathway, that is, the formation of the syntaxin–SNAP25 heterodimer (Connell et al, 2007). To assess the role of α-synuclein in the formation of this heterodimer in a cellular environment, we overexpressed the protein in the pheochromocytoma cell line PC12. These cells were transfected by electroporation, with more than 60% of the cells expressing α-synuclein (data not shown), allowing biochemical analysis. SNAP25 was immunoprecipitated and the amount of bound syntaxin 1 was estimated by western immunoblotting. Fig 2B shows that arachidonic acid treatment enhanced the interaction between syntaxin 1 and SNAP25, whereas overexpression of α-synuclein efficiently blocked the accelerated SNARE interaction in a cellular environment.
We noticed that the overexpressed α-synuclein did not interact with cellular SNAP25 and syntaxin 1, as assessed by immunoprecipitation (Fig 2B). To confirm that the action of α-synuclein does not involve a direct interaction with any of the SNARE proteins, we performed immunoprecipitation of α-synuclein from purified synaptic endings (Rickman & Davletov, 2005; Broersen et al, 2006). Fig 2C shows that endogenous α-synuclein did not interact with the SNARE proteins in the presence or absence of arachidonic acid (Fig 2C). These data suggest that α-synuclein affects SNARE function without direct interaction with the SNARE proteins, implying an interaction of α-synuclein with a SNARE regulator, such as arachidonic acid.
α-Synuclein acts by sequestering arachidonic acid
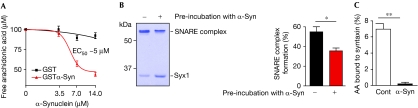
We investigated whether previous exposure of arachidonic acid solution to α-synuclein could still affect SNARE assembly. We immobilized α-synuclein through a glutathione S-transferase tag on Sepharose beads and incubated these beads with radioactive arachidonic acid. Fig 3A shows that immobilized α-synuclein was able to decrease free fatty acid concentration with an EC50 of 5 μM. Next we compared the effects of arachidonic acid solutions, exposed or not to α-synuclein, on SNARE assembly. Fig 3B shows that formation of the SNARE complex was downregulated when the fatty acid was exposed to α-synuclein, confirming that this protein can reduce free fatty acid levels to the point at which SNARE assembly is not stimulated.
Figure 3.
α-Synuclein sequesters arachidonic acid to modulate SNARE function. (A) Graph showing the dose dependence of arachidonic acid (AA) binding by α-Syn, immobilized through the glutathione S-transferase tag, on Sepharose beads. Efficient depletion of the radioactive AA (100 μM) takes place at an α-Syn concentration >5 μM. (B) Coomassie-stained gel (left) and a bar chart (right) showing that pre-treatment of the AA solution by immobilized α-Syn (7 μM) downregulates SNARE assembly. n=5; *P<0.05. (C) Bar chart showing the binding of AA to syntaxin (3 μM) in an equilibrium dialysis experiment. The presence of α-Syn (10 μM) in the opposite chamber abolishes binding of the fatty acid to syntaxin. **P<0.01, n=3. AA, arachidonic acid; SNAP25, synaptosomal-associated protein of 25 kDa; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; Syb, synaptobrevin 2; α-Syn, α-synuclein.
Among the three SNARE proteins, syntaxin is the most likely target of arachidonic acid (Connell et al, 2007). We therefore tested the effect of α-synuclein on the ability of syntaxin 1 to bind to arachidonic acid by equilibrium dialysis. Syntaxin was placed in the sample chamber, and, after equilibration with radioactively labelled arachidonic acid, the distribution of the fatty acid was estimated by scintillation counting. Fig 3C shows that arachidonic acid was enriched in the syntaxin-containing chamber, indicating that syntaxin bound to the fatty acid. Crucially, the addition of α-synuclein to the equilibration buffer in the opposite chamber prevented the binding of arachidonic acid to syntaxin (Fig 3C). As the two proteins were physically separated in two compartments, this result confirms that the inhibitory action of α-synuclein on SNAREs is mediated by its sequestration of arachidonic acid.
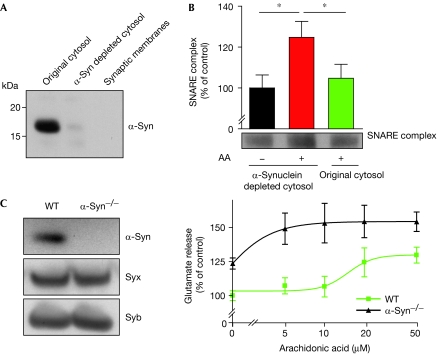
It was important to test SNARE assembly properties in the synaptic environment in which endogenous α-synuclein is highly enriched (Clayton & George, 1999). First, we separated the rat brain synaptic membranes, which contain the SNARE proteins, from the synaptic cytosol, which is enriched in α-synuclein (Fig 4A). Next, the cytosol was treated with a synuclein antibody immobilized on Sepharose beads, which allowed nearly complete removal of α-synuclein (Fig 4A; Broersen et al, 2006). Synaptic membranes were then exposed to arachidonic acid and cytosol preparations. The addition of arachidonic acid to synaptic membranes in the presence of synuclein-depleted cytosol led to a significant increase in the formation of the SNARE complex (Fig 4B). However, when we used the original cytosol, containing endogenous levels of α-synuclein, the effect of arachidonic acid was downregulated (Fig 4B). We further tested the link between the SNARE assembly and arachidonic acid in synaptic preparations obtained from mice lacking α-synuclein. The addition of arachidonic acid to the synaptic membranes in the presence of the synaptic cytosol taken from an α-synuclein−/− mouse led to an increase in SNARE assembly, whereas addition of the cytosol obtained from mice carrying α-synuclein had a downregulating effect (supplementary Fig S3 online). We conclude that endogenous α-synuclein can control formation of the SNARE complex mediated by arachidonic acid.
Figure 4.
Endogenous α-synuclein inhibits the formation of the SNARE complex. (A) Western immunoblot showing the amounts of endogenous α-Syn present in the synaptic cytosol before and after depletion of the protein. Washed synaptic membranes are devoid of α-Syn. (B) Anti-Syb immunoblot of synaptic membranes (bottom) and bar chart (top) showing the formation of the native SNARE complex in the absence and presence of arachidonic acid (AA; 100 μM) and corresponding cytosolic preparations. The stimulating effect of AA can be observed in the α-Syn-depleted but not in the original cytosol. n=9; *P<0.05. (C) Immunoblot showing levels of syntaxin, Syb and α-Syn in the synaptosomes obtained from wild-type (WT) or α-Syn−/− mice (left). Right: Graph showing glutamate release from α-Syn−/− or wild type synaptosomes (0.15 mg/ml final protein concentration), following a 5-min incubation with the indicated AA concentrations. Both the presence of AA and the absence of α-Syn enhance glutamate release. AA, arachidonic acid; SNAP25, synaptosomal-associated protein of 25 kDa; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; Syb, synaptobrevin 2; α-Syn, α-synuclein.
Finally, we analysed the effect of arachidonic acid on glutamate release from isolated synaptic endings (synaptosomes) obtained from the brain cortices of α-synuclein−/− and control mice (Fig 4C). For the measurement of glutamate release from synaptosomes, we used a fluorescent microplate assay that relies on the conversion of nicotinamide adenine dinucleotide phosphate to its reduced form by glutamate dehydrogenase (Darios et al, 2009). Consistent with recent studies of vesicle exocytosis (Almeida et al, 1999; Rigoni et al, 2005; Latham et al, 2007; Nemani et al, 2010), the unsaturated fatty acid increased glutamate release from the wild-type synaptic endings (Fig 4C). However, this increase was evident, in the case of α-synuclein ablation, at lower concentrations of arachidonic acid. Furthermore, the synaptic endings from α-synuclein−/− mice showed enhanced glutamate release even in the absence of exogenous fatty acid (Fig 4C). Together, these data suggest that wild-type nerve endings rely on α-synuclein to control the endogenous fatty acid levels, which can be intimately involved in the regulation of neurotransmitter release, for example, through syntaxin activation (Connell et al, 2007).
Several recent studies have suggested a role for α-synuclein in the regulation of vesicle transport and neurotransmitter release (Abeliovich et al, 2000; Yavich et al, 2004; Sousa et al, 2009), specifically affecting synaptic vesicle numbers or their clustering (Murphy et al, 2000; Cabin et al, 2002; Nemani et al, 2010). It was also proposed that α-synuclein inhibits neurotransmitter release, at a late stage, between docking and fusion of synaptic vesicles, possibly by affecting the function of the SNARE proteins (Chandra et al, 2005; Larsen et al, 2006). Here, we investigated in detail the relationship between α-synuclein and the SNARE proteins and now show that α-synuclein can modulate SNARE properties without direct binding. We show that α-synuclein can inhibit both exocytosis and the formation of the SNARE complex by decreasing the levels of free arachidonic acid available to the SNARE proteins. Arachidonic acid, which can be released from cellular membranes by the action of phospholipase A2 (Rossetto et al, 2006), is a well-characterized regulator of SNARE complex formation and exocytosis in endocrine cells and neurons (Freeman et al, 1990; Almeida et al, 1999; Connell et al, 2007; Latham et al, 2007). Our data show that endogenous cytosolic levels of α-synuclein can buffer arachidonic acid and thereby affect SNARE assembly, defining a possible physiological function for α-synuclein. Together, our data highlight the protein–fatty acid interaction that takes place in the regulation of SNARE-mediated vesicular exocytosis, and suggest a role for α-synuclein in this pathway. Interestingly, knockout of the α-synuclein gene produces changes in the total mass of the polyunsaturated fatty acids (Golovko et al, 2006) but results in only a mild phenotype, suggesting that the free fatty acid–exocytosis relationship can be modulated by other factors as well. It will now be important to investigate how other synaptic components such as CSPα (Chandra et al, 2005), Rab proteins (Cooper et al, 2006), complexins (Melia, 2007), Munc18 (Rickman & Davletov, 2005) and actin (Sousa et al, 2009) can contribute to or be influenced by the α-synuclein–fatty acid link.
Methods
Plasmids, antibodies and reagents. Plasmids encoding glutathione S-transferase fusions of rat SNAP25, syntaxin 1 (amino acids 1–261), synaptobrevin 2 (amino acids 1–96) and human α-synuclein have been described previously (Broersen et al, 2006; Connell et al, 2007). Human α-synuclein complementary DNA was subcloned into pcDNA3+ (Invitrogen) for expression in mammalian cells. Mouse monoclonal antibodies against syntaxin 1 (clone HPC-1), SNAP25 (clone SMI81), synaptobrevin 2 (clone 69.1) and α-synuclein (clone 42) were from Sigma, Covance, Synaptic Systems and BD Transduction Laboratories, respectively. Rabbit anti-SNAP25 has been previously described (Bajohrs et al, 2005). Arachidonic acid was from Sigma. [3H]-Arachidonic acid was from Perkin-Elmer.
Protein reactions and other experimental procedures. Isolation of recombinant proteins has been described previously (Broersen et al, 2006; Connell et al, 2007). All SNARE assembly reactions were performed in 20 mM HEPES, 100 mM NaCl, pH 7.3, at 22°C. Syntaxin 1 (1 μM) and SNAP25 were preincubated with α-synuclein and/or arachidonic acid for 5 min. SNARE complex formation was initiated by the addition of 1 μM synaptobrevin 2 and stopped, after 20 min, by the addition of SDS-containing sample buffer. The SNARE complex was visualized by Coomassie staining of SDS–polyacrylamide gel electrophoresis gels. The SNARE complex was quantified using the ChemiDoc XRS system and Quantity One software (BioRad).
The isolation of synaptic fractions, cell culture, secretion assay, immunoprecipitation, binding of arachidonic acid, SDS–polyacrylamide gel electrophoresis, western immunoblotting and statistical analyses are described in the supplementary information online. α-Synuclein−/− mice (C57BL/6S; Specht & Schoepfer, 2001) were obtained from Harlan, UK.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Michel Goedert for providing us with the recombinant α-synuclein plasmid. This work was supported by the Medical Research Council UK and the UK Parkinson's Disease Society (grant G-0903).
Footnotes
The authors declare that they have no conflict of interest.
References
- Abeliovich A et al. (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252 [DOI] [PubMed] [Google Scholar]
- Almeida T, Cunha RA, Ribeiro JA (1999) Facilitation by arachidonic acid of acetylcholine release from the rat hippocampus. Brain Res 826: 104–111 [DOI] [PubMed] [Google Scholar]
- Bader MF, Vitale N (2009) Phospholipase D in calcium-regulated exocytosis: lessons from chromaffin cells. Biochim Biophys Acta 1791: 936–941 [DOI] [PubMed] [Google Scholar]
- Bajohrs M, Darios F, Peak-Chew SY, Davletov B (2005) Promiscuous interaction of SNAP-25 with all plasma membrane syntaxins in a neuroendocrine cell. Biochem J 392: 283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG (2005) Lipid signaling in neural plasticity, brain repair, and neuroprotection. Mol Neurobiol 32: 89–103 [DOI] [PubMed] [Google Scholar]
- Ben Gedalya T, Loeb V, Israeli E, Altschuler Y, Selkoe DJ, Sharon R (2009) Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling. Traffic 10: 218–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broersen K, Brink D, Fraser G, Goedert M, Davletov B (2006) Alpha-synuclein adopts an alpha-helical conformation in the presence of polyunsaturated fatty acids to hinder micelle formation. Biochemistry 45: 15610–15616 [DOI] [PubMed] [Google Scholar]
- Cabin DE et al. (2002) Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci 22: 8797–8807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC (2005) Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123: 383–396 [DOI] [PubMed] [Google Scholar]
- Clayton DF, George JM (1999) Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res 58: 120–129 [PubMed] [Google Scholar]
- Connell E, Darios F, Broersen K, Gatsby N, Peak-Chew SY, Rickman C, Davletov B (2007) Mechanism of arachidonic acid action on syntaxin-Munc18. EMBO Rep 8: 414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR (2009) Alpha-synuclein and neuronal cell death. Mol Neurodegener 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA et al. (2006) Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313: 324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios F et al. (2009) Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis. Neuron 62: 683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EJ, Terrian DM, Dorman RV (1990) Presynaptic facilitation of glutamate release from isolated hippocampal mossy fiber nerve endings by arachidonic acid. Neurochem Res 15: 743–750 [DOI] [PubMed] [Google Scholar]
- Giner D, Lopez I, Neco P, Rossetto O, Montecucco C, Gutierrez LM (2007) Glycogen synthase kinase 3 activation is essential for the snake phospholipase A2 neurotoxin-induced secretion in chromaffin cells. Eur J Neurosci 25: 2341–2348 [DOI] [PubMed] [Google Scholar]
- Golovko MY, Rosenberger TA, Faergeman NJ, Feddersen S, Cole NB, Pribill I, Berger J, Nussbaum RL, Murphy EJ (2006) Acyl-CoA synthetase activity links wild-type but not mutant alpha-synuclein to brain arachidonate metabolism. Biochemistry 45: 6956–6966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Carroll J, Fedorovich S, Rickman C, Sukhodub A, Davletov B (2002) Vesicular restriction of synaptobrevin suggests a role for calcium in membrane fusion. Nature 415: 646–650 [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG (2002) Retrograde signaling by endocannabinoids. Curr Opin Neurobiol 12: 324–330 [DOI] [PubMed] [Google Scholar]
- Kubo S, Nemani VM, Chalkley RJ, Anthony MD, Hattori N, Mizuno Y, Edwards RH, Fortin DL (2005) A combinatorial code for the interaction of alpha-synuclein with membranes. J Biol Chem 280: 31664–31672 [DOI] [PubMed] [Google Scholar]
- Lang T, Halemani ND, Rammner B (2008) Interplay between lipids and the proteinaceous membrane fusion machinery. Prog Lipid Res 47: 461–469 [DOI] [PubMed] [Google Scholar]
- Larsen KE et al. (2006) Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci 26: 11915–11922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham CF, Osborne SL, Cryle MJ, Meunier FA (2007) Arachidonic acid potentiates exocytosis and allows neuronal SNARE complex to interact with Munc18a. J Neurochem 100: 1543–1554 [DOI] [PubMed] [Google Scholar]
- Melia TJ Jr (2007) Putting the clamps on membrane fusion: how complexin sets the stage for calcium-mediated exocytosis. FEBS Lett 581: 2131–2139 [DOI] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM (2000) Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci 20: 3214–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH (2010) Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65: 66–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RJ, Woods WS, Clayton DF, George JM (2001) Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J Biol Chem 276: 41958–41962 [DOI] [PubMed] [Google Scholar]
- Rickman C, Davletov B (2005) Arachidonic acid allows SNARE complex formation in the presence of Munc18. Chem Biol 12: 545–553 [DOI] [PubMed] [Google Scholar]
- Rigoni M, Caccin P, Gschmeissner S, Koster G, Postle AD, Rossetto O, Schiavo G, Montecucco C (2005) Equivalent effects of snake PLA2 neurotoxins and lysophospholipid–fatty acid mixtures. Science 310: 1678–1680 [DOI] [PubMed] [Google Scholar]
- Rossetto O, Morbiato L, Caccin P, Rigoni M, Montecucco C (2006) Presynaptic enzymatic neurotoxins. J Neurochem 97: 1534–1545 [DOI] [PubMed] [Google Scholar]
- Sharon R, Goldberg MS, Bar-Josef I, Betensky RA, Shen J, Selkoe DJ (2001) Alpha-synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc Natl Acad Sci USA 98: 9110–9115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE (1993) A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75: 409–418 [DOI] [PubMed] [Google Scholar]
- Sousa VL, Bellani S, Giannandrea M, Yousuf M, Valtorta F, Meldolesi J, Chieregatti E (2009) α-Synuclein and its A30P mutant affect actin cytoskeletal structure and dynamics. Mol Biol Cell 20: 3725–3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht CG, Schoepfer R (2001) Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci 2: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388: 839–840 [DOI] [PubMed] [Google Scholar]
- Wenk MR, De Camilli P (2004) Protein–lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA 101: 8262–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, Tanila H, Vepsalainen S, Jakala P (2004) Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci 24: 11165–11170 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.